Abstract
Introduction
Medical complications in pregnancy contribute significantly to maternal morbidity in sub-Saharan Africa. Anecdotally, obstetricians in Uganda do not feel equipped to treat complex medical cases, and receive little support from physicians.
Methods
The aim of the study was to quantify the burden of complex medical conditions on the obstetric high dependency unit at Mulago National Referral Hospital, and potential deficiencies in the referral of cases and training in obstetric medicine. A prospective audit was taken on the obstetric high dependency unit from April to May 2014. In addition, 50 trainees in obstetrics and gynaecology filled a nine-point questionnaire regarding their experiences.
Results
Complex medical disorders of pregnancy accounted for 22/106 (21%) admissions to the high dependency unit, and these cases were responsible for 51% of total bed occupancy, and had a case fatality rate of 6/22 (27.2%). Only 6/14 (43%) of referrals to medical specialties were fulfilled within 48 h. Of the six women who died due to medical conditions, three specialty referrals were made, none of which were fulfilled. Trainees reported deficiencies in obstetric medicine training and in referral of complex conditions. They were least confident addressing non-communicable conditions in pregnancy.
Discussion
Deficiencies exist in the care of women with complex medical conditions in pregnancy in Uganda. Frameworks of obstetric medicine training and referral of complex cases should be explored and adapted to the sub-Saharan African setting.
Keywords: Maternal–fetal medicine, maternal mortality, general medicine, infectious diseases
Introduction
There is increasing recognition that medical (or ‘indirect’) complications of pregnancy are a major contributor to maternal mortality and morbidity in low- and middle-income countries (LMICs).1 The WHO has reported that over a quarter (28%) of maternal deaths between 2003 and 2009 were due to such ‘indirect’ causes, compared with 27% and 14% due to haemorrhage and hypertensive disorders respectively.2,3 This constitutes a significant increase since the previous report between 1990 and 2002.
In sub-Saharan Africa this growing trend is fuelled by a combination of infectious, non-communicable and nutritional disorders. Whilst maternal deaths due to the HIV epidemic appear to be decreasing, women of reproductive age are suffering from more non-communicable disorders such as diabetes mellitus and hypertension.4,5 In Uganda, around 20% of women between 20 and 40 years have established hypertension,6 and the prevalence of metabolic disorders such as gestational diabetes mellitus (GDM) and obesity are likely to increase as the process of industrialisation and consumption of refined sugars increases.7,8 This emerging epidemic of non-communicable diseases is in line with trends seen across many LMICs in the 2010 Global Burden of Diseases report.2
This trend is likely to be an underestimation of the problem as ‘indirect’ causes of mortality are under-reported in LMICs. This may be because of under-diagnosis of medical conditions,9 lack of post-mortem autopsy information,10 and because maternal deaths due to pre-existing medical conditions often occur between six weeks and one year postpartum, outside the standard recognition period of a maternal death.4,11 Furthermore, there is increased recognition that medical co-morbidities in pregnancy play an important role in ‘direct’ causes of maternal death too.12 Examples include ruptured uterus due to macrosomia in untreated diabetes, severe pre-eclampsia due to pre-existing hypertension and death from obstetric haemorrhage in the context of anaemia.13 Medical conditions in pregnancy also have wider implications for reproductive health such as neonatal mortality and morbidity, and risk of chronic conditions in later life.14
Uganda is a low-income country in East Africa with a maternal mortality ratio (MMR) of 336 deaths per 100,000 live births. This is in decline from 524/100,000 in 2001.15 This would classify Uganda as stage II in the traditional model of the ‘obstetric transition’,16 a model based on the experience of high and middle-income countries over the last 100 years. This implies that the majority of maternal deaths are due to ‘direct’ causes, however, for reasons stated above this may not be an appropriate assumption in the sub-Saharan African context in 2018. The exact contribution of ‘direct’ and ‘indirect’ causes to Uganda’s MMR is unknown, as the depth of the data collected does not reflect the complexity of the multiple contributors to mortality. Some may view this as an insignificant issue in light of more pressing problems such as lack of access to basic healthcare infrastructure, low levels of health education and poor retention of healthcare personnel. However, an understanding of the profile of morbidity in pregnant women and the complex intersection between ‘direct’ and ‘indirect’ causes is likely to help direct service provision and clinical training.11,17
