Short abstract
Background
Multiple sclerosis is a central nervous system demyelinating disease that affects women of reproductive potential. It is important to identify the frequency and risk factors of unplanned or disease-modifying therapy-exposed pregnancies to create interventions to reduce these.
Methods
This retrospective, single-center, observational chart review study aims to identify risk factors for unplanned pregnancy to identify a target population for family counseling.
Results
In total, 63 live births in 45 patients (20 unplanned and 43 planned) were analyzed. The percentage of unplanned pregnancy was 32%. The proportion of those receiving family planning counseling was lower in the patients with unplanned pregnancies (p < 0.001). The main risk factors for unplanned pregnancy were younger age (p = 0.004), disease-modifying therapy exposure (p < 0.001), and being unmarried (p < 0.001). Overall, 16 pregnancies had disease-modifying therapy exposure and in a subsequent study the risk for disease-modifying therapy exposure was unplanned status (p < 0.001). Birth outcomes were not different between groups. There were more enhancing lesions in the post-partum magnetic resonance imaging of women with planned pregnancy (p < 0.04).
Conclusion
Prevention of unplanned pregnancy could lead to less disease-modifying therapy exposed pregnancies. This study suggests a targeted intervention of family planning counseling in younger, unmarried multiple sclerosis patients could potentially lead to less unintended in utero disease-modifying therapy exposure.
Keywords: Multiple sclerosis, pregnancy
Introduction
Multiple sclerosis (MS) is a chronic immune-mediated demyelinating disease of the central nervous system that leads to progressive permanent disability in most patients. In the USA, approximately 700,000 people live with MS1 and the prevalence is increasing.2,3 This increasing prevalence appears to be due to an increase mainly of the relapsing–remitting (RR) phenotype in women.2,4,5 The RR phenotype of MS is highly treatable, with regulatory approval of 16 disease-modifying therapies (DMT) that reduce relapses and potentially prevent or delay permanent neurologic disability.6,7 Evidence suggests early initiation of effective DMT leads to better outcomes in RRMS patients.8–10 Whereas emerging data suggest that some DMTs, particularly glatiramer acetate and interferon-beta, may be safe in the earliest stages of pregnancy, most DMT and symptomatic treatments being used in MS are not considered completely safe in women who are attempting to conceive, are pregnant, or nursing.11–15 Management of MS with DMT needs to be balanced with reproductive goals.
Several studies looking at unplanned pregnancy rates in various populations have been conducted in the teenage and military populations. In these studies, women who were younger than 30, unmarried, attained lower educational levels, economically disadvantaged, depressed, non-Caucasian, and used birth control incorrectly were more likely to have an unplanned pregnancy.16–19 Due to perceived stress, psychosocial problems, and lack of adequate prenatal care, these women were more likely to have preterm deliveries and infants with low birth rates.16 Moreover, women with unplanned pregnancies were more likely to have obstetric complications and infections.20
Limited data exist exploring the rate of unplanned pregnancy in MS patients. From a paper published by Henshaw et al. in 1998, estimates suggest that as many as 50% of pregnancies in the general population in the US are unplanned.21 Most citations in the literature source this study to quote a 50% unplanned pregnancy rate in MS.22 A few notable exceptions are the recently published studies that utilized population-based surveys and chart review techniques from Denmark, Germany, and Spain suggesting the unplanned pregnancy rates was as low as 10% and 16.6%, respectively.23,24 The rate of unplanned pregnancy is considered to be lower in western Europe compared to the USA.25 Therefore, it becomes important to look at the unplanned pregnancy rate in a US population. If the unplanned pregnancy rates in the USA also apply to the MS population, then there is reason for concern. Unintended in utero exposure to MS medications potentially can lead to teratogenic effects, long-term disability, and intellectual impairment in the offspring.26–29 It is critical that women are adequately counseled about family planning issues, especially while on DMT.30–33 By simply providing education and family planning counseling, the rate of contraceptive usage increased with a corresponding drop in the rate of unplanned pregnancies.17
A previous study showed that family planning issues are brought up by only 57% of physicians when starting a DMT in women of child-bearing potential.34 This retrospective, single-center, observational chart review study aims to identify risk factors for unplanned pregnancy to identify a target population for family counseling. Secondary aims are to examine the use of DMT during pregnancy, to determine the effect of unplanned pregnancy on the course of MS, and to determine outcomes of unplanned pregnancy in an MS population in the USA.
