Abstract
Pasteuria penetrans is a Gram-positive, endospore forming soil bacterium, infecting root-knot nematodes, Meloidogyne spp. Being obligate in nature, the bacterium is not easily grown in vitro, and the in vivo culturing technique is relied on the soil-based microcosm since long. Hence, culturing of P. penetrans using CYG germination pouches as a soil-less medium for plant growth, promises to provide a contamination free environment along with ease in isolation of infected females from the plant roots. Additionally, this method increases the percentage of P. penetrans infected nematode females as compared with the soil-based system. Schematic observation of all the life stages of P. penetrans was documented, which revealed chronological fragmentation of vegetative microcolony inside the nematode body demonstrating the formation of some stages not reported earlier. Further, germination of endospores attached to infective juveniles was found to be most likely asynchronous as single female nematode contained most of the developing stages of P. penetrans concurrently. Additionally, the effect of an antibiotic, streptomycin sulfate was evaluated for effects on the growth and development of the bacterium at different concentrations. Higher doses of antibiotic were found to exert a negative impact on the development of P. penetrans, which shows the incompatibility of Pasteuria and streptomycin sulfate.
Keywords: Life-cycle, Pasteuria penetrans, Soil-less culturing, Streptomycin sulfate
Pasteuria penetrans (Thorne) Sayre & Starr is a Gram-positive, mycelial, endospore forming bacterium that hyper-parasitizes the root-knot nematodes (RKN, Meloidogyne spp.) in natural environments. Endospores attached to the cuticle surface of the second-stage juveniles (J2s) give rise to a germ tube that penetrates the cuticle to form vegetative microcolony, and proliferates throughout the body of the developing nematode (Davies et al., 1988). The first report of an organism resembling Pasteuria was documented by Cobb (1906) from Dorylaimus bulbiferous, but erroneously reported as “perhaps monads.” The taxonomic identification of this bacterium remained obscured for decades until a formal legitimate name, P. penetrans was proposed by Sayre and Starr (1985) using the basis of conserved description of Pasteuria ramosa infecting cladocerans (Metchnikoff, 1888). Extensive and/or serendipitous scientific observations in subsequent periods could identify Pasteuria spp. from many other free living and plant-parasitic nematodes (Sayre et al., 1988, 1991; Kaplan, 1994; Subbotin et al., 1994; Sharma and Davies, 1996; Giblin-Davis et al., 2003; Bishop et al., 2007; Schmidt et al., 2010; Giblin-Davis et al., 2011), but in depth knowledge is still scanty for many of them. Keen inspection of the life stages of the bacterium has revealed the formation of small groups of vegetative sporangia, which subsequently give rise to quartets, doublets, and eventually produce endospores (Chen et al., 1997; Sayre and Starr, 1985). The obligate nature of Pasteuria has become an obvious obstacle for application of traditional classification approaches for the members of the genus and to study the developmental steps of life-cycle (Davies et al., 2011).
