Abstract
Vetiver, a nonhost grass for certain nematodes, was studied for the production of compounds active against the southern root-knot nematode, Meloidogyne incognita. In laboratory assays studying the effects on second-stage juvenile (J2) activity and viability, crude vetiver root and shoot extracts were nematotoxic, resulting in 40% to 70% J2 mortality, and were also repellent to J2. Vetiver oil did not exhibit activity against J2 in these assays. Gas chromatography-mass spectrometry analyses of three crude vetiver root ethanol extracts and a commercial vetiver oil determined that two of the major components in each sample were the sesquiterpene acid 3,3,8,8-tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid and the sesquiterpene alcohol 6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol. The acid was present in higher amounts in the extracts than in the oil. These studies demonstrating nematotoxicity and repellency of vetiver-derived compounds to M. incognita suggest that plant chemistry plays a role in the nonhost status of vetiver to root-knot nematodes, and that the chemical constituents of vetiver may be useful for suppressing nematode populations in the soil.
Keywords: Chemotaxis, management, marigold, Meloidogyne incognita, nematicide, nematotoxic, phytochemical, root-knot nematode, secondary metabolite, sesquiterpenoid, vetiver, vetiver extract, vetiver essential oil, Vetiveria zizanioides.
Vetiver (Vetiveria zizanioides (L.) Nash (synonym: Chrysopogon zizanioides (L.) Roberty), a member of the family Poaceae, is characterized by the production of dense biomass and growth of a large, strong, fibrous root system (Lim, 2016). This grass is resistant to various pests and diseases, and is tolerant to many environmental stresses, including flooding, drought, extreme temperatures, and heavy metals, and has long been used in land management (Maffei, 2002; Truong, 2002; Joy, 2009; Belhassen et al., 2015). Vetiver is also a source of numerous products, such as essential oil, fragrances, food, and medicinal compounds (Chomchalow, 2001; Belhassen et al., 2015; Lim, 2016). Consequently, vetiver is planted in more than 120 countries (Truong, 2000; Chou et al., 2016) for soil and water conservation, land stabilization, bioremediation, root oil production, and other uses (Chomchalow, 2001; Lim, 2016).
Owing to the many usages of vetiver and its products, studies have been conducted on the chemistry of this plant, with emphasis on vetiver root oil. Plant essential oils contain secondary metabolites that are lipophilic and volatile (Ríos, 2016). Vetiver essential oil is very complex and consists of more than 300 compounds; the primary constituents are sesquiterpenes and their derivatives, including sesquiterpene alcohols, hydrocarbons, and ketones (Champagnat et al., 2006; Leite, 2012; Belhassen et al., 2015; Lim, 2016). Root extracts contain secondary metabolites such as alkaloids, flavonoids, phenols, saponins, steroids, tannins, sesquiterpenes, terpenoids, and triterpenes (Subhadradevi et al., 2010; Aarthi et al. 2014; Krishnaveni, 2016; Kumar and Gayathri, 2016). The constituents extracted from the above-ground plant parts have also been identified, and include alkaloids, cholesterol, flavonoids, flavonolignans, glycosides, phenolic acids, phenylpropanoids, glycerols saponins, steroids, tannins, and many terpenoids (e.g. monoterpenes, sesquiterpenes, and a triterpene) (Huang et al., 2004; Gao et al., 2012; Prajna et al., 2013; Soni and Dahiya, 2015).
Vetiver-derived compounds have also been investigated for pest and pathogen management. Allelopathic or repellant activity was demonstrated against multiple organisms, including bacteria and fungi (Istianto and Emida, 2011; Vázquez-Sánchez et al., 2014; Soni and Dahiya, 2015), insects, ticks and a malarial parasite (Zhu et al., 2001a, 2001b; Ibrahim et al., 2004; Panella et al., 2005; Chauhan and Raina, 2006; Sujatha, 2010; Flor-Weiler et al., 2011; Aarthi et al., 2014; Campos et al., 2015), and plants (Mao et al., 2006). Additionally, research has been conducted on vetiver as a host for plant-parasitic nematodes. Vetiver is a host for the corn cyst nematode Heterodera zeae (Lal and Mathur, 1982). However, vetiver roots were resistant to infection by Meloidogyne arenaria, M. hapla, M. incognita, and M. javanica (West et al., 1996; Maffei, 2002; Fourie et al., 2007). This is of particular importance because Meloidogyne spp. (root-knot nematodes; RKN) attack many plant hosts and are economically important plant pathogens worldwide.
Despite the nonhost status of vetiver plants to RKN, few studies have been published on vetiver extracts, exudates, or oils and their effects on members of this genus. One investigation found that ethanol root extracts were nontoxic to M. incognita second-stage juveniles (J2) (Wiratno et al., 2009). Vetiver root exudates reduced motility of M. javanica J2, but the nematodes recovered after being removed from the treatments (Ahuja et al., 2014). This indicated nematostatic, rather than nematotoxic, activity of the exudates. These authors also noted that crude extracts from vetiver roots decreased M. javanica J2 motility, but they did not report on J2 recovery.