The majority of obstetric care in Uganda’s national referral hospital is delivered by obstetricians, with very little involvement from physicians. To qualify as a specialist obstetrician, trainees must complete a three-year Masters of Medicine (MMED) programme after their primary internship training (two years of general medical and surgical rotations). The obstetrics and gynaecology MMED programme is primarily a surgical training scheme, with little emphasis on the approach to complex medical complications of pregnancy. There is no sub-specialist training scheme in maternal–fetal medicine (MFM) yet, and doctors must go abroad to gain experience in this area. The obstetric high dependency unit (HDU) is a six-bed ward separate from the main post-natal ward. The unit cares for a minority of the women delivering in the hospital, but admits those who are thought to be suffering complex or unstable medical or surgical conditions. The unit does not offer classically defined high dependency therapies such as inotropic support or non-invasive ventilation, but offers closer nursing observation, superior documentation of clinical parameters, oxygen therapy and a dedicated clinical team (a specialist obstetrician, obstetric trainee and an intern doctor).
Obstetricians working at Mulago National Referral Hospital in Kampala anecdotally report a high burden of complex medical problems in pregnancy on the obstetric HDU and low confidence in approaching these conditions. They further suggest that the process of involving physicians in the care of these patients is suboptimal. These views were reflected by the author’s own experience working in the obstetric HDU. Since the claims to date had been informal and anecdotal in nature, the aim of this study was to formally quantify these issues based on the characteristics of admissions to the obstetric HDU and the opinions of those working within the unit.
Methods
A two-month prospective audit was conducted in the obstetric HDU at Mulago National Referral Hospital in Kampala during April and May 2014. This was done as part of a wider quality improvement project conducted by the author. Data were collected regarding basic demographics, admitting condition, length of stay on the unit and referral to medical specialities. The nature of data collected was decided in discussion with senior members of the department. All identifying information was removed. Data were compiled on a Microsoft Excel spreadsheet to create simple descriptive counts. No complex methods of statistical analysis were used.
A questionnaire (Figure 1) was circulated to the 61 trainees in obstetrics and gynaecology at Mulago Hospital in all three years of training, with nine questions addressing their experiences of medical complications of pregnancy in their place of work. The questionnaire was developed by the author, with the involvement of an expert reviewer. There was no known validated model addressing this area, and indeed this tool has not been tested previously. The questionnaires were distributed by one of the senior trainees in obstetrics and gynaecology at an opportune time in the trainees’ schedules, during teaching sessions. Some trainees were absent from these sessions resulting in a number of non-respondents. The questionnaires were filled in a confidential manner and the data were compiled by the senior trainee and returned to the author.
Figure 1.
Trainee questionnaire.
Results
Obstetric HDU audit
One hundred and six women were admitted to the obstetric HDU during the two-month period. This represents a small proportion of the 5000 women delivering at the institution during that same period (approximate value based on yearly total). The mean age was 26.1 years. 72/106 women were tested for HIV, of which 10/72 (14%) were HIV positive, and 84/106 (79%) of the admissions were due to common obstetric causes of morbidity; 30/106 (28%) were due to bleeding disorders (antepartum and postpartum haemorrhage), 25/106 (24%) due to hypertensive disorders of pregnancy (pre-eclampsia and eclampsia, but not haemolysis with elevated liver enzymes and low platelets (HELLP) syndrome), 21/106 (20%) due to obstructed labour and ruptured uterus, 3/106 (3%) due to sepsis and 5/106 (5%) due to various surgical gynaecological disorders, and 22/106 (21%) of the admissions were due to complex medical conditions. These included traditionally ‘indirect’ cause of maternal morbidity and HELLP syndrome. HELLP syndrome was included because, although technically a ‘direct’ cause of maternal morbidity, the cases had evidence of multi-organ failure requiring expert knowledge and management in excess of the routine obstetric care of pre-eclampsia. The potentially unhelpful nature of labelling conditions as ‘direct’ and ‘indirect’ is discussed later. The particular conditions and the amount of time that women spent on the HDU is summarised in Table 1.
