Abstract
Background
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart disease that causes heart failure and/or sudden cardiac death. Several desmosomal genes (DSC2, PKG, PKP2, DSP, and RyR2) are thought to be the causative gene involved in ARVC. Out of them, DSC2 mutations account for 2% of ARVC genetic abnormalities. This study aimed to clarify the effect of G790del mutation in DSC2 on the arrhythmogenic mechanism and cardiac function in a mouse model.
Result
Neither the heterozygous +/G790del nor homozygous G790del/G790del mice showed structural and functional defects in the right ventricle (RV) or lethal arrhythmia. The homozygous G790del/G790del 6-month-old mice slightly showed left ventricular (LV) dysfunction. Cell shortening decreased with prolongation of intracellular Ca2+ transient in cardiomyocytes isolated from the homozygous G790del/G790del mice, and spontaneous Ca2+ transients were frequently observed in response to isoproterenol.
Conclusions
G790del mutation in DSC2 was not relevant to the pathogenesis of ARVC, but showed a slight contractile dysfunction and Ca2+ dysregulation in the LV.
Keywords: Desmocollin-2 (DSC2), Arrhythmogenic right ventricular cardiomyopathy (ARVC)
Highlights
-
•
We successfully established the DSC2 KI mice of G790del.
-
•
Both heterozygous +/G790del and homozygous G790del/G790del mice showed no signs of ARVC.
-
•
Homozygous G790del/G790del mice revealed a slight LV dysfunction with aberrant Ca2+ release.
-
•
The G790del mutation in DSC2 alone is insufficient to develop ARVC in a mouse model..
1. Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart disease, characterized by myocyte loss and fibro-fatty tissue replacement [1]. To date, several genes have already known to cause ARVC including DSC, PKG, PKP2, DSP, and RyR2 [2]. Of these proteins, defect in desmocollin-2 (DSC2) has been reported to be a cause of familial arrhythmogenic right ventricular cardiomyopathy 11 (ARVC11) [3]. DSC2 and DSG2 are the cardiac isoforms of desmosomal cadherins known to have overlapping functions in binding to JUP and plakophilin-2 (PKP2). Several heterozygous mutations in both proteins have been described to cause dominant ARVC. G790del is one of the known mutations of DSC2 in patients with ARVC [4,5]. Although some investigators emphasized the role of G790del in the development of ARVC11, this remains to be further elucidated. We investigated the pathogenic effect of the G790del mutation on the heart structure and function in a DSC2 knock-in (KI) mouse model.
2. Methods
2.1. Animal model
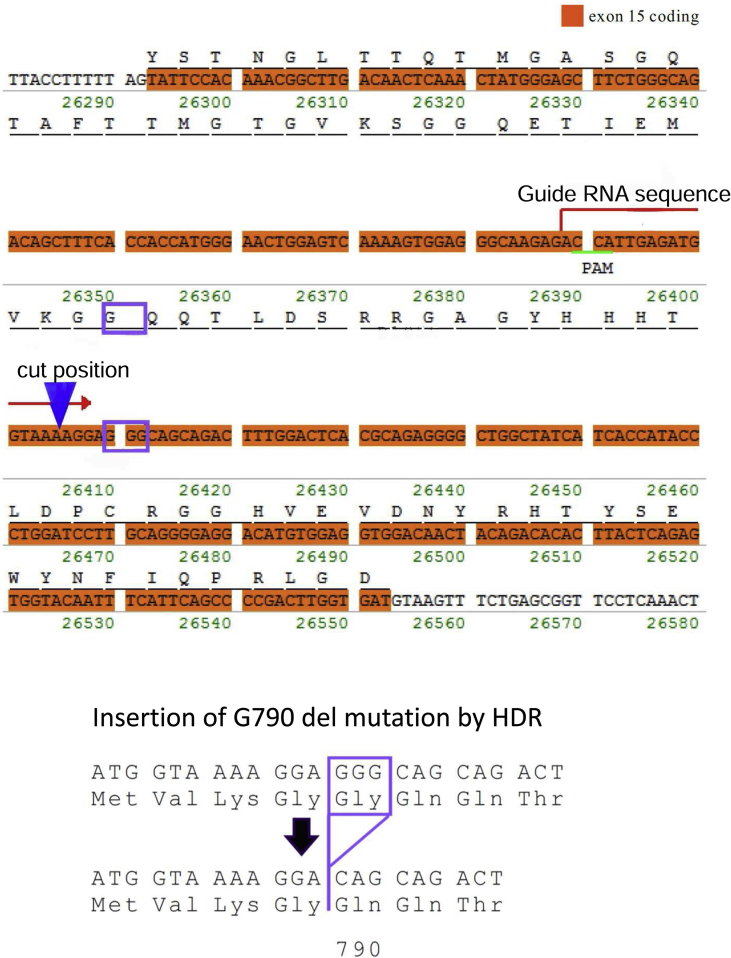
We obtained C57BL6 based G790del DSC2 KI mice using the CRISPR/Cas9 genome editing technique generated by Transgenic Inc (Fukuoka, Japan). Supplementary Fig. 1 shows the detailed methods of the mouse generation.
