Abstract
Background and purpose
We investigated how features relating to pelvic cavity anatomy and tumor hemodynamic factors may influence systemic failure in rectal cancer.
Materials and methods
Rectal cancer patients (207 women, 343 men), who had been prospectively enrolled onto six cohorts and given curative-intent therapy, were analyzed for the first metastatic event. In one of the cohorts, the diameter of the inferior mesenteric vein (IMV) was assessed on diagnostic abdominal computed tomography images (n = 113). Tumor volume (n = 193) and histologic response to neoadjuvant therapy (n = 445) were recorded from diagnostic magnetic resonance images and surgical specimens, respectively.
Results
More women than men developed lung metastasis (p = 0.037), while the opposite was the case for liver metastasis (p = 0.040). Wider IMV diameter correlated with larger tumor volume (r = 0.481, p < 0.001) and male sex (p < 0.001). Female sex was the only adverse prognostic factor for lung metastasis. When sex, tumor volume, and histologic response were taken into consideration, poor tumor response remained the only determinant for liver metastasis (p = 0.002).
Conclusions
In a diverse rectal cancer population given curative-intent treatment, women and men had different outcome with regard to the primary metastatic site. Tumor hemodynamic factors should be considered in rectal cancer risk stratification.
Keywords: Rectal cancer, Metastasis, Radiotherapy, Radiology, Sex
1. Introduction
Since the introduction of total mesorectal excision as the standard surgical technique for rectal cancer, and with the addition of neoadjuvant radiation for patients with locally advanced growth in the pelvic cavity, the local relapse rate is low [1]. However, as many as 30–40% of patients progress to distant metastasis [2], [3]. The liver is the most frequently affected organ followed by the lungs [4], and metastatic disease, particularly in abdominal cavity organs, remains the main cause of severe morbidity and poor survival [5].
For primary tumors of the colon, factors that impact on treatment and outcome have been extensively studied [6], [7], [8], [9], [10]. Tumor sidedness is associated with certain somatic mutations [6], [7], and the molecular subtype of the tumor has been identified as a marker of etiology and the probability of therapy response [11], [12]. For rectal cancer, however, much less is known. An association between the tumor distance from the anal verge and the propensity for developing lung or liver metastasis has been suggested [4], [13]. While colon cancer and orally placed rectal cancer primarily metastasize to the liver, probably due to mesenteric venous drainage into the portal vein, cancer located in the lower rectum seems more prone to primarily spread to the lungs [13].
It was recently shown that the diameter of the inferior mesenteric vein (IMV), assessed on computed tomography (CT) scans from patients with locally advanced rectal cancer before and after the completion of neoadjuvant chemoradiotherapy, was a surrogate marker of the short-term local tumor response to the treatment [14]. In order to elaborate on this finding, we analyzed six prospective rectal cancer cohorts from Norway and Denmark, covering stage I–III disease, for any sex differences in metastatic outcome. In one of the cohorts with available imaging data, we looked for any association between the IMV diameter and the development of liver or lung metastasis after the curative-intent treatment. Our hypothesis was that the IMV diameter may reflect hemodynamic factors of the tumor micro- and macroenvironment, including the pelvic cavity anatomy of both females and males, decisive for the long-term disease outcome in terms of dissemination to distant organs.
