Abstract
Background:
In therapeutic cancer vaccination, monocyte-derived dendritic cells (moDCs) efficiently activate specific T-cell responses; however, optimizing the activation of innate immune cells could support and improve the antitumor effects. A major disadvantage of moDCs matured with the standard cytokine cocktail (consisting of IL-1β, IL-6, TNFα, and PGE2) is their inability to secrete IL-12p70. IL-12 prominently activates natural killer (NK) cells, which are crucial in innate antitumor immunity, as they act as helper cells for the induction of a cytotoxic T lymphocyte (CTL) response and are also able to directly kill the tumor.
Methods:
Previously we have shown that triggering the NF-κB pathway in moDCs by transfection of mRNA encoding constitutively active IKKβ (caIKKβ) led to IL-12p70 secretion and improved the dendritic cells’ capability to activate and expand CTLs with a memory-like phenotype. In this study, we examined whether such dendritic cells could activate autologous NK cells.
Results:
moDCs matured with the standard cytokine cocktail followed by transfection with the caIKKβ-RNA were able to activate autologous NK cells, detected by the upregulation of CD54, CD69, and CD25 on the NK cells, their ability to secrete IFNγ, and their high lytic activity. Moreover, the ability of NK-cell activation was not diminished by simultaneous T-cell activation.
Conclusion:
The capacity of caIKKβ-DCs to activate both the adaptive and innate immune response indicates an enhanced potential for clinical efficacy.
Keywords: adoptive cellular immunotherapy, dendritic cells, interleukin-12, natural killer cells, NF-κB
Introduction
Dendritic cells (DCs) play a vital role in the immune system. They build the bridge between the adaptive and innate immune system because they can activate both T cells via major histocompatibility complex (MHC) presentation of antigens in conjunction with co-stimulatory signals1 and the innate immune system such as NK cells.2 Therefore, DCs have been used for therapeutic tumor vaccination with the primary goal of activating cytotoxic T lymphocytes (CTLs) to enable elimination of tumor cells.3 Recently, evidence emerged that not only adaptive immune responses, but also the activation of the innate immune system is important to fight against the malignant tissue.4,5 NK cells activated by vaccine DCs can: (a) induce the maturation of further DCs,6,7 which in turn leads to additional activation of CTLs in a CD4+ T cell-independent manner,8 (b) directly activate additional naïve T cells through IFNγ secretion,9 and (c) attack and directly kill tumor cells,10 which can then lead to a T-cell cross-presentation of released tumor material by DCs.11
The standard protocol for cancer vaccination generates DCs from monocytes by incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4 over 6 days.12,13 These immature DCs are usually matured using a standard cytokine cocktail consisting of TNFα, prostaglandin E2 (PGE2), IL-1β, and IL-6.14 However, so far the efficacy of tumor vaccination with these DCs, like other cancer vaccines, is limited and behind expectations when used as monotherapy.15 Therefore, different strategies for improvement are currently under investigation including combinations with checkpoint inhibitors, use of optimal tumor antigens, and increase of the immunostimulatory capacity of moDCs.
We16 and others17,18 have already observed, that a limitation of the standard maturation protocol is that the generated DCs spontaneously secrete only low concentrations of IL-12p70. This cytokine plays a pivotal role in the induction of T cell-mediated immune responses19 and also in the activation of NK cells.20 Consequently, additional factors either apart from or in addition to the standard maturation cocktail, are needed to more efficiently activate DCs.
A key player in the process of DC activation is the transcription factor NF-κB, which can be activated through the classical and the alternative pathways. The classical NF-κB pathway is induced through different danger signals, for example, via pro-inflammatory cytokines or activation of Toll-like receptors (TLRs),21 which then results in the activation of specific target genes. After receiving the activation signal, the IκB kinase (IKK) complex (IKKα, IKKβ, and IKKγ, the latter also called NEMO) phosphorylates IκB, which then releases NF-κB (consisting of RelA and p50).22 NF-κB then translocates into the nucleus to activate its target genes,23 such as for example, IL-12.
The standard maturation cocktail already activates the NF-κB pathway in DCs,24 but not to its full potential. Regarding the different strategies to improve DC vaccination, the NF-κB pathway is regularly involved, for example, through transfection of CD40 ligand25 or the use of different TLR agonists,26–29 the latter employing a combination of CD40 ligand, CD70 and constitutively active (ca)TLR4 (TriMix). Massa and co-authors used IFNγ together with monophosphoryl lipid A (MPLA) as an alternative maturation cocktail, which activates NF-κB, and led to DCs with the ability to secrete IL-12p70 and also to activate both innate and adaptive immune responses.17
We used a stabilized and constitutively active mutant of IKKβ as a direct and supplementary activation signal for the NF-κB pathway. To this end, we transfected caIKKβ-encoding mRNA by electroporation into DCs matured with the standard cytokine cocktail.16,30 This procedure resulted in DCs with an increased activation status and the ability to secrete IL-12p70. Moreover, these DCs activated T cells with a higher lytic capacity and a memory-like phenotype.16 In this study, we investigated whether the activation of the NF-κB pathway creates DCs that can also more effectively activate NK cells.
Materials and methods
Cells
Blood was obtained from healthy donors following informed consent and approval by the institutional review board (Ethikkommission der Friedrich-Alexander-Universität Erlangen Nürnberg, Ref. no. 4158), and peripheral blood mononuclear cells (PBMCs) were isolated using density centrifugation with Lymphoprep (Axis-Shield PoC AS, Oslo, Norway) as described previously.31 To generate moDCs, monocytes were separated first from the nonadherent fraction (NAF) by plastic adherence and differentiated to immature DCs over 6 days in DC medium consisting of RPMI 1640 (Lonza, Verviers, Belgium) supplemented with 1% nonautologous human plasma (Sigma-Aldrich, St. Louis, United States), 2 mM L-glutamine (Lonza), and 20 mg/l gentamycin (Lonza), adding fresh DC medium with GM-CSF (800 IU/ml; Miltenyi Biotec, Bergisch Gladbach, Germany) and IL-4 (250 IU/mL; Miltenyi Biotec) on days 1, 3, and 5, as described previously.31 On day 6, DCs were matured using the standard cytokine cocktail consisting of 200 IU/ml IL-1β (CellGenix, Freiburg, Germany), 1000 IU/ml IL-6 (Miltenyi Biotec), 10 ng/ml TNFα (Beromun, Boehringer Ingelheim Pharma, Germany), and 1 μg/ml PGE2 (Pfizer, Zurich, Switzerland). DCs were electroporated after 24 h of maturation.
NK cells were isolated from autologous PBMCs via negative selection using the Human NK cell Enrichment Set-DM (BD Biosciences, Heidelberg, Germany) according to the manufacturer’s description.
Cells were incubated at 37°C with 5% CO2 unless stated otherwise.
In vitro RNA transcription and electroporation of DCs
In vitro transcription of mRNA was carried out using the mMESSAGE mMACHINE™ T7 ULTRA Transcription Kit (Life Technologies, Carlsbad, CA, USA) and purified with an RNeasy Kit (Qiagen, Hilden, Germany) according to the manufacturers’ protocols. The RNA used for electroporation encoded a constitutively active mutant of IKKβ,16 which activates the classical NF-κB pathway. In a total volume of 100 µl, 6 × 106 DCs were electroporated with 30 µg caIKK-RNA or 5 µg EGFP-RNA or as a control mock electroporated using a square-wave pulse, 1 ms, and 1250 V/cm as recently described in detail.32
Co-cultures
Transfected DCs were harvested 2–4 h after electroporation and were either directly used for co-culture experiments or were pulsed with 10 µg/ml MelanA EAAGIGILTV-peptide (GenScript, Leiden, Netherlands) for 1 h and then used for co-culture. Donors employed for peptide pulsing were haplo-typed HLA-A0201.