Table 1.
Admissions, case fatality rates and bed hours on the obstetric HDU by condition.
| Condition | No. admissions (total = 106) | Case fatalities | Bed hours in HDU (total = 5365) | ||
|---|---|---|---|---|---|
| Bleeding disorders | Antepartum haemorrhage | 30 (28.3%) | 11 (10.3%) | 2 (6.7%) | 680 (12.6%) |
| Postpartum haemorrhage | 19 (17.9%) | ||||
| Hypertensive disorders | Pre-eclampsia | 25 (23.5%) | 8 (7.5%) | 0 (0%) | 1029 (19.2%) |
| Eclampsia | 17 (16%) | ||||
| Intra-partum obstruction | Obstructed labour | 21 (19.8%) | 2 (1.8%) | 0 (0%) | 500 (9.3%) |
| Ruptured uterus | 19 (17.9%) | ||||
| Other | Surgical disorders | 8 (7.5%) | 5 (4.7%) | 1 (12.5%) | 397 (7.4%) |
| Sepsis | 3 (2.8%) | ||||
| Complex medical conditions | Medical disorders | 22 (20.7%) | 14 (13.2%) | 6 (27.2%) | 2759 (51.4%) |
| HELLP syndrome | 8 (7.5%) | ||||
HDU: high dependency unit.
Fourteen referrals were made to medical specialties: five to renal medicine, four to infectious diseases, two to neurology, one to cardiology, one to respiratory medicine and one to endocrinology. Of these referrals, 6/14 (43%) were fulfilled within 48 h and 8/14 (57%) were fulfilled later than 48 h. The 22 cases of complex medical conditions are displayed in Table 2, with details of referral to medical specialities.
Table 2.
Complex medical conditions in pregnancy encountered on the obstetric HDU, with referrals and indication if patient was reviewed by physician team within 48 h.
| Age | HIV status | Gestation (wks) | Condition | Referral | Reviewed <48 h | |
|---|---|---|---|---|---|---|
| 29 | Unknown | 38 | Heart failure due to atrial septal defect and right–left shunt | Yes | No | |
| 26 | – | 36 | Acute severe asthma | – | – | |
| 32 | – | PP | Presumed pulmonary embolus postpartum | Yes | Yes | |
| 25 | + | 26 | Lobar pneumonia | Died | Yes | No |
| 16 | – | 18 | Acute hepatitis | – | – | |
| 25 | Unknown | 28 | Diabetic ketoacidosis | Yes | Yes | |
| 29 | Unknown | PP | Severe hyponatraemia (after postpartum haemorrhage) | – | – | |
| 19 | + | 34 | Viral encephalitis | Yes | No | |
| 24 | + | 27 | Bacterial meningitis | Died | Yes | No |
| 27 | + | 36 | Meningitis – clinical diagnosis | Died | – | – |
| 24 | – | 26 | Cerebral malaria | Died | Yes | No |
| 21 | – | PP | Encephalopathy post-eclamptic fit | Yes | No | |
| 24 | – | PP | Encephalopathy post-eclamptic fit | Yes | No | |
| 22 | – | PP | Hypoxic brain injury, cardiac arrest from high spinal anaesthesia | – | – | |
| 20 | – | 34 | HELLP syndrome | Yes | Yes | |
| 23 | – | 38 | HELLP syndrome | – | – | |
| 28 | Unknown | 34 | HELLP syndrome with PPH and DIC | Died | – | – |
| 27 | Unknown | PP | HELLP syndrome with PPH | – | – | |
| 32 | Unknown | PP | HELLP syndrome | – | – | |
| 26 | – | 37 | HELLP syndrome with PPH and DIC | Died | – | – |
| 28 | – | PP | HELLP syndrome | Yes | Yes | |
| 30 | – | 36 | HELLP syndrome | Yes | Yes |
PP: postpartum; PPH: postpartum haemorrhage; DIC: disseminated intravascular coagulation, HELLP: haemolysis with elevated liver enzymes and low platelets; HDU: high dependency unit.
