Summary
Growing evidence indicates a close link between energy metabolism and neural plasticity as obesity is associated with alterations of cognitive functions, memory, and hippocampal neurogenesis. However, it is still unknown whether obesity can affect low-level sensory plasticity. Here we investigated this issue by probing early visual plasticity induced by short-term (2 h) monocular deprivation in a group of adult volunteers with a wide range of Body Mass Index (BMI), from normal weight to morbid obesity. We found that the effect of monocular deprivation decreased with increasing BMI, and morbidly obese subjects (BMI>40) failed to show the homeostatic plasticity effect seen in normal-weight participants. In addition, morbidly obese subjects exhibited altered binocular rivalry dynamics compared with normal-weight observers. These results show for the first time that the impact of obesity observed at the neural and cognitive level extends to basic sensory processing and plasticity.
Subject Areas: Physiological State, Biological Sciences, Neuroscience, Sensory Neuroscience
Graphical Abstract
Highlights
-
•
Cognitive decline occurs in obesity suggesting altered high-level brain plasticity
-
•
Low-level sensory plasticity in adults declines with increasing body mass index
-
•
Morbidly obese subjects show altered visual plasticity and interocular interactions
Physiological State; Biological Sciences; Neuroscience; Sensory Neuroscience
Introduction
Obesity is one of the recognized worldwide health issues, with an increasing prevalence in both the developed and developing countries (Di Cesare et al., 2016). Besides weight gain and metabolic diseases, obesity is also associated with deficits in high-level brain processing and function (for review Guillemot-Legris and Muccioli, 2017). This suggests an interplay between peripheral metabolism, high-level brain functions, and hippocampal plasticity. However, it is still unknown whether obesity might affect lower-level sensory functions and plasticity. To address this issue, here we investigate the degree of short-term visual cortex plasticity in a group of healthy adult individuals belonging to different body weight categories, ranging from normal weight to morbid obesity.
Similar to previous studies on short-term visual plasticity, we quantified sensory eye dominance by means of binocular rivalry, a form of bistability that occurs when the two retinae are presented with dissimilar visual stimuli (Alais and Blake, 2005, Levelt, 1965). Normally, eye dominance is relatively balanced between the two eyes; however, after short-term (2 h to 2 h 30 min) monocular deprivation, eye dominance dramatically shifts in favor of the deprived eye (Lunghi et al., 2011). The deprivation effect usually decays within 1 h after eye-patch removal (but see Lunghi et al., 2013) and is thought to reflect a form of homeostatic plasticity. Homeostatic plasticity has been observed during development in animal models (Turrigiano and Nelson, 2004, Mrsic-Flogel et al., 2007, Maffei et al., 2010, Turrigiano, 2012) and in children during the critical period for binocularity (Lunghi et al., 2016) and is partially preserved in adult age (for a detailed discussion see Binda et al., 2018). It has been shown that this form of short-term plasticity is accompanied by transient changes of neuronal excitability in V1 (Begum and Ts’o, 2016, Binda et al., 2018, Lunghi et al., 2015a) and by a decrease of GABA measured by MR spectroscopy (Lunghi et al., 2015b). Thus, the demonstration of a change of homeostatic plasticity in obesity may provide strong evidence of the link between energy metabolism and basic sensory brain mechanisms.
Results
We measured the effect of short-term (2 h) monocular deprivation on sensory eye dominance by binocular rivalry on a group (N = 53) of adult healthy individuals with a wide range of body weight, from normal weight to morbid obesity. To avoid other potential factors that may also affect neuronal plasticity, we have included in the study only subjects with normal glucose tolerance. We computed the Body Mass Index (BMI) using the standard equation described in Equation 1 in the Transparent Methods section (Table 1). The ocular dominance plasticity was evaluated by the difference between sensory eye dominance (see Equation 2 in the Transparent Methods section) measured after and before 2 h of monocular deprivation (Figure 1A).
Table 1.