Methods
Patient identification
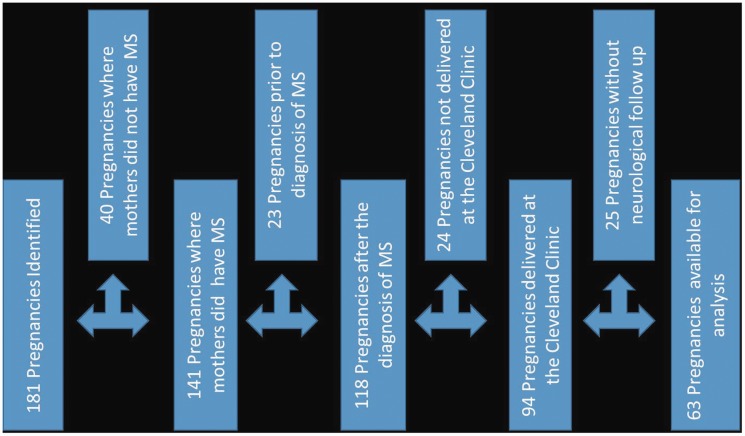
The study population comprised patients who received medical care at the Cleveland Clinic Mellen Center in Cleveland, Ohio, a large, quaternary MS specialty clinic that sees over 20,000 patient visits per year. Cleveland Clinic Institutional Research Board (IRB) approval was received to conduct a data query and chart review. After IRB approval, an automated electronic medical record (EMR) data search identified 181 pregnancies in 150 patients with International Classification of Diseases (ICD) 9 and 10 codes for both pregnancy and MS from 2000 to 2016. Comprehensive review was undertaken of the EMR of these 150 patients, including all office visits, telephone calls, and direct patient provider EMR messaging encounters. These data were extracted by a single reviewer (AS) to create a database. Patients were excluded from analysis if they did not have MS, the pregnancy occurred prior to being diagnosed with MS, they did not receive their obstetrics care within the Cleveland Clinic network, the pregnancy did not result in a live birth or they did not have a neurologic appointment at the Mellen Center before and after the pregnancy, resulting in 63 pregnancies in 43 women in the final analysis (Figure 1).
Figure 1.
Study population. For the study, 181 pregnancies in 150 patients with International Classification of Diseases (ICD) 10 codes for both pregnancy and multiple sclerosis (MS) from 2000 to 2016 were identified. Patients were excluded from analysis if they did not have MS, the pregnancy occurred prior to being diagnosed with MS, they did not deliver within the Cleveland Clinic network, or they did not have neurologic follow-up at the Mellen Center. These inclusion and exclusion criteria resulted in 63 pregnancies in 43 women for the final analysis.
Measurements of outcomes of interest
A pregnancy was defined as unplanned if it met one of the following two criteria. The first criterion required that pregnancy was documented as unplanned in the first obstetrics note. If this criterion was not met, then additional information in the chart was considered, including documentation elsewhere in the medical record indicating pregnancy was unplanned, evidence the patient required instruction on starting prenatal vitamins or folic acid, or asked the doctor what she needed to do after the discovery of the pregnancy. If either criterion were met, then the pregnancy was considered unplanned, otherwise, the pregnancy was considered planned. A pregnancy was also considered planned if there was documentation in the chart specifically stating that it was planned.
Measurement of exposure of interest
The primary exposure of interest was the presence of family planning counseling as documented in the EMR. If there was no documented family planning counseling, then it was assumed to have not occurred. Additionally, when family planning counseling occurred, the narrative of the note was explored to see if the counseling occurred after a patient inquiry about the topic. If it was not specified who initiated the conversation, then it was assumed that the provider initiated the conversation.
Measurement of covariates
For both analyses, several covariates were extracted from the patient’s EMR to determine their effects on outcome of interests for the purposes of modeling, specifically, demographic factors (age, race, marital status), obstetric history (pregnancy outcome, infant delivery weight, infant APGAR scores, previous pregnancies, previous pregnancy outcomes, complication with delivery, need for caesarian section, breast feeding choice, and breast feeding duration), age at delivery, smoking status, last measured pre-pregnancy body mass index, use of birth control prior to pregnancy, and MS-related clinical information (age of diagnosis of MS, use of DMT during pregnancy, time to restarting DMT post pregnancy, number of relapses the year before pregnancy, number of relapses during the pregnancy, number of relapses the year after and the number of new T2 and enhancing lesions on magnetic resonance imaging (MRI) pre-pregnancy and post-partum MRI).
Statistical analysis
Data were de-identified by removing all health-protected information. Characteristics of patients who had planned versus unplanned pregnancies were compared using Chi-square analysis and t-tests for categorical and continuous variables, respectively. Spearman plots with all the collected covariates were constructed from which a model was created with the variables that had the highest five correlation coefficients. From that model, a bidirectional stepwise logistic regression was performed to generate a model with the lowest Akaike information criterion value. Subsequently, the p-values of the natural log of the odds (Logit) were calculated using a t-test and Chi-squared test depending on the variable type. The Logit was used to calculate the odds ratio and 95% confidence intervals (CI) that each model component had on the likelihood of unplanned pregnancy. For each analysis, the statistical significance was defined as p < 0.05 and a Bonferroni correction was conducted to account for multiple comparisons.