Amongst the major emerging gridlocks in present day agriculture, Meloidogyne incognita (Kofoid and White) Chitwood is a widely adapted endoparasitic nematode species inflicting serious yield and quality losses on many different crops (Jones et al., 2013). Biological control, as an alternative to chemotherapeutics, promises to be a good and effective tool to reduce the nematode population density (Hallman et al., 2009; Collange et al., 2011) and P. penetrans is a potent candidate to be used as a biocontrol agent. But, several attempts of in vitro culturing of P. penetrans either by inoculating the spores or vegetative mycelial bodies onto a diverse range of media ended up with disappointment (Williams et al., 1989; Bishop and Ellar, 1991). However, Pasteuria Bioscience, Inc. (now Syngenta Crop Protection, LLC) has commercialized several bionematicides based on in vitro produced Pasteuria endospores as active ingredient. Notable amongst them are, Econem (Pasteuria usage against Belonolaimus longicaudatus), Naviva (Pasteuria sp. -Pr3 against Rotylenchulus reniformis), Clariva (P. nishizawae against Heterodera glycines) and formulations of P. penetrans (Kokalis-Burelle, 2015) (P. penetrans against M. incognita and Meloidogyne arenaria). All these commercial products are based on either clay-based or liquid formulation for seed and/or soil treatment. But, due to proprietary nature of the company research the Pasteuria scientists have no information about the actual technique or media (if any) to grow the endospores in vitro. Although in vitro production of Pasteuria endospores has been claimed by Pasteuria Biosciences Inc. and Gerber and White (2005), the most widely used method of raising the bacterial endospores is accomplished by in vivo techniques, where endospore encumbered juveniles of M. incognita are inoculated onto host plants and culture relies on the tri-trophic interaction in soil-based microcosms (Rao et al., 2012; Davies et al., 2011). Though the bacterium completes its life-cycle inside the developing nematode by ultimately turning the adult female into an endospore sac, concomitant contamination by other soil dwelling bacteria cannot be overruled (Mauchline et al., 2010). However, exploitation of CYG germination pouches (Mega International, St Paul, MN, USA) using adzuki bean (Vigna angularis (Willd.) Ohwi and Ohashi) as a host for RKN has been proposed to provide a clean and contamination free endospore production approach (Atkinson and Harris, 1989; Rao et al., 2012). This soil-free process also facilitates easy diagnosis of bacterial infection inside the developing nematode having methodological swiftness in isolation of the infected females of Meloidogyne sp. from the roots.
Antibiotics are considered as contaminants of environmental concern, often being biologically active and not biodegradable (Kummerer, 2009; Boutin et al., 2013; Gorokhova et al., 2015). However, despite obvious relevance, information on ecotoxicological effects of antibiotics on the naturally coevolved pathogen-hyperparasite system, as occurs in Pasteuria–RKN interaction, is scanty. Post-antibiotic susceptibility of Daphnia to infection by its pathogenic bacterium P. ramosa was examined previously (Zalewski et al., 2011). But, the effect of any antibiotic on the growth and development of P. penetrans is still largely unknown. The present study was aimed to re-investigate the developing stages of P. penetrans using soil-less CYG medium to have a comprehensive depiction of the life-cycle, along with determining the effect of an antibiotic on the establishment of the bacterium species.
Materials and methods
Maintenance of nematodes
A pure culture of an Indian isolate of M. incognita (Kofoid and White) Chitwood race 1 was multiplied on susceptible tomato plant (Solanum lycopersicum L. cv. Pusa ruby) in the glasshouse at ICAR-Indian Agricultural Research Institute, New Delhi, India. Nematode infected roots were washed free of soil, egg masses were hand-picked and kept for hatching in a Petri dish-wire gauze-tissue paper assembly with distilled water (Whitehead and Hemming, 1965). Freshly hatched juveniles (J2s) were used for experimental purpose.
Culturing of Pasteuria penetrans in soil-free CYG medium
The seeds of adzuki bean were sterilized by submerging in 3.25% sodium hypochlorite solution for 4 min; followed by washing with 70% ethanol for 3 min and then rinsed with double distilled water until traces of ethanol and sodium hypochlorite vanished completely. The seeds continued to be soaked in water for 6 hr and then transferred in Petri dishes containing moist filter paper to germinate at 28°C. Germinated seeds with 2.0 to 2.5 cm radical were transferred into CYG germination pouches and kept in a growth chamber maintained at 28°C, 70% RH and 16:8 hr light:dark photoperiod for proliferation of roots. Pasteuria penetrans (Strain AII-329: Pasteuria collection, ICAR-IARI, New Delhi, India) endospores were produced on adzuki bean with single egg mass culture of M. incognita as described earlier (Rao et al., 2012). Additionally, a seed tray (dimension: 540 mm × 280 mm; hole size: 60 × 30 × 55 mm3 each) was filled with sterilized soil, adzuki bean seeds were sown (one seed per hole) and maintained at growth chamber with aforesaid conditions. The plants, after attaining 6 cm height, were inoculated with endospore encumbered J2s with an inoculum level of 2 J2s/g soil and maintained for 45 days. This soil-based microcosm served as control for the CYG pouch-based system. The plants were supplemented with diluted Hoagland’s solution (1:500 stock solution). Endospore attachment (on J2 cuticle) was pursued by centrifugation method (Hewlett and Dickson, 1993) with a suspension of 2.5 × 106 endospores/ml.
