Abstract
The authors describe five new species of Pristionchus from Japan and Hongkong. Scarab beetle samplings in Hongkong identified P. hongkongensis sp. n. and P. neolucani sp. n., representing the first beetle-associated Pristionchus species from China. Surprisingly, samplings of millipedes in Japan revealed a previously unknown association of Pristionchus nematodes with these arthropods. Specifically, the authors found three previously known Pristionchus species, P. arcanus, P. entomophagus, and P. fukushimae on Japanese millipedes. In addition, the authors found three new Pristionchus species on millipedes, which are described as P. riukiariae sp. n., P. degawai sp. n., and P. laevicollis, sp. n., the latter of which was also found on stag beetles. These species are most closely related to P. maxplancki, P. japonicus, and P. quartusdecimus and belong to the pacificus species-complex. The authors describe all species based on morphology, morphometrics, and genome-wide sequence analysis. Mating experiments indicated that all species are reproductively isolated from each other and in contrast to the species of the “pacificus species-complex sensu stricto” they do not form F1 hybrids.
Keywords: Pristionchus pacificus, Millipedes, Hongkong, Japan, Stoma, scarab beetles
The genus Pristionchus (Kreis, 1932) is emerging as a model system for biodiversity research in nematodes and animals in general (Sommer, 2015). As Pristionchus species are often found in association with scarab beetles, detailed sampling of these insects has provided a comprehensive picture of the diversity of their associated nematodes (Ragsdale et al., 2015). Specifically, more than 30 species from around the world are known to form four well-defined groups of the genus. Two major strategies can be taken to explore Pristionchus taxonomy and phylogeny to eventually reach the saturation level of species identification. First, broad collecting efforts have to be conducted in continents where taxonomic discovery of Pristionchus is unsaturated. For example, samplings in East Asia and Taiwan in particular, revealed the identification of novel species in every new field trip as indicated in the accompanying publication (Yoshida et al., 2018). In contrast, the Pristionchus diversity in Europe has apparently reached saturation with collections in Western and Eastern Europe repeatedly finding a total of seven species in the “lheritieri” species-complex from soil and scarab beetle samplings (Herrmann et al., 2006). Similarly, collections in Africa and South-America have so far only identified the known hermaphroditic species of the genus (M.H., pers. Obs.). Thus, future studies should focus on East Asia, both looking at continental and island settings. Second, previous research had indicated that Pristionchus evolution involves host switching from scarab beetles to other insects including the Colorado potato beetle and fig wasps (D’Anna and Sommer, 2011; Susoy et al., 2016). Therefore, sampling efforts should include other arthropod taxa as potential sources of novel species.
Here, we describe five new Pristionchus species that result from field trips in 2016 and 2017 that aimed to explore new areas in East Asia and sampling of new arthropod species. Specifically, we found three new species on millipedes sampled in Japan, an Island that had previously shown saturation with regard to scarab beetle-associated Pristionchus species (Kanzaki et al., 2012; Kanzaki et al., 2014). In addition, we found two new Pristionchus species on scarab beetles in Hongkong, which represents the first scarab beetle sampling for Pristionchus nematodes in China. We describe all five new species by morphology, morphometrics, mating experiments, and molecular sequence analysis.
Materials and methods
Beetle sampling and nematode isolation
In addition to collecting millipedes in different parts of Japan, a field trip to Hongkong was conducted in June 2017. Basically all major areas in Hongkong, including the new territories have been visited and more than 250 specimens of scarabaeoid beetles belonging to 16 species in 13 genera were collected. Nematodes were isolated using standard procedures as previously described (Herrmann et al., 2006). In total, we isolated and characterized 12 strains of the Diplogastridae family with eight of these strains representing members of the genus Pristionchus. Three of the Pristionchus strains were hermaphroditic and were shown by sequencing of the full length SSU rRNA of 1.6kb using the primers RH5401 (5′- AAAGATTAAGCCATGCATG -3′) and SSU R2 (5′- GGTTCAAGCCACTGCGAT-3′) for PCR amplification, and RH5403 (5′- AGCTGGAATTACCGCGGCTG-3′), SSU R4 (5′- TGGTGCCCTTCCGTCAATTC-3′) and SSU R2 (5′- GGTTCAAGCCACTGCGAT-3′) for sequencing and mating experiments (see below) to represent new isolates of P. pacificus. Thus, these strains are not further considered in this study. Five strains of Pristionchus were gonochoristic and represent new species that will be described in this study. All new isolates are available as frozen stocks in the Department of Evolutionary Biology, Max Planck Institute (MPI) for Developmental Biology, Tübingen, Germany and can be provided to other researchers upon request.
Nematode cultivation
P. laevicollis sp. n. was isolated from an adult of Aegus subnitidus (Waterhouse, 1873) (Coleoptera: Lucanidae), formerly a subspecies of A. laevicollis Saunders, 1854, collected in Nagoya, Japan. The strain of P. hongkongensis sp. n. and P. neolucani sp. n. were isolated from two adults of Neolucanus championi, (Parry, 1864) (Coleoptera: Lucanidae) collected at Mui Tsz Lam, Hongkong. Pristionchus degawai sp. n. was isolated from the millipede Parafontaria laminata armigera (Verhoeff, 1936) (Diplopoda: Xystodesmidae) in Nagano, Japan and P. riukiariae sp. n. came from a millipede of the species Riukiaria holstii (Pocock, 1895) (Diplopoda: Xystodesmidae) from Okinawa, Japan. All strains have been kept in laboratory culture on NGM agar plates seeded with Escherichia coli strain OP50 under the following culture code and freezing voucher numbers RS5939 (P. laevicollis sp. n.), RS5957 (P. hongkongensis sp. n.), RS5949 (P. neolucani sp. n.), RS5938 (P. degawai sp. n.), and RS5937 (P. riukiariae sp. n.).
Morphological observation and preparation of type material
Light microscopic observations for drawings and morphometrics were conducted using live nematode material, which was handpicked from culture plates as described previously (Kanzaki, 2013).
Remarks concerning morphometric measurements: based on our repeated findings that measurements of certain nematode dimensions can change over time as a result to different culture conditions (Fonderie et al., 2013; Kanzaki et al., 2012a,2013; Ragsdale et al., 2015; Herrmann et al., 2016), we combine molecular, morphological, biological, and ecological information to characterize new material as broadly as possible.
Mating experiments
To examine reproductive isolation of the new species we crossed them with the most closely related species in the phylogeny. In the case of the four new species P. hongkongensis sp. n., P. neolucani sp. n., P. degawai sp. n., and P. riukiariae sp. n., we tested all possible combinations of all four species since they are closely related to one another. Mating experiments were performed as previously described with some modifications (Herrmann et al., 2006). Three virgin females of a strain were tested with six males of another strain on a mating plate that contained a small amount of E. coli OP50 bacteria. All crosses were performed reciprocally and in quadruplicate. To test for the production of viable F1 hybrids, crosses were set up between all possible pairs and F1 hybrids were tested for their ability to produce F2 progeny. We considered strains to belong to the same species if they produced viable and fertile offspring.
Molecular characterization and phylogenetic analysis
A species phylogeny of the complete Pristionchus genus was reconstructed as described in Rödelsperger et al. (2018). In the following, we give a brief summary about the generation of the Pristionchus phylogeny (please see Rödelsperger et al., 2018 for full details). For all cultivable Pristionchus species, worms were grown on NGM plates seeded with E. coli OP50 at 20°C and total RNA was isolated from 2 to 3 mixed-stage plates per species using standard Trizol extraction following the manufacturers’ instructions (Zymo Research, CA, USA). RNAseq libraries were prepared using TruSeq RNA library preparation kit v2 (Illumina Inc, CA, USA) according to the manufacturer’s instructions from 1 µg of total RNA in each sample and sequenced on the Illumina HiSeq3000 platform yielding a median of 14 million paired reads (2×150 bp) per species. Raw reads were submitted to the European nucleotide archive under the study accession PRJEB20959. RNA-seq. reads were assembled into transcriptomes using Trinity (version v2.2.0) (Grabherr et al., 2011). For further analysis, only the first reported isoform per gene was selected and the longest complete or partial ORF (≥60 amino acids) was called. Orthologous clusters were generated by orthAgogue (Ekseth et al., 2014) and protein sequences were aligned using the MUSCLE software (version 3.8.31) (Edgar, 2004). A total of 2,092 high quality alignments containing at least 14 species (without any duplication) and with at least 50 amino acid positions with coverage in all represented species were concatenated into a supermatrix spanning 350,000 amino acids. Based on previous analysis of dozens of gene families (Baskaran et al., 2015), we chose the LG substitution model to reconstruct a maximum-likelihood tree using RAxML (version 8.2.9, options -m PROTGAMMAILG -f a -N 100) (Stamatakis, 2014).
Results
Arthropod samplings in East Asia identify nine new Pristionchus species
We carried out field trips to Taiwan, Japan, and Hongkong in 2016 and 2017 as described in detail in “materials and methods” and in the accompanying publication (Yoshida et al., 2018). In Japan and Hongkong, we isolated a total of 12 gonochoristic Pristionchus strains, 10 strains from Japan, and 2 strains from Hongkong. These strains were first subjected to SSU rRNA sequence analysis, which suggested that they represent five new species of Pristionchus. Mating experiments with reference strains of available Pristionchus species with identical or closely related SSU sequences were performed confirming that these species are indeed reproductively isolated. Also, transcriptome analysis reported below confirmed these strains to represent novel species.
Mating experiments
We performed mating experiments to confirm reproductive isolation between P. laevicollis sp. n. and the related species P. maxplancki (Fig. 1). We observed some viable but sterile F1 individuals between the two species. In contrast, P. maxplancki did not produce viable F1 with species other than P. laevicollis sp. n. Next, we performed mating experiments between the other new species, P. neolucani sp. n., P. degawai sp. n., P. hongkongensis sp. n., and P. riukiariae sp. n., which form a new clade in the phylogenetic analysis (see below). All combinations of mating experiments produce no F1 hybrids. Thus, in contrast to the species in the pacificus species group sensu stricto, the species considered in this study largely do not form F1 hybrids.
Figure 1.
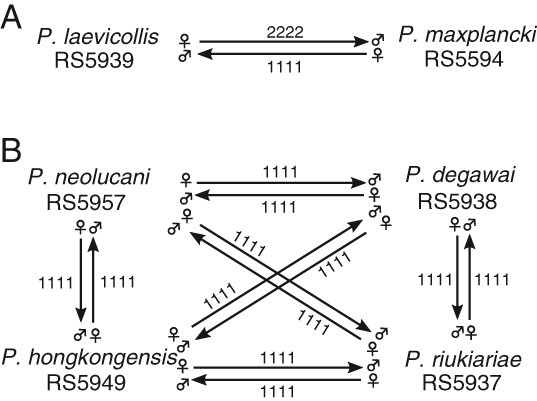
Results of mating experiments of all five new species with P. maxplancki. A: Mating between P. laevicollis sp. n. and P. maxplancki; B: Mating between the four new species, P. honkongensis sp. n., P. degawai sp. n., P. neolucani sp. n. and P. riukiariae sp. n. Numbers indicate the results of four replicates of mating experiments. Production of no F1 progeny is indicated as “1,” the formation of viable, sterile F1 progeny is indicated as “2.”