Obstetric trainee questionnaire
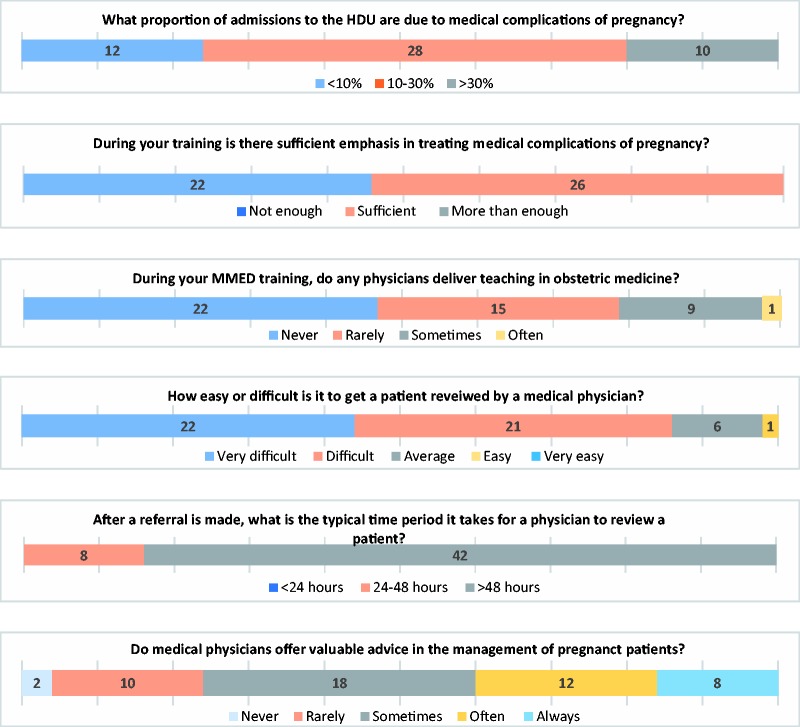
Of the 61 trainees, there were 50 respondents to the trainee questionnaire (20, 11 and 19 in their first, second and third years of training, respectively) and 11 non-respondents. Over half (56%) recognised that medical complications of pregnancy make up 10–30% of workload on the obstetric HDU. Interestingly, junior trainees underestimated this, with 7/20 (35%) of first years thinking that medical complications contribute <10% of workload compared with 3/30 (10%) of second and third years. In addition, 26/48 (54%) thought that there was sufficient emphasis on medical complications of pregnancy in their training, and 22/48 (46%) thought there was not; 37/47 (79%) of trainees said they ‘never’ or ‘rarely’ received training delivered by a physician. Trainees reported most confidence in approaching malaria, sepsis and sickle cell disease, and least confidence in addressing heart failure, diabetic ketoacidosis and thyroid disease in pregnancy. There did not appear to be a difference with the year of training. Moreover, 43/50 (86%) of trainees claimed it was ‘difficult’ or ‘very difficult’ to get a patient reviewed by a physician from a medical speciality, and 42/50 (84%) of trainees reported that it usually takes more than 48 h for a patient to be reviewed after a request for a consultation is made. There was a spread in opinion regarding whether the advice that physicians offered was valuable in the management of pregnant patients. Most consultation requests were made to the nephrology, neurology and cardiology departments. Trainees used various sources of information to aid in treating medical disorders of pregnancy; few used the RCOG (Royal College of Obstetrics and Gynaecology) Greentop guidance. Responses are reported in Figure 2 and Table 3.
Figure 2.
Responses from the trainee questionnaire.Note: Please refer to the online version of the article to view the figures in colour.
Table 3.