Subjects Demographics and Body Mass Index (BMI) Values
| Subject ID | Gender | Age (years) | BMI |
|---|---|---|---|
| S1 | F | 41 | 41.27 |
| S2 | F | 41 | 51.03 |
| S3 | F | 33 | 48.12 |
| S4 | F | 55 | 48.44 |
| S5 | F | 48 | 39.26 |
| S6 | F | 31 | 36.48 |
| S7 | F | 38 | 37.64 |
| S10 | F | 27 | 50.07 |
| S11 | M | 43 | 52.85 |
| S12 | F | 25 | 46.3 |
| S13 | F | 29 | 31.6 |
| S14 | F | 38 | 36.3 |
| S15 | M | 25 | 50.52 |
| S16 | F | 48 | 39 |
| S17 | F | 49 | 40.5 |
| S18 | F | 27 | 47.89 |
| S19 | F | 55 | 40.86 |
| S20 | M | 48 | 53.49 |
| S21 | F | 38 | 30.94 |
| S22 | M | 53 | 26.78 |
| S23 | M | 44 | 24.07 |
| C1 | F | 25 | 22.52 |
| C2 | F | 31 | 21.875 |
| C3 | F | 23 | 18.81 |
| C4 | F | 24 | 20.02 |
| C5 | M | 20 | 22.15 |
| C6 | F | 43 | 19.53 |
| C7 | M | 21 | 25.53 |
| C8 | M | 22 | 19.37 |
| C9 | F | 21 | 20.06 |
| C10 | F | 20 | 18.81 |
| C11 | F | 20 | 24.89 |
| C12 | M | 29 | 22.79 |
| C13 | F | 20 | 19.13 |
| C14 | M | 19 | 21.43 |
| C15 | F | 20 | 19.19 |
| C16 | F | 20 | 18.75 |
| C17 | M | 21 | 23.45 |
| C18 | F | 20 | 25.7 |
| S24 | F | 45 | 45.33 |
| S25 | F | 53 | 41.37 |
| S26 | F | 53 | 31.59 |
| S27 | F | 50 | 32.51 |
| S28 | F | 45 | 45.17 |
| S29 | F | 22 | 31.34 |
| S30 | F | 46 | 39.33 |
| S31 | M | 47 | 42.24 |
| S32 | F | 48 | 35.57 |
| S33 | M | 41 | 37.35 |
| S34 | F | 26 | 45.36 |
| S35 | M | 20 | 38.77 |
| S36 | F | 52 | 32.62 |
| S37 | M | 36 | 38.6 |
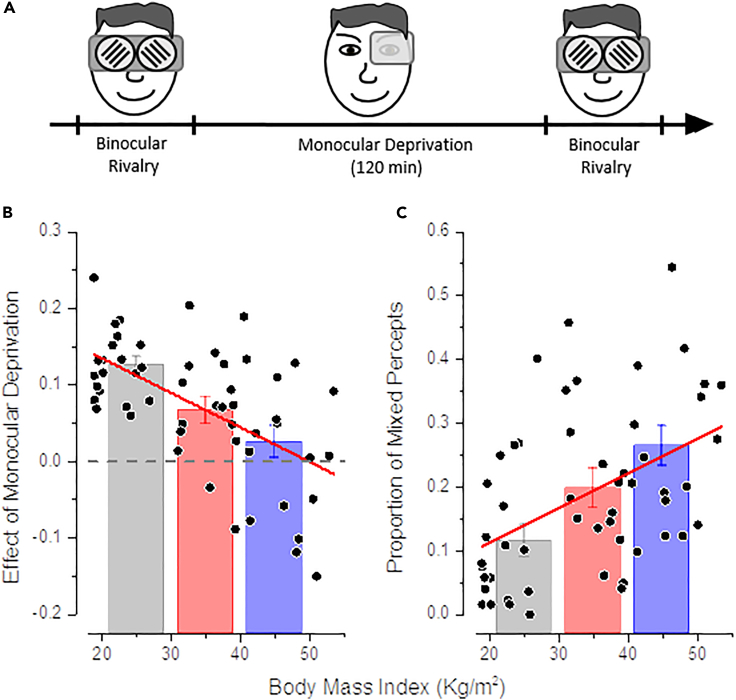
Figure 1.