Description
Description of common characters
Most Pristionchus species are typologically similar to each other and generally distinguished based on stomatal and male tail characters. Therefore, the general morphology (e.g., body shape and gonadal structures) is described first as common characters, and then the distinctive characters are described for each species. It is important to note that these common characters in the novel species are also identical to the typological characters of other Pristionchus species, such as those described in the accompanying publication (Yoshida et al., 2018). Therefore, the following description of common characters is highly redundant, but included here for completeness.
Adult
The adult body can be described as follows: Body cylindrical, stout; cuticle thick, with fine annulation and clear longitudinal striations; lateral field consisting of two lines, only weakly distinguishable from body striation with the presence of deirid; head without apparent lips, and with six short and papilliform labial sensillae; four small, papilliform cephalic papillae present in males, as typical for diplogastrid nematodes; amphidial apertures located on the lateral sector, slightly dorsally shifted, at level of margin of cheilo- and gymnostom; stomatal dimorphism present, with stenostomatous (narrow mouthed) and eurystomatous (wide mouthed) forms occurring in males and females, the latter (i.e. eurystomatous form), however, less frequent in males. Detailed stomatal morphology is described below. Dorsal pharyngeal gland clearly observed, penetrating dorsal tooth to gland opening; anterior part of pharynx (= pro and metacorpus) 1.5 times as long as posterior part (= isthmus and basal bulb); procorpus very muscular, stout, occupying half to two-thirds of corresponding body diameter; metacorpus very muscular, forming well-developed median bulb; isthmus narrow, not muscular; basal bulb glandular; pharyngo-intestinal junction clearly observed, well developed; nerve ring usually surrounding middle region of isthmus; excretory pore not conspicuous, ventrally located at level of isthmus to pharyngo-intestinal junction, excretory duct extending anteriad and reflexed back to position of pore; deirid observed laterally, located from slightly anterior to pharyngo-intestinal junction to a half body diameter posterior to the junction; hemizonid not clearly observed; lateral glands, small pores connected to secretory cell, present and observed on the lateral body surface, with positions inconsistent among individuals, numbering 5 to 8 for males and 9 to 13 for females.
Stenostomatous form
Cheilostom consisting of six per- and interradial plates. Incision between plates is not easily distinguished by light microscopy. Anterior end of each plate rounded and elongated to project from stomatal opening and form a small flap; gymnostom short, cuticular ring-like anterior end overlapping cheilostom internally; dorsal gymnostomatal wall slightly thickened compared to ventral side; stegostom separated into three subsections: pro-meso, meta, and telostegostom; pro-meso stegostom forming a weakly cuticularized ring surrounding the anterior edge of pharynx. Metastegostom bearing conspicuous and movable triangular or flint-shaped dorsal tooth with strongly sclerotized surface giving an appearance of an inverted V-shape in light microscopy in lateral view; pointed left subventral ridge with three minute adventitious denticles on a plate; pointed right subventral ridge, often with distinct distal adventitious denticle(s). Telostegostom forming a weakly sclerotized cup-like cavity connecting stoma and pharynx.
Eurystomatous form
Cheilostom divided into six distinctive per- and interradial plates. Anterior end of each plate rounded and elongated to project from stomatal opening, forming a small flap; gymnostom with thick cuticle, forming a short, ring-like tube being thicker posteriorly; anterior end of gymnostom internally overlapping posterior end of cheilostomatal plates; stegostom separated into three subsections: pro-meso, meta, and telostegostom; pro-mesostegostom forming a weakly cuticularized ring surrounding the anterior edge of pharynx; metastegostom bearing large claw-like dorsal tooth; right subventral tooth; ridge of left subventral denticles or cusps, varying in number and size; dorsal and right subventral teeth movable; no movement observed for left subventral denticles; telostegostom forming a weakly sclerotized cup-like cavity connecting stoma and pharynx.
Male
The male body can be described as follows: Ventrally arcuate, strongly ventrally curved at tail region when killed by heat. Testis single, ventrally located, anterior part reflexed to right side; spermatogonia arranged in three to five rows in reflexed part, then well-developed spermatocytes arranged as three to four rows in anterior two-thirds of the main branch, then mature amoeboid spermatids arranged in multiple rows in remaining, proximal part of gonad; vas deferens not clearly separated from other parts of gonad; three (two subventral and one dorsal) cloacal gland cells observed at distal end of testis and intestine; spicules paired, separate; spicules smoothly curved in ventral view, adjacent to each other for distal third of their length, each smoothly tapering to pointed distal end; spicule in lateral view smoothly ventrally arcuate, giving spicule about 100° curvature, oval manubrium present at anterior end, lamina/calomus complex (blade) clearly expanded slightly posterior to manubrium (ca 1/4 of blade length from anterior), then smoothly tapering to pointed distal end; gubernaculum conspicuous, about one-third of spicule length, broad anteriorly such that dorsal wall is slightly recurved with dorsal and ventral walls separate at 50 to 60° angle at posterior end; dorsal side of gubernaculum possessing single, membranous, anteriorly directed process and lateral pair of more sclerotized, anteriorly and obliquely ventrally directed processes. In lateral view, anterior half of gubernaculum with two serial curves separated by anteriorly and obliquely ventrally directed process, with anterior terminal curvature highly concave and almost closed, and with deep posterior curvature being one-third of gubernaculum length; posterior half forming tube-like process enveloping spicules. Thick cuticle around tail region, falsely appearing as a narrow leptoderan bursa in ventral view; cloacal opening (co) slit-like in ventral view; one small, ventral, single genital papilla (vs) on anterior cloacal lip; nine pairs of genital papillae (v1-v7, ad, pd) and a pair of phasmids (ph) present, three precloacal and six postcloacal pairs. Within those pairs, three distal ventral pairs (v5, v6, and v7) and a dorsal pair (pd) are close to each other around the posterior end of tail (just anterior to the root of spike). Anterior five pairs of papillae (v1-4 and ad) almost equal in size, rather large and conspicuous, v7 and pd papillae obviously smaller than anterior five pairs, v5 and v6 very small, sometimes difficult to observe with light microscope. Anterior two pairs of the ventral triplet papillae (v5 and v6) papilliform and borne from socket-like base, v7 simple or typical thorn-like in shape. Tip of v6 papillae split into two small papilla-like projections. Detailed arrangement of papillae and phasmid will be described for each species below. Tail conical, with long spike, about two to three cloacal body diameters (CDB) long; bursa or bursal flap absent.
Female
The female body can be described as follows: Relaxed or slightly ventrally arcuate when killed by heat. Gonad didelphic, amphidelphic; each gonadal system arranged from vulva/vagina as uterus, oviduct, and ovary; anterior gonad right of intestine, with uterus and oviduct extending ventrally and anteriorly on right of intestine and with a totally reflexed (= antidromous reflexion) ovary extending dorsally on left of intestine; oocytes mostly arranged in three to four rows in distal two-thirds of ovary and in double or single row in rest of ovary, distal tips of each ovary reaching oviduct of opposite gonad branch; anterior end of oviduct (= junction tissue between ovary and oviduct) consists of rounded cells; anterior part of oviduct consists of rounded cells, forming a simple tube; middle part of oviduct serving as spermatheca, consists of roundish and relatively large cells. Eggs in single to multiple-cell stage or even further developed at posterior part of oviduct (= uterus), in young females being composed of squared or angular cells, long enough to contain one well developed oocyte. Receptaculum seminis not observed, i.e., the organ is not independent, and a part of oviduct/uterus works as the organ; vaginal glands present but obscure; vagina perpendicular to body surface, surrounded by sclerotized tissue; vulva slightly protuberant in lateral view, pore-like in ventral view; rectum about one anal body diameter long, intestine/rectum junction surrounded by well-developed sphincter muscle; three anal glands (two subventral and one dorsal) present but not obvious; anus in form of dome-shaped slit, posterior anal lip slightly protuberant; phasmid about one to two anal body diameters posterior to anus; tail long, smoothly tapered, distal end variable from filiform to long and conical.
Description of species-specific characters
Diagnostic characters, i.e., stomatal morphology and male tail characters (arrangement of genital papillae) are herein described for each species including measurements for all species (Tables 1–3). Additionally, these typological characters applicable to species diagnosis of the pacificus group are summarized in Table 4.
Table 1.
Morphometrics of P. laevicollis sp. n.
| Character | P. laevicollis RS5939 stenostomatous male | Stenostomatous female |
|---|---|---|
| n | 10 | 10 |
| L | 834 ± 104 (662–971) | 1104 ± 209 (746–1325) |
| L’ | 705 ± 89.6 (566–836) | 908 ± 179 (595–1126) |
| a | 15 ± 1 (13–17) | 13 ± 0.8 (12–15) |
| b | 6.5 ± 0.5 (5.9–7.4) | 7.6 ± 0.8 (5.8–8.6) |
| c | 6.5 ± 0.4 (5.9–7.2) | 5.7 ± 0.7 (4.6–6.9) |
| c’ | 3.9 ± 0.4 (3–4.3) | 5.8 ± 0.7 (4.9–7.1) |
| T or V | 59 ± 2 (56–62) | 46 ± 2.1 (42–49) |
| Maximum body diam. | 57 ± 7.4 (47–66) | 83 ± 14.8 (57–97) |
| Stoma length | 9.4 ± 1 (7.7–10.6) | 11.1 ± 1.1 (9–12.5) |
| Stoma diam. | 5.6 ± 0.7 (4–6.4) | 6.4 ± 0.7 (5.5–8.0) |
| Pharynx length (head to base of pharynx) | 118 ± 9.5 (103–132) | 137 ± 15.5 (113–154) |
| Anterior pharynx (pro– + metacorpus) | 72 ± 6.7 (61–81) | 84 ± 8.7 (71–93) |
| Posterior pharynx (isthmus + basal bulb) | 46 ± 2.9 (42–51) | 53 ± 7.1 (42–63) |
| Ant/total pharynx % | 61 ± 1 (58–61) | 61 ± 1.2 (59–63) |
| Median bulb diam. | 20 ± 2.1 (16–22) | 27 ± 3.1 (22–30) |
| Terminal bulb diam. | 19 ± 1.7 (16–21) | 25 ± 3.8 (19–31) |
| Testis length | 490 ± 72.5 (370–570) | – |
| Ant. end to vulva | – | 504±86.2 (365–613) |
| Vulva to anus distance | – | 410 ± 78.9 (275–523) |
| Cloacal or anal body diam. | 33 ± 2.3 (28–36) | 34 ± 4.5 (32–60) |
| Tail length | 129 ± 17.4 (96–152) | 196 ± 47.1 (138–285) |
| Spicule length (curve) | 44 ± 2.5 (40–47) | – |
| Spicule length (chord) | 35 ± 2.1 (31–38) | – |
| Gubernaculum length | 16 ± 1 (14–17) | – |
Table 2.