This study conformed to the Guide for the Care and Use of Laboratory Animals published by the NIH (NIH Publications No. 8023, revised 1978). The care of the animals and the protocols used were in accordance with the guidelines established by the Animal Ethics Committee of Yamaguchi University School of Medicine.
2.2. Histological analysis
Hearts from WT, +/G790del KI, and G790del/G790del KI mice aged between 44 and 48 weeks were collected and fixed using 10% formalin. A complete, full-circumferential section, at the level of the left ventricular papillary muscles, was selected for morphometric analysis. Each section of the ventricle was stained with Hematoxylin-Eosin and Azan.
2.3. Echocardiography
Cardiac function was analyzed using an F37 ultrasound machine (Hitachi Medical, Netherlands) equipped with a 7.5-MHz probe (UST-5413). WT and KI mice were initially anesthetized with 4–5% isoflurane (mixed with oxygen) and maintained with 1–2% isoflurane during echocardiography.
2.4. Surface electrocardiogram (ECG)
The ECG was monitored in 24-month-old WT and KI mice in a conscious state using ECG telemetry. The transmitters (Data Sciences International, St. Paul, MN) were implanted in the backspace with subcutaneous electrodes in a lead II configuration. ECG was monitored for 24 h first followed byan exercise test performed using a treadmill for mice (Panlab, Barcelona, Spain). Finally, a drug challenge test using an adrenergic agonist with caffeine was performed. The ECG was recorded after the injection of epinephrine (1 mg/kg of body weight I.P.) and caffeine (100 mg/kg of body weight I.P.) and monitored for 30 min. The above-mentioned recording was performed in a subset of WT (n = 10), KI-hetero (n = 9), and KI- mice (n = 8).
2.5. Antibodies
Antibodies used in this experiment included DSC2 (anti DSC2_494–507 custom-made), DSG2 (Progen), PKG(SCB), PKP2(Progen), DSP(Santa Cruz), CX43(Sigma-Aldrich), Caspase-3 p17(SCB), TGF-β(SCB), collagen 6(Southern Biotech), and GAPDH(Sigma-Aldrich).
2.6. Western blotting
The membrane fraction of the heart from WT and KI mice was extracted using Mem-PER Plus Membrane Protein Extraction Kit (Thermo Fisher). Tissue membrane fraction samples were denatured in SDS-PAGE sample buffer. SDS-PAGE, blotting, and antibody detections were performed in the way we reported in our previous study [6].
2.7. Immunohistochemistry analysis of desmosome proteins
The hearts were fixed in 4% paraformaldehyde overnight at room temperature. Subsequently, the hearts were embedded in paraffin and sliced in 5 μm thick sections. Hematoxylin and eosin (HE) and Azan staining were performed. A BZ-9000 microscope was used for analyzing the HE and Azan stained specimens. Slices were deparaffinized using xylene and ethanol, and then stained overnight with the primary antibodies in 1% bovine serum albumin and 0.5% Triton X-100. After washing with PBS, slides were stained with the secondary antibodies for 4 h at room temperature. The LSM5 Exciter (Carl Zeiss Microscopy, Oberkochen, Germany) was used for the confocal analysis, and all images were processed with Zen software (Carl Zeiss Microscopy, Oberkochen, Germany).
2.8. Statistics
One-way ANOVA followed by a post hoc Dunnett's test was performed for statistical comparison of more than two groups. All data were expressed as mean ± SEM. P-value of less than 0.05 was considered statistically significant. To compare the occurrence rate of more than two groups, Chi-squared tests and Ryan's method were employed.