Short-term Monocular Deprivation Effect and Binocular Rivalry Dynamics as a Function of BMI
(A) Diagram of the experimental paradigm.
(B) The effect of monocular deprivation (difference between ocular dominance measured after and before 2 h of monocular deprivation) plotted as a function of subjects' Body Mass Index (BMI). The bars represent the average effect for three different BMI categories (gray, normal to overweight; red, obese class I and II; blue, obese class III), error bars represent 1 ± SEM. Black symbols represent single subjects' data.
(C) Same as (B) but for the proportion of the total time of mixed percepts measured during binocular rivalry before monocular deprivation.
The plasticity effect of monocular deprivation reduced significantly with increasing BMI (Figure 1B), as revealed by a univariate ANOVA (F(2,50) = 10.27, p < 0.001, η2 = 0.29) and confirmed by the strong correlation across subjects between the plasticity effect and BMI (Pearson's r = -0.58 [95% confidence interval (CI), −0.74 to −0.37], p < 0.001). When grouping the subjects in three categories according to their BMI, in normal to overweight subjects (group 1) we observed a significant shift of ocular dominance in favor of the deprived eye after deprivation (deprivation effect [mean ± SD] = 0.12 ± 0.05, t(19) = 11.6, p < 0.001), in line with previous reports (Binda and Lunghi, 2017, Lunghi et al., 2013, Lunghi et al., 2011). In subjects with moderate to severe obesity (group 2), the effect of deprivation was significantly smaller (t(34) = 3, p = 0.03) but still statistically different from 0 (mean ± SD = 0.07 ± 0.07, t(15) = 3.81, p = 0.01), whereas in morbidly obese subjects (group 3) sensory eye dominance did not change after monocular deprivation (mean ± SD = 0.02 ± 0.08, t(16) = 1.21, p = 0.99). The plasticity effect was significantly different between group 1 (normal to overweight) and group 3 (morbidly obese, t(35) = 4.46, p < 0.001), whereas there was no statistical difference between the two groups of obese patients (group 2 and 3, t(32) = 1.5, p = 0.86), indicating altered short-term visual plasticity in obese subjects.
A complementary measure of inter-ocular suppression is given by the proportion of time in which fusion of the two monocular images is perceived, usually referred to as mixed percepts. Strong fusion of inconsistent images is associated with weak inter-ocular inhibition (Klink et al., 2010, Robertson et al., 2015, Said and Heeger, 2013). We found that the proportion of mixed percepts remained similar before and after deprivation in both group 1 and group 2, whereas it showed a reduction in morbidly obese subjects (group 3, paired-samples t test, t(16) = 5.99, p < 0.001). Importantly, we also found that the proportion of mixed percepts before deprivation is related to and increases with increasing BMI (Figure 1C). This was revealed by a univariate ANOVA (F(2,50) = 7.47, p = 0.001, η2 = 0.23) and confirmed by the correlation across subjects between the proportion of mixed percepts and BMI (Pearson's r = 0.45 [95% CI, 0.21 to 0.64], p = 0.004). Post hoc tests showed that the proportion of mixed percepts was significantly larger in morbidly obese subjects (group 3) compared with normal to overweight subjects (t(35) = 3.86, p < 0.001), whereas there was no difference in mixed percepts proportion between moderately to severely obese subjects (group2) and either group 1 (t(34) = 2.16, p = 0.11) or group 3 (t(32) = 1.5, p = 0.39). Similar results were observed for the mean duration of mixed percepts that varied from 1.81 ± 0.24 s in normal-weight subjects to 2.93 ± 0.41 s in morbidly obese subjects, suggesting that the mean duration of mix percept varied with BMI (univariate ANOVA F(2,50) = 3.89, p = 0.027, η2 = 0.14, correlation Pearson's r = 0.27 [95% CI, −0.01 to 0.5], p = 0.05).