A secondary aim was to explore the proportion of DMT-exposed pregnancies in all planned and unplanned pregnancies. A Chi-squared test was done on the total pool of analyzed pregnancies in this study to compare DMT-exposed and non-DMT-exposed pregnancies. A logistic regression comparing DMT-exposed pregnancies to planning status and birth control usage was conducted. Next, a logistic regression analysis comparing pregnancy outcomes in planned versus unplanned pregnancies and DMT-exposed and non-DMT-exposed pregnancies was undertaken. Pregnancy outcomes were also compared between planned and unplanned pregnancies. R Studio was utilized to perform all statistical analysis.35
Results
Unplanned pregnancy rate and risk factors
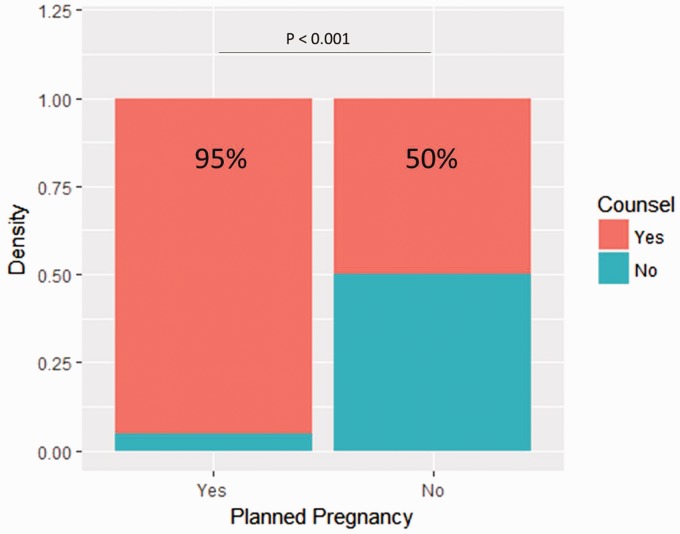
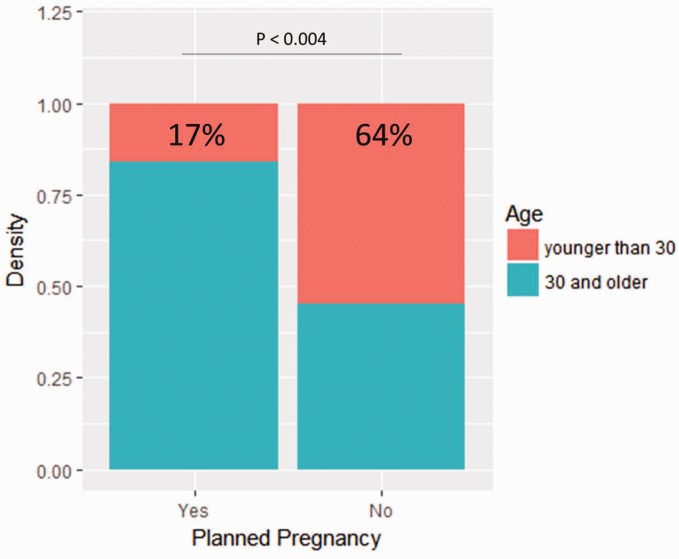
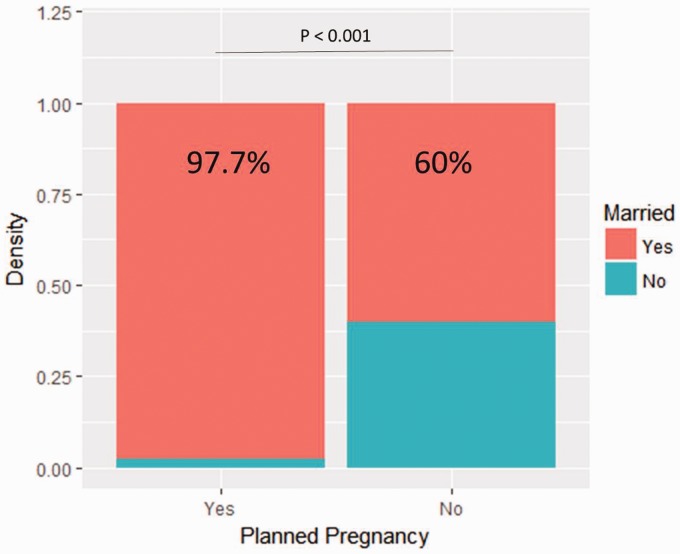
Baseline characteristics are summarized in Table 1. Compared to patients with planned pregnancies, patients with unplanned pregnancies were more frequently younger than 30 years old at delivery (11 (55%) versus seven (17%), p = 0.004, Figure 2), unmarried (12 (60%) versus 42 (98%), p < 0.001, Figure 3), treated with DMT at the time of conception (13 (65%) versus three (9%), p < 0.001), and reportedly on birth control (five (25%) versus 0 (0%), p < 0.001). Additionally, the proportion of those receiving family planning counseling was lower in patients with unplanned pregnancies (10 (50%) versus 41 (95%), p <0.001, Figure 4). Of those who received family planning counseling, women with unplanned pregnancies were less likely to initiate the conversation about reproductive goals than those who had planned pregnancies (four (40%) versus 38 (93%) p < 0.001). No other differences were significant after Bonferroni correction.
Table 1.
Baseline demographics comparing unplanned versus planned pregnancies.