3. Results
3.1. Structural characteristics of the G790del KI mice
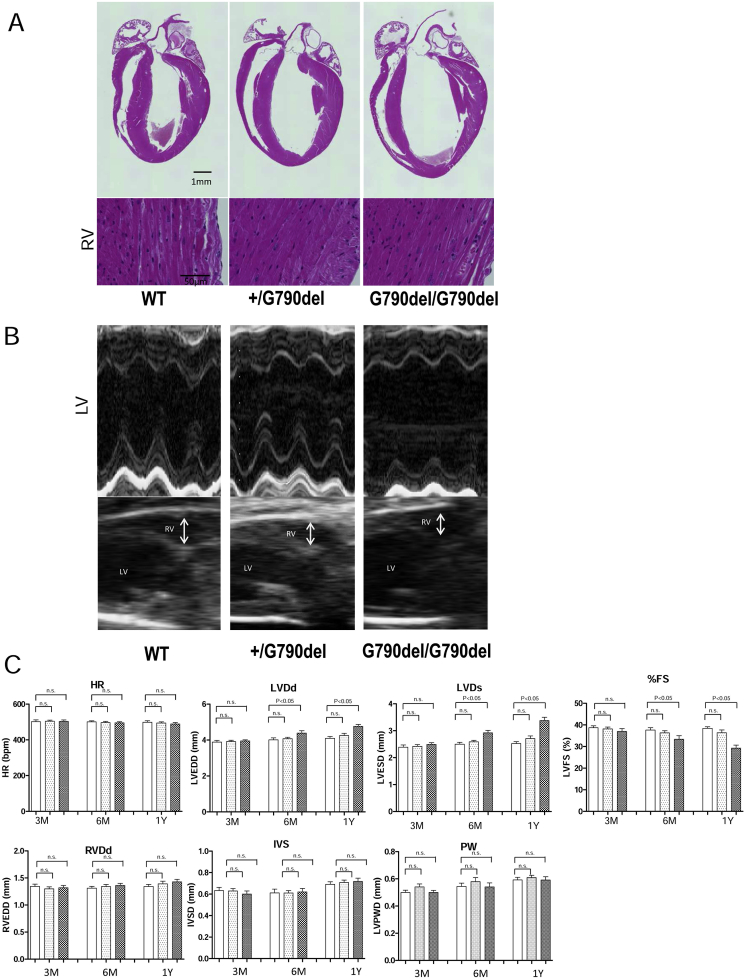
There were no significant structural differences observed in the RV between the WT and KI mice, and right ventricular free wall showed no fibrosis and fat infiltrations in the KI mice (Fig. 1A). In contrast, the chamber size of the LV was enlarged with a decrease in fractional shortening in the 6-month-old homozygous G790del/G790del mice (Fig. 1A–C).
Fig. 1.
Structural characterization of hearts from WT and Gly790del DSC2 knock-in (KI) mice. A. Representative images of hematoxylin/eosin-stained long axis section of hearts from WT and KI mice at the age of 1-year-old, and Azan stained RV wall. No right ventricular dilatations and fibrosis or fatty infiltration were observed even in homozygous KI mice. B, C. Representative images of echocardiogram and the summarized echocardiographic parameters in WT and KI mice. Only in homozygous KI mice, LV dilatation and decrease in % fractional shortening (FS) was observed after 6-month-old. Data represent means ± SEM of 9–12 mice.