2. Methods
2.1. Patient selection
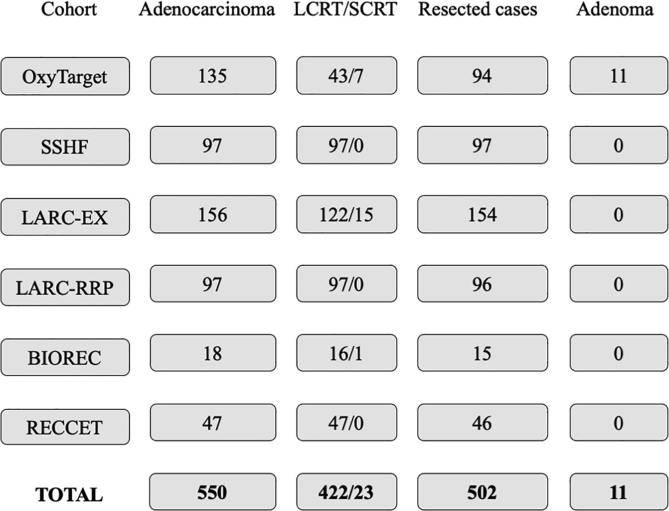
Five hundred and fifty patients with confirmed, non-metastatic rectal adenocarcinoma and 11 patients with benign rectal adenoma from six prospective studies were included in this post hoc analysis (Fig. 1, Fig. 2, Fig. 3, Supplementary Figs. 1–3). All patients had undergone rigid rectoscopy for measurement of the tumor distance from the anal verge as well as thoracic/abdominal CT and pelvic magnetic resonance imaging as a part of the diagnostic work-up. The patients were instructed to fast for 4 h prior to the CT scan. Patients who received neoadjuvant treatment had either short-course or long-course radiotherapy with or without concomitant or sequential chemotherapy. Three of the cohorts consisted of patients who had received study-specific intensified neoadjuvant treatment that included oxaliplatin (Supplementary Table 1). Patients with neoadjuvant therapy underwent pelvic surgery after its completion. The remaining individuals (n = 105) proceeded directly to surgery. Of the 550 cases, 46 were considered unresectable because of advanced age, comorbidity, or other factors related to the patient or cancer, and two patients declined surgery after neoadjuvant therapy due to personal opinions. Patient data collection and quality control were performed by the first author. The patient cohorts are detailed with demographic data and treatment regimens in the Supplementary Materials, including Supplementary Fig. 1.
Fig. 1.
The number of included patients in the various cohorts. The patient cohorts are detailed in the Supplementary Materials. Abbreviations: LCRT, long-course radiotherapy with or without concomitant or sequential chemotherapy; SCRT, short-course radiotherapy with or without sequential chemotherapy.
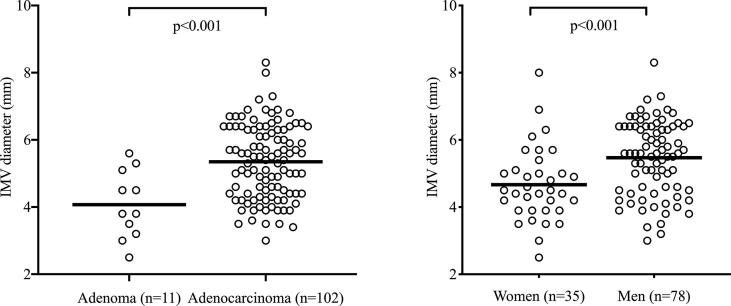
Fig. 2.
Inferior mesenteric vein (IMV) diameter in OxyTarget study patients. Lines indicate mean group values.
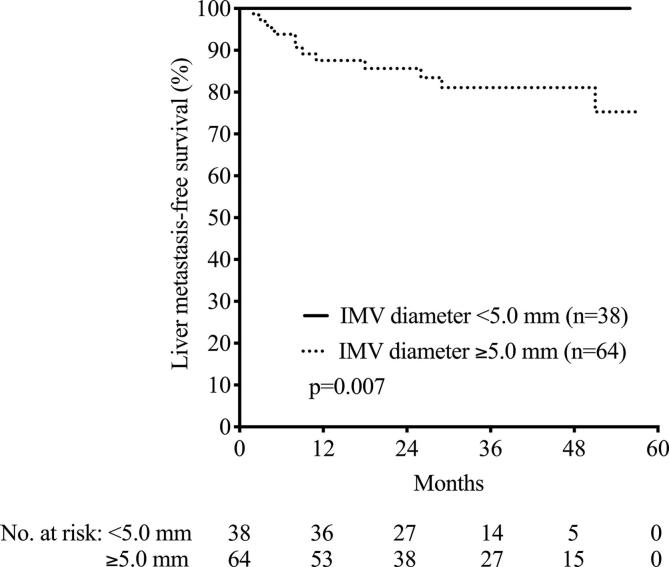
Fig. 3.
Inferior mesenteric vein (IMV) diameter and liver metastasis-free survival in OxyTarget study patients. The difference was calculated by log-rank test.
2.2. Tumor volume assessment
Tumor volume was calculated by two experienced specialists in radiology on the majority of patients (n = 193) included in two of the study cohorts (OxyTarget and LARC-RRP), as described previously [15], [16]. In brief, the tumor was manually contoured on T2-weighted magnetic resonance images. Whole-tumor volumes were then obtained by multiplying the cross-sectional tumor area in the individual slices by the sum of the slice thickness and the slice gap.