| Unplanned | Planned | p value | |
|---|---|---|---|
| N | 20 | 43 | |
| Married (n, (%)) | 12 (60) | 42 (98) | <0.001 |
| Pre-pregnancy body mass index (mean (sd)) | 26.74 (9.12) | 25.80 (5.83) | 0.63 |
| Age at MS diagnosis (mean (sd)) | 25.32 (4.96) | 27.41 (5.07) | 0.13 |
| Age at delivery (mean (sd)) | 29.48 (5.55) | 33.04 (4.33) | 0.007 |
| Number of women who had at least one relapse in the pre-pregnancy year (n (%)) | 6 (30) | 17 (40) | 0.472 |
| Average ARR pre-pregnancy year (mean (sd)) | 0.55 (0.83) | 0.63 (0.98) | 0.759 |
| Number of women who had at least one relapse within the first post-partum year (n (%)) | 12 (60) | 21 (49) | 0.417 |
| Average ARR post-partum year (mean (sd)) | 0.60 (0.68) | 0.65 (0.90) | 0.822 |
| Duration of maternal MS at delivery (mean (sd)) | 4.16 (3.53) | 5.63 (3.64) | 0.139 |
| Assisted reproductive therapy (n (%)) | 0.0 (0.0) | 5 (12) | 0.116 |
| In vitro fertilization (n (%)) | 0 (0.0) | 2 (5.0) | 0.335 |
| On DMT during pregnancy (n (%)) | 13 (65) | 3 (7) | <0.001 |
| DMT (n (%)) | <0.001 | ||
| None | 7 (35.0) | 40 (93.0) | |
| Interferon beta-1a intramuscular | 5 (25.0) | 0 ( 0.0) | |
| Interferon beta-1a subcutaneous | 1 ( 5.0) | 0 ( 0.0) | |
| Interferon beta-1b subcutaneous | 0 ( 0.0) | 1 ( 2.3) | |
| Glatiramer acetate | 3 (15.0) | 2 ( 4.7) | |
| Azathioprine | 1 ( 5.0) | 0 ( 0.0) | |
| Dimethyl fumarate | 1 ( 5.0) | 0 ( 0.0) | |
| Natalizumab | 2 (10.0) | 0 ( 0.0) | |
| Caesarean section (n (%)) | 5 (25) | 19 (44) | 0.149 |
| Birth weight (mean (sd)) | 3.31 (0.51) | 3.13 (0.55) | 0.244 |
| Gestational age (mean (sd)) | 38.75 (1.21) | 38.52 (1.69) | 0.59 |
| Age of menarche (mean (sd)) | 12.78 (1.64) | 12.04 (1.24) | 0.17 |
| Gravida (mean (sd)) | 1.95 (1.15) | 1.77 (1.07) | 0.539 |
| Breast feed (n (%)) | 7 (35) | 27 (63) | 0.04 |
| Duration of breastfeeding in days (mean (sd)) | 44 (87) | 100 (128) | 0.079 |
| Time to restart DMT in days after delivery (mean (sd)) | 392 (684) | 389 (525) | 0.984 |
| Smoking (n (%)) | 6 (30) | 5 (12) | 0.076 |
| Race (n (%)) | 0.792 | ||
| Black | 1 (5.0) | 4 (9.3) | |
| Hispanic | 1 (5.0) | 3 (7.0) | |
| White | 18 (90.0) | 36 (83.7) | |
| EDSS pre-pregnancy (median [IQR]) | 2.25 [1.00, 2.50] | 2 [0.50, 2.50] | 0.319 |
| Post-partum EDSS (median [IQR]) | 2 [1.38, 3.00] | 2 [1.50, 2.50] | 0.712 |
| Change in EDSS (mean (sd)) | 0.07 (0.86) | 0.31 (0.89) | 0.319 |
| New T2 lesions on post-partum MRI (mean (sd)) | 1.20 (1.74) | 2.05 (1.78) | 0.082 |
| Enhancing lesions (mean (sd)) | 0.30 (0.80) | 1.10 (1.54) | 0.034 |
| New T2 lesions prior to conception (mean (sd)) | 0.89 (1.45) | 1.45 (1.70) | 0.22 |
| Enhancing lesions pre-pregnancy (mean (sd)) | 0.68 (1.38) | 0.43 (1.06) | 0.431 |
ARR: annualized relapse rate; DMT: disease modifying therapies; EDSS: Expanded Disability Status Scale; IQR: interquartile range; MRI: magnetic resonance imaging; MS: multiple sclerosis.
Figure 2.
Proportion of planned pregnancies depending on whether the patient received family planning counseling. The number of people who had received family planning and had an unplanned pregnancy versus those who had not received family planning.
Figure 3.
Proportion of planned pregnancies based on age. The rate of planned pregnancy based on whether the patient was younger or older than 30 years of age.
Figure 4.
Proportion of planned pregnancies based on marital status. The number of planned pregnancies based on whether the patient was married.
Starting with the simplest logistic regression models, those patients who did not receive family planning counseling had a substantially greater likelihood of having an unplanned pregnancy than those who received counseling (odds ratio (OR) = 20, 95% CI 4.5–148, p < 0.0001). The correlation coefficient from this model was R2 = 0.339 (p < 0.001).
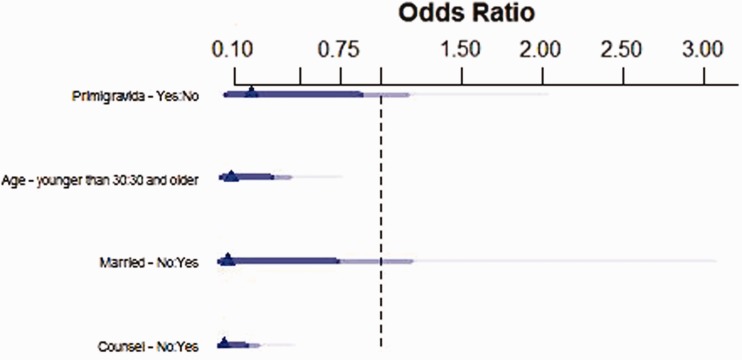
For the more complex model, the four covariates with the highest Spearman correlation coefficients where chosen: pregnancy number of the current pregnancy, counseling, marital status, and age ≥30 years at the time of delivery. In final models, those who did not receive family planning counseling had 0.028 times the odds of having a planned pregnancy than those who received counseling (p < 0.0011, Table 2 and Figure 5). Patients who were married at their first prenatal visit had 17.4 times the odds of having a planned pregnancy than those who were not married (p = 0.06). Those who entered their fourth decade of life had 13.9 times the odds of having a planned pregnancy compared to those who were younger (p < 0.01). Finally, patients who were pregnant for the first time had 0.2 times the odds of having a planned pregnancy compared to their multigravida counterparts (p = 0.07). The final model after a stepwise regression had a better predictive ability of unplanned pregnancy risk than the original model. The final model had an R2 of 0.634. This demonstrates that counseling was associated with a lower proportion of unplanned pregnancies.