DCs were co-cultured with fresh autologous PBMCs or purified NK cells at the indicated ratios and incubated in MLPC medium consisting of RPMI 1640 (Lonza), 10% nonautologous human serum (Sigma-Aldrich), 2 mM L-glutamine (Lonza), 20 mg/l gentamycin (Lonza), 10 mM HEPES (PAA Laboratories, GE Healthcare Life Sciences, Pasching/Linz, Austria), 1 mM sodium pyruvate (Lonza), and 1% nonessential amino acid (100×; Lonza), whereas PBMCs and NK cells only cultured in the respective medium served as control. For co-incubations at ratios of 1:2, 1 × 106 DCs/ml and 2 × 106 PBMCs/ml were seeded in a 24- or 48-well, depending on cell numbers, while for co-incubations at a ratio of 1:10 the final concentrations were 2 × 105 DCs/ml and 2 × 106 PBMCs/ml. DCs and NK cells were co-cultured at ratios of 5:1 and 1:1. For co-incubations at a ratio of 5:1, the final concentrations were 1 × 106 DCs/ml and 2 × 105 NK cells/ml. For co-incubations at a ratio of 1:1, the final concentrations were 1 × 106 DCs/ml and 1 × 106 NK cells/ml. PBMCs or NK cells cultured alone served as control.
Cells were harvested after 24 h, 48 h, and after 1 week of co-incubation, whereas supernatants were taken after 24 h and 48 h. Co-cultures over 1 week were split and fresh medium was added depending on their expansion rate.
Transwell analysis
To separate the cell populations from each other while allowing the transfer of soluble factors, transwell polycarbonate membrane cell culture inserts (Corning Incorporated, New York, United States) were used. Transfected DCs and freshly isolated PBMCs were counted and resuspended in 1 × 106 cells/ml and 2 × 106 cells/ml in MLPC medium, respectively. Of these suspensions, 350 µl DCs were seeded in a 24-well plate, either alone, adding 250 µl MLPC medium, or together with 250 µl PBMCs. After adding the membrane (pore size: 0.4 µm) 100 µl PBMCs were seeded in the upper compartment. We harvested cells after 48 h from both the upper and lower compartment and supernatant was taken.
Cell surface marker analysis
Cells were harvested after 24 h, 48 h, and 1 week. The expression of surface markers was analyzed by flow cytometry using anti-CD80-FITC, anti-CD70-PE, anti-CD40-PE and their corresponding isotype controls, and anti-CD56-FITC, anti-CD3-APC-Cy7 or anti-CD3-V500, anti-CD69-PE, anti-CD25-BV421 or anti-CD25-PE, and anti-CD54-APC or anti-CD54-PE (all from BD Biosciences) as recently described.33 Immunofluorescence was measured using a FACS Canto II (BD Biosciences), data were acquired with FACSDiva software (BD Biosciences) and evaluated with FCS Express software, version 5 (DeNovo Software). An average of approximately 6500 NK cells per measurement was acquired, with a minimum of 500 and a maximum of 23,000 cells.
MHC-tetramer staining
Co-cultures containing peptide-pulsed DCs and PBMCs at a ratio of 1:10 (final concentrations 2 × 105 DCs/ml and 2 × 106 PBMCs/ml) were harvested after 1 week. Cultures with DCs that had not been peptide-pulsed served as controls. T cells specific for the MelanA peptide were detected with HLA-A0201-PE ELAGIGILTV tetramer (produced in house according to Rodenko et al.34). Harvested cells were stained with the tetramer for 15 min, then anti-CD3-APC-H7, anti-CD4-AlexaFluor700, anti-CD8-PE-Cy7, anti-CD56-BV421, anti-CD16-FITC, anti-CD69-APC, and anti-CD27-BUV395 (all from BD Bioscience) were added and incubated for another 20 min. After washing twice with phosphate buffered saline, cells were acquired on a FACS Fortessa (BD Bioscience). CD8+ T cells and NK cells were distinguished and characterized via expression of CD3, CD56, CD8, and CD69. The gating strategy is depicted in Supplemental Figure S8.
Cytokine secretion analysis
The supernatants of the co-cultures were taken after 24 h and 48 h of incubation. Cytokine concentrations were determined using the Human Th1/Th2 Cytometric Bead Array Kit II (BD Biosciences) or the Human Inflammatory Cytometric Bead Array Kit (BD Biosciences) following the manufacturer’s instructions. Immunofluorescence was measured using a FACS Canto II (BD Biosciences), data were acquired with FACSDiva software (BD Biosciences) and evaluated with FCS Express software, version 5 (DeNovo Software).
To illustrate cytokine secretion on a per cell level, we normalized each cytokine concentration to cell number. We calculated the IL-12p70 secretion per 106 DCs as follows: the cytokine concentration of IL-12p70 was multiplied by 2 for the conditions Mock only, Mock 1:2, caIKKβ only, and caIKKβ 1:2, or multiplied by 10 for the conditions Mock 1:10 and caIKKβ 1:10. The TNFα and IFNγ secretion per 106 NK cells was calculated as follows: the average cytokine concentration of IFNγ and TNFα was multiplied by 2 for the conditions NK only, Mock 1:1, and caIKKβ 1:1, or multiplied by 10 for the conditions Mock 5:1 and caIKKβ 5:1.
Cytotoxicity assay
The cytolytic capacity of NK cells was determined after 1 week of co-incubation with DCs in a standard 4–6 h 51Cr release assay as described previously.35 Briefly, the target cell line K562 was labeled with 100 μCi of Na251CrO4/106 cells. Target cells were washed and subsequently cultured in 96-well plates (Thermo Fisher, Waltham, MA, USA) at 1000 cells/well. The labelled cells were then incubated with titrated amounts of effector cells (E:T ratios of 20:1, 6:1, 2:1, and 0.6:1) for 4–6 h, followed by collection of supernatants for measurement of released chromium concentrations using the Wallac 1450 MicroBeta plus Scintillation Counter (Wallac, Turku, Finland). The percentage of lysis was calculated using the following formula:
Statistical analysis
For the creation of graphs and statistical analysis GraphPad Prism, version 7 (GraphPad Software, La Jolla, USA) was employed. p values were determined comparing the respective conditions (for DC and PBMC co-cultures: Mock 1:2 + caIKKβ 1:2 and Mock 1:10 + caIKKβ 1:10; for DC and NK co-cultures: Mock 5:1 + caIKKβ 5:1, Mock 1:1 + caIKKβ 1:1) using the paired Student’s t test assuming a Gaussian distribution. It should be mentioned that not all formal requirements for the paired Student’s t test are fulfilled here: owing to our limited sample sizes, normal distribution cannot be tested. On the other hand, when we performed very similar experiments with more donors in the past, we usually observed a Gaussian distribution. In addition, Student’s t test is rather robust, even if this criterion is mildly violated.36
Results
Stimulation with caIKKβ-transfected mature DCs leads to the upregulation of activation markers on NK cells
Electroporation of caIKKβ mRNA in DCs matured with the standard cytokine cocktail leads to efficient activation of the classical NF-κB pathway, resulting in an enhanced activation state of mature DCs16 accompanied by a more long-lasting and higher stimulation capacity towards T cells.16 Transfection efficiency of DCs with mRNA is generally very robust with over 90% positive cells37 (Supplemental Figure S1A). The activation of the NF-κB pathway through caIKKβ-mRNA transfection is demonstrated by the upregulation of several activation markers such as CD70, CD80, and CD40 in the whole population of the DCs (Supplemental Figure S1B).