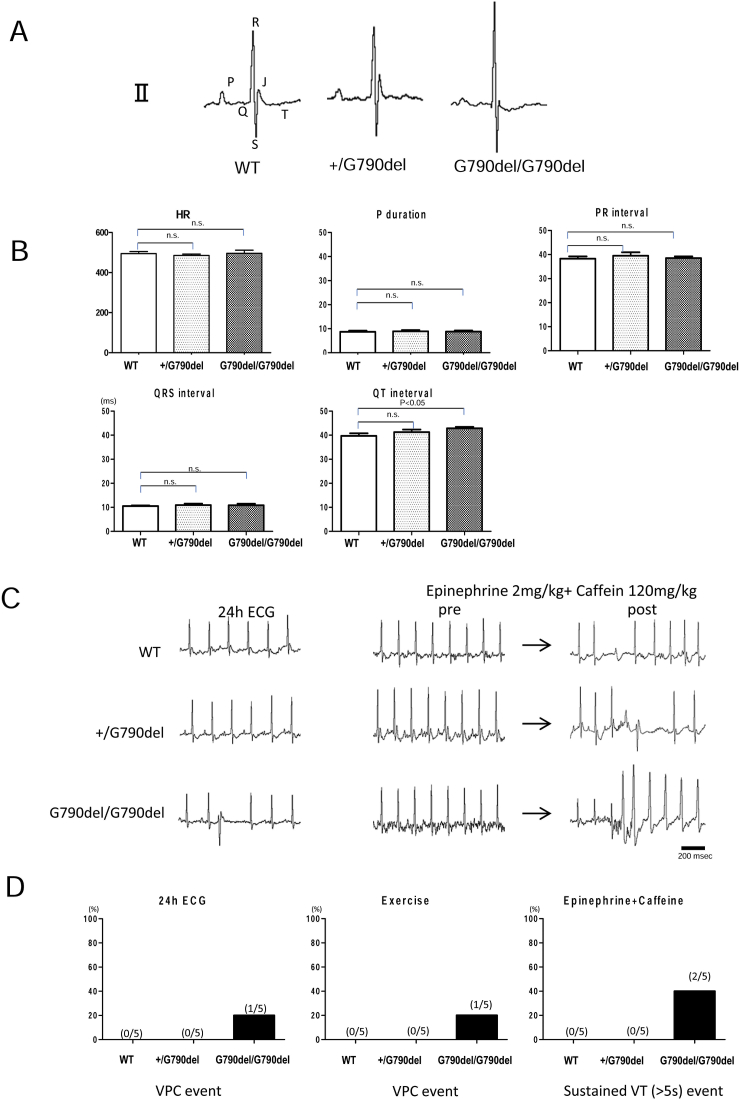
3.2. ECG in G790del mice
Fig. 2A and B shows the ECG in the 6-month-old mice. In the resting state, only the QT interval was slightly prolonged in the homozygous G790del/G790del mice. All parameters except the QT interval revealed no significant difference between the WT and KI mice. Fig. 2C depicts the representative ECG recordings during 24-h monitoring and those of drug challenge test. The summarized data are shown in Fig. 2D. The heterozygous +/G790del mice showed no arrhythmias in 24-h recording, exercise test, or drug challenge test. Homozygous G790del/G790del mice tended to show arrhythmias but demonstrated no significant difference in the occurrence of arrhythmias between WT and KI mice.
Fig. 2.
Electrocardiograms of WT and Gly790del DSC2 KI mice. A, B. Representative recordings of electrocardiogram (ECG) in WT and KI mice at baseline. The ECG was recorded and analyzed using a digital acquisition and analysis system (Power Lab/4SP). Data represent means ± SEM of 8–10 mice. C. (left) ECG recordings for 24 h. Ventricular arrhythmia was rarely seen in both WT and KI mice. (right) Incidence of ventricular tachyarrhythmia (VT) after intraperitoneal injection of epinephrine (2 mg/kg body weight) and caffeine (120 mg/kg body weight) (Epi/Caff). D. Summarized data of arrhythmia event. Only homozygous G790del/G790del mice showed arrhythmias but not frequently.