Table 2.
Odds ratio of model components that predict planned pregnancies
| Odds ratio (95% CI) | |
|---|---|
| No family planning counseling | 0.028 (0.0024, 0.20) |
| Married at first prenatal visit | 17.4 (1.30, 664) |
| Age ≥30 | 13.9 (2.57, 105) |
| Prior pregnancy | 0.20 (0.03, 1.03) |
CI: confidence interval.
Figure 5.
Odds ratios for unplanned pregnancy from the complex post stepwise regression model predicting planned pregnancies. The triangle represents the odds ratio, dark blue line represents 90% confidence interval, medium blue line represents 95% confidence interval, and light blue line represents 99% confidence interval.
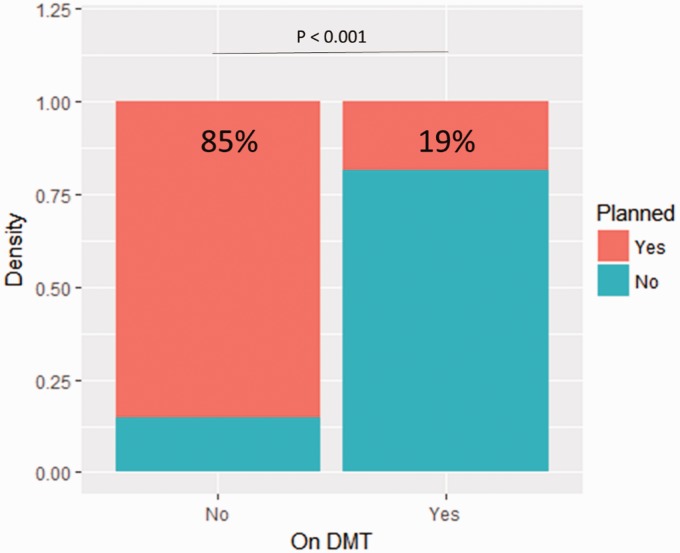
Figure 6.
Proportion of individuals on disease-modifying therapies (DMT) depending on their pregnancy status. The number of pregnancies exposed to DMT relative to whether the pregnancy was planned.
When examining the secondary outcome of being on DMT at pregnancy onset, those whose pregnancies were unplanned were 25 times (95% CI, 6.25–131) more likely to be on DMT while pregnant than those whose pregnancies were planned (p < 0.0001) (Figure 6). The DMTs used in the unplanned group consisted of nine patients on injectable therapies, one patient on azathioprine, one patient on dimethyl fumarate, and two on natalizumab. The three planned pregnancies were all exposed to injectable therapies. Of the 16 patients who became pregnant while on DMT, four were on birth control. When examining the birth outcomes of unplanned versus planned pregnancy status and DMT exposed and non-exposed patients, there were no statistically significant differences between the comparative groups.
Finally, when comparing the post-relapse rate and MRI outcomes, there was no difference in the rate of relapse whether the pregnancy was planned or unplanned (p = 0.11). Roughly 47% of women in the unplanned pregnancy group and 49% of women in the planned pregnancy group had an MRI within 1 year of their delivery. Among those receiving MRIs, there was an increased number of enhancing lesions in women with planned pregnancies (p = 0.034). Additionally, a higher percentage of women with planned pregnancies developed either new T2 lesions (p = 0.04) or enhancing lesions (p = 0.015) within a year after giving birth.
Discussion
MS is a disease that disproportionally affects women of reproductive potential. Therefore, research looking into reproductive health is a critical but sometimes forgotten aspect of clinical research and practice of medicine in general.36 The goal of the present study was to add to existing information concerning pregnancy and the importance of family planning for MS patients in a US population.
These results suggest an association exists between women who were counseled prior to becoming pregnant after an MS diagnosis and a lower proportion of unplanned pregnancies. One possible interpretation of the data would be that family planning counseling may lead to conscious choices by the patients to take steps to reduce their risk of unplanned pregnacies. However, the reverse interpretation may also be true, that MS patients who are already planning to become pregnant may be more likely to seek out family planning counseling. Given the retrospective design of this study and the limitations of a chart review, it is difficult to determine the temporal relationship between these variables.