No difference across body weight categories was observed for the other main parameter of binocular rivalry dynamics: mean phase durations were comparable across groups (univariate ANOVA [F(2,50) = 0.85, p = 0.44, η2 = 0.03], group1 mean ± SD = 3.5 ± 1.4, group2 mean ± SD = 4.2 ± 1.5, group3 mean ± SD = 3.8 ± 1.5). We also found a correlation between BMI and age (Pearson's r = 0.49 [95% CI, 0.25 to 0.67], p = 0.001, Bayes Factor [BF] = 94.1), as obese subjects were on average older than normal to overweight subjects. However, age did not correlate significantly with the effect of monocular deprivation (Pearson's r = −0.3 [95% CI, −0.53 to −0.08], p = 0.017, BF = 1.19). To disentangle the contribution of different factors (BMI, proportion of mix percepts and age) to the observed plasticity effect, we performed a multiple linear regression analysis using the three factors mentioned earlier as predictors of the deprivation effect. The analysis revealed that only the factor BMI was a significant predictor of the plasticity effect (t = −3.8, p < 0.001); both the factor proportion of mixed percepts (t = −1.9, p = 0.064) and the factor age (t = 0.17, p = 0.87) had no significant predictive value, implying that the apparently negative correlation between the effect of deprivation and the proportion of mixed percepts is a by-product of the correlation between both variables and BMI.
We asked whether the lack of short-term plasticity in morbidly obese subjects could result from a different dynamics of the effect—perhaps a faster decay. Figure 2 shows the decay of the plasticity effect (shift in ocular dominance) over the four experimental blocks acquired immediately after eye-patch removal. Ever since the first time point after eye-patch removal, morbidly obese subjects fail to show the ocular dominance shift, suggesting a total lack of effect after 2 h of monocular deprivation. A mixed model ANOVA (within-subjects factor TIME and the between subjects factor BMI GROUP) showed that there was no significant difference in the decay of the effect of deprivation: the interaction between the factors TIME and BMI GROUP was not significant (F(6,147) = 0.45, p = 0.85, η2 = 0.02).
Figure 2.
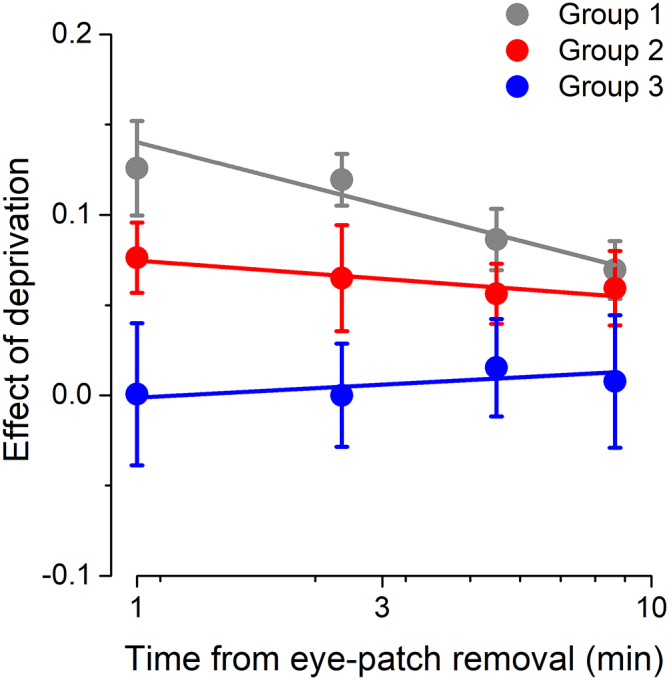
Decay of the Effect of Monocular Deprivation across Different BMI Groups
The effect of monocular deprivation is plotted for each of the four experimental blocks acquired after eye-patch removal and for the three different BMI groups (black symbols, normal weight to overweight subjects; red symbols, class I and class II obese subjects; blue symbols, class III obese subjects). Error bars represent 1 ± SEM.