2.3. IMV diameter measurement
The portovenous phase contrast-enhanced abdominal CT images recorded at the time of diagnosis of 113 patients enrolled in the primary cohort (OxyTarget) were reviewed to measure the IMV diameter. The IMV was identified in axial images by first locating the splenic vein and thereafter recognizing the veins draining into it. The descending course of the candidate vein was tracked and when it subdivided into the paired superior rectal veins, with a distinct pattern on the CT image, it was selected as the IMV. The image was magnified and the widest cross-sectional diameter of the vein was measured. The measurements were conducted blinded to clinicopathological results after training with an experienced abdominal radiologist.
2.4. Assessment of local treatment response
Histologic tumor regression grade (TRG) after neoadjuvant therapy was assessed on the resected tumor specimens according to local protocols at the different hospitals. Patients included in cohorts until 2010 were given a TRG between 1 and 5, where 1 represented complete eradication of tumor cells and 5 no treatment effect [17]. After 2010, complete response was given TRG 0 and no treatment effect TRG 3 [18]. The Danish cohort scores spanned from complete response at TRG 1 to no treatment response at TRG 4 [19]. To enable comparisons across the cohorts with a total of 445 patients given neoadjuvant therapy, all TRGs were converted to the same scale, spanning from complete response at TRG 1 to no treatment response at TRG 3, in consultation with an experienced specialist in pathology.
2.5. Statistical considerations
Analyses were performed using IBM SPSS Statistics for Mac version 25.0 or GraphPad Prism version 8.0.1. Continuous variables were transformed to logarithmic scale to ensure normal distribution. Groups were compared by Student’s t test. Categorical variables were compared by Chi-square test. Correlations were determined by Pearson product correlation analysis. In addition, multilinear regression analysis was applied to identify the strongest correlations. Follow-up consisted of CT scans and clinical examinations three months after surgery, followed by every 6 months for two years, and then every year until five years after surgery. Global distant metastasis-free survival (DMFS) and liver- and lung metastasis-free survival, respectively, were calculated from the time of study enrollment to the date of either liver or lung metastasis, death from any cause, or end of follow-up, whichever occurred first. Overall survival (OS) was measured from the date of enrollment to death of any cause or final censoring. Associations between variables and the survival outcomes were analyzed with univariable or multivariable Cox proportional hazards models, and results were presented as hazard ratio with 95% confidence interval. Determination of optimal cutoff for IMV diameter measures according to patient outcome, was done by receiver operations characteristics with equal weight on sensitivity and specificity. All tests were two-sided. p-values of<0.05 were considered statistically significant.