Microscopic study of life stages of Pasteuria penetrans
Developing nematodes were collected from the roots at 3 day intervals starting from 4 days after plant inoculation. The roots were teased very gently using a fine pointed needle and forceps under stereozoom binocular to take out the developing females. The dissected-out females were placed in Ringer’s solution on a cavity slide to prevent bursting. The nematode specimens were washed and surface sterilized following standard method (Davies et al., 2011). At each harvesting time, individual females were broken up on a clean glass slide using a cover slip by gentle pressure. On each occasion, 10 females were crushed for microscopic study of bacterial developmental stages. The growth stages of P. penetrans were monitored and photographed using a Zeiss Axiocam M2m compound microscope equipped with differential interference contrast (DIC) optics.
Exposure to streptomycin sulfate
To study the effectiveness of antibiotic treatment on the growth and development of P. penetrans, different concentrations, viz., 50, 100, 200, 500, 1000, and 5000 ppm of streptomycin sulfate was sprayed on the roots in the CYG germination pouches at 7 and 21 days post inoculation. Five replicates were maintained for each concentration with untreated control. Nematode inoculation and maintenance of pouches were pursued as described previously. The pouches were kept for 40 days in a growth chamber with aforesaid conditions for completion of life-cycle of the bacterium. At harvest, each pouch was observed carefully, documenting the occurrence of gelatinous matrix (with or without eggs). Females were then dissected out and individually checked for P. penetrans infection. The percentage of P. penetrans infected females was calculated as [(No. of infected females/(No. of infected females + No. of uninfected females)) × 100] considering each pouch as a replicate.
Statistical analysis
Bioassay data were subjected to linear regression analysis using SPSS software (version 24.0). The computed value of coefficient of determination (R2) was measured to evaluate the scatter of data points around the fitted regression line (P < 0.01).
Results
Review of life stages of Pasteuria penetrans
The developmental stages of P. penetrans inside M. incognita are schematically shown in Fig. 1. Inside the nematode, bacterial growth starts with the formation of germ tube from the endospore, which gives rise to vegetative microcolony within 8 to 10 days post penetration of juveniles. The “cauliflower like” mycelial microcolony then undergoes maturation and subsequent fragmentation. The fragmentation continues through formation of quartets, triplets, doublets, and eventual production of immature endospores at 25 to 28 days post inoculation. The pre-endospores then undergo maturation and ultimately become ellipsoidal cup-shaped when fully developed. Upon crushing the infected females after 38 to 40 days post inoculation, millions of endospores come out from the nematode body. The endospore filled females showed no trace of genital development as compared with the normal female (Fig. 2). The females with Pasteuria inside produced no egg mass, although in some cases very little gelatinous matrix was formed (Fig. 3).
Fig. 1.
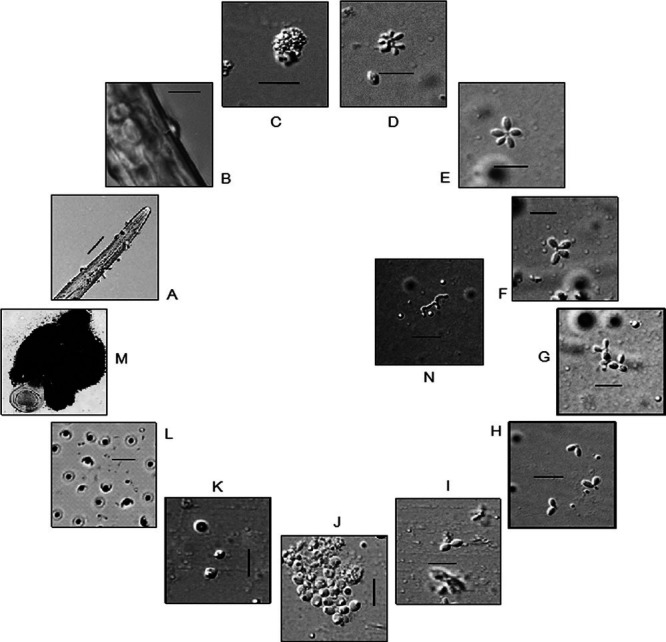
Life stages of Pasteuria penetrans infecting Meloidogyne incognita. A, Endospore encumbered J2; B, Endospore germination; C, Cauliflower-like microcolony; D, Fragmented microcolony; E, Star-shaped stage; F, Quartet; G, Triplet; H, Doublet; I–K, Developing endospores; L, Mature endospores; M, Mass of endospores coming out upon crushing of ♀ nematode (Scale bar: A = 20 µm; B–L = 5 µm).