Morphometrics of P. honkongensis sp. n. and P. neolucani sp. n.
| Character | P. hongkongensis RS5957 stenostomatous male | Stenostomatous female | P. neolucani RS5949 stenostomatous male | Stenostomatous female |
|---|---|---|---|---|
| n | 10 | 10 | 10 | 10 |
| L | 1014 ± 185 (700–1302) | 1181 ± 315 (918–2008) | 871 ± 49.6 (775–951) | 1060 ± 111.1 (930–1297) |
| L’ | 847 ± 184 (560–1125) | 940 ± 288 (703–1700) | 745 ± 50.7 (658–826) | 864 ± 103.2 (743–1080) |
| a | 14 ± 3.7 (11–24) | 13 ± 0.9 (12–15) | 15 ± 1 (14–16) | 14 ± 0.7 (13–15) |
| b | 6 ± 1 (4.6–7.7) | 6.6 ± 1.1 (5.6–9.5) | 5.4 ± 0.6 (4.7–6.6) | 5.5 ± 0.4 (4.8–6.5) |
| c | 6.1 ± 1.3 (5–8.5) | 4.8 ± 0.7 (4.3–6.5) | 7 ± 0.9 (6.1–9.2) | 5.4 ± 0.5 (4.5–6.1) |
| c’ | 4.2 ± 0.9 (3–5.9) | 6.3 ± 0.5 (5.1–6.9) | 3.7 ± 0.7 (2.6–4.9) | 6.1 ± 0.9 (5–7.8) |
| T or V | 53 ± 4.8 (51–68) | 45 ± 2.6 (42–50) | 53 ± 7.5 (33–59) | 47 ± 1.3 (45–48) |
| Maximum body diam. | 73 ± 14.9 (51–96) | 92 ± 29.4 (67–170) | 57 ± 5.8 (49–67) | 75 ± 9 (63–95) |
| Stoma length | 12.6 ± 1 (11.4–14.6) | 13.3 ± 1.6 (10.3–16) | 10.4 ± 1 (8.7–12) | 11 ± 0.9 (10.2–13) |
| Stoma diam. | 7.2 ± 0.7 (6.1–8.5) | 7.7 ±1.1 (6.5–9.7) | 6.0 ± 0.7 (5.3–7.6) | 7.1 ± 0.7 (6.3–8.4) |
| Pharynx length (head to base of pharynx) | 155 ± 9.7 (140–172) | 168 ± 14 (154-202) | 151 ± 11.9 (129-169) | 182 ± 14.9 (149-194) |
| Anterior pharynx (pro- + metacorpus) | 98 ± 5.4 (90–106) | 106 ± 7.4 (94–117) | 95 ± 10.2 (76–112) | 116 ± 9.2 (97–128) |
| Posterior pharynx (isthmus + basal bulb) | 57 ± 5.9 (49–66) | 62 ± 8.4 (56–85) | 56 ± 5.3 (51–69) | 66 ± 7.1 (52–75) |
| Ant/total pharynx % | 63 ± 2 (60–66) | 63 ± 2.5 (58–66) | 63 ± 3.2 (59–67) | 64 ± 1.8 (61– 66) |
| Median bulb diam. | 27 ± 2 (24–31) | 31 ± 3.6 (26–38) | 25 ± 1 (24–27) | 31 ± 2.9 (27–36) |
| Terminal bulb diam. | 26 ± 2.2 (22–28) | 29 ± 6.6 (24–47) | 25 ± 1.3 (22–27) | 29 ± 1.7 (27–33) |
| Testis length | 540 ± 141 (366–765) | – | 467±43 (425–561) | – |
| Ant. end to vulva | – | 539 ± 172 (384–995) | – | 493 ± 50.9 (420–581) |
| Vulva to anus distance | – | 419 ± 149 (320–815) | – | 371 ± 47 (290–447) |
| Cloacal or anal body diam. | 41 ± 5.4 (30–50) | 39 ± 8 (32–60) | 35 ± 4.8 (27–43) | 32 ± 3.8 (27–38) |
| Tail length | 167 ± 21.6 (140–206) | 241 ± 30 (215–308) | 125 ± 13.2 (99–148) | 196 ± 19 (175–228) |
| Spicule length (curve) | 53 ± 4.2 (44–57) | – | 47±2.3 (43–51) | – |
| Spicule length (chord) | 45 ± 3.7 (37–49) | – | 36 ± 1.7 (34–39) | – |
| Gubernaculum length | 19 ± 1.8 (17–22) | – | 17 ± 1.5 (14–19) | – |
Table 3.
Morphometrics of P. riukiariae sp. n. and P. degawai sp. n.
| Character | P. riukiariae RS5937 stenostomatous male | Stenostomatous female | P. degawai RS5938 stenostomatous male | Stenostomatous female |
|---|---|---|---|---|
| n | 10 | 10 | 10 | 10 |
| L | 918 ± 162 (663–1180) | 1362 ± 211 (1025–1700) | 872 ± 142.2 (710–1105) | 1184 ± 310.5 (811–1853) |
| L’ | 780 ± 151 (553–1010) | 1142 ± 201 (835–1466) | 727 ± 134.6 (585–961) | 986 ± 282 (652–1600) |
| a | 12 ± 2.5 (9.4–15) | 14 ± 1.5 (11–16) | 16 ± 2 (13–19) | 16 ± 3 (132–23) |
| b | 5.7 ± 0.8 (5–7.5) | 8.4 ± 1.5 (5.9–10.8) | 5.3 ± 0.6 (4.6–6.4) | 6.6 ± 1.2 (5.1–8.6) |
| c | 6.8 ± 1.2 (5.4–9.3) | 6.2 ± 0.7 (5–7.3) | 6 ± 0.8 (5.4–7.7) | 6 ± 0.9 (4.7–7.3) |
| c’ | 3.4 ± 0.4 (2.7–4.2) | 5.2 ± 0.6 (4.5–6.1) | 4.1 ± 0.5 (3.3–4.7) | 5.2 ± 1 (3.8–7.3) |
| T or V | 51 ± 5.3 (41–59) | 45 ± 11.1 (32–72) | 45 ± 5 (40–56) | 51 ± 5.5 (45–63) |
| Maximum body diam. | 78 ± 13.2 (62–97) | 99 ± 19.4 (65–128) | 56 ± 14.4 (40–80) | 80 ± 28.6 (37–138) |
| Stoma length | 11.1 ± 1 (9.7–12.5) | 11.8 ± 1.3 (9.2–14) | 11.4 ± 0.8 (10–12.3) | 12.1 ± 0.9 (11–13.4) |
| Stoma diam. | 6.5 ± 0.8 (5.4–7.6) | 7.3 ±0.7 (6.2–8.4) | 6.7 ± 0.3 (6.3–7) | 7.6 ± 0.6 (6.9–8.7) |
| Pharynx length (head to base of pharynx) | 143 ± 17.6 (111–167) | 156 ± 12.2 (136–178) | 152 ± 10.1 (143–176) | 169 ± 16.3 (148–207) |
| Anterior pharynx (pro– + metacorpus) | 94 ± 11 (78–111) | 97 ± 8.8 (86–116) | 95 ± 4.2 (89–103) | 106 ± 8.4 (96–122) |
| Posterior pharynx (isthmus + basal bulb) | 54 ± 5.8 (43–63) | 58 ± 7 (40–66) | 58 ± 7.1 (47–73) | 62 ± 9.6 (51–85) |
| Ant/total pharynx % | 67 ± 11.9 (59–100) | 62 ± 3.4 (60–71) | 62 ± 2.4 (59–67) | 64 ± 2.3 (60– 68) |
| Median bulb diam. | 27 ± 2.2 (23–31) | 31 ± 1.4 (29–33) | 26 ± 3 (22–32) | 30 ± 4.6 (24–41) |
| Terminal bulb diam. | 24 ± 2.8 (19–28) | 28 ± 2.3 (24–32) | 25 ± 4 (19–33) | 28 ± 4.8 (22–39) |
| Testis length | 468 ± 116.4 (295–677) | – | 397 ± 105.7 (285–580) | – |
| Ant. end to vulva | – | 624 ± 199 (415–1040) | – | 601 ± 173.3 (416–1043) |
| Vulva to anus distance | – | 510 ± 94.6 (332–665) | – | 365 ± 72.8 (255–487) |
| Cloacal or anal body diam. | 41 ± 4.5 (32–46) | 43 ± 6.1 (31–52) | 35 ± 5.6 (30–44) | 39 ± 8.7 (22–55) |
| Tail length | 137 ± 21.2 (104–170) | 220 ± 14.3 (190–241) | 145 ± 14.5 (125–180) | 198 ± 36.8 (150–253) |
| Spicule length (curve) | 53 ± 5.5 (40–60) | – | 47±7.6 (37–57) | – |
| Spicule length (chord) | 43 ± 3.4 (36–47) | – | 40 ± 6.9 (32–50) | – |
| Gubernaculum length | 12 ± 2.5 (9.4–15) | – | 16 ± 2.4 (13–19) | – |
Table 4.
Summary of diagnostic characters of the Pristionchus pacificus complex sensu stricto.
| Stenostomatous form | Eurystomatous form | ||||||
|---|---|---|---|---|---|---|---|
| Species | Reproductive mode | Right subventral plate | Left subventral plate | Right subventral tooth | Left subventral cusps | Genital papillae | Other characteristic feature(s) |
| pacificus | H | Single peak | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Exspectatus | G | Single peak | Three bumps | Claw-like | three large cusps each sometimes split into two (= 3-6 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Occultus | G | single peak | three bumps | claw-like | Three large cusps each sometimes split into two to three (= 3-9 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Sikae | G | Single peak | Three bumps | Claw-like with or without blunt projection | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Arcanus | G | Single peak | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 2: v1-v2d > v2d-v4; v2d and v3 are close to each other | |
| Kurosawai | G | Single peak | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Taiwanensis | G | Single peak | Three bumps | Claw-like with blunt projection | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Laevicollis n. sp. | G | Single peak | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 3: v1-v2 = v2-v4; v2 and v3d are separated | |
| maxplancki | G | 1-2 peaks | with minute denticles | claw-like | three large cusps each sometimes split into two (= 3-6 peaks) | Type 3: v1-v2 = v2-v4; v2 and v3d are separated | |
| Japonicus | G | Single peak | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 4: v1-v2 < v2-v4; v2 and v3d are separated | |
| Riukiariae n. sp. | G | Two peaks | Three bumps | Two large ridges or multiple small denticles | Three large cusps each sometimes split into two to three (= 3-9 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Neolucani n. sp. | G | Single peak | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | Type 1: v1-v2d = v2d-v4; v2d and v3 are close to each other | |
| Degawai n. sp. | G | Single peak | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | v1-v2d = v2d-v4; v2d and v3 are close to each other | v2 and v3 are very close, and sometimes third pair is directed sublaterally (v3d) |
| Hongkongensis n. sp. | G | Single peak | Three bumps | Claw-like | Three large cusps each split into three or more (= more than 9 peaks) | v1-v2 < v2-v4; v2 and v3d are separated | Comparatively large and slightly more barrel-shaped stoma in eurystomatous form |
| Quatrusdecimus | G | 1-2 peaks | Three bumps | Claw-like | Three large cusps each sometimes split into two (= 3-6 peaks) | v1-v2 < v2-v4; v2 and v3d are separated | |
Pristionchus laevicollis
sp. n. (Figs 2–5; Tables 1, 4, and 5).
Figure 2.