3. Results
3.1. Sex
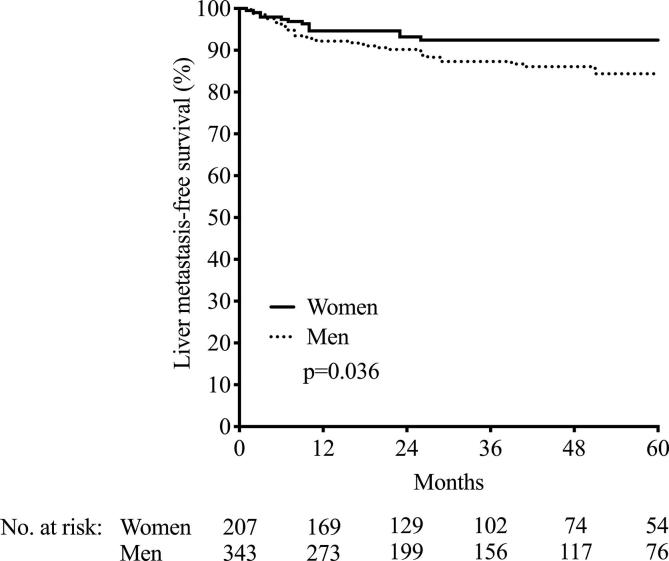
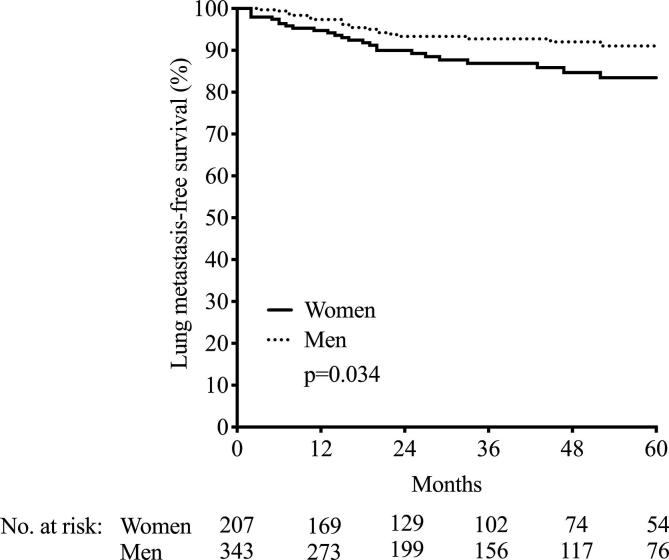
First, we analyzed the impact of female or male sex on outcome in the six cohorts of 550 rectal adenocarcinoma patients. There were no sex differences in the global DMFS or OS (p = 0.95 and p = 0.13, respectively; data not shown). Neither was there a sex difference in tumor distance from anal verge (p = 0.78; data not shown). However, men were more prone to develop liver metastasis (40 in men versus 13 in women, p = 0.040; Table 1 and visualized in Supplementary Fig. 2), while women were more likely to develop lung metastasis (27 in women versus 23 in men, p = 0.037; Table 1 and visualized in Supplementary Fig. 3). There was no sex difference in possible confounding factors such as tumor volume (p = 0.68), age (p = 0.068), or body mass index (BMI, p = 0.34), and men and women had similar time to surgery after neoadjuvant treatment (p = 0.57 for the difference).
Table 1.
Associations between patient and tumor parameters and development of liver or lung metastasis, calculated by univariable and multivariable Cox proportional hazards models. Abbreviations: BMI, body mass index; CI, confidence interval; HR, hazard ratio; IMV, inferior mesenteric vein; TRG, tumor regression grade (histologic tumor response to neoadjuvant therapy).
| Liver metastasis |
Lung metastasis |
|||||
|---|---|---|---|---|---|---|
| Univariable analysis | n | HR (95% CI) | p | n | HR (95% CI) | p |
| Sex (male as reference) | 545 | 1.93 (1.03–3.61) | 0.040 | 538 | 0.55 (0.32–0.97) | 0.037 |
| Increasing age | 545 | 0.36 (0.09–1.38) | 0.14 | 538 | 1.05 (0.22–4.94) | 0.95 |
| Increasing BMI | 225 | 2.07 (0.18–24.3) | 0.56 | 215 | 1.75 (0.09–35.0) | 0.72 |
| Increasing IMV diameter | 102 | 23.21 (1.06–507) | 0.046 | 88 | 0.13 (0.02–7.52) | 0.32 |
| Increasing tumor distance from the anal verge | 376 | 0.99 (0.95–1.04) | 0.80 | 370 | 0.99 (0.94–1.03) | 0.57 |
| Increasing tumor volume | 189 | 1.86 (1.20–2.87) | 0.005 | 170 | 0.86 (0.51–1.45) | 0.57 |
| TRG (1 as reference) | 315 | 3.69 (1.66–8.23) | 0.001 | 313 | 1.09 (0.56–2.10) | 0.80 |
| Liver metastasis |
||||||
| Multivariable analysis |
n |
HR (95% CI) |
p |
|||
| Sex (male as reference) | 121 | 2.88 (0.95–8.73) | 0.062 | |||
| Increasing tumor volume | 121 | 1.01 (0.61–1.68) | 0.75 | |||
| TRG (1 as reference) | 121 | 4.67 (1.76–12.4) | 0.002 | |||
A certain number of patients died or were lost to follow-up without occurrence of a metastatic event or available clinical data varied for the different calculations, which explains patient numbers (n) different from the total of 550. In addition, BMI, tumor distance from the anal verge, and TRG were missing for one of the cohorts, and tumor volume was determined only for selected patients in two of the cohorts. IMV diameter was determined in only one cohort.