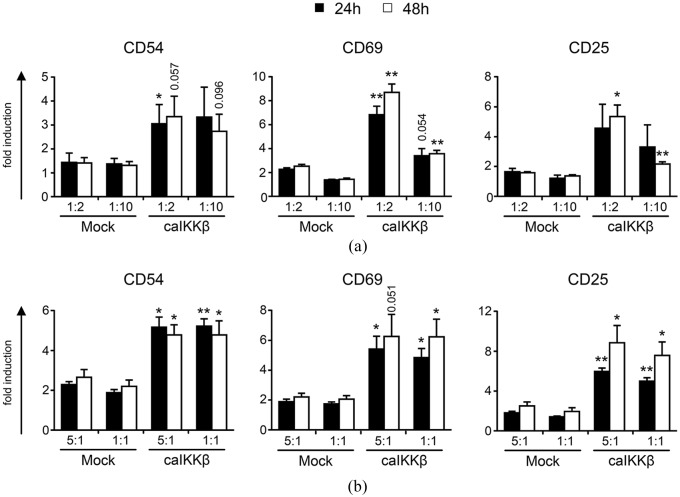
To analyze whether DCs transfected with caIKKβ could also trigger the activation of NK cells, caIKKβ-transfected DCs or mock-transfected DCs were co-cultured with PBMCs at a cell ratio of 1:2 and 1:10, for 24 h, 48 h, and 1 week. PMBCs cultured in the absence of DCs served as control. At the indicated time points cells were stained with antibodies directed against CD56 and CD3 to define CD3–CD56+ NK-cells as indicated in Supplemental Figure S2. NK-cell activation was assessed by measuring the expression of CD25, CD54, and CD69 by flow cytometry. These markers are well known to be upregulated on activated NK cells.38,39
On NK cells stimulated with caIKKβ-DCs, the activation markers CD54, CD69, and CD25 were upregulated significantly, in comparison with NK cells stimulated with mock-electroporated DCs (Figure 1(a) and Supplemental Figure S3). The expression of CD54 on NK cells stimulated with caIKKβ-transfected DCs was about twice as high as on NK cells stimulated with mock-electroporated DCs at both cell ratios of 1:2 and 1:10, reaching significance after 24 h at a DC/PBMC ratio of 1:2 (Figure 1(a)). In conditions with a ratio of 1:2, the expression of CD25 was also significantly higher on NK cells stimulated with caIKKβ-DCs after 48 h. The strongest effect was observed for CD69 with up to eightfold increased expression levels on NK cells co-incubated with caIKKβ-transfected DCs at a DC/PBMC ratio of 1:2. In contrast, the CD69 expression on NK cells co-cultured with mock-DCs only increased up to twofold (Figure 1(a)) when compared with the background mean fluorescence intensity (MFI) of PBMCs that were cultured alone. The expression of CD69 and CD25 on NK cells stimulated with caIKKβ-DCs at a cell ratio of 1:10 was upregulated to a lesser extent, and reached significance after 48 h (Figure 1(a)).
Figure 1.
Stimulation with caIKKβ-transfected mature dendritic cells (DCs) results in the upregulation of activation markers on NK cells.
Cytokine-matured DCs were electroporated either with caIKKβ-RNA or as a control were mock electroporated. (a) Transfected DCs were co-cultured with fresh autologous peripheral blood mononuclear cells (PBMCs) 2–4 h after electroporation at a ratio of 1:2 (final concentrations: 1 × 106 DCs/ml and 2 × 106 PBMCs/ml) or 1:10 (final concentrations: 2 × 105 DCs/ml and 2 × 106 PBMCs/ml). To determine background levels, PBMCs were cultured alone. Cells were harvested after 24 h or 48 h and the expression of the surface markers CD54, CD69, and CD25 was determined via flow cytometry (using the gating strategy shown in Supplemental Figure S2). All values show the upregulation of each surface marker, calculated relative to the mean fluorescence intensity (MFI) of PBMCs alone. The average fold induction of four different donors with the SEM is shown; for original data, see Supplemental Table S1. Each donor was analyzed in independent experiments. (b) DCs were co-cultured with fresh autologous NK cells at a ratio of 5:1 (final concentrations: 1 × 106 DCs/ml and 2 × 105 NK cells/ml) or 1:1 (final concentrations: 1 × 106 DCs/ml and 1 × 106 NK cells/ml). To determine background levels, NK cells were cultured alone. Cells were analyzed as described in (a). Average fold induction (relative to MFI of NK cells alone) is shown from four different donors with SEM; for original data see Supplemental Table S2. p values were calculated to the respective mock condition with the paired Student’s t test using the specific MFI values, **p ⩽ 0.01, *p ⩽ 0.05, numbers indicate p value of 0.05 ⩽ p ⩽ 0.1.
After showing that caIKKβ-DCs activated NK cells in co-culture with PBMCs, we studied whether this activation was also possible with purified NK cells, or if bystander cells were necessary. For this, transfected DCs were co-cultured with purified NK cells at a cell ratio of 5:1 and 1:1 or, to measure background expression levels, NK cells were cultured alone. Indeed, again all three activation markers on the NK cells were highly and significantly upregulated by stimulation through caIKKβ-DCs (Figure 1(b) and Supplemental Figure S4).
The upregulation of the activation markers CD54, CD69, and CD25 on NK cells was preserved up to 1 week of co-incubation with caIKKβ-DCs, however only reaching significance for CD54 at a ratio of 1:1 (Supplemental Figure S5A). After 1 week of incubation, NK cells incubated alone did not sufficiently survive, whereas NK cells co-cultured with mock or caIKKβ-DCs were able to persist during this period. After 1 week of co-culture, NK cells stimulated with caIKKβ-DCs changed their morphology and increased in size (Supplemental Figure S5B). In addition, the intensity of the CD56 expression increased (Supplemental Figure S5C) indicating a superior activation state of these NK cells, compared with the controls. Thus, caIKKβ-DCs were well capable of activating NK cells and this activation occurred independently of bystander cells.
The presented data support the hypothesis that caIKKβ-DC enhance NK-cell activation, based on CD25, CD54, and CD69 expression, but further research is necessary to determine the direct effects of caIKKβ-DCs on the expression CD16 and natural cytotoxicity receptors, including NKG2D.
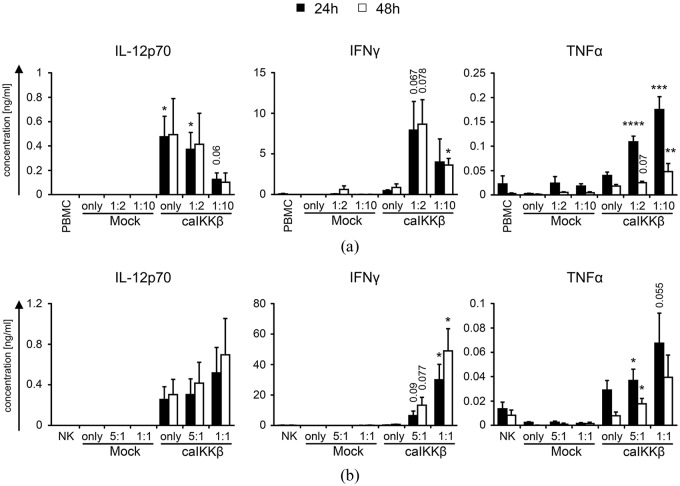
Stimulation of NK cells with caIKKβ-DCs leads to the secretion of pro-inflammatory cytokines
To determine which cytokines are involved in the stimulation of NK cells by cytokine-matured DCs, in which the NF-κB pathway was activated, caIKKβ-electroporated DCs or mock-electroporated DCs were either cultured alone, or co-cultured with PBMCs at a DC/PBMC ratio of 1:2 and 1:10. As an additional control, PBMCs were cultured in the absence of DCs. Supernatants were harvested after 24 h or 48 h and the secretion of different cytokines was determined via a cytometric bead array.
We16 and others17 have previously shown that DCs matured with the standard cytokine cocktail do not sufficiently secrete IL-12p70. However, activation of the NF-κB pathway in such DCs enabled the secretion of this cytokine was shown by our group16 and also in Figure 2(a) and (b). As IL-12p70 is an important cytokine for the activation of NK cells,17 we studied whether caIKKβ-DCs have a positive effect on NK-cell activation. A reason for the lower IL-12p70 concentration in the DC/PBMC co-cultures at a cell ratio of 1:10 compared with the DC/PBMC co-cultures at a cell ratio of 1:2 could simply be the presence of fewer DCs in the former. To demonstrate this, the IL-12p70 production was normalized to DC numbers which showed a constant production of approximately 1 ng IL-12p70 per 106 DCs (Supplemental Figure S6A).
Figure 2.
caIKKβ-DCs induce NK cells to secrete pro-inflammatory cytokines.