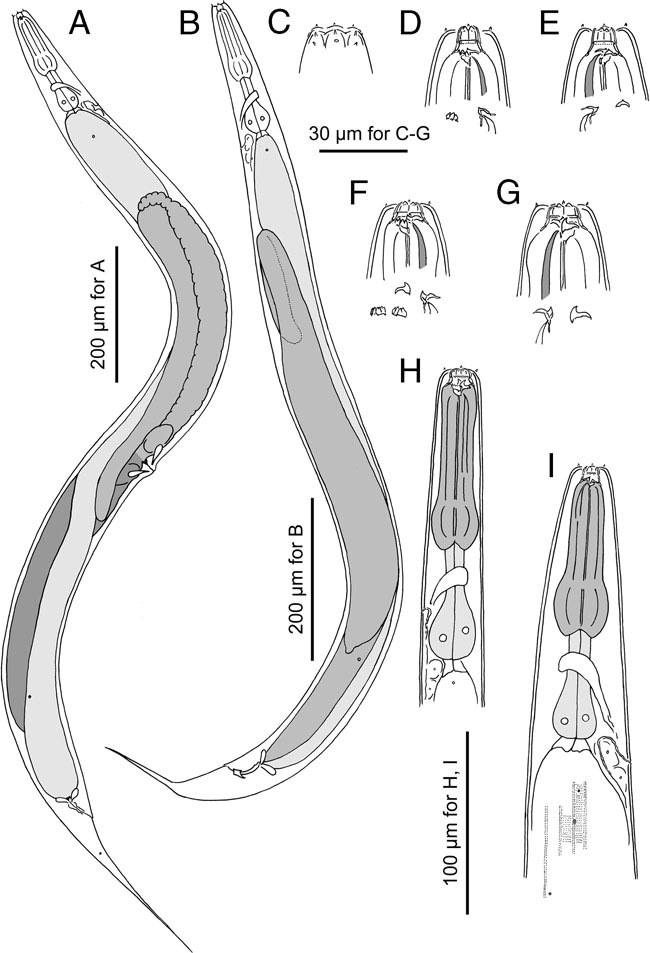
Adults of Pristionchus laevicollis sp. n. A: Female in right lateral view; B: Male in left lateral view; C: Surface of male lip region in left lateral view; D: Stomatal region of stenostomatal male in left lateral view; E: Stomatal region of stenostomatal male in right lateral view; F: Stomatal region of eurystomatal female in left lateral view; G Stomatal region of eurystomatal female in right lateral view; H: Anterior part of eurystomatous female in left lateral view; I: Anterior region of stenostomatous female in right lateral view.
Figure 5.
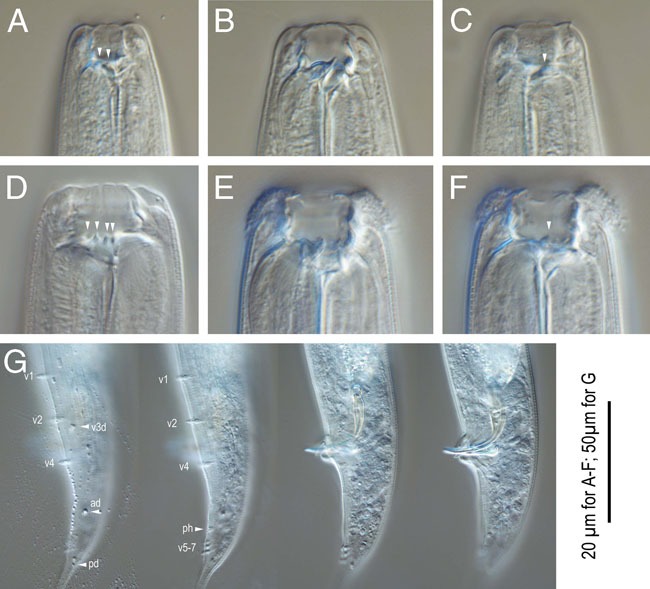
Pristionchus laevicollis n. sp. A-C: Stomatal region of stenostomatous form showing left subventral denticles (arrowheads in A), dorsal tooth (B) and right subventral ridge (arrowhead in C); D-F: Stomatal region of eurystomatous form showing left subventral cusps (arrowheads in D), Dorsal tooth (E) and right subventral tooth (arrowhead in F); G: Male tail in four different focal planes showing genital papillae (v+number), phasmid (ph), and spicule and gubernaculum shape. 20 µm for A-F; 50 µm for G.
Table 5.
The table shows the number of molecular differences in the 1.6kb SSU rRNA sequence for every pairwise comparison in the pacificus species-complex.
| P. pacificus | P. exspectatus | P. occultus | P. sikae | P. arcanus | P. kurosawai | P. taiwanensis | P. laevicollis sp. n. | P. maxplancki | P. riukiariae sp. n. | P. neolucani sp. n. | P. degawai sp. n. | P. hongkongensis sp. n. | P. quartusdecimus | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P. pacificus | 0 | 8 | 5 | 10 | 6 | 3 | 6 | 21 | 16 | 35 | 25 | 31 | 42 | 36 |
| P. exspectatus | – | 0 | 6 | 12 | 8 | 5 | 8 | 23 | 16 | 34 | 27 | 33 | 43 | 38 |
| P. occultus | – | – | 0 | 1 | 4 | 2 | 5 | 18 | 14 | 27 | 21 | 27 | 38 | 33 |
| P. sikae | – | – | – | 0 | 10 | 1 | 4 | 18 | 14 | 32 | 21 | 27 | 38 | 33 |
| P. arcanus | – | – | – | – | 0 | 3 | 4 | 19 | 14 | 31 | 24 | 30 | 41 | 35 |
| P. kurosawai | – | – | – | – | – | 0 | 3 | 18 | 13 | 28 | 22 | 28 | 39 | 33 |
| P. taiwanensis | – | – | – | – | – | – | 0 | 19 | 14 | 29 | 24 | 30 | 41 | 35 |
| P. laevicollis sp.n. | – | – | – | – | – | – | – | 0 | 18 | 35 | 34 | 36 | 47 | 42 |
| P. maxplancki | – | – | – | – | – | – | – | – | 0 | 29 | 28 | 30 | 41 | 39 |
| P. riukiariae sp.n. | – | – | – | – | – | – | – | – | – | 0 | 5 | 4 | 14 | 19 |
| P. neolucani sp.n. | – | – | – | – | – | – | – | – | – | – | 0 | 6 | 17 | 18 |
| P. degawai sp.n. | – | – | – | – | – | – | – | – | – | – | – | 0 | 14 | 19 |
| P. hongkongensis sp.n. | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 23 |
| P. quartusdecimus | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 |
Figure 3.
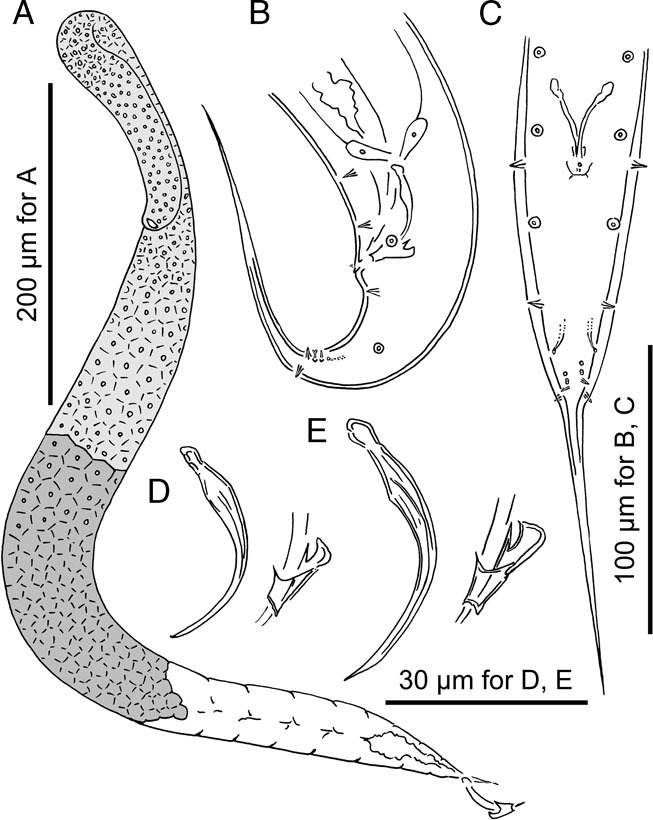
Adult male of Pristionchus laevicollis sp. n. A: Gonadal system in right lateral view; B, C: Tail region in left lateral (B) and ventral (C) views; D, E: Spicule and gubernaculum showing the variation in the size among individuals.
Figure 4.
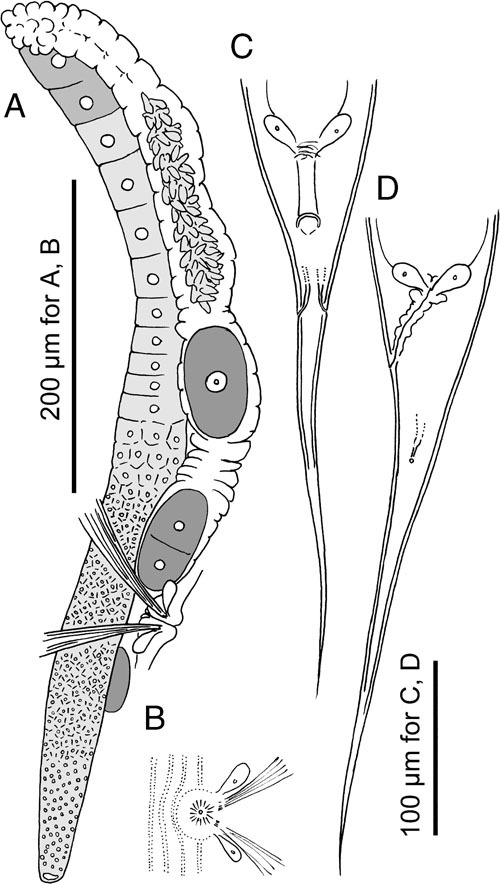
Adult female of Pristionchus laevicollis sp. n. A: Gonadal system in right lateral view; B: Ventral view of vulval region; C, D: ventral (C) and left lateral (D) and views.
The species name is derived from the type host (carrier) insect of the nematode species, Aegus laevicollis (Coleoptera: Lucanidae).
Measurements
Measurements have been summarized in Table 1.
Stenostomatous form
Metastegostom bearing conspicuous and movable triangular or flint-shaped dorsal tooth with strongly sclerotized surface giving an appearance of an inverted V-shape in light microscopy in lateral view; pointed left subventral ridge with three minute adventitious denticles on a plate; pointed right subventral ridge, often with distinct distal adventitious denticle.
Eurystomatous form
Metastegostom bearing large claw-like dorsal tooth; large claw-like right subventral tooth; ridge of left subventral denticles or cusps, varying in number and size, i.e., three large cusps, the tip of two lateral cusps sometimes split into two small tips. Dorsal and right subventral teeth movable. No movement observed for left subventral denticles.
Arrangement of genital papillae and phasmid
The paired papillae and the phasmid are arranged as <v1, v2, v3d, co, v4, ad, ph, (v5, v6, v7), pd> according to the nomenclature of Sudhaus and Fürst von Lieven (2003), where v1 located about 1 CBD anterior to co; v2 and v3d close to, but clearly separated from each other and located at slightly anterior to co; v4 at less than 1 CBD posterior to co; ad laterally located at the midway between co and the root of tail spike or slightly posterior to the midpoint; ph subventrally located, opening in between ad and v5; ventral v5-v7 forming triplet just anterior to the root of tail spike; and subdorsal pd slightly posterior to v7.