3.2. IMV diameter
IMV diameter was wider in patients with verified adenocarcinoma (n = 102) than in those with benign adenoma (n = 11; p < 0.001) and in men compared to women (p < 0.001; Fig. 2). Wider IMV diameter correlated with larger tumor volume (r = 0.444, p < 0.001); also, wider IMV diameter was weakly associated with higher BMI (r = 0.188, p = 0.046) and longer tumor distance from the anal verge (r = 0.205, p = 0.031) but not with age (Table 2). The rectal tumor distance from the anal verge did not correlate with tumor volume (r = 0.006, p = 0.94). In multilinear regression analysis, increasing tumor volume was the only factor remaining as correlated with increasing IMV diameter (p < 0.001; Table 2). Receiver operating characteristics analysis identified IMV diameter of ≥ 5 mm for patients at risk of developing liver metastasis (area under the curve = 0.70, sensitivity = 1.00, specificity = 0.43, p = 0.030), illustrated by a significantly better liver metastasis-free survival for patients with IMV diameter of < 5 mm (p = 0.007; Fig. 3).
Table 2.
Correlations between the inferior mesenteric vein (IMV) diameter and patient and tumor parameters, calculated by Pearson’s correlation (r) analysis supplemented with multilinear regression analysis with backward elimination (the rightmost column).
| Increasing IMV diameter |
||||
|---|---|---|---|---|
| n | r | p | p | |
| Increasing age | 113 | −0.079 | 0.41 | 0.30 |
| Increasing body mass index | 113 | 0.188 | 0.046 | 0.053 |
| Increasing tumor distance from the anal verge | 111 | 0.205 | 0.031 | 0.059 |
| Increasing tumor volume | 88 | 0.444 | <0.001 | <0.001 |
3.3. Outcome
In line with previous research [20], [21], [22], [23], [24], larger tumor volume was associated with worse TRG (p < 0.001), while tumors closer to the anal verge obtained better TRG (p = 0.008); there was no association between TRG and the IMV diameter in the 41 subjects with both measures (Table 3). In the six cohorts, men presented with lower T-stage than women (p = 0.016) and as a consequence [25], men also obtained better TRG score (p = 0.008; data not shown). As shown in Table 1, male sex as well as wider IMV diameter, larger tumor volume, and TRG 2–3 were associated with development of liver metastasis (p = 0.040, p = 0.046, p = 0.005, and p = 0.001 respectively; by Cox proportional hazards analysis). Female sex was the only adverse prognostic factor for lung metastasis, with a hazard ratio almost twice as high as for men. In multivariable Cox regression, TRG 2–3 remained the only adverse prognostic factor for liver metastasis (p = 0.002), with a hazard ratio higher than 4 compared to the TRG 1 outcome. The IMV diameter was left out of the multivariable analysis due to small numbers (only measured in one cohort).
Table 3.
Associations between tumor regression grade (TRG; histologic tumor response to neoadjuvant therapy) and patient and tumor parameters, calculated by Student’s t-test. Other abbreviations: BMI, body mass index; SD, standard deviation.
| n | Mean (SD) | p | ||
|---|---|---|---|---|
| Tumor volume (ccm3) | TRG 1 | 82 | 24.47 (31.09) | |
| TRG 2–3 | 54 | 46.48 (47.82) | <0.001 | |
| Distance from the anal verge (cm) | TRG 1 | 157 | 6.21 (3.93) | |
| TRG 2–3 | 144 | 7.27 (4.50) | 0.008 | |
| IMV diameter (mm) | TRG 1 | 18 | 5.52 (0.97) | |
| TRG 2–3 | 23 | 5.58 (1.15) | 0.93 | |
| BMI (kg/m2) | TRG 1 | 91 | 25.07 (3.43) | |
| TRG 2–3 | 59 | 25.74 (4.65) | 0.45 |
4. Discussion
Building on the hypothesis that hemodynamic features related to the pelvic cavity anatomy may influence failure of rectal cancer treatment with curative intent, we explored sex differences in outcome for patients included in six prospective cohorts from Norway and Denmark. Although no differences were found for the global DMFS or OS, men were more prone to develop liver metastasis, while females more frequently developed lung metastasis as the primary distant site. This is in line with findings in a Swedish Cancer Registry report [4]. Moreover, wider IMV diameter correlated with larger tumor volume, and the IMV diameter was wider in men. Being female was the only adverse prognostic indicator for development of lung metastasis. For development of liver metastasis, on the other hand, a poor histologic tumor response to neoadjuvant therapy was the only adverse prognostic determinant when male sex and large tumor volume also were accounted for.