The overall proportion of unplanned pregnancies in this cohort was 32%. Prior to this study, the number the unplanned pregnancies was assumed to be 50% in the US,37 based on the national average from non-MS patients. Given the make-up of this cohort, the age-corrected unplanned pregnancy rate in US MS patients should be 28.2%.38 Other known risk factors for unplanned pregnancy, such as age, race, marital status, and whether there was a prior pregnancy, were included in the model. Still, the importance of counseling patients remained. However, half of those patients with unplanned pregnancies were counseled. This observation suggests that either counseling alone is not effective, or that effective counseling needs to be discussed multiple times with the patient instead of mainly just at initial diagnosis and start of a new DMT. In this sample, most of those with unplanned pregnancies who were counseled only had family planning counseling documented once in the chart. Therefore, a possible intervention to address infrequent family planning counseling would be to include a brief review of reproductive goals during all clinical follow-up visits of reproductively able MS patients.
Another important result is the low proportion of birth control use among women on DMT who became pregnant. Of those who were on DMT when they became pregnant, 81% of those pregnancies were unplanned. Only four of 16 women who had DMT exposure during pregnancy were documented as being on highly effective forms of birth control (the pill or an intra-uterine device). This probably underestimates the true birth control usage rate in the unplanned pregnancy group due to not counting the use of barrier methods. This observation highlights the importance of not only discussing family planning and use of birth control when they are placed on DMT but also ensuring patients are using birth control correctly. Although neurologists may not be familiar with all of the options for birth control and their correct usage, they can either encourage patients to discuss these issues with their gynecologist or primary-care physician or make an appropriate referral. With most clinics implementing EMRs in regular practice, ensuring adequate birth control use while on DMT could be automated. For example, an automated alert could be triggered during an encounter when a woman is on or is prescribed a DMT or other teratogenic medication to remind the physician to provide family planning counseling.
We also found the rate of MRI activity within a year after giving birth appeared to be higher in the patients who had planned pregnancies. This result could suggest that women who stop DMT prior to pregnancy in order to conceive are more likely to have a relapse post-partum. Another explanation could be secondary to the higher rate of breastfeeding in the group with planned pregnancies as opposed to unplanned pregnancies. However, this question warrants further study. Additionally, we also found the percentage of patients having a relapse was greater in the year post partum when compared to the year prepartum in the studied population (37% versus 52%). Potential explanations of this difference could be due to multiple factors. The first was that 25% of the patients were on DMT at the time of pregnancy. The second is that the delay to restarting DMT was on average over a year. Considering that the pre- and post-annual relapse rate (ARR) were 0.605 and 0.634 respectively, this could suggest that annualized relapse rates were not substantially different between the two time epochs. Finally, there was no difference between planned and unplanned groups in regards to the percentage of patients who relapsed in the post-partum year and the ARR.
Another finding is that there were no differences in birth outcomes between the unplanned and planned pregnancies and between DMT exposed versus non-exposed pregnancies. This finding is unexpected due to the previously reported literature of the negative consequences of unplanned pregnancies.16 This may be due to the study being underpowered to detect these effects.
This study has several limitations. The most critical is that this was a single-center, retrospective chart review. The analysis depended on data available in the chart, which might be incomplete or inaccurate, thus leading to misclassification bias. For example, published evidence suggests that preventive counseling rates may be underreported in the medical chart by as much as as 30% compared to what is observed during a clinical encounter.39–41 Similarly, the difference in patient-initiated reproductive goals discussion may have led to a recording bias in charting family planning counseling among those actively planning to conceive. Another issue is that, although this was an extensive chart review, it only included the records in the Cleveland Clinic Health system. Moreover, because the charts were selected by ICD 9 and 10 codes for pregnancy and MS, this may have caused pregnanies that did not lead to live birth to be excluded. Some of the non-live birth outcomes, if they occurred early enough in the pregnancy, could have been coded only under ICD codes related to spontaneous or elective abortions and not under pregnancy ICD codes and therefore were missed in this retrospective data collection. Additionally, these non-live births could also have been missed if the care for these pregancies occurred at an outside facility. Another issue was that it was not possible to measure any family planning counseling that the patient may have received from outside sources, such as a pharmaceutical company patient assistance program, pharmicist, or other provider. It is also possible the relationship between family planning and unplanned pregnancy is non-causal. The presence of family planning as noted in the medical records analyzed may be a confounder related to women who are planning a pregnancy. Besides the concerns of errors and bias in the medical record notation when using chart extraction to measure the rate of preventitive counseling done in the clinic, some data were impossible to extract from the chart review. Ideally, these issues could be addressed by following a prospective cohort, which would allow data to be collected in a more controlled and systematic fashion. Furthermore, it would allow for increased recruitment due to patients not being required to receive all of their care within one medical system. Another limitation is that the cohort was small, and may have been underpowered to detect an effect for some of the exposures of interest such as birth outcomes and disease state outcomes. However, it should be noted that national pregnancy databases in patients with MS generally only number hundreds of patients, with Brazil’s database including 142 patients and Germany’s database including 335 patients.42,43 Therefore, for a single-center study to include data on 63 pregnancies in patients with MS does not seem too far out of line. The lower pregnancy number may be secondary to various issues such as women having completed their family goals prior to the diagnosis of MS or deciding not to become pregnant because of their disease or other social reasons.44 A final issue is that the study population that comes to a terteriary center may differ from the general MS clinic. In this retrospective study, approximately 26% of identified pregnancies were excluded due to lack of neurologic follow-up data. The relatively high drop-out rate reduces the overall strength of study findings. The fact that many patients had to be censored due to incomplete records could also introduce selection bias. These data did not allow estimation of population-based incidence and prevalence rates and the results therefore may suffer from selection bias, due to this patient population having potentially more aggressive disease, limiting generalizability.