Fig. 2.
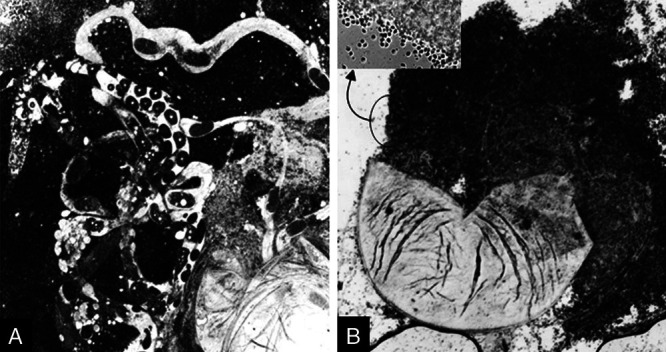
Crushed female of Meloidogyne incognita. A Uninfected female showing the genital tracts with developing eggs inside; B, Infected female without formation of reproductive tract and filled with endospores (Inset: magnified view of spore mass with free endospores).
Fig. 3.
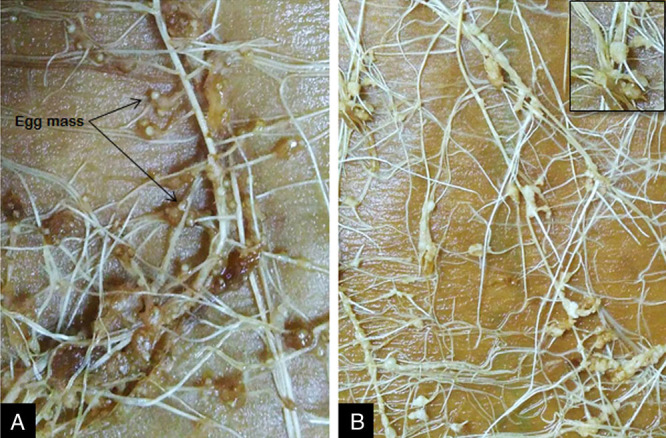
Roots of adzuki bean inoculated with Meloidogyne incognita in CYG germination pouch. A, Uninfected female showing the production of egg mass; B, Infected female without production of egg mass (Inset: magnified view of galls without egg mass).
Establishment of Pasteuria penetrans in CYG germination pouches
The production of P. penetrans endospores was found to be significantly (P < 0.05) higher in the CYG germination pouches as compared with the soil-based microcosms. Approximately 81.65 ± 5.78 percent infected females were observed in the pouch system, whereas in soil, the infection was recorded to be 60.05 ± 10.94 percent.
Effect of Streptomycin sulfate on Pasteuria penetrans establishment
The impact of streptomycin sulfate was found variable according to the administered concentrations. Application of 50, 100, and 200 ppm of the antibiotic resulted in 81.44 ± 4.73, 78.60 ± 3.14, and 80.61 ± 5.90 percent infected females respectively, not significantly (P < 0.01) different with the untreated control (81.65±5.78). However, the percent infected females were reduced to 66.34 ± 3.45, 45.92 ± 4.23, and 19.04 ± 5.28 against an exposure dosage of 500, 1000, and 5000 ppm of the antibiotic. Analysis of the data revealed that, higher doses of the antibiotic reduced the establishment of the bacterium (Fcal = 136.37; P < 0.01) (Fig. 4).
Fig. 4.