Diagnosis and relationship
The species-specific (diagnostic) characters are summarized in Table 4. The new species is characterized by the pointed left subventral ridge with three minute adventitious denticles on a plate; pointed right subventral ridge often bearing a distinct distal adventitious denticle in the stenostomatous form; a large claw-like right subventral tooth and a ridge of left subventral denticles or cusps varying in number and size, i.e., three large cusps, the tip of two lateral cusps which sometimes split into two small tips; and the relative position of the anterior four pairs of genital papillae, i.e., v1-v2 and v2-v4 are almost equal and v2 and v3d are separated (not adjusted to each other). In addition to the species-specific characters, the new species is molecularly characterized by the barcoding sequence (NCBI accession number MH114986).
The new species is both typologically and phylogenetically close to P. maxplancki. Pristionchus laevicollis sp. n. and P. maxplancki share two distinctive characters: the tips of left subventral cusps of the eurystomatous form often split into multiple small tips; and the relative position of the anterior four pairs of genital papillae, i.e., the distance between v1-v2 and v2-v4 are almost equal, and v3d is located slightly posterior to v2 (Kanzaki et al., 2013; Ragsdale et al., 2015). However, P. laevicollis sp. n. is distinguished from P. maxplancki by the shape of the stenostomatous form; the right subventral plate with single vs one or two denticles, and the left subventral plate with two bumps vs minute denticles. The species is distinguished from all other species by mating experiments and by its unique SSU rRNA sequence (Table 5).
Type host (carrier) and locality
The culture from which the type specimens were obtained was originally isolated by N. Kanzaki from an adult A. laevicollis collected at the Nagoya University, Nagoya, Aichi, Japan in June 2015.
Other host (carrier) and locality
Besides its type carrier and locality, the species was isolated from a millipede species, Parafontaria laminata armigera collected at Nobeyama, Nagano, Japan in September 2016.
Type material, type strain, and nomenclatural registration
One slide holotype male; two slides, each with paratypes one male and one female, 28530-28532, deposited in the University of California Riverside Nematode Collection (UCRNC), CA, USA. Two slides, each with paratypes one male and one female (SMNHType-8989 and 8990) deposited in the Swedish Natural History Museum, Stockholm, Sweden. Two slides, each with paratypes one male and one female (SMNK-NEMA-T-0143 and 0144) deposited in the Natural History Museum Karlsruhe, Germany. The strains are available as living cultures and as frozen stocks under culture codes RS5939 (type strain isolated from stag beetle) and RS5941 (millipede isolate) in the Department of Evolutionary Biology, MPI for Developmental Biology, Tübingen, Germany, and can be provided to other researchers upon request. The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier [urn:lsid:zoobank.org:act:A6797D55-6BDA-4AE6-BC5E-1D0D7A010808].
Pristionchus neolucani
sp. n. (Figs 6 and 7; Tables 2, 4, and 5).
Figure 6.
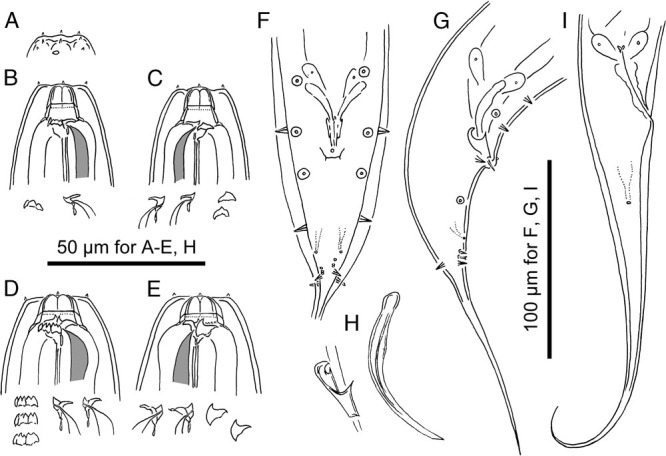
Adults of Pristionchus neolucani sp. n. A: Surface of male lip region in right lateral view; B: Stomatal region of stenostomatal male in left lateral view; C: Stomatal region of stenostomatal male in right lateral view; D: Stomatal region of eurystomatal female in left lateral view; E Stomatal region of eurystomatal female in right lateral view; F, G: Male tail region in ventral (F) and right lateral (G) views; H: Spicule and gubernaculum in right lateral view; I: Female tail region in right lateral view.
Figure 7.
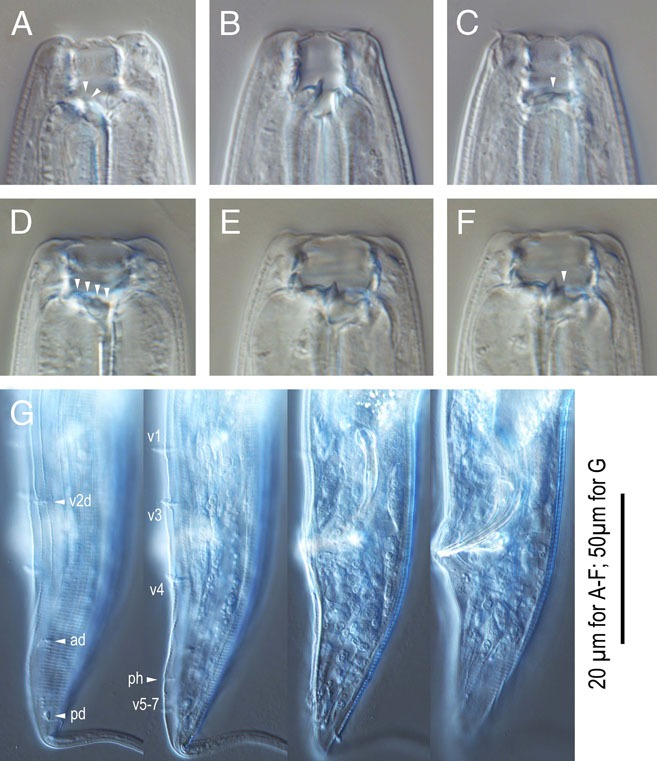
Pristionchus neolucani n. sp. A-C: Stomatal region of stenostomatous form showing left subventral denticles (arrowheads in A), dorsal tooth (B) and right subventral ridge (arrowhead in C); D-F: Stomatal region of eurystomatous form showing left subventral cusps (arrowheads in D), Dorsal tooth (E) and right subventral tooth (arrowhead in F); G: Male tail in four different focal planes showing genital papillae (v+number), phasmid (ph), and spicule and gubernaculum shape. 20 µm for A-F; 50 µm for G.
The species name is derived from the genus name of type host (carrier) of the nematode, Neolucanus championi (Coleoptera: Lucanidae).
Measurements
Measurements have been summarized in Table 2.
Stenostomatous form
Metastegostom bearing conspicuous and movable triangular or flint-shaped dorsal tooth with strongly sclerotized surface giving an appearance of an inverted V-shape in light microscopy in lateral view; pointed left subventral ridge with three minute adventitious denticles on a plate; pointed right subventral ridge, often with distinct distal adventitious denticle.
Eurystomatous form
Metastegostom bearing large claw-like dorsal tooth; large claw-like right subventral tooth; ridge of left subventral denticles or cusps, varying in number and size, (i.e., three large cusps), the tip of two lateral cusps often split into two small tips. Dorsal and right subventral teeth movable. No movement observed for left subventral denticles.
Arrangement of genital papillae and phasmid
The paired papillae and the phasmid are arranged as <v1, (v2d, v3), co, v4, ad, ph, (v5, v6, v7), pd>, where v1 located at ca 1 CBD anterior to co; v2d and v3 close to, or almost overlapping to each other and located at less than 1/2 CBD anterior to co; v4 slightly, less than 1/3 CBD posterior to co; ad laterally located at the midway between co and the root of tail spike; ph subventrally located in between ad and v5; ventral v5-v7 forming triplet just anterior to the root of tail spike; and subdorsal pd overlapping with-, or slightly posterior to v7.
Diagnosis and relationship
The new species is characterized by the pointed left subventral ridge with three minute adventitious denticles on a plate, the right subventral ridge bearing distinct distal adventitious denticles in the stenostomatous form; a large claw-like right subventral tooth and three left subventral cusps, which sometimes split at the tip into two small denticles; and the relative position of the anterior four pairs of genital papillae, i.e. v1-v2d and v2d-v4 are almost equal and v2d and v3 are very close to each other. In addition, the new species is molecularly characterized by the barcoding sequence (NCBI accession number MH114987).
The new species is typologically similar to and a cryptic species group with several species in the pacificus complex sensu stricto (Yoshida et al., 2018), namely, P. pacificus, P. exspectatus, and P. occultus, as well as P. kurosawai and P. degawai n.sp. i.e., the stomatal morphology in stenostomatous and eurystomatous forms are typical to the group (Table 4). Further, the arrangement of the anterior four pairs of papillae is similar to each other, i.e., v2d and v3 are close to each other, and located around the midway between v1 and v4 (Sommer et al., 1996; Kanzaki, Ragsdale, Herrmann, Mayer, and Sommer, 2012; Kanzaki et al., 2013; Ragsdale et al., 2015; Herrmann et al., 2016; Yoshida et al., 2018). However, P. neolucani n. sp. is distinguished from P. pacificus by its reproductive mode, gonochoristic vs. hermaphroditic. P. neolucani n. sp. is also distinguished from P. degawai n. sp. by the relative position of the second and third pair of genital papillae. Specifically, although these two pairs are very close to each other in both species, the second pair is always directed sublaterally (v2d) vs. sometimes the third papillae are directed laterally forming v3d (see below). The species is distinguished from all other species by mating experiments and by its unique SSU rRNA sequence (Table 5).
Type host (carrier) and locality
The culture from which the type specimens were obtained was originally isolated by M. Herrmann from an adult stag beetle, Neolucanus championi collected at Mui Tsz Lam, Hongkong, China in June 2017.
Type material, type strain, and nomenclatural registration
One slide holotype male; two slides, each with paratypes one male and one female, 28521-28523, deposited in the UCRNC, CA, USA. Two slides, each with paratypes one male and one female (SMNHType-8983 and 8984) deposited in the Swedish Natural History Museum, Stockholm, Sweden. Two slides, each with paratypes one male and one female (SMNK-NEMA-T-0137 and 0138) deposited in the Natural History Museum Karlsruhe, Germany. The strain is available as living culture and as frozen stock under culture code RS5949 in the Department of Evolutionary Biology, MPI for Developmental Biology, Tübingen, Germany, and can be provided to other researchers upon request. The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier [urn:lsid:zoobank.org:act:6B6DE4C3-5F44-46ED-A8B4-E8CC3FBE0643].
Pristionchus degawai
sp. n. (Figs 8 and 9; Tables 3–5)
Figure 8.