Although these studies with RKN did not find nematotoxic activity from vetiver constituents, the results must be considered with the knowledge that there has been little published work in this area, and that research on plant-derived compounds is affected by a complex web of factors. Vetiver age, plant part (such as stems vs. roots), vetiver cultivar and genes, environmental variation during plant growth, microbial populations in the rhizome and rhizosphere, and extraction methods used to obtain compounds can all influence the chemical components accumulated in or extracted from vetiver (Martinez et al., 2004; Adams et al., 2008; Belhassen et al., 2015; Lim, 2016). Deregistration of many synthetic nematicides has led to a need for new management agents for these plant pathogens, and the large number of compounds produced by vetiver, activity against numerous organisms, and the nonhost status to RKN all indicate that further research on vetiver activity against nematodes is warranted. Consequently, the current study was conducted to determine the effects of vetiver oil and of crude vetiver root and shoot extracts on activity and viability of M. incognita J2 in laboratory assays, and to investigate whether the oil or selected extracts would attract or repel J2.
Materials and methods
Plant materials and extraction
In the U.S., vetiver cv. Sierra was purchased from Agriflora Tropicals, Caguas, Puerto Rico and grown in the greenhouses of the Mycology and Nematology Genetic Diversity and Biology Laboratory (2-mon-old plants) and the Invasive Insect Biocontrol and Behavior Laboratory (4-yr-old plants) at USDA ARS, Beltsville, Maryland. Vetiver plants that were harvested after 2 mon of growth had been planted in Promix PGX (Premier Tech Horticulture, Quakertown, PA) in one-gallon (3.8 L) pots and maintained at 24°C to 29°C, with natural and supplemental lighting combined for a 16-hr daylength. Vetiver plants that were harvested after 4 yr of growth had been planted in Promix BX in 19 gallon pots (72 L) and maintained at 18°C to 24°C, with natural and supplemental lighting combined for a 16- to 18-hr daylength. Roots were chopped into 1 cm pieces and dried at room temperature (23-25°C) for 5 to 7 d. The dried material was ground by a milling machine (Thomas-Wiley, Laboratory Mill Model 4, Swedesboro, NJ) and passed through a sieve with a pore size of 2 mm. The powders were stored at 4°C until use.
In Thailand, 1-yr-old roots of field-grown vetiver cv. Songkha 3 were collected from plants grown at the Land Development Department, Nakhon Ratchasima province, Thailand. The vetiver plants were cultivated in Pak Chong series, red brown earth loam soil. In addition, because French marigold (Tagetes patula L.) has been widely studied for nematode management, plants of cv. Durango Mix (AGA Agro Co., Ltd.) were purchased from an orchard (Phu Ruea District, Loei Province, Thailand) to compare their activity with vetiver. Roots of vetiver and French marigold were chopped into 1 cm pieces and dried at room temperature (28-30°C) for 5 to 7 d. The dried material was ground by a milling machine (Hammer Mill, Department of Farm Machinery, Kasetsart University, Thailand) and passed through a sieve with a pore size of 2 mm. The powders were stored at room temperature until use.
Shoot and root extracts from 2-mon-old and 4-yr-old vetiver plants were prepared from cv. Sierra in the U.S. Since vetiver shoots can be harvested and used as soil mulch (Lim, 2016), studies with shoots focused on water-soluble compounds that might more readily leach into the soil. Research on root extracts was conducted with aqueous extracts, and with ethanol extracts that would provide material for GC-MS investigation of secondary metabolites. Procedures for making extracts were similar to those described in Meyer et al. (2006). To summarize, water-soluble compounds were extracted from dried, powdered shoots and roots (10% dry weight plant material/volume water) on a mechanical rotary shaker (VWR, Advanced Digital Shaker, Radnor, PA) at 100 rpm for 24 hr at room temperature (25°C). The mixture was filtered through eight layers of cheesecloth, centrifuged for 10 min at 3,000 g, and the supernatants were then sequentially filtered through syringe filters: 1.0 µm, 0.45 µm (Whatman, Clifton, NJ) and 0.2 µm (Nalgene, Rochester, NY), and stored at 4°C until use.
To prepare ethanol extracts, dried root powder was immersed in 95% ethanol (10% dry weight plant material/volume ethanol) and placed on a shaker as described above. The solution was then vacuum filtered through Whatman No. 1 filter paper, and the filtered solution was evaporated in a rotary evaporator at 45°C, 172 bar (Heidolph 2 Rotavac, Schwabach, Germany) to nearly dry. The small amount of remaining ethanol was air dried and the extracts were stored at -20°C.
Haitian vetiver oil (VO), also from V. zizanioides, was purchased as a commercial product (Texarome, Inc. TX, USA). The vetiver oil had been extracted by water distillation, with 99% purity.