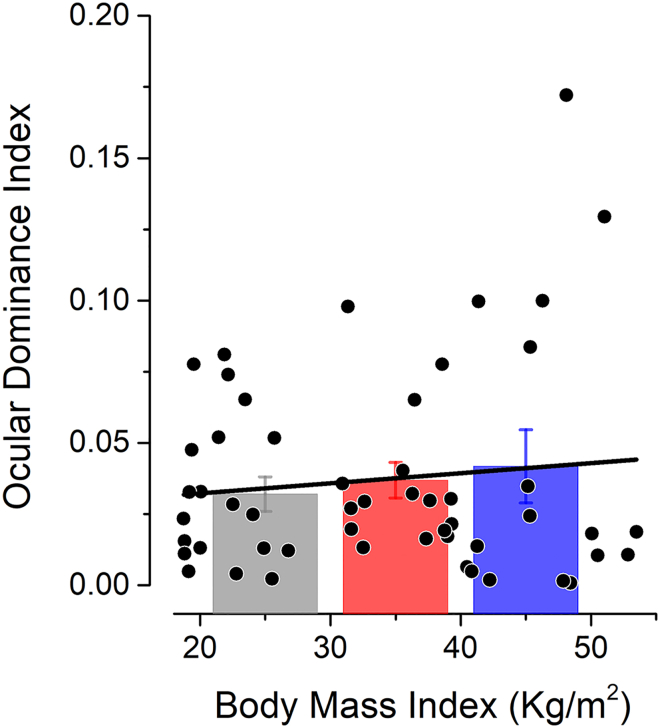
Finally, the effect of monocular deprivation might be masked by strong natural ocular dominance. This potential confound is particularly important since we always deprived the dominant eye, which gives the largest deprivation effects in typical subject (Lunghi et al., 2011). However, we found that natural ocular dominance was not systematically different in morbidly obese subjects (Figure 3). The ocular dominance index measured before deprivation did not differ across BMI groups (univariate ANOVA, F(2,50) = 0.34, p = 0.71, η2 = 0.01), nor did it correlate with BMI across subjects (Pearson's r = 0.12, p = 0.43).
Figure 3.
Ocular Dominance as a Function of BMI
The ocular dominance index measured before deprivation is plotted as a function of subjects' BMI. Individual subjects are represented by black symbols. Average ocular dominance values for each BMI group are represented by the bars (same color code as in Figure 2). Error bars represent 1 ± SEM.
Discussion
We found that the degree of residual homeostatic visual plasticity and binocular rivalry dynamics were altered in morbidly obese adult subjects. Both homeostatic plasticity (Binda et al., 2018, Lunghi et al., 2015a) and binocular rivalry (Lee et al., 2007, Leopold and Logothetis, 1996) occur at the earliest stages of visual processing, at the level of the primary visual cortex. Interestingly, homeostatic plasticity (Lunghi et al., 2015b) and binocular rivalry dynamics (Mentch et al., 2019, Robertson et al., 2015, van Loon et al., 2013) are both mediated by GABAergic inhibition, suggesting that intracortical excitation/inhibition balance might be altered in obese individuals in favor of a stronger GABAergic inhibition.