Cytokine-matured dendritic cells (DCs) were either electroporated with RNA encoding caIKKβ or as a control were mock electroporated. (a) Transfected DCs were co-cultured 2–4 h after electroporation with fresh autologous peripheral blood mononuclear cells (PBMCs) at a ratio of 1:2 (final concentrations: 1 × 106 DCs/ml and 2 × 106 PBMCs/ml) or 1:10 (final concentrations: 2 × 105 DCs/ml and 2 × 106 PBMCs/ml). As controls, PBMCs and DCs were cultured alone. Secretion of IL-12p70, TNFα, and IFNγ was measured in the supernatant by Cytometric Bead Array after 24 h and 48 h of co-incubation. Average cytokine concentrations with SEM are shown from 7 (24 h) or 4 (48 h) different donors; for original data see Supplemental Table S3. (b) Transfected DCs were co-cultured with fresh autologous NK cells at a ratio of 5:1 (final concentrations: 1 × 106 DCs/ml and 2 × 105 NK cells/ml) or 1:1 (final concentrations: 1 × 106 DCs/ml and 1 × 106 NK cells/ml). As controls, NK cells and DCs were cultured alone. Cytokine secretion was measured as described in (a). Average cytokine concentrations are shown from 5 (24 h) or 4 (48 h) different donors; for original data see Supplemental Table S4. p values were calculated to the respective mock condition with the paired Student’s t test, ****p ⩽ 0.0001, ***p ⩽ 0.001, **p ⩽ 0.01, *p ⩽ 0.05, numbers indicate p values of 0.05 ⩽ p ⩽ 0.1.
As IFNγ and TNFα are strongly secreted by activated NK cells, it was analyzed whether both cytokines were secreted after NK cell stimulation with caIKKβ-DCs, known to produce intermediate amounts of TNFα, but not IFNγ.16,30 Both cytokines were barely secreted in co-cultures with mock-transfected DCs, whereas high and significant quantities were detected in co-cultures with caIKKβ-DCs (Figure 2(a)). IFNγ was hardly secreted by caIKKβ-DCs alone, or by PBMCs alone, but was highly secreted in co-cultures stimulated with caIKKβ-DCs after 24 h and 48 h, especially at a DC/PBMC ratio of 1:2, barely missing the level of significance (Figure 2(a)). After 24 h, a low concentration of TNFα was detected in co-cultures with mock-transfected DCs, caIKKβ-DCs, and PBMCs alone, but it was strongly and significantly secreted in co-cultures with caIKKβ-electroporated DCs, at both DC/PBMC ratios of 1:2 and 1:10 (Figure 2(a)). After 48 h TNFα secretion decreased (Figure 2(a)). IL-10 was only secreted in marginal amounts in each condition (data not shown).
To address whether bystander cells produced the IFNγ and TNFα, transfected DCs were co-cultured with purified NK cells at a DC/NK cell ratio of 5:1 and 1:1 and cytokine concentrations were determined in the supernatant. In co-cultures with purified NK cells and caIKKβ-electroporated DCs, IFNγ was still very efficiently and significantly secreted at a cell ratio of 1:1 (Figure 2(b)). TNFα was still secreted at higher concentrations in co-cultures of purified NK cells with caIKKβ-DCs (barely missing significance at a ratio of 1:1 after 24 h) compared with NK cells with mock DCs, but in lower concentrations compared with co-incubations of PMBCs and caIKKβ-DCs (Figure 2(b)). The large difference in cytokine secretion levels between DC/NK co-cultures at a cell ratio of 5:1 and 1:1 could possibly be a result of fewer NK cells present at a cell ratio of 5:1. Therefore, the cytokine production was normalized to NK cell numbers, demonstrating that IFNγ was indeed constantly secreted at a concentration of approximately 60 ng (24 h) to 100 ng (48 h) per 106 NK cells (Supplemental Figure S6B). It is noteworthy that the secretion of TNFα per 106 NK cells was clearly higher if more DCs were seeded together with the same amount of NK cells (Supplemental Figure S6B). These data indicate that the NK cells are probably the main sources for IFNγ secretion, but we cannot say for sure which cells are primarily responsible for TNFα secretion. Another possibility is that through a DC/NK cell crosstalk, the activated NK cells trigger additional secretion of pro-inflammatory cytokines in the DCs. In summary, the interaction of NK cells with caIKKβ-transfected DCs triggered the production of large quantities of IFNγ, and also small, but significant quantities of TNFα, proving an active interaction of the caIKKβ-DCs with the NK cells.
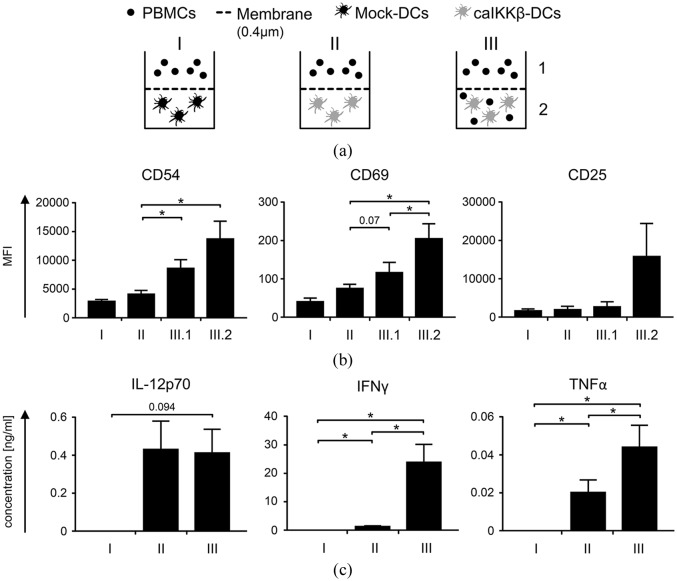
The cell–cell interaction between caIKKβ-transfected DCs and NK cells is necessary for optimal NK-cell activation
In addition to IL-12p70 a variety of other cytokines are induced by NF-κB-activation of calKKβ-transfected DCs.16,30 Therefore, it was investigated whether the secreted soluble molecules by the caIKKβ-DCs were sufficient to activate the NK cells or if direct cell–cell interaction is needed. Mock-transfected and calKKβ-transfected DCs were subjected to a transwell assay, which prevents cell contact of PBMCs and DCs, but allows soluble factors to pass (Figure 3(a)). DCs and PBMCs were either completely separated from each other (Figure 3(a); I + II), or DCs and PBMCs were co-cultured and separated from further PBMCs (Figure 3(a); III). To measure NK-cell activation, the expression of CD54, CD25, and CD69 was determined after 48 h of incubation (using the gating strategy shown in Supplemental Figure S2, to gate on NK cells). The expression of all three surface markers was the highest when DCs and PBMCs had direct cell–cell contact (Figure 3(b); III.2 and Supplemental Figure S7). For CD54 and CD69, the differences to NK cells separated from pure caIKKβ-DC were significant. Interestingly, CD54 and also slightly CD69 expression was upregulated on PMBCs that were separated from the PBMC/caIKKβ-DC co-culture (Figure 3(b); III.1), although not as high when PBMCs and DCs were in direct contact. This result indicates that a DC/NK interaction resulted in the release of soluble factors with some NK-cell activation capacity.
Figure 3.
Cell–cell interaction is necessary for best NK-cell activation by caIKKβ-DCs.
(a) Cytokine-matured dendritic cells (DCs) were electroporated with caIKKβ-RNA or, as a negative control, were mock electroporated. A transwell assay was carried out, to analyze whether cell–cell interaction between DCs and NK cells was required, using a membrane allowing transfer of soluble factors while separating cell populations, 2–4 h after electroporation. DCs and fresh autologous peripheral blood mononuclear cells (PBMCs) were either completely separated through a 0.4 µm pore sized membrane (I = mock DCs, II = caIKKβ-DCs) or were co-cultured in the lower compartment (III.2) and separated from further PBMCs in the upper (III.1). Each condition was incubated for 48 h. (b) Surface marker expressions (CD54, CD69, and CD25) on NK cells (using the gating strategy shown in Supplemental Figure S2) were determined for each condition as described in (a) by flow cytometry. Average values of 4 (I) or 5 (II and III) different donors with SEM are shown; for original data, see Supplemental Table S5. (c) The concentrations of IL-12p70, TNFα, and IFNγ in the supernatants from each condition were measured by Cytometric Bead Array. Average values of 4 (I) or 5 (II and III) different donors with SEM are shown; for original data, see Supplemental Table S6. All donors were analyzed in independent experiments. p values were evaluated using paired Student’s t test, *p ⩽ 0.05, numbers indicate p values of 0.05 ⩽ p ⩽ 0.1 in (b) and (c).