Responses from the trainee questionnaire indicating the conditions which trainees feel most confident and least confident in treating.
|
Which of these conditions do you feel MOST and LEAST confident in treating? | |||
|---|---|---|---|
| Most confident | Responses | Least confident | Responses |
| Malaria | 43 | Heart failure | 22 |
| Sepsis | 37 | DKA | 22 |
| Sickle cell disease | 18 | Thyroid disease | 22 |
| HELLP syndrome | 15 | Acute hepatitis | 20 |
| Heart failure | 9 | Venous thrombosis | 18 |
| Asthma | 8 | Tuberculosis | 10 |
| DKA | 7 | HIV complications | 10 |
| Venous thrombosis | 5 | Sickle cell disease | 9 |
| HIV complications | 1 | HELLP syndrome | 4 |
| Tuberculosis | 0 | Asthma | 2 |
| Acute hepatitis | 0 | Sepsis | 2 |
| Thyroid disease | 0 | Malaria | 0 |
DKA: Diabetic ketoacidosis.
Discussion
Limitations
The data of admissions to the HDU are by no means an epidemiological representation of the profile of maternal morbidity and mortality in Uganda, but provide a brief snapshot of the workload faced by obstetricians in the country’s tertiary referral centre. The data collected were simple in nature and may not accurately reflect the complex nature of the conditions faced. The short sample period (two months) may have led to an incomplete sample. The data cannot be extrapolated to other levels of healthcare facility within Uganda, as others have the option of onward referral. All we may conclude is that obstetricians are dealing with a significant number of complex medical conditions in pregnancy at this specific hospital, and that there is a sub-optimal referral system between the departments of obstetrics and internal medicine. Of course, one may hypothesise that a similar situation is faced in other large maternity centres in sub-Saharan Africa, and we would welcome this feedback.
The questionnaire of obstetric trainees is, to our knowledge, the first of its kind. We recognise that the questionnaire is simple in its design and does not conform to the rigorous design of in-depth qualitative research; however, we hope that the involvement of an expert in obstetric medicine training in creating the tool adds some validity. Not all trainees responded to the questionnaire which may produce some bias, though 50/61 (82%) seems a representative sample. We also recognise that there may be cultural differences in the way that trainees respond to such questionnaires; whether Ugandan trainees are more likely to emphasise or understate certain deficiencies in their training or knowledge-base is unknown. We hope that the anonymity of the questionnaire and distribution by a local trainee helped to mitigate these issues.
Inferences
The audit data collected from the obstetric HDU and the trainee questionnaire appear to reflect a common theme; that complex medical disorders make a major contribution to both admissions and maternal death on the obstetric HDU in this setting, and that there are difficulties in referring cases to physicians from medical specialities. Indeed, we note that all women who died due to complex medical conditions were either not reviewed by a physician, or they were reviewed more than 48 h after the referral was made. It would be interesting to explore the reasons for this disconnect; whether it is due to knowledge deficiency, physical distance or politics between departments, or perhaps resistance to a decision-sharing culture, we do not yet know. The trainees reported that physicians are rarely involved in their education, and they are least confident in approaching conditions associated with non-communicable disease. There is therefore clearly scope for the improvement in the care of women with complex medical conditions in pregnancy. The importance of this is supported by a growing body of evidence that suggests women in LMICs are suffering from an increasing incidence of non-communicable disease (NCDs),18,19 which may predispose them to developing complex medical disorders in pregnancy.
Improvements may be directed at the local to the national level and may be generalised to other LMICs:
Improvements in training for obstetric trainees: Modules in obstetric medicine could form part of the formal training pathway for obstetricians and include more education around unfamiliar subjects such as NCDs in pregnancy. Engagement with physicians to deliver education may be explored. External training courses such as the Global ALSO© and Maternal Collapse© courses may be adapted to address the conditions faced and the resources available in sub-Saharan Africa. Such a drive could reflect moves being made in Latin America.17 The situation in South Africa is currently being explored.20,21
Creation of context-specific guidelines: Most obstetric medicine guidelines are based on the experiences in the high income setting and are not suited for use where the majority of medical complications of pregnancy occur worldwide. These could be adapted and made context-specific. Most doctors in sub-Saharan Africa carry a smartphone with widespread access to fast 3G internet; guidelines could be developed online or into a smartphone application. The use of linked online education tools adapted to this setting may be explored.22
Development of multi-disciplinary care at the tertiary level: We have learnt from the high resource setting that multi-disciplinary care is crucial to the care of pregnant women with complex medical disorders.11 Improved communication between the obstetric and medical departments should be developed, with a better framework for referrals of critically unwell patients. With this objective in mind, the senior management team are currently exploring the option of employing two in-house physicians at the new specialised women’s hospital in Kampala. Work has also begun to establish the first joint clinic between obstetricians and cardiologists from The Heart Institute.