Ethanol root extracts from French marigold and from 1-yr-old vetiver plants (cv. Songkha 3) were prepared in Thailand. Dried powder from vetiver roots or French marigold roots was immersed in 95% ethanol (20% dry weight plant material/volume ethanol) for 7 d in the dark at room temperature (28-30°C) without shaking. The solution was then vacuum filtered through a Whatman No. 1 filter paper and concentrated in a rotary evaporator at 50°C, 200 bar (Heidolph Hei-VAP Value, Schwabach, Germany). The concentrated extract was then transferred to a separatory funnel and partitioned with dichloromethane (Sac Science-Eng, Ltd, Thailand) at a ratio of 1:3. The dichloromethane phase was kept and re-evaporated at 50°C, dried with nitrogen, and finally stored at −20°C (procedure modified from Laksanaphisut, 2010).
Root-knot nematode culture
Meloidogyne incognita (Kofoid & White) Chitwood Race 1 (originally isolated in Maryland) was maintained on pepper (Capsicum annuum L.) cv. PA-136 in a greenhouse with temperatures and lighting as described above for vetiver plants. Procedures for J2 collection were like those in Meyer et al. (2016). Pepper roots from 2-mon-old plants infected with M. incognita were collected from the greenhouse; egg masses were handpicked from roots and surface sterilized in 0.6% sodium hypochlorite for 3.5 min, rinsed in sterile distilled water (SDW), and transferred to a hatching chamber (25-µm-diam. Spectra/Mesh Nylon Filter, Spectrum Laboratories Inc., Rancho Dominguez, CA) in a storage dish. The hatching chamber was incubated at 27°C and 40 rpm in a refrigerated incubator shaker (Innova 4230, New Brunswick Scientific, Edison, NJ) for 3 d, and the J2 were then collected and used for assays. J2 were adjusted to a final concentration of 20 per 10 µl.
Microwell assays
Laboratory assays with aqueous and ethanol extracts were conducted in 96-well polystyrene plates, following the procedures in Meyer et al. (2006). Approximately 20 J2 were added to each well in 10 µl SDW, and then 190 µl of extract, or of vetiver oil, was added to each well. The microwell plates were covered by a plastic adhesive sealing film (Excel Scientific, Inc., Victorville CA) and the lids were sealed with Parafilm (Bemis, Neenah, WI). The plates were incubated at 26°C.
For microwell assays of vetiver shoot aqueous (VSA) extracts and vetiver root aqueous (VRA) extracts from 2-mon-old plants, 100 µl of a 50.0 mg/ml streptomycin sulfate (Sigma-Aldrich, St. Louis, MO) stock solution was added to 9.9 ml of 100% vetiver aqueous extract (100% was the undiluted extract, and was filtered through a 0.2 µm syringe filter prior to addition of the antibiotic). The streptomycin sulfate was used to eliminate the growth of microbes that sometimes occurred in the aqueous extract assays despite sterile filtering. The extract solutions were then diluted to 75%, 50%, and 25% with SDW, and 190 µl of each extract was added to 10 µl J2 in SDW in each well. There were eight aqueous extract treatments: VSA and VRA at final concentrations in the wells of 94%, 71%, 47%, and 24%. Control treatments were SDW and the highest concentration of the antibiotic, equivalent to that in the 94% treatment: 0.5 mg/ml streptomycin sulfate, also diluted with 10 µl J2 in SDW in each well for a final streptomycin sulfate concentration of 0.48 mg/ml. Treatments were replicated in eight wells in each of the two trials, for a total of 16 wells. Active J2 (those exhibiting any movement within 5 seconds) and inactive J2 (no movement after 5 sec) were counted after 1 and 2 d (Days 1 and 2) incubation in the treatments. Following the Day 2 count, the J2 were rinsed two times with SDW and incubated in the second SDW rinse. Active vs. inactive J2 (those exhibiting body movements and those that were not) were counted on Day 3. J2 not active after the water rinse were considered dead.
Vetiver root ethanol (VRE) extracts and French marigold root ethanol (FMRE) extracts were prepared at 0.1 and 0.01 mg/ml concentrations in a solvent (referred to as CTD) of equal parts castor oil (BASF, Ludwigshafen, Germany), Tween 80 (Sigma, St. Louis, MO), and dimethyl sulfoxide (DMSO: Fisher, Fairlawn, NJ). To dissolve the extracts, a stock solution was prepared of 10 mg of crude ethanol extract in 1.0 ml of 100% CTD. To prepare the extract concentration of 0.1 mg/ml, 100 µl of this solution was then added to 9.9 ml SDW and was sequentially filtered through 1.0 µm, 0.45 µm, and 0.2 µm sterile filters. To prepare the 0.01 mg/ml extract, 1 ml of the 0.1 mg/ml filtered solution was added to 9.0 ml SDW. After addition of 190 µl extract to 10 µl J2 in SDW, the final extract concentrations in the wells were 0.095 mg/ml and 0.0095 mg/ml, respectively. The treatments at each of the two concentrations were (1) 2-mon VRE, (2) 1-yr VRE, (3) 4-yr VRE, (4) VO (vetiver oil), and (5) FMRE; these comprised treatments 1 through 10. Control treatments were: (11) SDW; (12) 1% CTD (which was 0.95% after dilution in the wells); and 13) 0.1% CTD (0.095% after dilution in the wells). Treatments were each replicated in eight wells for each of the two trials, for a total of 16 wells per treatment. Counts were performed as described above.