Experiments on animal models demonstrated that homeostatic plasticity is a particular form of experience-dependent plasticity that operates during the critical period to shape organization of binocular mechanisms in primary visual cortex (Turrigiano and Nelson, 2004). Both homeostatic plasticity (Maffei et al., 2010) and long-term plasticity (Fagiolini and Hensch, 2000), mainly relying on Hebbian cellular mechanisms (Cooke and Bear, 2014), depend on the net excitation/inhibition ratio in the visual cortex, which changes dramatically during the critical period for visual development. Thus, in spite of being different mechanisms (for discussion see Binda et al., 2018), these two forms of plasticity may be expected to co-vary in many pathological conditions that modify the excitatory/inhibitory homeostasis. Interestingly, an example of correlation between the two forms of plasticity has been observed in amblyopic children, where homeostatic plasticity is a predictive measure of the recovery of visual acuity in the amblyopic eye after standard occlusion therapy (Lunghi et al., 2016). Another example of interaction between the two forms of plasticity is provided by the long-term recovery of visual acuity in adult amblyopic subject after inducing repetitively homeostatic plasticity (Lunghi et al., 2019, Zhou et al., 2019). So, although the effect reported here is transient, a large corpus of the literature on animal models and humans suggests that homeostatic plasticity may be an important biomarker of the brain's ability to respond to environmental changes (Turrigiano, 2017).
Here we find that, morbidly obese subjects not only fail to show homeostatic plasticity for ocular dominance, but also have a different dynamics of binocular rivalry. The period of fusion of the two inconsistent monocular stimuli is longer than in normal-weight subjects, suggesting a decrease of interocular inhibition (Klink et al., 2010, Robertson et al., 2015, Said and Heeger, 2013). At first sight, this result seems to contradict the conclusion of increased GABAergic inhibition in obese patients, based on the lack of homeostatic ocular dominance shift after deprivation. However, a recent study (Mentch et al., 2019) showed that the duration of mixed percepts and that of exclusive dominance during binocular rivalry are modulated differently by GABA-A and GABA-B agonists, suggesting that the two measures may reflect independent inhibitory mechanisms that are both atypical in morbidly obese subjects. In addition, this population shows an atypical reduction of mixed percept after deprivation, not observed in normal-weight subjects, and in contrast with the result of a recent study (Sheynin et al., 2019). These authors classified mixed percept on a finer scale, estimating separately the mixed percepts with a relative dominance for either eye. They found that the balanced mixed percepts showed no change, consistently with our results in normal-weight individuals. Overall, morbidly obese individuals have alterations of three properties of binocular rivalry (baseline mixed percepts, the deprivation effect on ocular dominance, and its effect on mixed percepts), implicating abnormal interocular interactions.
Growing evidence indicates that obesity is not only associated with metabolic diseases, but also affects high-level brain processing and function. Morbidly obese subjects are more liable to develop neurodegenerative diseases such as Alzheimer and Parkinson disease (Mazon et al., 2017). At the functional level, it has been shown that obese individuals have impaired cognitive and executive functions, reduced spatial and recognition memory, impaired spatial learning and altered temporal integration (Scarpina et al., 2016). At the neural level, obesity is associated with severe neuroinflammation, reduced gray and white matter volume, damage in the hippocampus (reduced volume and hippocampal neurogenesis), structural changes in the frontal and temporal lobe, and altered connectivity and dopamine release in the neural circuits related to reward processing (reviewed in Guillemot-Legris and Muccioli, 2017, Matikainen-Ankney and Kravitz, 2018, Stice and Burger, 2019). Our results suggest that similar mechanisms might mediate ocular dominance plasticity. Interestingly, it has been shown that neuroinflammation induced by obesity can be mended by physical exercise. We have recently reported that visual homeostatic plasticity can be boosted by physical exercise both in normal sighted (Lunghi and Sale, 2015) and in amblyopic subjects (Lunghi et al., 2019). Therefore, we hypothesize that reduced physical activity can contribute to the loss of homeostatic plasticity in obese individuals, although it may be difficult to disentangle the two factors. However, both explanations strongly point to a role of body metabolism in mediating visual plasticity.
Our results indicate that both early sensory plasticity and visual processing are altered in non-diabetic obese individuals, showing for the first time that the deficits observed both at the neural and functional level for higher cognitive functions extend to basic sensory processing.