Development of sub-speciality training in MFM: This is currently being explored by the department of Obstetrics and Gynaecology at Mulago Hospital. Established competencies and curricula are defined elsewhere.23,24
National screening for NCDs in pregnancy: The health infrastructure in Uganda is modelled on the treatment of episodic issues, such as infections, or around delivery in pregnancy. There are few systems which support on-going longitudinal management of chronic conditions such as diabetes and hypertension. The exception is HIV care, which remains reliant on funding from large non-governmental donors, the likes of which we are yet to see in the area of NCDs. If we take diabetes in pregnancy as an example, basic work still needs to be done regarding the suitability of certain screening tests to the population and infrastructure. Only then can we build an epidemiological picture of the incidence of conditions such as GDM. It is recognised that the early identification and risk stratification of women with pre-existing co-morbidities lies at the heart of safe pregnancy and healthy neonatal life.13 The task is large, but work has begun in the area of diabetes at the MRC/UVRI & LSHTM Uganda Research Institute which is currently conducting a large observational study to describe the risk factors and outcomes from GDM.
Further work
Clearly more comprehensive data are needed to make a robust case for investment in training and services in obstetric medicine across sub-Saharan Africa. Having said this, steps are already being taken at Mulago Hospital as the case appears clear. It may be helpful to change the way that maternal mortality data is collected and presented; up to now much data collection has been too simplistic by concentrating on ‘mortality’ (without including wider ‘morbidity’)25 and by considering ‘direct’ and ‘indirect’ causes of mortality as distinct entities. This way of presenting data reduces the complex sequence of events that may lead to a maternal death to a single data point that does not reflect the close inter-dependent nature of a woman’s medical co-morbidities during pregnancy and the process of childbirth. There is then a danger that the contribution of early and treatable medical co-morbidities may be ignored due to focus on the late and grave consequences that are later faced by the surgically-focussed obstetrician. The high rate of eclampsia we see in Kampala, a condition that may be avoided with robust antenatal surveillance, illustrates this point well. It would also be short-sighted to train more surgeons to address mortality caused by ruptured uterus whilst ignoring programmes to develop screening for GDM and macrosomia for example. This focus on mortality is being addressed, as data collection is increasingly utilising indices such as ‘severe maternal morbidity’ or ‘near-miss events’26 to gain a more comprehensive picture of various medical contributions, rather than just the simplistic final event of mortality.27,28 The classification system of ‘direct’ and ‘indirect’ deaths is likely to remain for now. Perhaps, we should consider generating terms that describe obstetric conditions that are predictable by the recognition of co-morbidities earlier in pregnancy, or those which require knowledge of multi-organ physiology; whether or not the condition is specific to pregnancy seems satisfying but inconsequential. Whether a condition can be identified early and treated to avoid a peripartum emergency seems more important,29 as is whether a woman is eventually treated by a doctor with the appropriate knowledge and skills.