Chemotaxis assays
Chemotaxis assays were conducted with methods modified from Laznik and Trdan (2013). For our studies, 1.4% water agar (Noble agar, Difco, Leeuwarden, The Netherlands) was poured into plastic plates that were sold as lid sizes of 100- and 60-mm diam. The corresponding plate bases were 85-mm diam. (used for ethanol extracts; 25 ml agar added per plate), and 53-mm diam. (used for aqueous extracts, 10 ml agar per plate). Dried aqueous extracts were dissolved in SDW to obtain 0.1 g/ml. The treatments tested with aqueous extracts were: (1) 2-mon VRA and (2) 2-mon VSA. SDW was the control. Dried ethanol extracts were dissolved in 95% ethanol to obtain 0.1 g/ml. The treatments tested with ethanol extracts were: (1) 2-mon VRE, (2) 1-yr VRE, (3) 4-yr VRE, (4) VO, (5) FMRE, and (6) 95% ethanol. SDW was the control. Each plate was divided into three areas (Figure 1): the starting area, the treatment area, and the control area. Extract treatments (10 µl) were gently pipetted onto the agar at the treatment point, 10 µl of live J2 (ca. 20 J2) onto the starting point, and 10 µl of water onto the control point. Ethanol-dissolved treatments and the ethanol control were air dried for 10 min to allow ethanol to dissipate. The plates were incubated at 26°C for 24 hr. Each treatment was replicated with 5 plates in each of the two trials, for a total of 10 plates. The J2 were counted in each area and the chemotaxis index was calculated using the formula: CI = (number of nematodes in the treatment area – number of nematodes in the control area)/total number of nematodes in the assay. The interpretations of the CI values for the treatments are summarized as follows (from Laznik and Trdan, 2013): ≥0.2 indicated an attractant; between 0.2 and 0.1, a weak attractant; from 0.1 to -0.1, without effect; between -0.1 and -0.2, a weak repellent; and ≤-0.2, a repellent.
Figure 1.
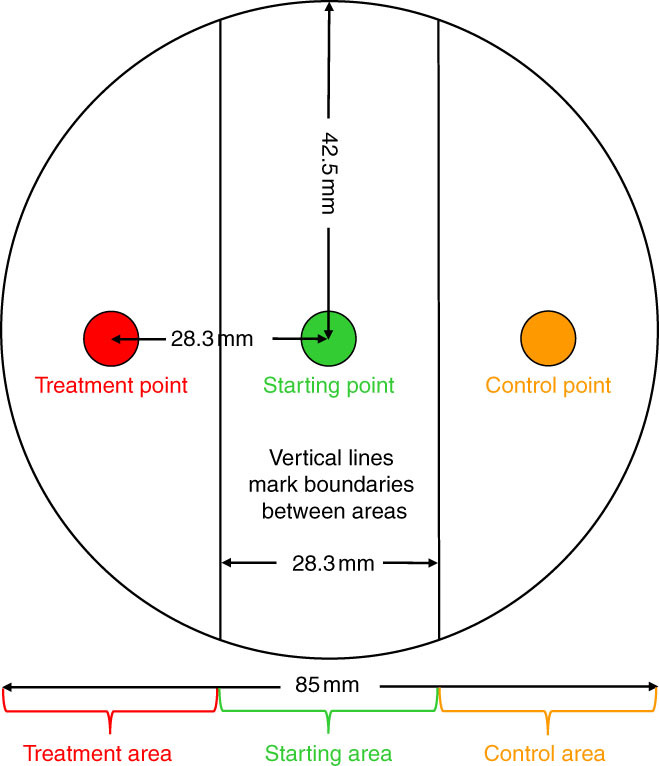
Diagram of a chemotaxis assay. Each plastic plate was divided into three areas: the treatment area (consisting of the treatment point and the surrounding area), the starting area (consisting of the starting point and the surrounding area), and the control area (consisting of the control point and the surrounding area). J2 were placed on the agar at the starting point, treatments were applied to the agar at the treatment point, and sterile distilled water was applied to the agar at the control point. Measurements are given for a plate with an 85-mm-diam. cup (100-mm-diam. lid).
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS analysis of the volatile constituents of VRE and VO was carried out using an Agilent 6890 N GC instrument coupled with a 5973 mass selective detector. The instrument was equipped with a DB-5 capillary column of length 30 m, internal diam. 0.25 mm, and film thickness 0.25 µm. The carrier gas was helium at a constant flow rate of 2.0 ml/min. The oven temperature was maintained at 50°C for 5 min and ramped to 280°C at 10°C/min and held there for 3 min. Diluted samples (in methanol) of 2 µl were injected in the split mode with a split ratio of 25:1 and the inlet temperature was 280°C. The mass detector scanned from 4.5 min to 30 min at a mass range from 40 to 400 (EI, 70 eV). The MS ion source temperature was 230°C and the quadrupole temperature was 150°C. The components were identified by comparing mass spectra with the NIST mass spectra library in the GC/MS data system.