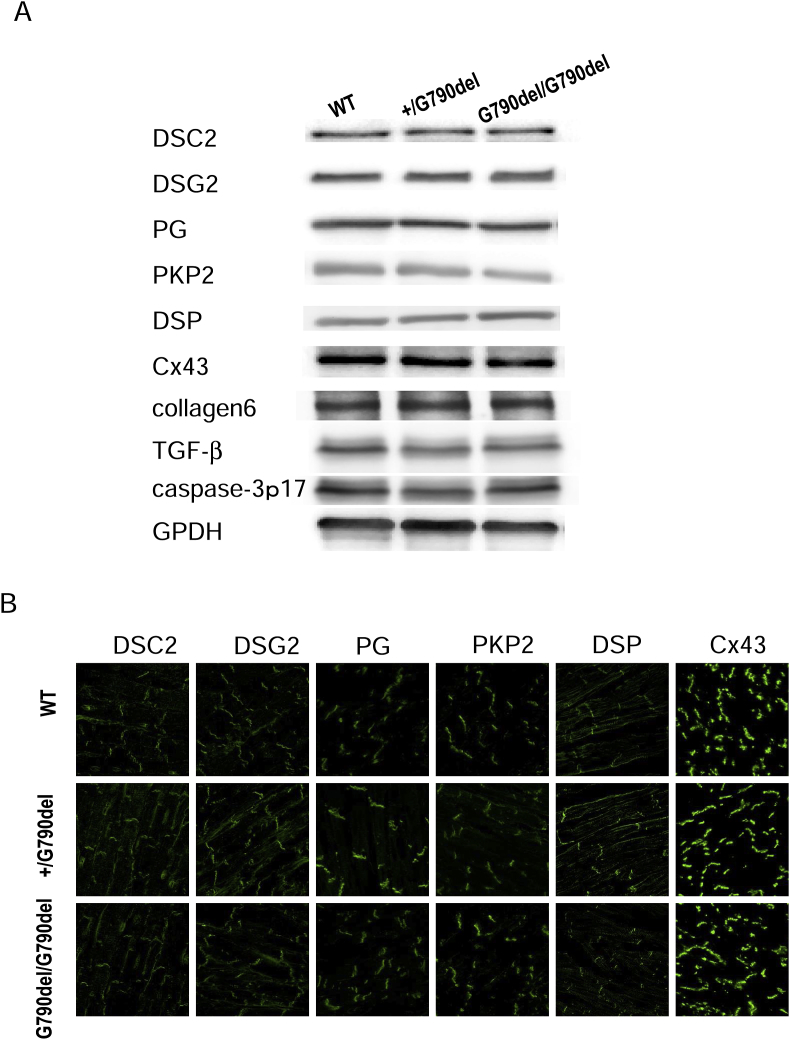
3.3. Desmosomal protein expression in the G790del mice
Western blot analysis of desmosomal proteins in the membrane fraction of the hearts was performed in 6-month-old WT, +/G790del, and G790del/G790del mice. The result revealed no major difference in DSC2, DSG2, PKG, PKP2, DSP, and CX43 between the groups (Fig. 3A). The immunofluorescent study also demonstrated no remarkable difference in the amount and structure of the desmosomal proteins between the groups (Fig. 3B).
Fig. 3.
Immunofluorescence analysis and expression level of desmosome proteins A. Western blot analysis of desmocollin-2 (DSC2), desmoglein-2 (DSG2), plakoglobin (PG), plakophilin-2 (PKP2), desmoplakin (DSP), connexin-43 (CX43), collagen 6, TGF-b, and caspase 3 p17 in membrane fraction of the hearts from 6-month-old WT and KI mice. There were no difference in the amount of proteins. B. Immunofluorescence analysis of DSC2, DSG2, PKG, PKP2, DSP, and CX43 in WT and KI mice hearts. There were no difference in the localization of proteins.
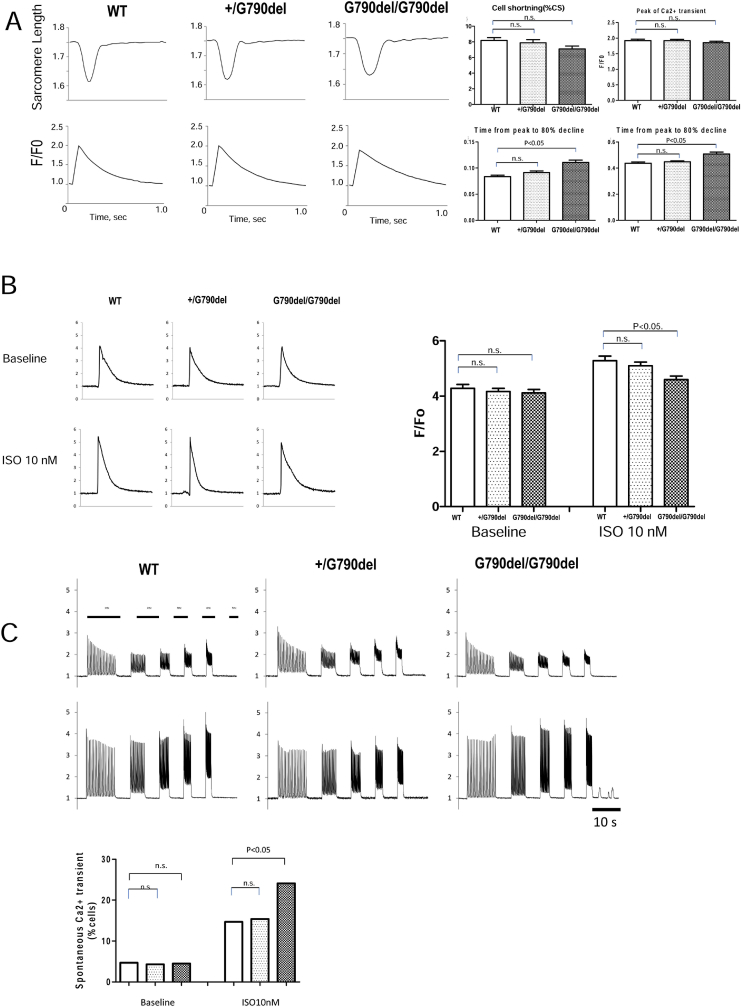
3.4. Sarcomere shortening and Ca2+ transient of mice cardiomyocytes
Cardiomyocyte shortening was measured in WT, heterozygous +/G790del, and homozygous G790del/G790del mice. Homozygous G790del/G790del mice demonstrated a slight decrease in cell shortening and prolongation of the relaxation period (Fig. 4A). The peak of the intracellular Ca2+ transient was also slightly decreased with a slower decline of the Ca2+ transient in homozygous G790del/G790del mice (Fig. 4A). In response to isoproterenol, the sarcoplasmic reticular Ca2+ content measured by the caffeine administration was slightly decreased in the homozygous mice (Fig. 4B). The frequency of spontaneous Ca2+ transients increased in homozygous G790del/G790del mice (Fig. 4C).
Fig. 4.