Many think of the future development of maternal healthcare in sub-Saharan Africa in terms of the model of ‘the obstetric transition’.16 This suggests that in the context of high MMR there should remain a focus on ‘direct’ causes of maternal mortality, and that the contribution of non-communicable diseases and ‘indirect’ causes of mortality only become relevant later in the development of the health infrastructure. However, as we have seen with this small study, and many more comprehensive studies in the literature,30,31 this may not be the case in low-income countries. The model of the obstetric transition is based on experiences of very different settings around the world over the past 100 years (e.g. the UK and Brazil), where the emergence of non-communicable disorders such as diabetes and hypertension caused by various socio-economic changes coincided with the rapid development of a state-of-the-art health infrastructure capable of addressing this new epidemic. The situation in sub-Saharan Africa is quite different where we see a similar emergence of NCDs due to urbanisation and consumption of refined sugars, but long before the basic health infrastructure is able to cope with managing chronic conditions and the traditional ‘direct’ causes of maternal mortality simultaneously. By referring to a model which is not representative of sub-Saharan Africa, we risk unjustified focus on surgical causes of mortality rather than wider medical co-morbidities.
What seems clear is that complex medical disorders in pregnancy are not confined to the high resource setting, but affect women worldwide. Indeed, pregnant women in sub-Saharan Africa today are at risk from multiple contributing factors: the pregnancy itself, infectious diseases, and now metabolic and cardiovascular disorders. Future strategies should address these in conjunction rather than as discrete entities, and throughout the entirety of the antenatal period. Obstetricians working in these settings should be supported in the areas of obstetric medicine that they feel unconfident in addressing and may not have been given priority during their training. A case for engaging and training sub-Saharan Africa’s first obstetric physicians is now evolving, and we would welcome the involvement of the International Society of Obstetric Medicine in this ongoing venture.
Acknowledgements
The authors thank Dr Josaphat Byamuguisha for supporting the project as chair of the department; Dr Tabulay Jackson for distribution, collection and compilation of the questionnaire responses and Professor Catherine Nelson-Piercy for expert advice regarding design of the trainee questionnaire tool.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
The data included in this article were collected as part of usual quality improvement programme activities. Ethical approval for publication was granted in retrospect by the Mulago National Referral Hospital Institutional Review Board (IRB). The IRB did not see it necessary to have written consent from patients as this work was part of usual quality improvement programme activities. Permission was granted by the IRB in retrospect on behalf of their clients.
Guarantor
JMM.
Contributorship
JMM was responsible for the conception and design of the work, and drafting of the manuscript. AN contributed with interpretation, critical revision and final approval of the manuscript.
References
- 1.Firoz T, Ateka-Barrutia O, Rojas-Suarez JA, et al. Global obstetric medicine: collaborating towards global progress in maternal health. Obstet Med 2015; 8: 138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassebaum NJ, et al. Global, regional, and national levels of maternal mortality, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1775–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014; 2: 323–333. [DOI] [PubMed] [Google Scholar]
- 4.WHO, UNICEF, UNFPA, et al. Trends in maternal mortality: 1990 to 2015: estimates by WHO, UNICEF, UNFPA, world bank group and the united nations population division. Geneva: World Health Organization, 2015. [Google Scholar]
- 5.Yusuf S, Reddy S, Ôunpuu S, et al. Global burden of cardiovascular diseases part I: general considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation 2001; 104: 2746–2753. [DOI] [PubMed] [Google Scholar]
- 6.Guwatudde D, Mutungi G, Wesonga R, et al. The epidemiology of hypertension in Uganda: findings from the national non-communicable diseases risk factor survey. PloS One 2015; 10: e0138991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atun R, et al. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol 2017; 5: 622–667. [DOI] [PubMed] [Google Scholar]
- 8.Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab 2008; 93: 9–30. [DOI] [PubMed] [Google Scholar]
- 9.Ronsmans C, Graham WJ; Group LMSSs. Maternal mortality: who, when, where, and why. Lancet 2006; 368: 1189–1200. [DOI] [PubMed] [Google Scholar]
- 10.Cross S, Bell JS, Graham WJ. What you count is what you target: the implications of maternal death classification for tracking progress towards reducing maternal mortality in developing countries. Bull World Health Org 2010; 88: 147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight M, Nair M, Tuffnell D, et al. ; on behalf of MBRRACE-UK. Saving lives, improving mothers’ care – surveillance of maternal deaths in the UK 2011–2013 and lessons learned to inform maternity care from the UK and Ireland confidential enquiries into maternal deaths and morbidity 2009–2014. Oxford: University of Oxford, 2016.