Secretion of IFNγ was only sufficiently and significantly detectable when DCs and PBMCs were allowed to interact directly (Figure 3(c)). TNFα was strongly secreted when caIKKβ-DCs and PBMCs were co-cultured, intermediately when caIKKβ-DCs and PBMCs were separated and not at all in the mock condition (Figure 3(c)). These data show that caIKKβ-DCs and NK cells require direct cell–cell interaction for improved NK-cell activation. Activated NK cells seem to induce the activation of further NK cells, independently of direct cell contact.
caIKKβ-DCs can simultaneously activate both CD8+ T cells and NK cells
The classical function of DCs in therapeutic tumor vaccination is the activation of tumor-specific T cells that attack the tumor. Hence, it is essential that the DCs’ ability to activate CD8+ T cells is not diminished. On the other hand, it may be possible that the T cells that are stimulated by the DCs compete with the NK cells for the DC-mediated activation. To investigate this, we analyzed whether NK cells and T cells were in competition with one another or if they could both be activated simultaneously in a caIKKβ-DC/PBMC co-culture. Therefore, caIKKβ-RNA-electroporated and mock-electroporated DCs were loaded with a CD8+ T-cell epitope from the melanoma antigen MelanA, or were left untreated as a control. These DCs were co-cultured with autologous PBMCs at a cell ratio of 1:10. After 1 week of stimulation, cells were stained with antibodies directed against CD56, CD3, and CD8 to distinguish between CD3–/CD56+ NK cells and CD8+/CD3+/CD56– T cells (using the gating strategy in Supplemental Figure S8). caIKKβ-DCs loaded with the MelanA peptide were able to expand MelanA-specific CD8+ T cells on average to 0.75% of all CD8+, whereas mock-electroporated DCs were able to yield an average of 0.16% MelanA-specific CD8+ T cells. A representative donor out of four is shown in Figure 4A; data from all donors are depicted in Supplemental Table S7. To display NK-cell activation the expression of CD69 was determined (Figure 4B). The expression of CD69 on NK cells stimulated by caIKKβ-DCs with MelanA peptide was almost exactly as high as on NK cells stimulated by caIKKβ-DCs without a peptide (Figure 4B). These data indicate that at least in this model system, caIKKβ-DCs were able so specifically activate CD8+ T cells, while simultaneously interacting with NK cells. In addition, NK-cell activation was not diminished in the presence of a tumor antigen-derived T-cell epitope, indicating no competitive effects between T-cell and NK-cell activation.
Figure 4.
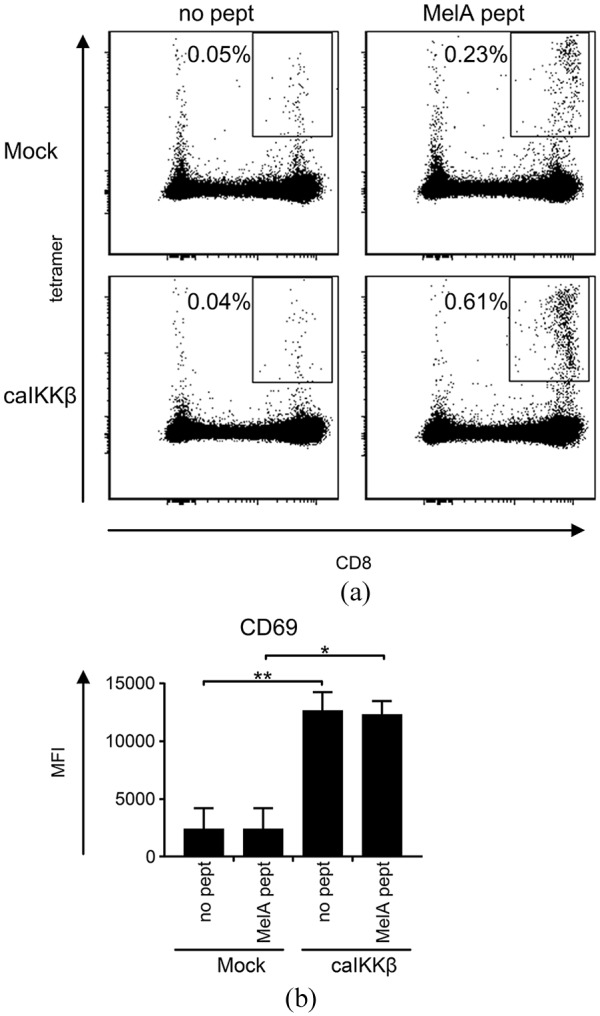
Stimulation of peripheral blood mononuclear cells (PBMCs) with caIKKβ-DCs leads to activation of both NK cells and CD8+ T cells.
Cytokine-matured dendritic cells (DCs) were electroporated either with caIKKβ-RNA or, as a control, were mock electroporated. Transfected DCs were then either loaded with a CD8+ T-cell epitope from the melanoma antigen MelanA (MelA pept) or were left untreated (no pept). These DCs were co-cultured with fresh autologous PMBCs at a ratio of 1:10 (final concentrations: 2 × 105 DCs/ml and 2 × 106 PBMC/ml) and incubated for 1 week. (a) MelanA-specific CD8+ T cells were measured by peptide-HLA-tetramer staining. To identify CD8+ T cells the gating strategy shown in Supplemental Figure S8A–E was used. The percentage of MelanA-specific CD8+ T cells out of all CD8+ T cells was calculated. Dot plots from a representative donor out of four individual donors is shown; for all original data, see Supplemental Table S7. (b) The expression of CD69 on NK cells (using the gating strategy shown in Supplemental Figure S8A–D to identify NK cells) was determined for each condition via flow cytometry. The average MFI of four different donors with the SEM is shown; for original data, see Supplemental Table S8. p values were calculated to the respective mock condition with paired Student’s t test. **p ⩽ 0.01, *p ⩽ 0.05.
caIKKβ-electroporated mature DCs induce NK cells that can lyse K562 target cells
One of the most desirable properties of DCs for their use in tumor vaccination is their ability to activate effector cells to initiate tumor killing. We could previously show that CD8+ T cells stimulated with caIKKβ-DCs were activated with a superior lytic capacity towards tumor cells compared with DCs matured with the standard protocol.16 As NK cells could also eliminate tumor cells, a standard cytotoxicity assay was performed to determine whether caIKKβ-DCs could also stimulate NK cells to lyse tumor cells. Therefore, caIKKβ- or mock-transfected DCs were co-cultured with autologous PMBCs at a cell ratio of 1:2 and 1:10 (Figure 5(a)) or with autologous purified NK cells at a cell ratio of 5:1 and 1:1 (Figure 5(b)) for 1 week. The resulting cell population was then used in a cytotoxicity assay against K562 cells with a target-to-effector ratios of 1:20, 1:6, 1:2, and 3:2.
Figure 5.
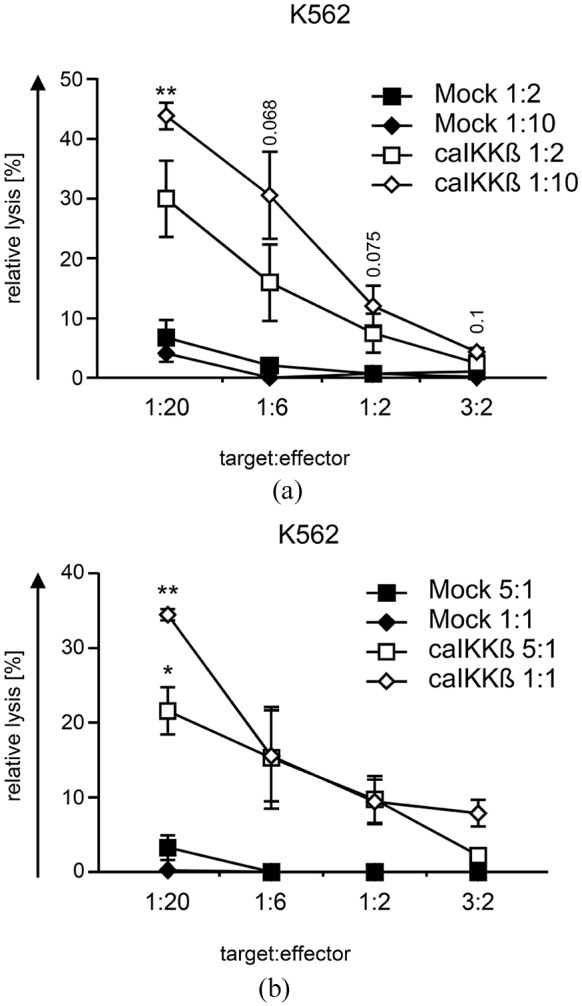
NK cells stimulated with caIKKβ-DCs can kill K562 cells.