Limitations of the Study
One limitation of the current study is the relatively small sample size due to the difficulty of recruiting morbidly obese individuals without associated metabolic conditions.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This research was funded by the European projects ERA-NET Neuro-DREAM, ECSPLAIN (European Research Council, Seventh Framework Program, FPT/2007–2013, n.338866), and PUPILTRAITS (European Research Council, Horizon 2020 Research and Innovation Program, n.801715) and the Italian Ministry of University and Research under the project “PRIN 2015”and funded by University of Pisa under the PRA 2015 “Progetto di Ricerca di Ateneo”.
Author Contributions
C.L., G.D., M.C.M., and S.D.P. designed the experiment; G.D., A.D., G.C., and F.S. recruited the patients and performed clinical examinations; C.L. and P.B. collected and analyzed the data; C.L., G.D., P.B., S.D.P., and M.C.M. discussed the results and wrote the paper.
Declaration of Interests
The authors declare no competing interests.
Published: December 20, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.11.027.
Supplemental Information
References
- Alais D., Blake R. MIT Press; 2005. Binocular Rivalry. [Google Scholar]
- Begum, M., Ts’o, D.., 2016. Shifts in interocular balance resulting from short-term monocular deprivation in adult macaque visual cortex are not magno-dominated, in: Vision Sciences Society Annual Meeting. Journal of Vision, St. Pete’s Beach, FL, p. 1328.
- Binda P., Kurzawski J.W., Lunghi C., Biagi L., Tosetti M., Morrone M.C. Response to short-term deprivation of the human adult visual cortex measured with 7T BOLD. Elife. 2018;7 doi: 10.7554/eLife.40014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binda P., Lunghi C. Short-term monocular deprivation enhances physiological pupillary oscillations. Neural Plast. 2017;13 doi: 10.1155/2017/6724631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke S.F., Bear M.F. How the mechanisms of long-term synaptic potentiation and depression serve experience-dependent plasticity in primary visual cortex. Philos. Trans. R. Soc. B Biol. Sci. 2014;369 doi: 10.1098/rstb.2013.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cesare M., Bentham J., Stevens G.A., Zhou B., Danaei G., Lu Y., Bixby H., Cowan M.J., Riley L.M., Hajifathalian K. Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagiolini M., Hensch T.K. Inhibitory threshold for critical-period activation in primary visual cortex. Nature. 2000;404:183–186. doi: 10.1038/35004582. [DOI] [PubMed] [Google Scholar]
- Guillemot-Legris O., Muccioli G.G. Obesity-induced neuroinflammation: beyond the hypothalamus. Trends Neurosci. 2017;40:237–253. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- Klink P.C., Brascamp J.W., Blake R., van Wezel R.J.A. Experience-driven plasticity in binocular vision. Curr. Biol. 2010;20:1464–1469. doi: 10.1016/j.cub.2010.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.-H.H., Blake R., Heeger D.J. Hierarchy of cortical responses underlying binocular rivalry. Nat. Neurosci. 2007;10:1048–1054. doi: 10.1038/nn1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold D.A., Logothetis N.K. Activity changes in early visual cortex reflect monkeys’ percepts during binocular rivalry. Nature. 1996;379:549–553. doi: 10.1038/379549a0. [DOI] [PubMed] [Google Scholar]
- Levelt W.J.M. Institution for Perception, Soesterberg; 1965. On Binocular Rivalry. [Google Scholar]
- Lunghi C., Berchicci M., Morrone M.C., Di Russo F. Short-term monocular deprivation alters early components of visual evoked potentials. J. Physiol. 2015;593:4361–4372. doi: 10.1113/JP270950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., Burr D.C., Morrone C. Brief periods of monocular deprivation disrupt ocular balance in human adult visual cortex. Curr. Biol. 2011;21:R538–R539. doi: 10.1016/j.cub.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Lunghi C., Burr D.C., Morrone M.C. Long-term effects of monocular deprivation revealed with binocular rivalry gratings modulated in luminance and in color. J. Vis. 2013;13 doi: 10.1167/13.6.1. [DOI] [PubMed] [Google Scholar]