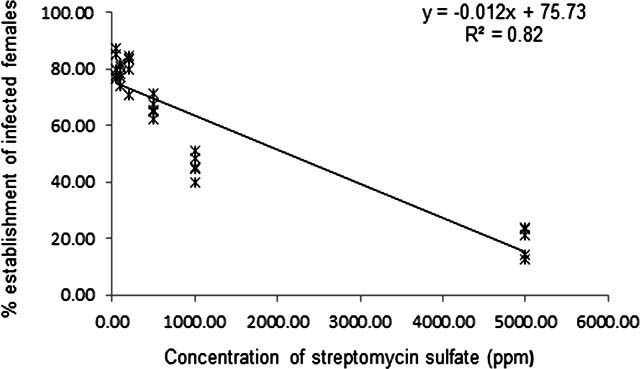
Effect of Streptomycin sulfate on establishment of Pasteuria penetrans .
Discussion
The schematic life-cycle of P. penetrans has been studied earlier (Imbriani and Mankau, 1977; Sayre and Wergin, 1977) and majority of the subsequent descriptions are in compliance with them. According to the previous reports, the germ tube develops from the attached endospore giving rise to a “cauliflower like” vegetative mycelial microcolony, eventually forming quartets and then doublets of the developing sporangia (Sayre and Starr, 1989). Finally, endospores are formed due to sporogenesis and proliferate throughout the body of the female nematode turning it into a “spore-filled sac.” A single M. incognita female has been reported to contain ~2000,000 endospores inside its body (Davies et al., 1988). The germination of endospores is triggered by some unknown cue(s) and takes place before the first molt of the J2s inside the root, after establishing the permanent feeding site (Sayre and Wergin, 1977; Davies et al., 1988). Earlier, the life-cycle of Pasteuria was divided into three phases (Williams, 1960) and similar kind of phase division has also been retained later with fair modification (Davies et al., 2011).
In the present study, certain observations were recorded about the life-cycle of P. penetrans infecting M. incognita that were hitherto not depicted. Based on this study, it can be assumed that the newly formed microcolony first undergoes maturation and systematically fragmented before entering sporogenesis. Instead of direct formation of quartets and doublets from the microcolony (through fragmentation), a “star-shaped” structure was found to be formed with many single sporangium attached together. These vegetative bodies may presumably break into smaller fragments or single developing sporangium may detach, ultimately leading to premature endospore development. The formation of “star-shaped” vegetative body between microcolony and quartet and/or “triplet” stage between quartet and doublet has not been reported earlier. Generation of these stages hints for segregation of single sporangium containing endospore at an early stage, and their further progression toward maturation. Crushing of a single developing female at 25 days postinoculation revealed the presence of most of the life stages, starting from the “star-shaped” stage to doublets. The simultaneous occurrence of all these stages together in a single female possibly indicates the asynchrony in germination of the attached endospores. This suggests that the endospores on the J2 nematode cuticle germinate at various time points and proceed toward formation of vegetative microcolony. The genesis of premature endospores was found at around 25 to 28 days after feeding site development by M. incognita, and fully developed ellipsoidal and cup-shaped endospores were formed at 38 to 40 days after nematode invasion. The immature endospores contained a rhomboid exosporium and at maturity became morphologically distinguishable that could be detected through light microscopy. The comparison of sporogenesis in Bacillus and Pasteuria has been overlaid in many earlier publications (Sayre and Starr, 1985; Stragier and Losick, 1996; Chen et al., 1997). Recently it has been suggested that the sporogenesis phenomenon commences in the granular mass composed of aggregated rod-shaped body of Pasteuria (Davies et al., 2011). Similar types of aggregated rod-shaped structures were also seen in the developing nematode body. The rod-shaped bodies have been proposed to grow rapidly leading to the development of aggregated granular masses within the female nematode, directed to exponential growth of P. penetrans (Davies et al., 2011). The whole process of sporulation has been discovered to be unidirectional, committed and controlled by signaling cascades in Bacillus spp. and a similar trend with involvement of cascades have also been detected in Pasteuria spp. (Hoch, 2000; Kojetin et al., 2005). As the formation of endospores progresses, the female reproductive system is completely destroyed and whole body comes out to be full of endospores along with lipid granules.