- 12.Nair M, Kurinczuk J, Brocklehurst P. Factors associated with maternal death from direct pregnancy complications: a UK national case-control study. BJOG 2015; 122: 653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussein J. Non-communicable diseases during pregnancy in low and middle income countries. Obstet Med 2016; 10: 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baird J, Jacob C, Barker M, et al. Developmental origins of health and disease: a lifecourse approach to the prevention of non-communicable diseases. Healthcare (Basel) 2017; 5: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uganda Bureau of Statistics (UBOS). Demographic and health survey (DHS), available at: https://www.ubos.org/onlinefiles/uploads/ubos/pdf%20documents/Uganda_DHS_2016_KIR.pdf (accessed 29 October 2018).
- 16.Souza J, Tunçalp Ö, Vogel J, et al. Obstetric transition: the pathway towards ending preventable maternal deaths. BJOG 2014; 121: 1–4. [DOI] [PubMed] [Google Scholar]
- 17.Rojas-Suarez J, Suarez N and, Ateka-Barrutia O. Developing obstetric medicine training in Latin America. Obstet Med 2017; 10: 16–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Bank. The growing danger of non-communicable diseases. Acting now to reverse the course. World Bank Report, Washington, USA, 2011. [Google Scholar]
- 19.WHO. Non-communicable diseases: a priority for women’s health and development, www.who.int/pmnch/topics/maternal/2011_women_ncd_report.pdf.pdf (2011, accessed 27 September 2018).
- 20.Acquah L, Burton R. Obstetric medicine: Interlinking obstetrics and internal medicine. S Afr Med J 2014; 104: 636–639. [DOI] [PubMed] [Google Scholar]
- 21.Wium L, Vannevel V, Bothma S. Obstetric medical care and training in South Africa. Obstet Med 2018; 1–4 [ePub only]. [DOI] [PMC free article] [PubMed]
- 22.Cumyn A, Gibson P. Validation of content of clinical cases in obstetric medicine for a shared web-based education tool. Obstet Med 2018; 1--7 [ePub only]. [DOI] [PMC free article] [PubMed]
- 23.Cumyn A, Gandhi S, Gibson P, et al. Defining competencies for training in obstetric medicine. Obstet Med 2014; 7: 137–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cumyn A, Gibson P. CanCOM obstetric medicine curriculum, http://gemoq.ca/wp-content/uploads/2011/08/CanCOM-competencies-for-Obstetric-Medicine.pdf (2015, accessed 27 September 2018).
- 25.Firoz T, Chou D, von Dadelszen P, et al. Measuring maternal health: focus on maternal morbidity. Bull World Health Organ 2013; 91: 794–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Say L, Souza JP, Pattinson RC, et al. Maternal near miss – towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol 2009; 23: 287–296. [DOI] [PubMed] [Google Scholar]
- 27.Hogan MC, Saavedra-Avendano B, Darney BG, et al. Reclassifying causes of obstetric death in Mexico: a repeated cross-sectional study. Bull World Health Organ 2016; 94: 362–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koblinsky M, Chowdhury ME, Moran A, et al. Maternal morbidity and disability and their consequences: neglected agenda in maternal health. J Health Popul Nutr 2012; 30: 124–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Akker T, et al. Maternal mortality: direct or indirect has become irrelevant. Lancet Glob Health 2017; 5: 1181–1182. [DOI] [PubMed] [Google Scholar]
- 30.Chaves SC, Cecatti JG, Carroli G, et al. Obstetric transition in the World Health Organization multicountry survey on maternal and newborn health: exploring pathways for maternal mortality reduction. Rev Panam Salud Pública 2015; 37: 203–210. [PubMed] [Google Scholar]
- 31.Souza JP. Maternal mortality and development: the obstetric transition in Brazil. Rev Bras Ginecol Obstet 2013; 35: 533–535. [DOI] [PubMed] [Google Scholar]