- Lunghi C., Emir U.E., Morrone M.C., Bridge H. Short-term monocular deprivation alters GABA in the adult human visual cortex. Curr. Biol. 2015;25:1496–1501. doi: 10.1016/j.cub.2015.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., Morrone M.C., Secci J., Caputo R. Binocular rivalry measured 2 hours after occlusion therapy predicts the recovery rate of the amblyopic eye in anisometropic children. Invest. Ophthalmol. Vis. Sci. 2016;57:1537–1546. doi: 10.1167/iovs.15-18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., Sale A. A cycling lane for brain rewiring. Curr. Biol. 2015;25:R1122–R1123. doi: 10.1016/j.cub.2015.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunghi C., Sframeli A.T., Lepri A., Lepri M., Lisi D., Sale A., Morrone M.C. A new counterintuitive training for adult amblyopia. Ann. Clin. Transl. Neurol. 2019;6:274–284. doi: 10.1002/acn3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A., Lambo M.E., Turrigiano G.G. Critical period for inhibitory plasticity in rodent binocular V1. J. Neurosci. 2010;30:3304–3309. doi: 10.1523/JNEUROSCI.5340-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matikainen-Ankney B.A., Kravitz A.V. Persistent effects of obesity: a neuroplasticity hypothesis. Ann. N. Y. Acad. Sci. 2018;1428:221–239. doi: 10.1111/nyas.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazon J.N., de Mello A.H., Ferreira G.K., Rezin G.T. The impact of obesity on neurodegenerative diseases. Life Sci. 2017;182:22–28. doi: 10.1016/j.lfs.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Mentch J., Spiegel A., Ricciardi C., Robertson C.E. GABAergic inhibition gates perceptual awareness during binocular rivalry. J. Neurosci. 2019;39:8398–8407. doi: 10.1523/JNEUROSCI.0836-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrsic-Flogel T.D., Hofer S.B., Ohki K., Reid R.C., Bonhoeffer T., Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Robertson C.E., Ratai E.M., Kanwisher N. Reduced GABAergic action in the autistic brain. Curr. Biol. 2015 doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Said C.P., Heeger D.J. A model of binocular rivalry and cross-orientation suppression. PLoS Comput. Biol. 2013;9:e1002991. doi: 10.1371/journal.pcbi.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpina F., Migliorati D., Marzullo P., Mauro A., Scacchi M., Costantini M. Altered multisensory temporal integration in obesity. Sci. Rep. 2016;6 doi: 10.1038/srep28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheynin Y., Proulx S., Hess R.F. Temporary monocular occlusion facilitates binocular fusion during rivalry. J. Vis. 2019;19:23. doi: 10.1167/19.5.23. [DOI] [PubMed] [Google Scholar]
- Stice E., Burger K. Neural vulnerability factors for obesity. Clin. Psychol. Rev. 2019;68:38–53. doi: 10.1016/j.cpr.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect. Biol. 2012;4:a005736. doi: 10.1101/cshperspect.a005736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G.G. The dialectic of hebb and homeostasis. Philos. Trans. R. Soc. B Biol. Sci. 2017;372 doi: 10.1098/rstb.2016.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano G.G., Nelson S.B. Homeostatic plasticity in the developing nervous system. Nat. Rev. Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Loon A.M., Knapen T., Scholte H.S., St John-Saaltink E., Donner T.H., Lamme V.A. GABA shapes the dynamics of bistable perception. Curr. Biol. 2013;23:823–827. doi: 10.1016/j.cub.2013.03.067. [DOI] [PubMed] [Google Scholar]
- Zhou J., He Z., Wu Y., Chen Y., Chen X., Liang Y., Mao Y., Yao Z., Lu F., Qu J., Hess R.F. Inverse occlusion: a binocularly motivated treatment for Amblyopia. Neural Plast. 2019;2019:5157628. doi: 10.1155/2019/5157628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.