Statistical analysis
Data were analyzed with the statistical package JMP Version 12.1.0 (SAS Institute, Inc., 2015). Differences among treatments were determined by analysis of variance (ANOVA) and means were compared using Tukey–Kramer’s adjustment for multiple comparisons (P ≤ 0.05).
Results
Microwell assays
An initial screening of 2-mon VSA, 2-mon VRA, and 4-yr VRA indicated that aqueous extracts from the younger plants showed greater activity against M. incognita J2 than extracts from the older plants (unpubl. data), so aqueous extract studies focused on the 2-mon-old plants. On Day 1, J2 inactivity in all 47%, 71%, and 94% treatments was significantly higher than in the streptomycin sulfate or water controls (Table 1). The 94% and 71% VRA had a greater effect than any of the other treatments, with ca. 5 times more inactive J2 than in the streptomycin sulfate control. On Day 2, 94% VRA again caused the highest J2 inactivity (4½ times greater than in the streptomycin sulfate control), with results similar to 94% VSA and 71% VRA. All treatments except 24% VRA resulted in higher J2 inactivity than in the streptomycin sulfate control. On Day 3, following a 1-d rinse in water, every treatment had significantly higher mortality than in the controls (Table 1). Compared with the streptomycin sulfate control, mortality ranged from 2 times greater (in 24% VRA) to more than 3 times greater (47% VSA and all 71% and 94% treatments). For all but one treatment, the J2 mortality in the water rinse was no different from the percentage inactive J2 on Day 2 (Table 1). Only 47% VSA resulted in significantly more nonviable J2 on Day 3 than inactive J2 on Day 2. No treatment showed a significant increase in J2 activity following the water rinse.
Table 1.
Percentage inactive or dead Meloidogyne incognita second-stage juveniles (J2) in Vetiveria zizanioides (vetiver) shoot aqueous (VSA) extracts and vetiver root aqueous (VRA) extracts.
| Percentage inactive J2 | Percentage dead J2 | ||
|---|---|---|---|
| Treatmenta | Day 1 | Day 2 | Day 3 rinsed |
| VSA 94% | 53.4 bAb | 70.4 abA | 68.2 aA |
| VRA 94% | 79.6 aAB | 86.7 aA | 71.9 aA |
| VSA 71% | 46.2 bB | 63.5 bcA | 71.1 aA |
| VRA 71% | 74.4 aA | 72.1 abA | 68.0 aA |
| VSA 47% | 39.8 bcB | 49.3 cdB | 67.6 aA |
| VRA 47% | 50.3 bA | 49.8 cdA | 53.4 abA |
| VSA 24% | 23.7 cdB | 38.3 dAB | 56.3 abA |
| VRA 24% | 16.6 deB | 34.3 deA | 43.6 bA |
| 0.48 mg/ml streptomycin sulfate | 14.7 deA | 18.7 efA | 22.3 cA |
| Water | 6.7 eB | 9.8 fAB | 17.5 cA |
aAll extracts were from shoots or roots of greenhouse-grown, 2-mon-old vetiver cv. Sierra. Treatments were prepared from 100% extracts (undiluted extracts) containing streptomycin sulfate, diluted to 75%, 50%, and 25% with water, and then added to J2 suspensions in the wells. Final extract dilutions in the wells are presented in the table.
bValues are means of eight replications in each of the two trials, for a total of N = 16. Differences among treatments were determined by analysis of variance (ANOVA) and means were compared using Tukey-Kramer’s adjustment for multiple comparisons (P ⩽ 0.05). Similar lower case letters indicate that means are not significantly different within a column; similar upper case letters indicate that means are not significantly different within a row.
The aqueous extracts used for the microwell assays were not dried to determine weights. However, weights were estimated based on the weights of the dried aqueous extracts used for the chemotaxis assays. For 30 g of dry, powdered plant material, the dry weight of the crude aqueous shoot extract (384 mg) was almost twice the weight of the crude aqueous root extract (207 mg). An estimate of the extract weights used in the microwell assays would be: 94% VSA (9.02 mg/ml), 94% VRA (4.87 mg/ml), 71% VSA (6.82 mg/ml), 71% VRA (3.68 mg/ml), 47% VSA (4.51 mg/ml), 47% VRA (2.43 mg/ml), 24% VSA (2.30 mg/ml), and 24% VRA (1.24 mg/ml). The 24% VRA was likely about half the concentration of the 24% VSA, which might have resulted in this root extract causing significantly lower mortality than the highest root and shoot concentrations.