The use of CYG germination pouches increased the percentage establishment of P. penetrans as compared with the method relied on soil-based microcosm. The higher number of infected females may be a result of assured penetration brought by direct inoculation of endospore encumbered juveniles on roots, which may not be achieved in soil due to uncontrolled infection. Being an obligate parasite, the bacterium cannot be cultured in artificial media and exposure to antibiotic was brought indirectly, i.e., application on germination pouches. Streptomycin sulfate is a systemic aminoglycoside antibiotic and binds irreversibly to bacterial ribosomes inhibiting the protein synthesis (Levin et al., 2000). The bactericide can affect the microbial population even at a concentration of 50 µg/ml (Kogut and Harris, 1969; Yashiro and McManus, 2012). The effect of streptomycin sulfate was found to be concentration dependent on successful establishment of P. penetrans in CYG germination pouches, where higher concentrations (500–5000 ppm) had a negative impact. The observed response of P. penetrans at higher doses may be due to the indirect way of exposure unlike supplementing it directly in the growth media as followed for culturable bacteria. Production of clean and contamination free endospores has been one of the major hindrances so far that affected the basic research on interaction of P. penetrans and M. incognita. Thus, the occasional use of streptomycin sulfate (at low concentration) in CYG germination pouches is advantageous to get clean Pasteuria endospores in high quantity, for any downstream basic research.
Acknowledgments
The authors are thankful to Dr. Gautam Chawla, Division of Nematology, ICAR-Indian Agricultural Research Institute, New Delhi, India for providing the microscope facility.
Literature cited
- Atkinson, H.J., and Harris, P.D.. 1989. Changes in nematode antigens recognized by monoclonal antibodies during early infections of soya beans with the cyst nematode Heterodera glycines. Parasitology 98: 479–487. [Google Scholar]
- Bishop, A.H., and Ellar, D.J.. 1991. Attempts to culture Pasteuria penetrans in vitro. Biocontrol Science and Technology 1: 101–114. [Google Scholar]
- Bishop, A.H., Gowen, S.R., Pembroke, B., and Trotter, J.R.. 2007. Morphological and molecular characteristics of a new species of Pasteuria parasitic on Meloidogyne ardenensis. Journal of Invertebrate Pathology 96: 28–33. [DOI] [PubMed] [Google Scholar]
- Boutin, S., Bernatchez, L., Audet, C., and Derome, N.. 2013. Network analysis highlights complex interactions between pathogen, host and commensal microbiota. PLOS ONE 8: e84772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Z.X., Dickson, D.W., Freitas, L.G., and Preston, J.F.. 1997. Ultrastructure, morphology and sporogenesis of Pasteuria penetrans. Phytopathology 87: 273–283. [DOI] [PubMed] [Google Scholar]
- Cobb, N.A.. 1906. Fungus maladies of the sugar cane, with notes on associated insects and nematodes, 2nd ed. Hawaiian Sugar Planters Association, Experimental Station. Division of Pathology and Physiology Bulletin 5: 163–195. [Google Scholar]
- Collange, B., Navarrete, M., Peyre, G., Mateille, T., and Tchamitchian, M.. 2011. Root-knot nematode (Meloidogyne) management in vegetable crop production: the challenge of an agronomic system analysis. Crop Protection 30: 1251–1262. [Google Scholar]
- Davies, K.G., Kerry, B.R., and Flynn, C.A.. 1988. Observations on the pathogenicity of Pasteuria penetrans, a parasite of root-knot nematodes. Annals of Applied Biology 112: 1491–1501. [Google Scholar]
- Davies, K.G., Rowe, J., Manzanilla-Lopez, R., and Opperman, C.H.. 2011. Re-evaluation of the life-cycle of the nematode-parasitic bacterium Pasteuria penetrans in root-knot nematodes, Meloidogyne spp. Nematology 13: 825–835. [Google Scholar]