We postulated that the IMV diameter on diagnostic CT images might be a surrogate marker for hemodynamic differences related to female or male sex. In patients with confirmed adenocarcinoma or benign adenoma, the IMV diameter was significantly wider in individuals with cancer and in men, the latter shown previously [26]. The diameter correlated strongly with tumor volume, suggesting that a dilated IMV may reflect an increased venous return from a growing tumor. Further following the findings that wider IMV diameter was associated with large size of an invasive carcinoma, our data support the hypothesis that patients who developed liver metastasis in these cohorts had tumors that drained into the IMV, but this will need further confirmation.
The results also demonstrated that the IMV diameter had a positive, albeit weak correlation with the tumor’s distance from the anal verge, which may indicate less blood load onto the IMV for aborally placed rectal tumors. Of further note, the direct drainage into the systemic circulation, via the inferior rectal vein into the internal iliac vein and vena cava, is often perceived as an explanation of why tumors of the lower rectum often metastasize to the lungs as the primary organ [27]. The only factor directly associated with the development of lung metastasis in our cohorts was being female. The inconsistency of worse TRG result in female patients who at the same time were less prone to liver metastasis is somewhat puzzling, but could possibly be explained by more treatment-related acute organ toxicity and the resulting dose reduction in women, shown to be associated with favorable long-term outcome [28].
As the IMV may enlarge along its ascending course when receiving branches, it is plausible to expect that measurement of its diameter might partially depend on anatomical variations of branch vein drainage into the it, adding uncertainty to the results in this study. Also, the variables included in this study exhibited great variance in coverage across the cohorts; for example, the treatment regimens were not uniform, which might have affected outcome. Still, the IMV diameter is an easily obtainable marker, accessible at multiple time points during most cancer treatments, motivating further confirmation of our current findings. Further, metastatic progression occurred regardless of the variance in the treatment regimens the patients had received, supporting the theory of dissemination of tumor cells to distant organs at an early disease stage [29].
5. Conclusion
In this study, we found a possible association between sex and the primary metastatic site in rectal cancer. Histologic tumor response following neoadjuvant therapy was strongly associated with the probability of developing liver metastasis, while female sex was the only adverse prognostic marker for lung metastasis. The IMV diameter was associated with tumor volume and the development of liver metastasis. Liver and lung metastases seem to be different entities with regard to primary tumor and host biology, and should therefore be reported as separate DMFS end points in clinical studies. We also believe that various aspects related to the pelvic cavity anatomy and tumor hemodynamic factors should be further explored in terms of risk factors for distant metastasis in rectal cancer. This may be particularly important when considering the implementation of total neoadjuvant treatment in rectal cancer, including all neoadjuvant modalities to reduce the risk of metastatic dissemination [30].
6. Role of funding sources
The work presented in this paper has been funded by the South-Eastern Norwegian Health Authority with grants no. 2015048 (KRR), 2015033, 2017109, 2018054 (AHR). We are thankful for the contribution to our research, and guarantee that the funding sources had no part in the process leading to the writing of this paper.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2019.11.006.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
References
- 1.Aklilu M., Eng C. The current landscape of locally advanced rectal cancer. Nat Rev Clin Oncol. 2011;8(11):649–659. doi: 10.1038/nrclinonc.2011.118. Epub 2011/08/10. PubMed PMID: 21826084. [DOI] [PubMed] [Google Scholar]
- 2.Bosset J.F., Calais G., Mineur L., Maingon P., Stojanovic-Rundic S., Bensadoun R.J. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–190. doi: 10.1016/S1470-2045(13)70599-0. Epub 2014/01/21. PubMed PMID: 24440473. [DOI] [PubMed] [Google Scholar]