On Day 1 in the ethanol extracts, J2 activity was similar among the 0.95 mg/ml FMRE and VRE (crude extract) treatments, with more than twice as many inactive J2 than in SDW or the 0.95% CTD control (Table 2). In contrast, 0.95 mg/ml VO treatment resulted in only 20% inactive J2, which was not significantly different from the controls. Unlike the higher concentration of vetiver extracts and FMRE, no treatment at the lower concentration (0.095 mg/ml) was active against J2. Results on Day 2 were similar to those on Day 1, with the 0.95 mg/ml FMRE and VRE all resulting in more than 3 times as many inactive J2 as in 0.95% CTD. The 0.95 mg/ml VO again had no effect on J2 activity. Most of the 0.095 mg/ml treatments, including FMRE, were not effective against J2. At 0.095 mg/ml, only 2-mon VRE was antagonistic to J2, with 2½ times more inactive J2 than in 0.095% CTD. On Day 3, after incubation in a water rinse, the 0.95 mg/ml FMRE and vetiver extract treatments all resulted in greater mortality than the VO or the SDW and 0.95% CTD controls. The 0.95 mg/ml FMRE treatment was the most nematotoxic (Table 2), increasing J2 mortality by more than 6 times compared with mortality in 0.95% CTD. The 0.95 mg/ml VRE treatments increased J2 mortality by more than three times compared with 0.95% CTD. Mortality in FMRE was significantly affected by extract concentration, and was twice as great in the higher concentration of FMRE. However, mortality in 0.095 mg/ml VRE and VO treatments was not significantly lower than in the comparable 0.95 mg/ml VRE and VO treatments. Among the 0.095 mg/ml treatments, all but vetiver oil and extract from 2-mon-old cv. Sierra plants increased mortality compared with the comparable CTD control.
Table 2.
Percentage inactive or dead Meloidogyne incognita second-stage juveniles (J2) in Vetiveria zizanioides (vetiver) root ethanol (VRE) extracts, Tagetes patula (French marigold cv. Durango Mix) root ethanol (FMRE) extract, and in vetiver oil (VO). Extracts were prepared from greenhouse-grown, 2-mon-old and 4-yr-old vetiver cv. Sierra, and from field-grown, 1-yr-old cv. Songkha 3.
| Percentage inactive J2 | Percentage dead J2 | ||
|---|---|---|---|
| Treatmenta | Day 1 | Day 2 | Day 3 rinsed |
| FMRE 0.95 mg/ml | 34.9 aBb | 30.7 aB | 69.1 aA |
| 2-mon VRE 0.95 mg/ml | 24.9 abcB | 28.0 abB | 38.3 bcA |
| 1-yr VRE 0.95 mg/ml | 27.6 abB | 26.5 abB | 42.4 bA |
| 4-yr VRE 0.95 mg/ml | 29.7 abB | 30.0 aB | 41.9 bA |
| VO 0.95 mg/ml | 20.3 bcdA | 12.6 cdA | 17.1 deA |
| 0.95% CTD | 12.9 dA | 8.0 dA | 11.3 eA |
| FMRE 0.095 mg/ml | 13.7 cdB | 17.0 bcdB | 35.0 bcA |
| 2-mon VRE 0.095 mg/ml | 16.2 cdA | 20.2 abcA | 24.0 cdeA |
| 1-yr VRE 0.095 mg/ml | 16.2 cdB | 13.9 cdB | 30.5 bcdA |
| 4-yr VRE 0.095 mg/ml | 14.2 cdB | 11.0 cdB | 27.8 bcdA |
| VO 0.095 mg/ml | 9.7 dA | 8.6 cdA | 9.8 eA |
| 0.095% CTD | 12.8 dA | 8.0 dA | 11.8 eA |
| Sterile distilled water | 11.8 dA | 12.4 cdA | 15.2 deA |
a2-mon VRE = vetiver root ethanol extracts from greenhouse-grown, 2-mon-old cv. Sierra; 1-yr VRE = vetiver root ethanol extracts from field-grown, 1-year-old cv. Songkha 3; 4-yr VRE = vetiver root ethanol extracts from greenhouse-grown, 4-yr-old cv. Sierra; VO = commercial vetiver oil. Treatments were prepared from 1.0 mg/ml and 0.1 mg/ml extracts that were dissolved in a solvent of equal parts castor oil, Tween 80, and dimethyl sulfoxide (CTD) and then added to J2 suspensions in the wells. Final extract dilutions in the wells are presented in the table.
bValues are means of eight replications in each of the two trials, for a total of N = 16. Differences among treatments were determined by analysis of variance (ANOVA) and means were compared using Tukey-Kramer’s adjustment for multiple comparisons (P ⩽ 0.05). Similar lower case letters indicate that the means are not significantly different within a column; similar upper case letters indicate that the means are not significantly different within a row.
J2 activity and mortality were also compared among days to determine if the treatments were nematostatic or nematotoxic. No difference in J2 activity was found between Day 1 and Day 2 for any treatment. However, on Day 3, J2 mortality increased compared with inactive J2 on Day 2 in almost all extracts (Table 2). The one exception was 0.095 mg/ml VRE from 2 mon-old plants, in which J2 inactivity on Day 2 was the same as J2 mortality after the rinse on Day 3. There were also no significant differences in percent inactive or dead J2 among Days 1, 2, and 3 in vetiver oil, in CTD, or in the SDW control.