Cell shortening and Ca2+ kinetics. A. Representative recordings of sarcomere shortening and Calcium transient during electrical stimulation in WT and KI cells isolated from 6-month-old mice. Data represent means ± SD of 24–33 cells from 3 preparations. B. SR calcium content measured by caffeine application, with or without 10 nM isoproterenol. Data represent means ± SEM of 12–20 cells from 3 preparations. C. Representative recordings of spontaneous Ca2+ transients during sequential pacing of isolated cardiomyocytes. Spontaneous Ca2+ transients were observed in homozygous KI mice. The occurrence of evoked Ca2+ transients was shown in the bar graph. Data represent means ± SD of 188–246 cells from 3 to 4 preparations.
4. Discussion
The major finding of this study is that a single amino-acid deletion within DSC2 at AA790 does not appear to be involved in the development of ARVC, although a slight LV dysfunction with aberrant Ca2+ release was observed in the homozygous G790del/G790del mice.
Here, we focus on two important questions: (1) Is the G790del mutation in DSC2 causative for ARVC? (2) What is the influence of the G790del mutation in DSC2 on the heart?
DSC2 is known to be a causative protein in the development of ARVC. It has been reported that DSC2 mutation are observed as a pathogenic cause of ARVD/C in 1–2% of the ARVC patients [2]. Heuser et al. [3] reported the occurrence of a heterozygous splice-acceptor–site mutation in intron 5 (c.631-2A→G) of the DSC2 gene in ARVD/C patients. They provided further evidence that this mutation causes ARVC by reproducing it in a zebra fish model. Liu et al. [7] reported the detection of a heterozygous V364 M mutation of DSC2 in a Chinese ARVC family. In contrast, Kapplinger et al. [8] recently pointed out that the prevalence of the DSC2 mutation in ARVC cohorts is similar to that of the control cohorts. Interestingly, Bhuiyan et al. [9] found that mutations in either DSG2 or DSC2 are less prevalent (10%) than the PKP2 mutations (40%) in the Dutch task force criteria (TFC)+ARVD/C patients. Collectively, it is suggested that while some of mutations in DSC2 might be causative factors in ARVD/c, single DSC2 mutations are less likely to result in a full-blown ARVD/C phenotype.
Then, what does the G790del of DSC2 mean?