- 3.Rodel C., Graeven U., Fietkau R., Hohenberger W., Hothorn T., Arnold D. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2015;16(8):979–989. doi: 10.1016/S1470-2045(15)00159-X. Epub 2015/07/21. PubMed PMID: 26189067. [DOI] [PubMed] [Google Scholar]
- 4.Riihimaki M., Hemminki A., Sundquist J., Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765. doi: 10.1038/srep29765. PubMed PMID: 27416752; PubMed Central PMCID: PMCPMC4945942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadden W.J., de Reuver P.R., Brown K., Mittal A., Samra J.S., Hugh T.J. Resection of colorectal liver metastases and extra-hepatic disease: a systematic review and proportional meta-analysis of survival outcomes. HPB (Oxford) 2016;18(3):209–220. doi: 10.1016/j.hpb.2015.12.004. Epub 2016/03/29. PubMed PMID: 27017160; PubMed Central PMCID: PMCPMC4814625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aljehani M.A., Morgan J.W., Guthrie L.A. Association of primary tumor site with mortality in patients receiving bevacizumab and cetuximab for metastatic colorectal cancer. JAMA Surg. 2017 doi: 10.1001/jamasurg.2017.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karim S., Brennan K., Nanji S., Berry S.R., Booth C.M. Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.1016. PubMed PMID: 28594974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrelli F., Tomasello G., Borgonovo K., Ghidini M., Turati L., Dallera P. Prognostic survival associated with left-sided vs right-sided colon cancer: a systematic review and meta-analysis. JAMA Oncol. 2016 doi: 10.1001/jamaoncol.2016.4227. Epub 2016/10/28. PubMed PMID: 27787550. [DOI] [PubMed] [Google Scholar]
- 9.Taieb J., Kourie H.R., Emile J.F., Le Malicot K., Balogoun R., Tabernero J. Association of prognostic value of primary tumor location in stage III colon cancer with RAS and BRAF mutational status. JAMA Oncol. 2017 doi: 10.1001/jamaoncol.2017.3695. Epub 2017/11/24. PubMed PMID: 29167892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tejpar S., Stintzing S., Ciardiello F., Tabernero J., Van Cutsem E., Beier F. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2016;3(2):194–201. doi: 10.1001/jamaoncol.2016.3797. PubMed PMID: 27722750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guinney J., Dienstmann R., Wang X., de Reynies A., Schlicker A., Soneson C. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21(11):1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loree J.M., Pereira A.A.L., Lam M., Willauer A.N., Raghav K., Dasari A. Classifying colorectal cancer by tumor location rather than sidedness highlights a continuum in mutation profiles and consensus molecular subtypes. Clin Cancer Res. 2018;24(5):1062–1072. doi: 10.1158/1078-0432.CCR-17-2484. Epub 2017/11/29. PubMed PMID: 29180604; PubMed Central PMCID: PMCPMC5844818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augestad K.M., Keller D.S., Bakaki P.M., Rose J., Koroukian S.M., Oresland T. The impact of rectal cancer tumor height on recurrence rates and metastatic location: a competing risk analysis of a national database. Cancer Epidemiol. 2018;53:56–64. doi: 10.1016/j.canep.2018.01.009. Epub 2018/02/08. PubMed PMID: 29414633. [DOI] [PubMed] [Google Scholar]
- 14.Ivan C.V., Mullineux J.H., Verma R., Shah V., De A., Elabassy M. Assessment of the inferior mesenteric vein diameter as a surrogate marker to evaluate response to neoadjuvant chemoradiotherapy for locally advanced rectal adenocarcinoma. Colorectal Dis. 2017;19(12):1076–1080. doi: 10.1111/codi.13811. Epub 2017/07/12. PubMed PMID: 28696522. [DOI] [PubMed] [Google Scholar]
- 15.Bakke K.M., Hole K.H., Dueland S., Groholt K.K., Flatmark K., Ree A.H. Diffusion-weighted magnetic resonance imaging of rectal cancer: tumour volume and perfusion fraction predict chemoradiotherapy response and survival. Acta Oncol. 2017;56(6):813–818. doi: 10.1080/0284186X.2017.1287951. PubMed PMID: 28464745. [DOI] [PubMed] [Google Scholar]