Chemotaxis assays
The chemotaxis assay used to evaluate the repellent/attractant effects of crude aqueous extracts from vetiver shoots and roots demonstrated that J2 were repelled by 0.1 g/ml VRA and VSA (Table 3). The two aqueous extracts had similar chemotaxis indices. In chemotaxis assays with VO and crude ethanol extracts from French marigold and vetiver roots, the ethanol control did not attract or repel J2 (Table 4). French marigold and vetiver root extract treatments were repellent, although the 1-yr VRE was not significantly different from the water control (Table 4). The root extract from 2-mon VRE (cv. Sierra, greenhouse, U.S.) was twice as repellent as extracts from 1-yr VRE (cv. Songkha 3, field, Thailand) and 4-yr VRE (cv. Sierra, greenhouse, U.S.), but was only significantly greater than the effect of 1-yr VRE. Repellency by French marigold root extracts was similar to repellency with all of the vetiver root extract treatments. VO was not a repellent or an attractant, with a CI that was not significantly different from 1-yr VRE, 4-yr VRE, water, or ethanol in its effects on J2.
Table 3.
Repellent activity of aqueous Vetiveria zizanioides (vetiver) extracts on Meloidogyne incognita second-stage juveniles. The chemotaxis index (CI) was recorded after 24 hr.
| Treatmenta | CIb |
|---|---|
| 2-mon VRA 0.1 g/ml | −0.5 bc |
| 2-mon VSA 0.1 g/ml | −0.6 b |
| Control sterile distilled water | 0.0 a |
aVRA = vetiver root aqueous extracts from greenhouse-grown, 2-mon-old cv. Sierra; VSA = vetiver shoot aqueous extracts from greenhouse-grown, 2-mon-old cv. Sierra.
bChemotaxis index (CI): ⩾0.2 indicated an attractant; between 0.2 and 0.1, a weak attractant; 0.1 to −0.1, without effect; between −0.1 and −0.2, a weak repellent; and ⩽−0.2, a repellent.
cValues are means of five replications in each of the two trials, for a total of N = 10. Differences among treatments were determined by analysis of variance (ANOVA) and means were compared using Tukey-Kramer’s adjustment for multiple comparisons (P ⩽ 0.05). Similar lower case letters indicate that means are not significantly different.
Table 4.
Repellent activity of Vetiveria zizanioides (vetiver) oil, vetiver root ethanol extracts, and Tagetes patula (French marigold cv. Durango Mix) root ethanol extracts on Meloidogyne incognita second-stage juveniles. The chemotaxis index (CI) was recorded after 24 hr.
| Treatmenta | CIb |
|---|---|
| FMRE 0.1 g/ml | −0.4 dec |
| 2-mon VRE 0.1 g/ml | −0.6 e |
| 1-yr VRE 0.1 g/ml | −0.3 bcd |
| 4-yr VRE 0.1 g/ml | −0.3 cde |
| VO 0.1 g/ml | −0.1 abc |
| Ethanol | 0.1 a |
| Sterile distilled water | 0.0 ab |
a2-mon VRE = vetiver root ethanol extracts from greenhouse-grown, 2-mon-old cv. Sierra; 1-yr VRE = vetiver root ethanol extracts from field-grown, 1-yr-old cv. Songkha 3; 4-yr VRE = vetiver root ethanol extracts from greenhouse-grown, 4-yr-old cv. Sierra; VO = commercial vetiver oil.
bChemotaxis Index (CI): ≥0.2 indicated an attractant; between 0.2 and 0.1, a weak attractant; 0.1 to −0.1, without effect; between −0.1 and −0.2, a weak repellent; and ⩽−0.2, a repellent.
cValues are means of five replications in each of the two trials, for a total of N = 10. Differences among treatments were determined by analysis of variance (ANOVA) and means were compared using Tukey–Kramer’s adjustment for multiple comparisons (P ⩽ 0.05). Similar lower case letters indicate that the means are not significantly different.
Gas chromatography-mass spectrometry (GC-MS) analysis
The major component peaks of the three VRE extracts and the vetiver oil (VO) were detected and identified by GC/MS analysis including MS library matching (Table 5, Figs. 2 and 3). The VRE extracts, which represented different vetiver cultivars, growing conditions, and ages, contained similar constituents that differed mainly in their concentrations. Two of the major components in all three extracts and the VO were the sesquiterpene acid 3,3,8,8-tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid and the sesquiterpene alcohol 6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol (Fig. 2A-D; 3 A,B and Table 5). Among the extracts, the percentage area of 3,3,8,8-tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid was lowest in the extract from 4-yr, greenhouse-grown vetiver. However, it was even lower in the VO, with the percentage areas in the extracts being 20 to 60 times greater than in the VO. Unlike the extracts from the greenhouse-grown cv. Sierra, the extract from 1-yr, field-grown vetiver cv. Songkha 3 had two other major components, which are esters of fatty acids: ethyl hexadecanoate (ethyl palmitate) and ethyl 9,12-octadecadienoate (ethyl linoleate) (Figs. 2B; 3C,D and Table 5). Vetiver oil had a major component that differed from those found in the extracts: the sesquiterpene alcohol γ-costol (2-(4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-2-yl)-prop-2-en-1-ol) (Fig. 3E, Table 5).