- Gerber, J.F., and White, J.H.. 2005. Materials and methods for the efficient production of Pasteuria. U.S. Patent 6: 919,197 B2. [Google Scholar]
- Giblin-Davis, R.M., Nong, G., Preston, J.F., Williams, D.S., Center, B.J., Brito, J.A., and Dickson, D.W.. 2011. ‘Candidatus Pasteuria aldrichii’, an obligate endoparasite of the bacterivorous nematode Bursilla. International Journal of Systematic and Evolutionary Microbiology 61: 2073–2080. [DOI] [PubMed] [Google Scholar]
- Giblin-Davis, R.M., Williams, D.S., Bekal, S., Dickson, D.W., Brito, J.A., Becker, J.O., and Preston, J.F.. 2003. ‘Candidatus Pasteuria usgae’ sp. nov., an obligate endoparasite of the phytoparasitic nematode Belonolaimus longicaudatus. International Journal of Systematic and Evolutionary Microbiology 53: 197–200. [DOI] [PubMed] [Google Scholar]
- Gorokhova, H., Rivetti, C., Furuhagen, S., Edlund, A., Ek, K., and Breitholtz, M.. 2015. Bacteria-mediated effects of antibiotics on Daphnia nutrition. Environmental Science and Technology 49: 5779–5787. [DOI] [PubMed] [Google Scholar]
- Hallman, J., Davies, K.G., and Sikora, R.. 2009. “Biological control using microbial pathogens, endophytes and antagonists”, in Perry, R.N., Moens, M., and Starr, J.L. (Eds), Root-knot Nematodes, CAB International, Oxfordshire, 380–411. [Google Scholar]
- Hewlett, T.E., and Dickson, D.W.. 1993. A centrifugation method for attaching endospores of Pasteuria spp. to nematodes. Supplement to the Journal of Nematology 25: 785–788. [PMC free article] [PubMed] [Google Scholar]
- Hoch, J.A.. 2000. Two-component and phosphorelay signal transduction. Current Opinion in Microbiology 3: 165–170. [DOI] [PubMed] [Google Scholar]
- Imbriani, J.L., and Mankau, R.. 1977. Ultrastructure of the nematode pathogen, Bacillus penetrans. Journal of Invertebrate Pathology 30: 337–347. [Google Scholar]
- Jones, J.T., Haegeman, A., Danchin, E.G.J., Gaur, H.S., Helder, J., Jones, M.G.K., Kikuchi, T., Manzanilla-López, R., Palomares-Rius, J.E., Wesemael, W.M., and Perry, R.N.. 2013. Top 10 plant-parasitic nematodes in molecular plant pathology. Molecular Plant Pathology 14: 946–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, D.T.. 1994. Partial characterization of a Pasteuria sp. attacking the citrus nematode, Tylenchulus semipenetrans, in Florida. Fundamental and Applied Nematology 17: 509–512. [Google Scholar]
- Kogut, M., and Harris, M.. 1969. Effects of Streptomycin in bacterial cultures growing at different rates; interaction with bacterial ribosomes in vivo. European Journal of Biochemistry 9: 42–49. [DOI] [PubMed] [Google Scholar]
- Kojetin, D.J., Thompson, R.J., Benson, L.M., Naylor, S., Waterman, J., Davies, K.G., Opperman, C.H., Stephenson, K., Hoch, J.A., and Cavanagh, J.. 2005. Structural analysis of divalent metals binding to the Bacillus subtilis response regulator Spo0F: The possibility for in vitro metalloregulation in the initiation of sporulation. Biometals 18: 449–466. [DOI] [PubMed] [Google Scholar]
- Kokalis-Burelle, N.. 2015. Pasteuria penetrans for control of Meloidogyne incognita on tomato and cucumber, and M. arenaria on snapdragon. Journal of Nematology 47: 207–2013. [PMC free article] [PubMed] [Google Scholar]
- Kummerer, K.. 2009. Antibiotics in the aquatic environment-a review-part I. Chemosphere 75: 417–434. [DOI] [PubMed] [Google Scholar]
- Levin, B.R., Perrot, V., and Walker, N.. 2000. Compensatory mutations, antibiotic resistance and the population genetics of adaptive evolution in bacteria. Genetics 154: 985–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauchline, T.H., Mohan, S., Davies, K.G., Schaff, J.E., Opperman, C.H., Kerry, B.R., and Hirsch, P.R.. 2010. A method for release and multiple strand amplification of small quantities of DNA from endospores of the fastidious bacterium Pasteuria penetrans. Letters in Applied Microbiology 50: 515–521. [DOI] [PubMed] [Google Scholar]