To address these shortcomings, the next step would be to validate the results in a broader clinical setting. Replicating these data in different centers would strengthen the generalizibility of the results. Ultimately, a prospective multicenter study in the USA would be optimal to define and control factors of interest in patients with MS with planned or unplanned pregancies. Ideally, this can be accomplished by establishing a new pre-pregnancy registry in patients with MS or partnering with an existing research collaborative.
Conflict of Interests
Dr Andrew Smith serves as a consultant or speaker for Bayer, Biogen, Genentech, Genzyme, EMD Serono and Novartis.
Jeffrey Cohen reports personal compensation for consulting for Alkermes, Biogen, Convelo, EMD Serono, ERT, Gossamer Bio, Mapi, Novartis, Pendopharm, and ProValuate; speaking for Mylan and Synthon; and serving as a Co-Editor of Multiple Sclerosis Journal – Experimental, Translational and Clinical.
Dr. Mary Rensel serves as a consultant or speaker for Biogen, Teva, Genzyme, Serono and Novartis. She receives grant funding from National Multiple Sclerosis Society (NMSS).
Dr Daniel Ontaneda reports receiving received research support from National Multiple Sclerosis Society, National Institutes of Health, Patient Centered Outcomes Research Institute, Race to Erase MS Foundation, Genentech, Novartis, and Genzyme. He has also received consulting fees from Biogen Idec, Genentech/Roche, Genzyme, and Merck.
Funding
This research was funded by National Multiple Sclerosis Society through the Sylvia Lawry Physician Fellowship.
ORCID iDs
Andrew L Smith https://orcid.org/0000-0001-8499-4820 Jeffrey A Cohen https://orcid.org/0000-0001-9245-9772 Daniel Ontaneda https://orcid.org/0000-0002-2838-9148
References
- 1.Ma VY, Chan L, Carruthers KJ. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch Phys Med Rehabil 2014; 95: 986–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol 2010; 9: 520–532. [DOI] [PubMed] [Google Scholar]
- 3.Wallin MT, Culpepper WJ, Campbell JD, et al. The prevalence of MS in the United States: A population-based estimate using health claims data. Neurol 2019; 92: e1029–e1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orton SM, Herrera BM, Yee IM, et al. Sex ratio of multiple sclerosis in Canada: A longitudinal study. Lancet Neurol 2006; 5: 932–936. [DOI] [PubMed] [Google Scholar]
- 5.Kalincik T, Vivek V, Jokubaitis V, et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain 2013; 136: 3609–3617. [DOI] [PubMed] [Google Scholar]
- 6.Bruck W, Stadelmann C. Inflammation and degeneration in multiple sclerosis. J Neurol Sci 2003; 24: S265–267. [DOI] [PubMed] [Google Scholar]
- 7.McKay KA, Kwan V, Duggan T, et al. Risk factors associated with the onset of relapsing-remitting and primary progressive multiple sclerosis: A systematic review. Biomed Res Int 2015; 2015: 817238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leray E, Yaouanq J, Le Page E, et al. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010; 133: 1900–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Confavreux C, Vukusic S, Adeleine P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain 2003; 126: 770–782. [DOI] [PubMed] [Google Scholar]
- 10.Freedman MS . Multiple sclerosis therapeutic strategies: Use second-line agents as first-line agents when time is of the essence. Neurol Clinical Practice 2011; 1(1): 66–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thiel S, Langer-Gould A, Rockhoff M, et al. Interferon-beta exposure during first trimester is safe in women with multiple sclerosis: A prospective cohort study from the German Multiple Sclerosis and Pregnancy Registry. Mult Scler 2016; 22: 801–809. [DOI] [PubMed] [Google Scholar]
- 12.Geissbuhler Y, Vile J, Koren G, et al. Evaluation of pregnancy outcomes in patients with multiple sclerosis after fingolimod exposure. Ther Adv Neurol Disord 2018; 11: 1756286418804760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tisovic K, Amezcua L. Women's health: Contemporary management of MS in pregnancy and post-partum. Biomedicines 2019; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbstritt S, Langer-Gould A, Rockhoff M, et al. Glatiramer acetate during early pregnancy: A prospective cohort study. Mult Scler 2016; 22: 810–816. [DOI] [PubMed] [Google Scholar]
- 15.Sandberg-Wollheim M, Neudorfer O, Grinspan A, et al. Pregnancy outcomes from the branded Glatiramer Acetate Pregnancy Database. Int J MS Care 2018; 20: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shah PS, Balkhair T, Ohlsson A, et al. Intention to become pregnant and low birth weight and preterm birth: A systematic review. Matern Child Health J 2011; 15: 205–216. [DOI] [PubMed] [Google Scholar]
- 17.Goyal V, Borrero S, Schwarz EB. Unintended pregnancy and contraception among active-duty servicewomen and veterans. Am J Obstet Gynecol 2012; 206: 463–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zapata LB, Tregear SJ, Tiller M, et al. Impact of reminder systems in clinical settings to improve family planning outcomes: A systematic review. Am J Prev Med 2015; 49: S57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moss DA, Snyder MJ, Lu L. Options for women with unintended pregnancy. Am Fam Physician 2015; 91: 544–549. [PubMed] [Google Scholar]