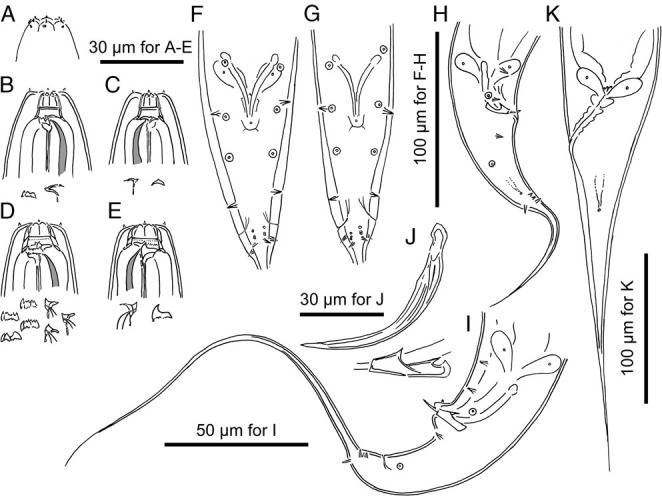
Adults of Pristionchus degawai sp. n. A: Surface of male lip region in right lateral view; B: Stomatal region of stenostomatal male in left lateral view; C: Stomatal region of stenostomatal male in right lateral view; D: Stomatal region of eurystomatal female in left lateral view; E Stomatal region of eurystomatal female in right lateral view; F, G: Male tail region in ventral view showing the variation in the arrangement of genital papillae; H, I: Male tail region in right lateral (H) and left lateral (I) views showing the variation in body size and arrangement of genital papillae; J: Spicule and gubernaculum in left lateral view; K: Female tail region in left lateral view.
Figure 9.

Pristionchus degawai n. sp. A-C: Stomatal region of stenostomatous form showing left subventral denticles (arrowheads in A), dorsal tooth (B) and right subventral ridge (arrowhead in C); D-F: Stomatal region of eurystomatous form showing left subventral cusps (arrowheads in D), Dorsal tooth (E) and right subventral tooth (arrowhead in F); G: Male tail in four different focal planes showing genital papillae (v+number), phasmid (ph), and spicule and gubernaculum shape. 20 µm for A-F; 50 µm for G.
The species name is derived from Dr. Yousuke Degawa, a mycologist from the University of Tsukuba, who provided 400 live specimens of millipedes for the present study.
Measurements
Measurements have been summarized in Table 3.
Stenostomatous form
Metastegostom bearing conspicuous and movable triangular or flint-shaped dorsal tooth with strongly sclerotized surface giving an appearance of an inverted V-shape in light microscopy in lateral view; pointed left subventral ridge with three minute adventitious denticles on a plate; pointed right subventral ridge, often with distinct distal adventitious denticle.
Eurystomatous form
Metastegostom bearing large claw-like dorsal tooth; large claw-like right subventral tooth; ridge of left subventral denticles or cusps, varying in number and size, (i.e., three large cusps), the tip of two lateral cusps sometimes split into two small tips. Dorsal and right subventral teeth movable. No movement observed for left subventral denticles.
Arrangement of genital papillae and phasmid
The paired papillae and phasmid are usually arranged as <v1, (v2d, v3), co, v4, ad, ph, (v5, v6, v7), pd>, where v1 located at ca 1 CBD anterior to co; v2d and v3 close to, or almost overlapping to each other and located slightly anterior to co, in few cases, the third papilla on one side is directed laterally forming v3d; v4 at less than 1 CBD posterior to co; ad laterally located ph at the midway between co and the root of tail spike or a little posterior to the midpoint; subventrally located between ad and v5; ventral v5-v7 forming triplet just anterior to the root of tail spike; and subdorsal pd overlapping with v6 or v7, or slightly posterior to v7.
Diagnosis and relationship
The new species is characterized by the pointed left subventral ridge with three minute adventitious denticles on a plate, a right subventral ridge bearing a distinct distal adventitious denticle in the stenostomatous form; a large claw-like right subventral tooth and three left subventral cusps which sometimes split at the tip into two small denticles; and the relative position of the anterior four pairs of genital papillae, i.e., v1-v2d and v2d-v4 are almost equal and v2d and v3 are extremely close to each other, and sometimes the third pair is directed sublaterally forming v3d. In addition, the new species is molecularly characterized by the barcoding sequence (NCBI accession number MH114984).
The species forms a cryptic species-complex with several species in the P. pacificus complex s. s., namely, P. pacificus, P. exspectatus, and P. occultus, as well as P. kurosawai and P. neolucani sp. n., where the stomatal morphology in stenostomatous and eurystomatous forms are typical of the group, and the arrangement of the anterior four pairs of papillae is also similar to each other (Sommer et al., 1996; Kanzaki, Ragsdale, Herrmann, Mayer, and Sommer, 2012; Kanzaki et al., 2013; Ragsdale et al., 2015; Herrmann et al., 2016; Yoshida et al., 2018). However, P. degawai n. sp. is distinguished from P. pacificus by its reproductive mode. In addition, P. degawai n. sp. is distinguished from the other four species by the variation in the relative position of the second and third pairs of genital papillae. Although the second pair is usually directed sublaterally forming v2d, the third papillae are sometimes directed sublaterally forming v3d in P. degawai n. sp. vs the second pair is always directed sublaterally in the other four species (Table 4). In addition to the typological characters, the species is distinguished from all other species by mating experiments and by its unique SSU rRNA sequence (Table 5).
Type host (carrier) and locality
The culture from which the type specimens were obtained was originally isolated by N. Kanzaki from an adult millipede, Parafontaria laminata armigera collected at Nobeyama, Nagano, Japan in September 2016.
Type material, type strain, and nomenclatural registration
One slide holotype male; two slides, each with paratypes one male and one female, 28527-28529, deposited in the UCRNC, CA, USA. Two slides, each with paratypes one male and one female (SMNHType-8985 and 8986) deposited in the Swedish Natural History Museum, Stockholm, Sweden. Two slides, each with paratypes one male and one female (SMNK-NEMA-T-0141 and 0142) deposited in the Natural History Museum Karlsruhe, Germany. The strain is available as living culture and as frozen stock under culture code RS5938 in the Department of Evolutionary Biology, MPI for Developmental Biology, Tübingen, Germany, and can be provided to other researchers upon request. The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier [urn:lsid:zoobank.org:act:430C36BC-DD11-4CF5-A4F4-A1EF478CEFB8].
Pristionchus hongkongensis
sp. n. (Figs 10 and 11; Tables 2,4, and 5).
Figure 10.
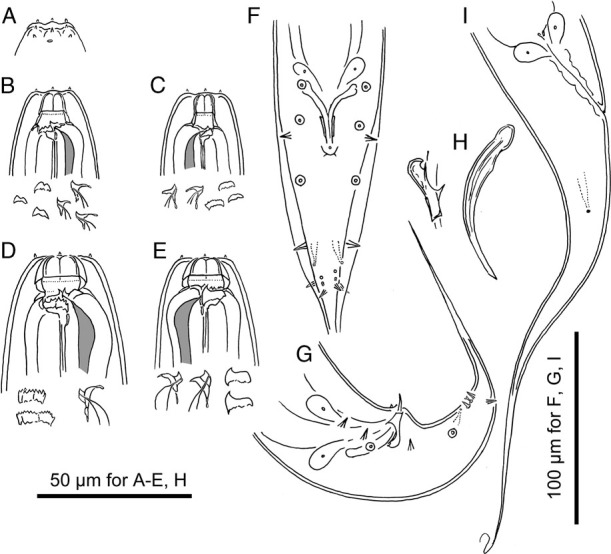
Adults of Pristionchus hongkongensis sp. n. A: Surface of male lip region in right lateral view; B: Stomatal region of stenostomatal male in left lateral view; C: Stomatal region of stenostomatal male in right lateral view; D: Stomatal region of eurystomatal female in left lateral view; E Stomatal region of eurystomatal female in right lateral view; F, G: Male tail region in ventral (F) and right lateral (G) views; H: Spicule and gubernaculum in right lateral view; I: Female tail region in right lateral view.
Figure 11.
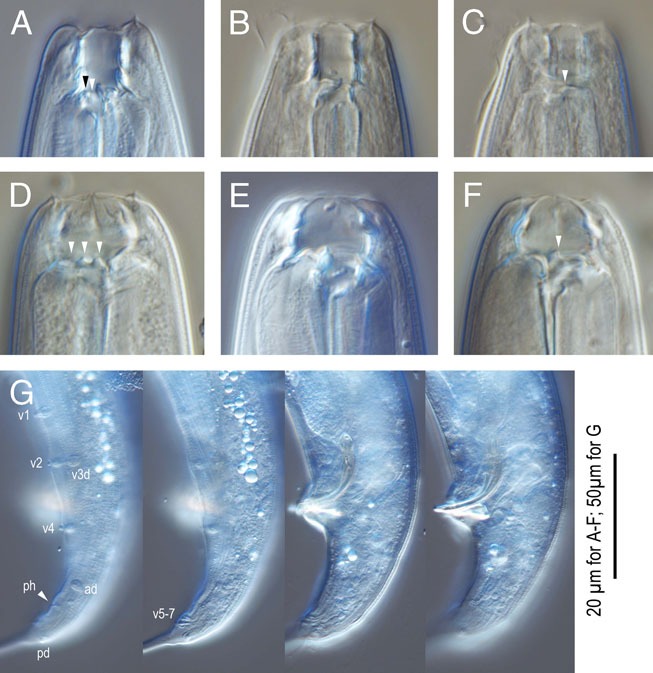
Pristionchus hongkongensis n. sp. A-C: Stomatal region of stenostomatous form showing left subventral denticles (arrowheads in A), dorsal tooth (B) and right subventral ridge (arrowhead in C); D-F: Stomatal region of eurystomatous form showing left subventral cusps (arrowheads in D), Dorsal tooth (E) and right subventral tooth (arrowhead in F); G: Male tail in four different focal planes showing genital papillae (v+number), phasmid (ph), and spicule and gubernaculum shape. 20 µm for A-F; 50 µm for G.
The species name is derived from the type locality of the nematode, Hongkong, China.
Measurements
Measurements have been summarized in Table 2.
Stenostomatous form
Metastegostom bearing conspicuous and movable triangular or flint-shaped dorsal tooth with strongly sclerotized surface giving an appearance of an inverted V-shape in light microscopy in lateral view, the distal end pointed and curved to direct anteriorly; pointed left subventral ridge with three minute adventitious denticles on a plate; pointed right subventral ridge, often with one or more distinct distal adventitious denticle(s).
Eurystomatous form
Cheilostomatal plates very thick. Anterior end of each plate rounded and elongated to project from stomatal opening, forming a small flap. Each cheilostomatal plate slightly inclined inwardly, giving an appearance that the whole stoma is narrowing anteriorly. Gymnostom with very thick cuticle, forming a short, ring-like tube being thicker posteriorly, and whole gymnostomatal ring widening anteriorly. Anterior end of gymnostom internally overlapping the posterior end of cheilostomatal plates. Thus, the cheilo and gymnostomatal regions form a barrel-shape in lateral view. Metastegostom bearing large claw-like dorsal tooth; large claw-like right subventral tooth; ridge of left subventral denticles or cusps, varying in number and size, (i.e., three large cusps), the tip of these cusps often split into three or more small tips, sometimes forming serrated plate. Dorsal and right subventral teeth movable. No movement observed for left subventral denticles.
Arrangement of genital papillae and phasmid
The paired papillae and the phasmid are arranged as <v1, v2, v3d, co, v4, ad, ph, (v5, v6, v7), pd>, where v1 located at ca 1 CBD anterior to co; v2 and v3d close to each other and located at less than 1/2 CBD anterior to co; v4 slightly, ca 1/2 CBD posterior to co; ad laterally located at the 2/3 way from co to the root of tail spike; ph subventrally located between ad and v5; ventral v5-v7 forming triplet just anterior to the root of tail spike; and subdorsal pd overlapping with v6 or v7.