Cytokine-matured DCs were electroporated either with caIKKβ-RNA or, as a control, were mock electroporated. (a) Transfected dendritic cells (DCs) were co-cultured with fresh autologous peripheral blood mononuclear cells (PBMCs) at a ratio of 1:2 (final concentrations: 1 × 106 DCs/ml and 2 × 106 PBMCs/ml) or 1:10 (final concentrations: 2 × 105 DCs/ml and 2 × 106 PBMCs/ml) and incubated for 1 week. The cytolytic capacity of the resulting cell population was determined in a 51chromium release assay. The K562 cell line was used as target at the indicated effector to target ratios. Average values ± SEM of three independent donors, each analyzed in triplicates, are shown; for original data see Supplemental Table S9. (b) Transfected DCs were co-cultured with fresh autologous NK cells at a ratio of 5:1 (final concentrations: 1 × 106 DCs/ml and 2 × 105 NK cells/ml) and 1:1 (final concentrations: 1 × 106 DCs/ml and 1 × 106 NK cells/ml) and incubated for 1 week. The lytic capacity of the resulting NK cells was determined as depicted in (a). Average values ± SEM of three independent donors, each analyzed in triplicates, are shown; for original data, see Supplemental Table S10. p values were calculated to the respective mock condition using the paired Student’s t test, **p ⩽ 0.01, *p ⩽ 0.05, numbers indicate p values of 0.05 ⩽ p ⩽ 0.1.
Mock-electroporated DCs could not sufficiently activate NK cells as they were not able to lyse the target cells (Figure 5A, B). In contrast, the caIKKβ-DCs were able to stimulate PBMCs and also purified NK cells, resulting in NK cells that efficiently lysed the K562 cell line (Figure 5). Stimulated PMBCs were able to lyse K562 cells at a target-to-effector ratio of up to 1:2, reaching significance at a target-to-effector ratio of 1:20, when DCs and PMBCs had been co-cultured at a cell ratio of 1:10 (Figure 5(a)). caIKKβ-DC/PBMC co-cultures at a cell ratio of 1:2 also led to a cell population with an enhanced ability to lyse K562 cells, however, without reaching significance. Regarding purified NK cells, both caIKKβ/NK cell ratios of 5:1 and 1:1 were able to equip these NK cells with the ability to significantly lyse K562 cells at a target-to-effector ratio of 1:20. Purified NK cells that were stimulated with caIKKβ DCs were able to lyse K562 cells at a target-to-effector ratio of up to 1:2, when co-incubated at a ratio of 5:1 and even up to 1:0.6 when co-incubated at a ratio of 1:1.
Discussion
The DCs currently used for tumor vaccination were mainly optimized for induction of potent tumor-specific T cells, but the clinical efficacy observed after treatment with DCs as monotherapy suggested that an improvement of this approach is required. Therefore, next to new combinatorial approaches, it is of great importance to generate DCs with immunostimulatory functions beyond CTL induction.
Our group has established a method to enhance the activation of monocyte-derived DCs matured with the standard cytokine cocktail through subsequent transfection with a caIKKβ in order to additionally activate the NF-κB pathway. This strategy generated DCs with several advantageous features: (i) the activation of NF-κB led to an increased activation status of DCs by upregulation of several activation markers, while their ability to migrate towards lymphatic tissue remained intact; (ii) they spontaneously and continuously secreted IL-12p70; and, thus, (iii) activate CD8+ T cells that displayed a memory-like phenotype characterized by an upregulation of CD27 with a superior lytic capacity.16 DCs matured with only the standard cocktail required CD4+ T cell help to secrete IL-12p70 and to induce CD8+ T cells with similar features.40
In the study described here, we show that caIKKβ-DCs strongly activate NK cells in contrast to DCs generated with the standard protocol. Following contact with caIKKβ-DCs, activated NK cells were able to secrete high amounts of IFNγ and also some TNFα (Figure 2), which can promote further activation of DCs,9 and naïve T cells6 for induction of robust cytotoxic T-cell responses. Indeed, a clearly increased expansion of tumor antigen-specific CD8+ T cells by the caIKKβ-DC was found when compared with conventional DCs in presence of NK-cells (Figure 4). Nevertheless, in theory NK cells and T cells might compete for the DCs, thus resulting in a lower NK activation when the DCs had to stimulate both types of effector cells simultaneously. However, the fact that loading caIKKβ-DCs with an antigen resulted in the generation of specific CD8+ T cells and that this did not influence NK-cell activation (Figure 4) indicated that no competition between T-cell and NK-cell activation occurred in the utilized model system.
In addition, the DC-activated NK cells by themselves were able to effectively lyse the classical HLA-negative NK-cell target K562 (Figure 5). Hence, the simultaneous activation of CTL and NK cells would allow an attack on the tumor via tumor antigens presented in HLA class I and efficiently preempt the immune escape mechanism of HLA class I loss. The mechanisms by which the NK cells exert this killing remains to be further investigated; so far, we excluded degranulation as well as production of IFNγ and TNFα via CD107a and intracellular staining (data not shown) suggesting that cell-surface interaction might to play a role here.
The observation that even immature moDCs are in principle able to activate NK cells was made many years ago, but the classical maturation cocktail did not increase this ability.41 Therefore, other groups have focused on creating improved protocols using alternative maturation mixtures, mostly containing different TLR agonists,17,27,28 inducing DCs to more efficiently activate effector cells. Anguille et al. used so-called IL-15-DCs by replacing IL-4 with IL-15 during the differentiation of DCs and then using TNFα, IFNγ, PGE2, and R-848 (a TLR-7/8 agonist) for maturation.27 The maturation cocktail used by Massa et al. contained IFNγ and MLPA,17 which is a ligand for TLR-4, whereas Mailliard et al. used a maturation mixture consisting of IFNα, IFNγ, TNFα, IL-1β, and a TLR-3 agonist (p-I:C), creating so-called α-type-1-polarized DCs (αDC1).28
The caIKKβ-DCs, like the IL-15-DCs, αDC1, and DCs matured with MLPA and IFNγ were all able to effectively activate NK cells as shown in the upregulation of CD69 (Figure 1),17,42,43 CD25 (Figure 1),17,43 CD54 (Figure 1), and further activation markers.43 caIKKβ DCs were able to activate both NK cells in co-cultures with PBMCs and also with purified NK cells showing that bystander cells were not necessary for NK-cell activation. NK cells activated through caIKKβ-DCs or MPLA and IFNγ matured DCs were both able to secrete IFNγ (Figure 2).17 Both these DCs and also IL-15-DCs were able to induce NK cells to effectively kill certain tumor cell lines (Figure 5).17,43 Cytotoxicity of NK cells activated by αDC1s was not analyzed.42 Regarding IFNγ production, IL-15-DCs alone were already able to secrete IFNγ themselves, whereas in IL-15-DC/NK co-culture the secretion of IFNγ did not increase significantly.43 αDC1 were able to induce IFNγ production by NK cells, but only when αDC1 were co-cultured with PBMCs (or together with CD40L stimulation). In αDC1/NK cell co-cultures, neither IFNγ secretion was detectable, nor was an upregulation of CD69 seen, showing that co-factors (such as CD40L) are needed for NK-cell activation with αDC1.42 In this context, it is noteworthy that CD40L is a bona fide activator of the NF-κB pathway.