- 20.Connery HS, Albright BB, Rodolico JM. Adolescent substance use and unplanned pregnancy: Strategies for risk reduction. Obstet Gynecol Clin North Am 2014; 41: 191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henshaw SK. Unintended pregnancy in the United States. Fam Plann Perspect 1998; 30: 24–29, 46. [PubMed] [Google Scholar]
- 22.Lu E, Wang BW, Guimond C, et al. Disease-modifying drugs for multiple sclerosis in pregnancy: A systematic review. Neurol 2012; 79: 1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rasmussen PV, Magyari M, Moberg JY, et al. Patient awareness about family planning represents a major knowledge gap in multiple sclerosis. Mult Scler Relat Disord 2018; 24: 129–134. [DOI] [PubMed] [Google Scholar]
- 24.Hellwig K, Brune N, Haghikia A, et al. Reproductive counselling, treatment and course of pregnancy in 73 German MS patients. Acta Neurol Scand 2008; 118: 24–28. [DOI] [PubMed] [Google Scholar]
- 25.Sedgh G, Singh S, Hussain R. Intended and unintended pregnancies worldwide in 2012 and recent trends. Stud Fam Plann 2014; 45: 301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harden CL, Meador KJ, Pennell PB, et al. Management issues for women with epilepsy: Focus on pregnancy (an evidence-based review): II. Teratogenesis and perinatal outcomes: Report of the Quality Standards Subcommittee and Therapeutics and Technology Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009; 50: 1237–1246. [DOI] [PubMed] [Google Scholar]
- 27.Veroniki AA, Rios P, Cogo E, et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: A systematic review and network meta-analysis. BMJ Open 2017; 7: e017248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughn C, Bushra A, Kolb C, et al. An update on the use of disease-modifying therapy in pregnant patients with multiple sclerosis. CNS Drugs 2018; 32: 161–178. [DOI] [PubMed] [Google Scholar]
- 29.Ebrahimi N, Herbstritt S, Gold R, et al. Pregnancy and fetal outcomes following natalizumab exposure in pregnancy: A prospective, controlled observational study. Mult Scler 2015; 21: 198–205. [DOI] [PubMed] [Google Scholar]
- 30.Gerard EE, Meador KJ. Managing epilepsy in women. Continuum (Minneap Minn) 2016; 22: 204–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernandez Liguori N, Klajn D, Acion L, et al. Epidemiological characteristics of pregnancy, delivery, and birth outcome in women with multiple sclerosis in Argentina (EMEMAR study). Mult Scler 2009; 15: 555–562. [DOI] [PubMed] [Google Scholar]
- 32.Friend S, Richman S, Bloomgren G, et al. Evaluation of pregnancy outcomes from the Tysabri(R) (natalizumab) pregnancy exposure registry: A global, observational, follow-up study. BMC Neurol 2016; 16: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kieseier BC, Benamor M. Pregnancy outcomes following maternal and paternal exposure to teriflunomide during treatment for relapsing-remitting multiple sclerosis. Neurol Ther 2014; 3: 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wundes A, Pebdani RN, Amtmann D. What do healthcare providers advise women with multiple sclerosis regarding pregnancy? Mult Scler Int 2014; 2014: 819216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.RRStudio T.: Integrated Development for R. 3.5.3 ed.: RStudio, Inc., 2018.
- 36.Gawron LM, Hammond C, Keefer L. Documentation of reproductive health counseling and contraception in women with inflammatory bowel diseases. Patient Educ Couns 2014; 94: 134–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karlsson G, Francis G, Koren G, et al. Pregnancy outcomes in the clinical development program of fingolimod in multiple sclerosis. Neurol 2014; 82: 674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mosher WD, Jones J, Abma JC. Intended and unintended births in the United States: 1982–20 . Natl Health Stat Report 2012: 1–28. [PubMed] [Google Scholar]
- 39.Dresselhaus TR, Peabody JW, Lee M, et al. Measuring compliance with preventive care guidelines: Standardized patients, clinical vignettes, and the medical record. J Gen Intern Med 2000; 15: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Conroy MB, Majchrzak NE, Silverman CB, et al. Measuring provider adherence to tobacco treatment guidelines: A comparison of electronic medical record review, patient survey, and provider survey. Nicotine Tob Res 2005; 7: S35–43. [DOI] [PubMed] [Google Scholar]
- 41.Luck J, Peabody JW, Dresselhaus TR, et al. How well does chart abstraction measure quality? A prospective comparison of standardized patients with the medical record. Am J Med 2000; 108: 642–649. [DOI] [PubMed] [Google Scholar]
- 42.Finkelsztejn A, Fragoso YD, Ferreira ML, et al. The Brazilian database on pregnancy in multiple sclerosis. Clin Neurol Neurosurg 2011; 113: 277–280. [DOI] [PubMed] [Google Scholar]
- 43.Hellwig K, Haghikia A, Rockhoff M, et al. Multiple sclerosis and pregnancy: Experience from a nationwide database in Germany. Ther Adv Neurol Disord 2012; 5: 247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alwan S, Yee IM, Dybalski M, et al. Reproductive decision making after the diagnosis of multiple sclerosis (MS). Mult Scler 2013; 19: 351–358. [DOI] [PubMed] [Google Scholar]