- 16.Seierstad T., Hole K.H., Groholt K.K., Dueland S., Ree A.H., Flatmark K. MRI volumetry for prediction of tumour response to neoadjuvant chemotherapy followed by chemoradiotherapy in locally advanced rectal cancer. Br J Radiol. 2015;88(1051):20150097. doi: 10.1259/bjr.20150097. Epub 2015/04/23. PubMed PMID: 25899892; PubMed Central PMCID: PMCPmc4628535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bouzourene H., Bosman F.T., Seelentag W., Matter M., Coucke P. Importance of tumor regression assessment in predicting the outcome in patients with locally advanced rectal carcinoma who are treated with preoperative radiotherapy. Cancer. 2002;94(4):1121–1130. Epub 2002/03/29. PubMed PMID: 11920483. [PubMed] [Google Scholar]
- 18.Bateman A.C., Jaynes E., Bateman A.R. Rectal cancer staging post neoadjuvant therapy–how should the changes be assessed? Histopathology. 2009;54(6):713–721. doi: 10.1111/j.1365-2559.2009.03292.x. Epub 2009/05/15. PubMed PMID: 19438746. [DOI] [PubMed] [Google Scholar]
- 19.Mandard A.-M., Dalibard F., Mandard J.-C., Marnay J., Henry-Amar M., Petiot J.-F. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73(11):2680–2686. doi: 10.1002/1097-0142(19940601)73:11<2680::aid-cncr2820731105>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 20.Garland M.L., Vather R., Bunkley N., Pearse M., Bissett I.P. Clinical tumour size and nodal status predict pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer. Int J Colorectal Dis. 2014;29(3):301–307. doi: 10.1007/s00384-013-1821-7. Epub 2014/01/15. PubMed PMID: 24420737. [DOI] [PubMed] [Google Scholar]
- 21.Bitterman D.S., Resende Salgado L., Moore H.G., Sanfilippo N.J., Gu P., Hatzaras I. Predictors of complete response and disease recurrence following chemoradiation for rectal cancer. Front Oncol. 2015;5:286. doi: 10.3389/fonc.2015.00286. Epub 2016/01/07. PubMed PMID: 26734570; PubMed Central PMCID: PMCPMC4686647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Probst C.P., Becerra A.Z., Aquina C.T., Tejani M.A., Wexner S.D., Garcia-Aguilar J. Extended Intervals after neoadjuvant therapy in locally advanced rectal cancer: the key to improved tumor response and potential organ preservation. J Am Coll Surg. 2015;221(2):430–440. doi: 10.1016/j.jamcollsurg.2015.04.010. Epub 2015/07/25. PubMed PMID: 26206642; PubMed Central PMCID: PMCPMC5014360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Zhang Z., Jiang G., Zhao K. Gross tumor volume is the prognostic factor for squamous cell esophageal cancer patients treated with definitive radiotherapy. J Thorac Dis. 2016;8(6):1155–1161. doi: 10.21037/jtd.2016.04.08. Epub 2016/06/14. PubMed PMID: 27293832; PubMed Central PMCID: PMCPMC4886004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Felice F., Izzo L., Musio D., Magnante A.L., Bulzonetti N., Pugliese F. Clinical predictive factors of pathologic complete response in locally advanced rectal cancer. Oncotarget. 2016;7(22):33374–33380. doi: 10.18632/oncotarget.8133. Epub 2016/03/19. PubMed PMID: 26992214; PubMed Central PMCID: PMCPMC5078102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maas M., Nelemans P.J., Valentini V., Das P., Rodel C., Kuo L.J. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11(9):835–844. doi: 10.1016/S1470-2045(10)70172-8. Epub 2010/08/10. PubMed PMID: 20692872. [DOI] [PubMed] [Google Scholar]
- 26.Wu C.C., Lee R.C., Chang C.Y. Prediction of lymphovascular invasion in rectal cancer by preoperative CT. AJR Am J Roentgenol. 2013;201(5):985–992. doi: 10.2214/AJR.12.9657. Epub 2013/10/24. PubMed PMID: 24147468. [DOI] [PubMed] [Google Scholar]
- 27.Sakorafas G.H., Zouros E., Peros G. Applied vascular anatomy of the colon and rectum: clinical implications for the surgical oncologist. Surg Oncol. 2006;15(4):243–255. doi: 10.1016/j.suronc.2007.03.002. Epub 2007/05/29. PubMed PMID: 17531744. [DOI] [PubMed] [Google Scholar]
- 28.Wolff H.A., Conradi L.-C., Beissbarth T., Leha A., Hohenberger W., Merkel S. Gender affects acute organ toxicity during radiochemotherapy for rectal cancer: Long-term results of the German CAO/ARO/AIO-94 phase III trial. Radiother Oncol. 2013;108(1):48–54. doi: 10.1016/j.radonc.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Hu Z., Ding J., Ma Z., Sun R., Seoane J.A., Scott Shaffer J. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat Genet. 2019 doi: 10.1038/s41588-019-0423-x. Epub 2019/06/19. PubMed PMID: 31209394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fokas E., Allgauer M., Polat B., Klautke G., Grabenbauer G.G., Fietkau R. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019:Jco1900308. doi: 10.1200/JCO.19.00308. Epub 2019/06/01. PubMed PMID: 31150315. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.