The standard maturation cocktail contains PGE2 as it has been shown that it is important for the DCs’ ability to migrate to the lymph nodes (LNs).44,45 However, PGE2 interferes with the IL-12p70 secretion by DCs.46,47 Through electroporation of caIKKβ-RNA in DCs matured with the standard maturation cocktail, this problem could be overcome, as these DCs still could migrate towards the LN, but had the ability to secrete high amounts of IL-12p70.16 For IL-15-DCs PGE2 was contained in their maturation cocktail, indeed creating DCs that could migrate towards the LN. However, these DCs were not able to secrete IL-12p70 when left alone, only gaining this ability when co-cultured with CD40L-transfected 3T3 mouse fibroblasts, representing the CD40–CD40L interaction between DCs and helper T cells.27 Even though PGE2 was not included in the maturation cocktail to create αDC1s, these DCs were still able to migrate towards the corresponding chemokine, although not quite as well as DCs matured with the standard protocol.28 Despite strong CCR7 expression on DCs matured with MPLA and IFNγ, these DCs did not show efficient migratory capacity towards CCL21, indicating a low potency to migrate towards the LN.48 Both αDC1 and DCs matured with MPLA and IFNγ were able to secrete IL-12p70.17,28
The ability to secrete IL-12p70 is one of the most favorable features for vaccine DCs. IL-12p70 plays a crucial role in the development of a CD8+ T-cell memory,49 and it is also important for a Th1 response.50 Massa et al. showed that NK cells are highly dependent on IL-12p70 for the production of IFNγ, whereas IL-12p70 does not play a central role in the cytotoxicity of NK cells.17 In line with others,2,20 we observed that the soluble factors secreted by the caIKKβ-DC, including IL-12p70, did not induce NK cells to secrete IFNγ, but that direct cell–cell interaction was required. An interesting observation was that once NK cells had become activated via direct interaction with caIKKβ-DCs, further NK cells that could not directly interact with these DCs were also slightly activated, as indicated by upregulation of CD54 and slightly CD69, but not CD25 (Figure 3). It is possible that IFNγ in concert with other cytokines produced by activated NK cells, led to the stimulation of further NK cells (as reviewed by Boehm et al.51). This may indicate a positive feedback mechanism for NK cell recruitment but this process as well as the induced activation program within those NK cells requires further investigations.
In conclusion, caIKKβ-DCs meet many features for an optimal vaccination: they can migrate towards lymphatic tissue, secrete IL-12p70 for more than 2 days, activate CTL with a memory-like phenotype and NK cells. The possibility to activate the NF-κB pathway by mRNA electroporation is another advantage as this is a safe method approved and tested for clinical use.15,31 Therefore, we believe that caIKKβ-DCs are a powerful tool for anticancer vaccination and we are about to start testing these DCs in a phase I clinical trial.
Supplemental Material
Supplemental material, Raw_data_used_for_this_manuscript_15.08.19 for NF-κB activation triggers NK-cell stimulation by monocyte-derived dendritic cells by Naomi C. Bosch, Reinhard E. Voll, Caroline J. Voskens, Stefanie Gross, Barbara Seliger, Gerold Schuler, Niels Schaft and Jan Dörrie in Therapeutic Advances in Medical Oncology
Supplemental Material
Supplemental material, Supplemental_Figures_15.08.19 for NF-κB activation triggers NK-cell stimulation by monocyte-derived dendritic cells by Naomi C. Bosch, Reinhard E. Voll, Caroline J. Voskens, Stefanie Gross, Barbara Seliger, Gerold Schuler, Niels Schaft and Jan Dörrie in Therapeutic Advances in Medical Oncology
Acknowledgments
We want to thank Dennis Harrer for fruitful discussions and Carmen Lorenz and Annett Hamann for technical assistance. We also thank Ton Schumacher for his help and advice concerning the production of peptide-HLA-tetramers and for providing the HLA-expression construct. We also express our gratitude to the voluntary blood donors and the medical staff for the acquisition of the blood. The present work was performed in fulfillment of the requirements for obtaining the degree of Dr. med. at the Martin-Luther University Halle-Wittenberg by Naomi C. Bosch.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Preparatory work for this project was supported by grants from the German Cancer Aid (Deutsche Krebshilfe e.V; grant number 110265 to Jan Dörrie, Beatrice Schuler-Thurner, and Niels Schaft; grant number 111105 to Barbara Seliger and Chiara Massa; grant number 113311 to Barbara Seliger).
Conflict of interest statement: The authors declare the following potential conflict of interest: REV, GS, NS, and JD are named as inventors on a patent on caIKK-RNA-electroporated DCs (WO/2012/055551).
ORCID iD: Jan Dörrie  https://orcid.org/0000-0002-3478-0741
https://orcid.org/0000-0002-3478-0741
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Naomi C. Bosch, Institute of Medical Immunology, Martin-Luther University Halle-Wittenberg, Halle (Saale), Germany Department of Dermatology, Universitätsklinikum Erlangen, Erlangen, Germany.
Reinhard E. Voll, Department of Rheumatology and Clinical Immunology, Medical Center – University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany
Caroline J. Voskens, Department of Dermatology, Universitätsklinikum Erlangen, Erlangen, Germany
Stefanie Gross, Department of Dermatology, Universitätsklinikum Erlangen, Erlangen, Germany.
Barbara Seliger, Institute of Medical Immunology, Martin-Luther University Halle-Wittenberg, Halle (Saale), Germany.
Gerold Schuler, Department of Dermatology, Universitätsklinikum Erlangen, Erlangen, Germany.
Niels Schaft, Department of Dermatology, Universitätsklinikum Erlangen, Erlangen, Germany.
Jan Dörrie, Department of Dermatology, Universitätsklinikum Erlangen, Research Campus, Hartmannstraße 14, Erlangen, 91052, Germany.