Interestingly, homozygote G790del/G790del mice showed a slight LV dysfunction with aberrant intracellular Ca2+ release. Fressart et al. analyzed 135 ARVD/C patients, and identified 41 disease-causing mutations [10]. In this report, three DSC2 heterozygous mutations, Glu114Gly fsX7, R132C, and G790del, were indicated as disease causing mutations. To understand the clinical significance of G790del, we first investigated the conservation at G790. G790 is conserved in humans, mice, rats, pigs, and monkeys (Supplementary Fig. 2). According to http://exac.broadinstitute.org/variant/18-28648997-GTCC-G, 1.8% of the East Asian population has harbors this mutation. If the percentage in this report is accurate, G790del might be a benign SNP. In this study, we observed that the heterozygous +/G790del mice indeed showed no significant effect on LV and RV function, but homozygous G790/G790del mice showed a slight LV dysfunction with aberrant Ca2+ release. Moreover, two studies have reported about DSC2 mutations in which only a homozygous mutation showed the ARVC phenotype. Lorenzon et al. [11] reported the detection of the D179G mutation in 5.3% of the 94 arrhythmogenic cardiomyopathy (ACM) patients evaluated. According to their report, only homozygous D197G mutation demonstrated the phenotype. Gerull et al. [12] also reported that the homozygous Q554X mutation in DSC2 was a causative factor in ACM. However, the reported G790del mutation in the ARVD/C patients is a heterozygous one. According to our study, G790del in DSC2 alone is insufficient to develop ARVC in mice. There might be a possibility that G790del mutation affects different in humans. Another possibility is that unknown second gene abnormality is required to develop ARVD/C.
5. Conclusions
We successfully established the DSC2 KI mice of G790del. Both heterozygous +/G790del and homozygous G790del/G790del mice showed no signs of ARVC, although homozygous G790del/G790del mice revealed a slight LV dysfunction with aberrant Ca2+ release. Thus, our study indicated that the G790del mutation in DSC2 alone is insufficient to develop the pathogenesis of ARVC in a knock-in mouse model.
Sample Credit author statement
Yoriomi Hamada: Data curation. Takeshi Yamamoto: Conceptualization, Methodology, Writing- Original draft preparation. Yoshihide Nakamur: Data curation of the western blots. Yoko Sufu-Shimizu: Helping data curation. Takuma Nanno: Helping data curation. Masakazu Fukuda: Helping data curation. Makoto Ono: Helping data curation. Tesuro Oda: Helping data curation. Shinichi Okuda: Helping data curation. Takeshi Ueyama: Supervision. Shigeki Kobayash: Supervision. Masafumi Yano: Writing- Reviewing and Editing.
Acknowledgements
This work was supported by grants-in-aid for scientific research from The Ministry of Education in Japan(grant Nos. 18K15890 to MF, 18K08108 to TY)
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2019.100711.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Design of Gly790del mutation in desmocollin-2 in miceIn
exon 15 of C57BL6 mice desmocollin-2, G790del was inserted using CRISPR/Cas9 genome editing technique. Twenty-seven F0 mice were analyzed by direct sequence and
14 mice were found to have G790del. Three of them were used to get F1 mice.
Supplementary Fig. 2.
Aliment analysis of desmocollin-2 amino acid sequence of various animals.
Mouse, rat, pig, monkey and human have Gly790, however, rabbit and horse does not have Gly790.
References
- 1.Corrado D., Wichter T., Link M.S., Hauer R.N.W., Marchlinski F.E., Anastasakis A., Bauce B., Basso C., Brunckhorst C., Tsatsopoulou A., Tandri H., Paul M., Schmied C., Pelliccia A., Duru F., Protonotarios N., Estes N.M., Mckenna W.J., Thiene G., Marcus F.I., Calkins H. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Circulation. 2015 doi: 10.1161/CIRCULATIONAHA.115.017944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McNally E., MacLeod H., Dellefave-Castillo L. Arrhythmogenic right ventricular cardiomyopathy. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., Genetic Counseling, editors; Ledbetter N., editor. University of Washington, Seattle; Seattle (WA): 1993-2019. (GeneReviews® [Internet]). Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH. initial Posting: April 18, 2005; Last Update: May 25, 2017. [Google Scholar]