Diagnosis and relationship
Pristionchus hongkongensis n. sp. is characterized by its stomatal morphology, i.e., the stenostomatous form has a pointed left subventral ridge with three minute adventitious denticles on a plate and a right subventral ridge bearing distinct distal adventitious denticles; and the eurystomatous form has a barrel-shaped stoma consisting of six very thick cheilostomatous plates without a divided anterior edge, anteriorly widened short tube or ring-like gymnostom, and a stegostom with claw-like right subventral tooth and three left subventral cusps with a tip split into three or more small denticles. In addition, the new species is molecularly characterized by the barcoding sequence (NCBI accession number MH114985).
This species is typologically distinctive from the other members of the pacificus complex s. s. by its species-specific apomorphy, i.e. the barrel-shaped stoma. A similar stomatal shape has been reported in Parapristionchus giblindavisi, the P. triformis group (P. triformis, P. hoplostomus and P. fukushimae), and P. fissidentatus (Kanzaki et al., 2012b, 2012c; Ragsdale et al., 2013). However, P. hongkongensis sp. n. is readily distinguished from P. giblindavisi by the number of stomatal plates in both stenostomatous and eurystomatous forms, six vs 12, and the arrangement of genital papillae, v2d and v3 are close to each other vs v2d is clearly separated from v3. Pristionchus hongkongensis sp. n. is distinguished from the P. triformis group by its cheilostomatal plates, forming six plates vs sometimes partially or totally separated forming 6 to 12 plates. In addition, the anterior end of the gymnostom and pro-mesostegostom, are not serrated vs. the anterior end of both sections are serrated. Pristionchus hongkongensis sp. n. is distinguished from P. fissidentatus with its right subventral tooth in the eurystomatous form, having a large claw-like tooth vs a large ridge with multiple peaks and a triangular tooth-like projection most ventrally. In addition to its typological characters, P. hongkongensis n. sp. is distinguished from all other species by mating experiments and by its unique SSU rRNA sequence (Table 5).
Type host (carrier) and locality
The culture from which the type specimens were obtained was originally isolated by M. Herrmann from an adult Neolucanus championi collected at Mui Tsz Lam, Hongkong, China in June 2017.
Type material, type strain, and nomenclatural registration
One slide holotype male; two slides, each with paratypes one male and one female, 28518-28520, deposited in the UCRNC, CA, USA. Two slides, each with paratypes one male and one female (SMNHType-8981 and 8982) deposited in the Swedish Natural History Museum, Stockholm, Sweden. Two slides, each with paratypes one male and one female (SMNK-NEMA-T-0135 and 0136) deposited in the Natural History Museum Karlsruhe, Germany. The strain is available as living culture and as frozen stock under culture code RS5957 in the Department of Evolutionary Biology, MPI for Developmental Biology, Tübingen, Germany, and can be provided to other researchers upon request. The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier [urn:lsid:zoobank.org:act:C29A0FB6-D5C4-49D2-88D1-2596732974FB].
Pristionchus riukiariae
sp. n. (Figs 12 and 13; Tables 3-5).
Figure 12.
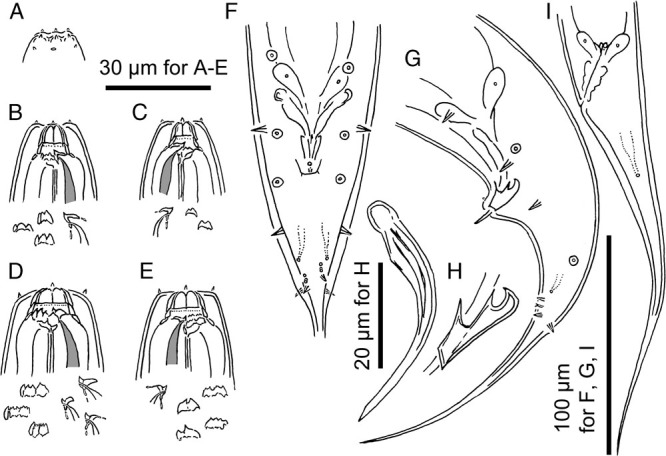
Adults of Pristionchus riukiariae sp. n. A: Surface of male lip region in right lateral view; B: Stomatal region of stenostomatal male in left lateral view; C: Stomatal region of stenostomatal male in right lateral view; D: Stomatal region of eurystomatal female in left lateral view; E Stomatal region of eurystomatal female in right lateral view; F, G: Male tail region in ventral (F) and left lateral (G) views; H: Spicule and gubernaculum in left lateral view; I: Female tail region in left lateral view.
Figure 13.
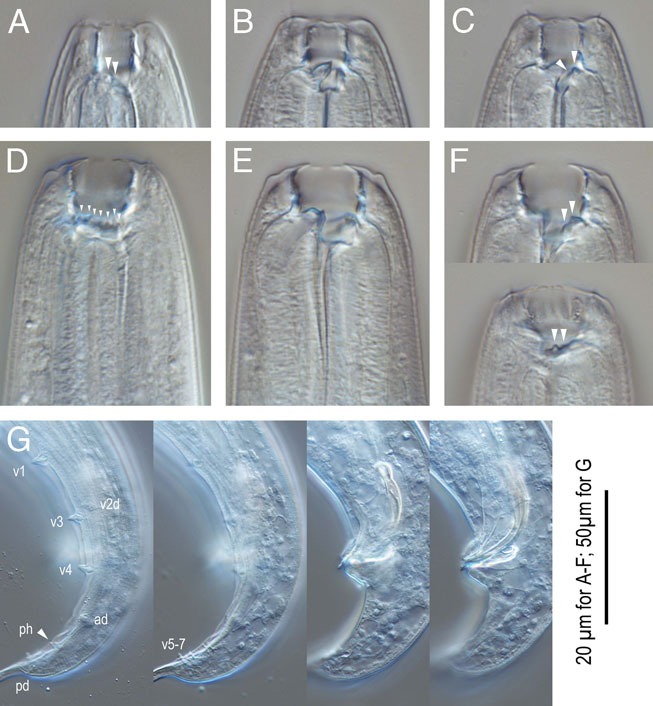
Pristionchus riukiariae n. sp. A-C: Stomatal region of stenostomatous form showing left subventral denticles (arrowheads in A), dorsal tooth (B) and right subventral ridge (arrowhead in C); D-F: Stomatal region of eurystomatous form showing left subventral cusps (arrowheads in D), Dorsal tooth (E) and right subventral dinticles in two different focal planes (arrowheads in F); G: Male tail in four different focal planes showing genital papillae (v+number), phasmid (ph), and spicule and gubernaculum shape. 20 µm for A-F; 50 µm for G.
Figure 14.
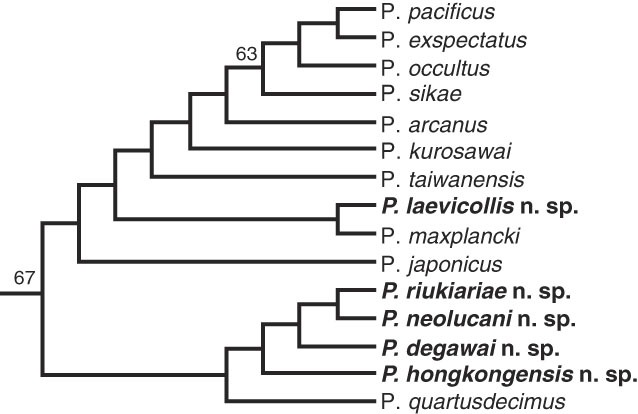
Phylogenetic relationship among all members in the pacificus species-complex. The schematic tree is part of a phylogeny that was generated from transcriptome data of all cultivable Pristionchus species (Rödelsperger et al., 2018). Inner nodes are labeled with bootstrap support values and stars indicate full bootstrap support (100 replicates).
The species name is derived from the genus name of type host (carrier) of the nematode, Riukiaria holstii.
Measurements
Measurements have been summarized in Table 3.
Stenostomatous form
Metastegostom bearing conspicuous and movable triangular or flint-shaped dorsal tooth with strongly sclerotized surface giving an appearance of an inverted V-shape in light microscopy in lateral view; pointed left subventral ridge with three minute adventitious denticles on a plate; right subventral plate with two distinct distal adventitious denticles.
Eurystomatous form
Metastegostom bearing large claw-like dorsal tooth; large right subventral tooth forming various shaped ridge, i.e., the tip is split into two large ridges or multiple small denticles; ridge of left subventral denticles or cusps, varying in number and size, (i.e., three large cusps), the tip of two lateral cusps sometimes split into two small tips. Dorsal and right subventral teeth movable. No movement observed for left subventral denticles.
Arrangement of genital papillae and phasmid
The paired papillae and the phasmid are usually arranged as <v1, (v2d, v3), co, v4, ad, ph, (v5, v6, v7), pd>, where v1 located at a little more than 1 CBD anterior to co; v2d and v3 close to, or almost overlapping to each other and located at slightly anterior to co; v4 slightly, less than 1/3 CBD posterior to co; ad laterally located at the midway between co and the root of tail spike; ph subventrally located just anterior to v5; ventral v5-v7 forming triplet just anterior to the root of tail spike; and subdorsal pd overlapping with, or slightly posterior to v7.
Diagnosis and relationship
Pristionchus riukiariae n. sp. is characterized by the pointed left subventral ridge with three minute adventitious denticles on a plate, the right subventral ridge bearing two distinct distal adventitious denticles in the stenostomatous form; the right subventral tooth forming two large ridges with each ridge being split at the tip forming multiple small denticles, and three left subventral cusps, which sometimes split at the tip into two small denticles; and the relative position of anterior four pairs of genital papillae, i.e., v1-v2d and v2d-v4 are almost equal and v2d and v3 are extremely close to each other. In addition, the new species is molecularly characterized by the barcoding sequence (NCBI accession number MH114988). The species is typologically distinctive from the other Pristionchus spp. by its species-specific apomorphy: in the eurystomatous form the right subventral tooth (ridge) forms a plate with multiple tips (a large ridge with multiple peaks). Similar right subventral ridges have been reported in P. fissidentatus (Kanzaki, Ragsdale, Herrmann, and Sommer, 2012), however, P. riukiariae sp. n. is distinguished from P. fissidentatus with its whole stomatal morphology, tube-like vs. barrel-shaped, and the arrangement of genital papillae, v2d and v3 are clearly separated from v4 vs v2-v4 are very close to each other around the cloacal opening, and the spicule and gubernaculum shape, the spicule is stouter and gubernaculum is wider in P. fissidentatus (Kanzaki, Ragsdale, Herrmann, and Sommer, 2012). P. riukiariae sp. n. is distinguished from all other species by mating experiments and by its unique SSU rRNA sequence (Table 5).
Type host (carrier) and locality
The culture from which the type specimens were obtained was originally isolated by N. Kanzaki from an adult millipede, Riukiaria holstii collected at Nago, Okinawa in January 2017.
Type material, type strain, and nomenclatural registration
One slide holotype male; two slides, each with paratypes one male and one female, 28524-28526, deposited in the UCRNC, CA, USA. Two slides, each with paratypes one male and one female (SMNHType-8987 and 8988) deposited in the Swedish Natural History Museum, Stockholm, Sweden. Two slides, each with paratypes one male and one female (SMNK-NEMA-T-0139 and 0140) deposited in the Natural History Museum Karlsruhe, Germany. The strain is available as living culture and as frozen stock under culture code RS5937 in the Department of Evolutionary Biology, MPI for Developmental Biology, Tübingen, Germany, and can be provided to other researchers upon request. The new species binomial has been registered in the ZooBank database (zoobank.org) under the identifier [urn:lsid:zoobank.org:act:DB937AF5-0107-4C71-BEDC-8E92B332D368].