- Metchnikoff, M.E.. 1888. Pasteuria ramosa, un representant des bacteries a division longitudinale. Annales de l’Institut Pasteur 2: 165–170. [Google Scholar]
- Rao, U., Mauchline, T.H., and Davies, K.G.. 2012. The 16S rRNA gene of Pasteuria penetrans provides an early diagnostic of infection of root-knot nematodes (Meloidogyne spp.). Nematology 14: 799–804. [Google Scholar]
- Sayre, R.M., and Starr, M.P.. 1985. Pasteuria penetrans (ex. Thorne, 1940) nom. rev., comb. n., sp. n., a mycelial and endospore-forming bacterium parasitic in plant-parasitic nematodes. Proceedings of Helminthological Society of Washington 52: 149–165. [Google Scholar]
- Sayre, R.M., and Starr, M.P.. 1989. Genus Pasteuria Metchnikoff, 1888, in Williams, S.T., Sharp, M.E., and Holt, J.G. (Eds), Bergey’s Manual of Systematic Bacteriology 1, Williams and Wilkins, Philadelphia, PA, 2601–2615. [Google Scholar]
- Sayre, R.M., and Wergin, W.P.. 1977. Bacterial parasite of a plant nematode: Morphology and ultrastructure. Journal of Bacteriology 129: 1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayre, R.M., Starr, M.P., Golden, A.M., Wergin, W.P., and Endo, B. Y.. 1988. Comparison of Pasteuria penetrans from Meloidogyne incognita with a related mycelial and endospore-forming bacterial parasite from Pratylenchus brachyurus. Proceedings of Helminthological Society of Washington 55: 28–49. [Google Scholar]
- Sayre, R.M., Wergin, W.P., Schmidt, J.M., and Starr, M.P.. 1991. Pasteuria nishizawae sp. nov., a mycelial and endospore forming bacterium parasitic on cyst nematodes of genera Heterodera and Globodera. Research in Microbiology 142: 551–564. [DOI] [PubMed] [Google Scholar]
- Schmidt, L.M., Hewlett, T.E., Green, A., Simmons, L.J., Kelley, K., Doroh, M., and Stetina, S.R.. 2010. Molecular and morphological characterization and biological control capabilities of a Pasteuria ssp. parasitizing Rotylenchulus reniformis, the reniform nematode. Journal of Nematology 42: 207–217. [PMC free article] [PubMed] [Google Scholar]
- Sharma, S.B., and Davies, K.G.. 1996. Characterisation of Pasteuria isolated from Heterodera cajani using morphology, pathology and serology of endospores. Systematic and Applied Microbiology 19: 106–112. [Google Scholar]
- Stragier, P., and Losick, R.. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annual Review of Genetics 30: 297–341. [DOI] [PubMed] [Google Scholar]
- Subbotin, S.A., Sturhan, D., and Ryss, A.Y.. 1994. Occurrence of nematode-parasitic bacteria of genus Pasteuria in the former USSR. Russian Journal of Nematology 2: 61–64. [Google Scholar]
- Whitehead, A.G., and Hemming, J.R.. 1965. A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Annals of Applied Biology 55: 25–38. [Google Scholar]
- Williams, A.B., Stirling, G.R., Hayward, A.C., and Perry, J.. 1989. Properties and attempted culture of Pasteuria penetrans, a bacterial parasite of root-knot nematode (Meloidogyne javanica). Journal of Applied Microbiology 67: 145–156. [Google Scholar]
- Williams, J.R.. 1960. Studies on the nematode soil fauna of sugarcane fields in Mauritius. 5. Notes upon a parasite of root-knot nematodes. Nematologica 5: 37–42. [Google Scholar]
- Yashiro, E., and McManus, P.S.. 2012. Effect of Streptomycin treatment on bacterial community structure in the apple phyllosphere. PLOS ONE 7: e37131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski, A., Wagner, N.D., and Frost, P.C.. 2011. Antibiotics affect the growth responses of Daphnia magna to poor food quality. Aquatic Ecology 45: 493–504. [Google Scholar]