- 3.Heuser A., Plovie E.R., Ellinor P.T., Grossmann K.S., Shin J.T., Wichter T., Basson C.T., Lerman B.B., Sasse-Klaassen S., Thierfelder L., MacRae C.A., Gerull B. Mutant desmocollin-2 causes arrhythmogenic right ventricular cardiomyopathy. Am. J. Hum. Genet. 2006;79:1081–1088. doi: 10.1086/509044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng D., Johnston J.J., Teer J.K., Singh L.N., Peller L.C., Wynter J.S., Lewis K.L., Cooper D.N., Stenson P.D., Mullikin J.C., Biesecker L.G. Interpreting secondary cardiac disease variants in an exome cohort. Circ. Cardiovasc. Genet. 2013 doi: 10.1161/CIRCGENETICS.113.000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fedida J., Fressart V., Charron P., Surget E., Hery T., Richard P., Donal E., Keren B., Duthoit G., Hidden-Lucet F., Villard E., Gandjbakhch E. Contribution of exome sequencing for genetic diagnostic in arrhythmogenic right ventricular cardiomyopathy/dysplasia. PLoS One. 2017 doi: 10.1371/journal.pone.0181840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakamura Y., Yamamoto T., Kobayashi S., Tamitani M., Hamada Y., Fukui G., Xu X., Nishimura S., Kato T., Uchinoumi H., Oda T., Okuda S., Yano M. Ryanodine receptor–bound calmodulin is essential to protect against catecholaminergic polymorphic ventricular tachycardia. JCI Insight. 2019 doi: 10.1172/jci.insight.126112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J.S., Fan L.L., Li J.J., Xiang R. Whole-Exome sequencing identifies a novel mutation of desmocollin 2 in a Chinese family with arrhythmogenic right ventricular cardiomyopathy. Am. J. Cardiol. 2017 doi: 10.1016/j.amjcard.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 8.Kapplinger J.D., Landstrom A.P., Salisbury B.A., Callis T.E., Pollevick G.D., Tester D.J., Cox M.G.P.J., Bhuiyan Z., Bikker H., Wiesfeld A.C.P., Hauer R.N.W., Van Tintelen J.P., Jongbloed J.D.H., Calkins H., Judge D.P., Wilde A.A.M., Ackerman M.J. Distinguishing arrhythmogenic right ventricular cardiomyopathy/dysplasia- associated mutations from background genetic noise. J. Am. Coll. Cardiol. 2011 doi: 10.1016/j.jacc.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhuiyan Z.A., Jongbloed J.D.H., Van Der Smagt J., Lombardi P.M., Wiesfeld A.C.P., Nelen M., Schouten M., Jongbloed R., Cox M.G.P.J., Van Wolferen M., Rodriguez L.M., Van Gelder I.C., Bikker H., Suurmeijer A.J.H., Van Den Berg M.P., Mannens M.M.A.M., Hauer R.N.W., Wilde A.A.M., Van Tintelen J.P. Desmoglein-2 and Desmocollin-2 mutations in Dutch arrhythmogenic right ventricular dysplasia/cardiomypathy patients : results from a multicenter study. Circ. Cardiovasc. Genet. 2009 doi: 10.1161/CIRCGENETICS.108.839829. [DOI] [PubMed] [Google Scholar]
- 10.Fressart V., Duthoit G., Donal E., Probst V., Deharo J.C., Chevalier P., Klug D., Dubourg O., Delacretaz E., Cosnay P., Scanu P., Extramiana F., Keller D., Hidden-Lucet F., Simon F., Bessirard V., Roux-Buisson N., Hebert J.L., Azarine A., Casset-Senon D., Rouzet F., Lecarpentier Y., Fontaine G., Coirault C., Frank R., Hainque B., Charron P. Desmosomal gene analysis in arrhythmogenic right ventricular dysplasia/cardiomyopathy: spectrum of mutations and clinical impact in practice. Europace. 2010 doi: 10.1093/europace/euq104. [DOI] [PubMed] [Google Scholar]
- 11.Lorenzon A., Pilichou K., Rigato I., Vazza G., De Bortoli M., Calore M., Occhi G., Carturan E., Lazzarini E., Cason M., Mazzotti E., Poloni G., Mostacciuolo M.L., Daliento L., Thiene G., Corrado D., Basso C., Bauce B., Rampazzo A. Homozygous desmocollin-2 mutations and arrhythmogenic cardiomyopathy. Am. J. Cardiol. 2015 doi: 10.1016/j.amjcard.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 12.Gerull B., Kirchner F., Chong J.X., Tagoe J., Chandrasekharan K., Strohm O., Waggoner D., Ober C., Duff H.J. Homozygous founder mutation in desmocollin-2 (DSC2) causes arrhythmogenic cardiomyopathy in the hutterite population. Circ. Cardiovasc. Genet. 2013 doi: 10.1161/CIRCGENETICS.113.000097. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.