Molecular characterization and phylogenetic analysis
RNAseq libraries were prepared for all new species (presented in this study and the two accompanying manuscripts) and the 30 Pristionchus species currently in culture (see Methods for details). More than 2,000 high quality alignments containing at least 14 species (without any duplication) and with at least 50 amino acid positions with coverage in all represented species were concatenated into a supermatrix spanning 350,000 amino acids. The phylogenetic relationships among all members in the pacificus species-complex are shown in Figure 9. For the species described in this study, P. laevicollis sp. n. forms a new sister group relationship with P. maxplancki. In contrast, the species P. honkongensis sp. n., P. degawai sp. n., P. neolucani sp. n., and P. riukiariae sp. n are closest to P. quartusdecimus forming a new subclade within the pacificus species-complex.
Discussion
In contrast to many animal or plant groups, nematode taxonomy suffers from a paucity of morphological features, resulting in two major shortcomings. First, while nematodes are believed to represent the most species-rich phylum among animals with an estimated species number in the range of 1 to 10 million, less than 30,000 species are currently described (Lambshead, 1993). Second, little is known about the total number of species in most nematode taxa, because extinction and speciation processes are largely unknown and species catalogues are far from completion. In addition, many studies in nematode taxonomy rely on morphology and molecular barcode analysis only, but do not involve mating experiments, often because species cannot be kept in culture under laboratory conditions. In free-living and parasitic nematodes alike total species assessments are not available, neither at the taxonomic, nor the genetic level. Thus, the presumably largest animal phylum lacks a reasonable species and biodiversity assessment. As a result, the diversity and magnitude of nematode species often goes unnoticed in general zoology. Similarly, other than for organisms such as Caenorhabditis elegans and its relatives, little is known about population structure in nematodes. To overcome this situation, suitable nematode taxa, in which species assessments can be performed, should be established. The selection of such taxa should be guided by the easiness of isolating nematodes in the wild on a global scale and the possibility to study isolates under laboratory conditions. A pragmatic collection of half a dozen or a dozen nematode taxa, representing the major phylogenetic branches of nematodes, could help to work toward a first global species assessment for nematodes.
In the genus Pristionchus, interest in taxonomy and phylogeny has been increasing in the last decade with the development of P. pacificus as a model system in comparative and evolutionary biology (Sommer and McGaughran, 2013; Sommer, 2015). Given the fact that Pristionchus nematodes are often found in soil and in association with scarabaeoid beetles, and that most known species can be cultured under laboratory conditions, this genus might represent one potential model for nematode biodiversity studies and species assessments. That is, the identification of close to all species in the genus in addition to Caenorhabditis. While all nematode taxa are adapted to their specific niche and the scarab beetle association might have resulted in specific, unusual features that are not representative for other nematode groups, the pure fact that (i) scarabaeoid beetles can easily be isolated from around the world, (ii) protocols for Pristionchus isolation from soil, beetles, and other arthropod groups are simple and can even be conducted at bachelor and high school level, and most importantly (iii) mating experiments and molecular sequencing analyses give reliable results on species designations, make Pristionchus one such candidate for nematode species assessments.
This study shows that East Asia represents a hotspot of Pristionchus diversification. New territories, such as Hongkong, but also the analysis of new potential host arthropods, such as millipedes in Japan, revealed five new Pristionchus species. In particular, the identification of Pristionchus on millipedes was surprising, as similar previous attempts in other areas of the world did not identify Pristionchus-associations with millipedes. Together, these findings support the usefulness of Pristionchus nematodes for species assessments as well as indicate that the hotspot of speciation in East Asia is far from complete sampling.
Acknowledgments
The authors are very grateful for getting support permits and help for this biodiversity project from various authorities and colleagues: Professor Ming Bai from the Institute of Zoology, Chinese Academy of Science, Dr Ying-Ming Lee, Dr Ka-Hong Cheung, Dr Kin-Fung Chan, and Man-Hin Leung from the Beetle Working Group, Agriculture, Fisheries and Conservation Department, Hong Kong SAR Government; Dr Y. Kanana from the Schmalhausen Institute in Kiev (Ukraine) for help with the morphometric measurements. Finally, the authors would like to thank the beetle expert and long-standing expedition collaborator Dr M. J. Paulsen from the University of Nebraska State Museum. Natsumi Kanzaki and Matthias Herrmann contributed equally to this work.
References
- Baskaran P., Rödelsperger C., Prabh N., Serobyan V., Markov G. V., Hirsekorn A., and Diederich C.. 2015. Ancient gene duplications have shaped developmental stage-specific expression in Pristionchus pacificus. BMC Evolutionary Biology 15: 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Anna I., and Sommer R. J.. 2011. Pristionchus uniformis – should I stay or should I go? Recent host range expansion in a European nematode. Ecology and Evolution 1: 468–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R. C. 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acid Research 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekseth O. K., Kuiper M., and Mironov V.. 2014. orthAgogue: an agile tool for the rapid prediction or orthology relations. Bioinformatics 30: 734–736. [DOI] [PubMed] [Google Scholar]
- Fonderie P., Steel H., Moens T., and Bert W.. 2013. Experimental induction of intraspecific morphometric variability in a single population of Halicephalobus cf. gingivalis may surpass total interspecific variability. Nematology 15: 529–544. [Google Scholar]
- Grabherr M. G., Haas B. J., Yassour M., Levin J. Z., Thompson D. A., Amit I., Adiconis X., Fan L., Raychowdhury R., Zeng Q., et al. 2011. Full length transcriptomics assembly from RNA-seq data without a reference genome. Nature Biotechnology 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M., Mayer W. E., and Sommer R. J.. 2006. Nematodes of the genus Pristionchus are closely associated with scarab beetles and the Colorado potato beetle in Western Europe. Zoology 109: 96–108. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Weiler C., Rödelsperger C., Kanzaki N., and Sommer R. J.. 2016. Two new Pristionchus Species (Nematoda: Diplogastridae) from Taiwan are part of a species-cluster representing the closest known relatives of the Model Organism P. pacificus. Zoological Studies 55: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N., Ragsdale E. J., Herrmann M., Mayer W. E., and Sommer R. J.. 2012. Description of three Pristionchus species (Nematoda: Diplogastridae) from Japan that form a cryptic species complex with the model organism P. pacificus. Zoological Science 29: 403–417. [DOI] [PubMed] [Google Scholar]
- Kanzaki N., Ragsdale E. J., Herrmann M., Röseler W., and Sommer R. J.. 2013. Two new species of Pristionchus (Nematoda: Diplogastridae) support the biogeographic importance of Japan for the evolution of the genus Pristionchus and the model system P. pacificus. Zoological Science 30: 680–692. [DOI] [PubMed] [Google Scholar]
- Kanzaki N., Ragsdale E., Herrmann M., and Sommer R. J.. 2012. Two new species of Pristionchus (Rhabditida: Diplogastridae) P. fissidentatus n.sp. from Nepal and La Réunion island and P. elegans n.sp. from Japan. Journal of Nematology 44: 80–90. [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N., Ragsdale E., Herrmann M., and Sommer R. J.. 2014. Two new and two recharacterized species resulting froma radiation of Pristionchus (Nematoda: Diplogastridae) in. European Journal of Nematology 46: 60–74. [PMC free article] [PubMed] [Google Scholar]
- Kanzaki N., Ragsdale E., Herrmann M., Mayer W. E., Tanaka R., and Sommer R. J.. 2012. Parapristionchus giblindavisi n. gen, n. sp. (Rhabditida: Diplogastridae) isolated from stag beetles (Coleoptera: Lucanidae) in Japan. Nematology 14: 933–947. [Google Scholar]
- Kreis H. A. 1932. Beiträge zur Kenntnis pflanzenparasitischer Nematoden. Zeitschrift für Parasitenkunde 5: 184–194. [Google Scholar]
- Lambshead P. J. D. 1993. Recent developments in marine benthic biodiversity research. Oceanis 19: 5–24. [Google Scholar]
- Parry F. J. S. 1864. A catalogue of Lucanoid Coleoptera; with illustrations and descriptions of various new and interesting species. Transactions of the Entomological Society of London 3: 1–113. [Google Scholar]
- Ragsdale E. J., Kanzaki N., and Herrmann M.. 2015. Taxonomy and natural history: the genus Pristionchus, in Sommer R. J. (Ed.), Pristionchus Pacificus – a nematode model for comparative and evolutionary biology, BRILL, Leiden, 77–120. [Google Scholar]
- Ragsdale E., Kanzaki N., Roeseler W., Herrmann M., and Sommer R. J.. 2013. Three new species of Pristionchus (Nematoda: Diplogastridae) show morphological divergence through evolutionary intermediates of a novel feeding polymorphism. Zoological Journal of the Linnean Society 168: 671–698. [Google Scholar]
- Rödelsperger C., Röseler W., Prabh N., Yoshida K., Weiler C., Herrmann M., and Sommer R. J.. 2018. Phylotranscriptomics of Pristionchus nematodes revealed parallel gen loss in six hermaphroditic lineages. Current Biology 28: 3123–3127.. [DOI] [PubMed] [Google Scholar]
- Sommer R. J. 2015. Integrative evolutionary biology and mechanistic approaches in comparative biology, in Sommer R. J. (Ed.), Pristionchus Pacificus – a nematode model for comparative and evolutionary biology, BRILL, Leiden, 19–41. [Google Scholar]
- Sommer R. J., Carta L. K., Kim S. Y., and Sternberg P. W.. 1996. Morphological, genetic and molecular description of Pristionchus pacificus n. sp. (Nematoda: Neodiplogastridae). Fundamental and Applied Nematology 19: 511–521. [Google Scholar]
- Stamatakis A. 2014. RaxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhaus W., and Fürst von Lieven A.. 2003. A phylogenetic classification and catalogue of the Diplogastridae (Secernentea, Nematoda). Journal of Nematode Morphology and Systematics 6: 43–90. [Google Scholar]
- Susoy V., Herrmann M., Kanzaki N., Kruger M., Nguyen C. N., Rödelsperger C., Röseler W., Weiler C., Giblin-Davis R. M., Ragsdale E. J., and Sommer R. J.. 2016. Large-scale diversification without genetic isolation in nematode symbionts of figs. Science Advances 2: e1501031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer R. J., and McGaughran A.. 2013. The nematode Pristionchus pacificus as a model system for integrative studies in evolutionary biology. Mol. Ecol 22: 2380–2393. [DOI] [PubMed] [Google Scholar]
- Verhoeff K. W. 1936. Über Diplopoden aus Japan gesammelt von Herrn Y. Takakuwa. Transactions of the Sapporo Natural History Society 14: 148–172. [Google Scholar]
- Waterhouse C. O. 1873. On the pectinicorn Coleoptera of Japan, with description of three new species. The Entomologists Monthly Magazine 9: 277–278. [Google Scholar]
- Yoshida K., Herrmann M., Kanzaki N., Weiler C., Rödelsperger C., and Sommer R. J.. 2018. Two new Pristionchus Species of (Nematoda: Diplogastridae) from Taiwan and the definition of the pacificus species-complex sensu stricto. (submitted). [DOI] [PMC free article] [PubMed]