References
- 1. Guermonprez P, Valladeau J, Zitvogel L, et al. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol 2002; 20: 621–667. [DOI] [PubMed] [Google Scholar]
- 2. Fernandez NC, Lozier A, Flament C, et al. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nat Med 1999; 5: 405–411. [DOI] [PubMed] [Google Scholar]
- 3. Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013; 39: 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nat Rev Immunol 2002; 2: 957–964. [DOI] [PubMed] [Google Scholar]
- 5. Lion E, Smits EL, Berneman ZN, et al. NK cells: key to success of DC-based cancer vaccines? Oncologist 2012; 17: 1256–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gerosa F, Baldani-Guerra B, Nisii C, et al. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med 2002; 195: 327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mocikat R, Braumuller H, Gumy A, et al. Natural killer cells activated by MHC class I(low) targets prime dendritic cells to induce protective CD8 T cell responses. Immunity 2003; 19: 561–569. [DOI] [PubMed] [Google Scholar]
- 8. Adam C, King S, Allgeier T, et al. DC-NK cell cross talk as a novel CD4+ T-cell-independent pathway for antitumor CTL induction. Blood 2005; 106: 338–344. [DOI] [PubMed] [Google Scholar]
- 9. Martin-Fontecha A, Thomsen LL, Brett S, et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 2004; 5: 1260–1265. [DOI] [PubMed] [Google Scholar]
- 10. Caligiuri MA. Human natural killer cells. Blood 2008; 112: 461–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Krebs P, Barnes MJ, Lampe K, et al. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood 2009; 113: 6593–6602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med 1994; 180: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med 1994; 179: 1109–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol 1997; 27: 3135–3142. [DOI] [PubMed] [Google Scholar]
- 15. Schuler G. Dendritic cells in cancer immunotherapy. Eur J Immunol 2010; 40: 2123–2130. [DOI] [PubMed] [Google Scholar]
- 16. Pfeiffer IA, Hoyer S, Gerer KF, et al. Triggering of NF-kappaB in cytokine-matured human DCs generates superior DCs for T-cell priming in cancer immunotherapy. Eur J Immunol 2014; 44: 3413–3428. [DOI] [PubMed] [Google Scholar]
- 17. Massa C, Seliger B. Fast dendritic cells stimulated with alternative maturation mixtures induce polyfunctional and long-lasting activation of innate and adaptive effector cells with tumor-killing capabilities. J Immunol 2013; 190: 3328–3337. [DOI] [PubMed] [Google Scholar]
- 18. Pedersen AE, Thorn M, Gad M, et al. Phenotypic and functional characterization of clinical grade dendritic cells generated from patients with advanced breast cancer for therapeutic vaccination. Scand J Immunol 2005; 61: 147–156. [DOI] [PubMed] [Google Scholar]
- 19. Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev 2002; 13: 155–168. [DOI] [PubMed] [Google Scholar]
- 20. Yu Y, Hagihara M, Ando K, et al. Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells. J Immunol 2001; 166: 1590–1600. [DOI] [PubMed] [Google Scholar]
- 21. Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death–a new approach to cancer therapy. J Clin Invest 2005; 115: 2625–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li ZW, Chu W, Hu Y, et al. The IKKβ subunit of IkappaB kinase (IKK) is essential for nuclear factor kappaB activation and prevention of apoptosis. J Exp Med 1999; 189: 1839–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol 2004; 25: 280–288. [DOI] [PubMed] [Google Scholar]
- 24. Tas SW, de Jong EC, Hajji N, et al. Selective inhibition of NF-kappaB in dendritic cells by the NEMO-binding domain peptide blocks maturation and prevents T cell proliferation and polarization. Eur J Immunol 2005; 35: 1164–1174. [DOI] [PubMed] [Google Scholar]
- 25. Calderhead DM, DeBenedette MA, Ketteringham H, et al. Cytokine maturation followed by CD40L mRNA electroporation results in a clinically relevant dendritic cell product capable of inducing a potent proinflammatory CTL response. J Immunother 2008; 31: 731–741. [DOI] [PubMed] [Google Scholar]
- 26. Boullart AC, Aarntzen EH, Verdijk P, et al. Maturation of monocyte-derived dendritic cells with Toll-like receptor 3 and 7/8 ligands combined with prostaglandin E2 results in high interleukin-12 production and cell migration. Cancer Immunol Immunother 2008; 57: 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anguille S, Smits EL, Cools N, et al. Short-term cultured, interleukin-15 differentiated dendritic cells have potent immunostimulatory properties. J Transl Med 2009; 7: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mailliard RB, Wankowicz-Kalinska A, Cai Q, et al. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res 2004; 64: 5934–5937. [DOI] [PubMed] [Google Scholar]
- 29. Bonehill A, Tuyaerts S, Van Nuffel AM, et al. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther 2008; 16: 1170–1180. [DOI] [PubMed] [Google Scholar]
- 30. Gerer KF, Erdmann M, Hadrup SR, et al. Preclinical evaluation of NF-κB-triggered dendritic cells expressing the viral oncogenic driver of Merkel cell carcinoma for therapeutic vaccination. Ther Adv Med Oncol 2017; 9: 451–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schaft N, Dorrie J, Thumann P, et al. Generation of an optimized polyvalent monocyte-derived dendritic cell vaccine by transfecting defined RNAs after rather than before maturation. J Immunol 2005; 174: 3087–3097. [DOI] [PubMed] [Google Scholar]
- 32. Gerer KF, Hoyer S, Dorrie J, et al. Electroporation of mRNA as universal technology platform to transfect a variety of primary cells with antigens and functional proteins. Methods Mol Biol 2017; 1499: 165–178. [DOI] [PubMed] [Google Scholar]
- 33. Schaft N, Dorrie J, Muller I, et al. A new way to generate cytolytic tumor-specific T cells: electroporation of RNA coding for a T cell receptor into T lymphocytes. Cancer Immunol Immunother 2006; 55: 1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rodenko B, Toebes M, Hadrup SR, et al. Generation of peptide-MHC class I complexes through UV-mediated ligand exchange. Nat Protoc 2006; 1: 1120–1132. [DOI] [PubMed] [Google Scholar]
- 35. Hofflin S, Prommersberger S, Uslu U, et al. Generation of CD8+ T cells expressing two additional T-cell receptors (TETARs) for personalised melanoma therapy. Cancer Biol Ther 2015; 16: 1323–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salkind N. Encyclopedia of research design. Los Angeles: Sage, 2010. [Google Scholar]
- 37. Schaft N, Wellner V, Wohn C, et al. CD8+ T-cell priming and boosting: more antigen-presenting DC, or more antigen per DC? Cancer Immunol Immunother 2013; 62: 1769–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Clausen J, Vergeiner B, Enk M, et al. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology 2003; 207: 85–93. [DOI] [PubMed] [Google Scholar]
- 39. Robertson MJ, Caligiuri MA, Manley TJ, et al. Human natural killer cell adhesion molecules. Differential expression after activation and participation in cytolysis. J Immunol 1990; 145: 3194–3201. [PubMed] [Google Scholar]
- 40. Hoyer S, Prommersberger S, Pfeiffer IA, et al. Concurrent interaction of DCs with CD4+ and CD8+ T cells improves secondary CTL expansion: it takes three to tango. Eur J Immunol 2014; 44: 3543–3559. [DOI] [PubMed] [Google Scholar]
- 41. Ferlazzo G, Tsang ML, Moretta L, et al. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med 2002; 195: 343–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gustafsson K, Ingelsten M, Bergqvist L, et al. Recruitment and activation of natural killer cells in vitro by a human dendritic cell vaccine. Cancer Res 2008; 68: 5965–5971. [DOI] [PubMed] [Google Scholar]
- 43. Anguille S, Van Acker HH, Van den Bergh J, et al. Interleukin-15 dendritic cells harness NK cell cytotoxic effector function in a contact- and IL-15-dependent manner. PLoS One 2015; 10: e0123340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Luft T, Jefford M, Luetjens P, et al. Functionally distinct dendritic cell (DC) populations induced by physiologic stimuli: prostaglandin E(2) regulates the migratory capacity of specific DC subsets. Blood 2002; 100: 1362–1372. [DOI] [PubMed] [Google Scholar]
- 45. Scandella E, Men Y, Gillessen S, et al. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 2002; 100: 1354–1361. [DOI] [PubMed] [Google Scholar]
- 46. Kalinski P, Schuitemaker JH, Hilkens CM, et al. Prostaglandin E2 induces the final maturation of IL-12-deficient CD1a+CD83+ dendritic cells: the levels of IL-12 are determined during the final dendritic cell maturation and are resistant to further modulation. J Immunol 1998; 161: 2804–2809. [PubMed] [Google Scholar]
- 47. Kalinski P, Hilkens CM, Snijders A, et al. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J Immunol 1997; 159: 28–35. [PubMed] [Google Scholar]
- 48. Massa C, Thomas C, Wang E, et al. Different maturation cocktails provide dendritic cells with different chemoattractive properties. J Transl Med 2015; 13: 175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pearce EL, Shen H. Generation of CD8 T cell memory is regulated by IL-12. J Immunol 2007; 179: 2074–2081. [DOI] [PubMed] [Google Scholar]
- 50. Kalinski P, Hilkens CM, Wierenga EA, et al. T-cell priming by type-1 and type-2 polarized dendritic cells: the concept of a third signal. Immunol Today 1999; 20: 561–567. [DOI] [PubMed] [Google Scholar]
- 51. Boehm U, Klamp T, Groot M, et al. Cellular responses to interferon-gamma. Annu Rev Immunol 1997; 15: 749–795. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Raw_data_used_for_this_manuscript_15.08.19 for NF-κB activation triggers NK-cell stimulation by monocyte-derived dendritic cells by Naomi C. Bosch, Reinhard E. Voll, Caroline J. Voskens, Stefanie Gross, Barbara Seliger, Gerold Schuler, Niels Schaft and Jan Dörrie in Therapeutic Advances in Medical Oncology
Supplemental material, Supplemental_Figures_15.08.19 for NF-κB activation triggers NK-cell stimulation by monocyte-derived dendritic cells by Naomi C. Bosch, Reinhard E. Voll, Caroline J. Voskens, Stefanie Gross, Barbara Seliger, Gerold Schuler, Niels Schaft and Jan Dörrie in Therapeutic Advances in Medical Oncology