Table 5.
Chemical composition of major constituents in three Vetiveria zizanioides (vetiver) root crude extracts and a commercial vetiver oil, determined by GC/MS analysis.
| Sourcea | Compound nameb | Retention time | Component percentage under peakc | Molecular formula | Molecular weight |
|---|---|---|---|---|---|
| 2-mon VRE | 3,3,8,8-Tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid (a sesquiterpene acid) | 20.11 | 42.8 | C15H22O2 | 234 |
| 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol (a sesquiterpene alcohol) | 19.46 | 11.2 | C15H24O | 220 | |
| 1-yr VRE | 3,3,8,8-Tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid | 20.12 | 26.1 | C15H22O2 | 234 |
| 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol | 19.47 | 3.0 | C15H24O | 220 | |
| Ethyl hexadecanoate (ethyl palmitate) | 21.86 | 32.3 | C18H36O2 | 284 | |
| Ethyl 9,12-Octadecadienoate (ethyl linoleate) | 23.42 | 18.9 | C20H36O2 | 308 | |
| 4-yr VRE | 3,3,8,8-Tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid | 20.17 | 14.1 | C15H22O2 | 234 |
| 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol | 19.46 | 9.9 | C15H24O | 220 | |
| VO | 3,3,8,8-Tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid | 20.15 | 0.7 | C15H22O2 | 234 |
| 6-Isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol | 19.47 | 17.1 | C15H24O | 220 | |
| 2-(4a,8-Dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-2-yl)-prop-2-en-1-ol(the sesquiterpene alcohol γ-costol) | 19.95 | 10.5 | C15H24O | 220 |
a2-mon VRE = vetiver root ethanol extracts from greenhouse-grown, 2-mon-old cv. Sierra; 1-yr VRE = vetiver root ethanol extracts from field-grown, 1-yr-old cv. Songkha 3; 4-yr VRE = vetiver root ethanol extracts from greenhouse-grown, 4-yr-old cv. Sierra; VO = commercial vetiver oil.
bThe components were identified based on the NIST mass spectra library.cRepresents highest area peak of a given compound, and does not include any further isomers.
Figure 2.
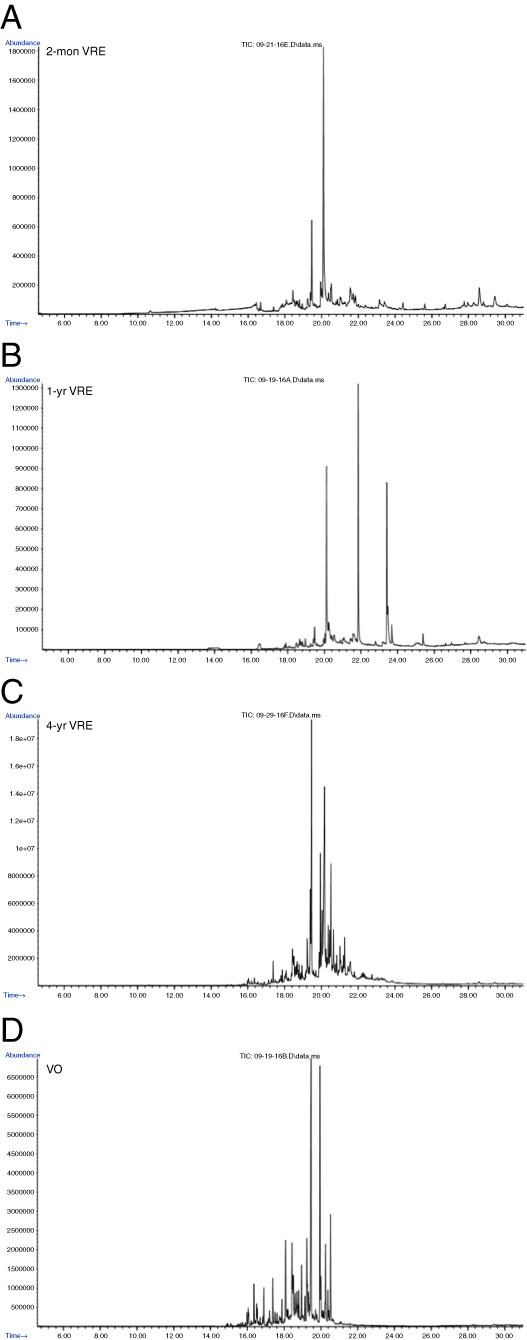
GC-MS spectra of crude Vetiveria zizanioides (vetiver) root ethanol (VRE) extracts and commercial vetiver oil (VO). (A) VRE from greenhouse-grown, 2-mon-old cv. Sierra; (B) VRE from field-grown, 1-yr-old cv. Songkha 3; (C) VRE from greenhouse-grown, 4-yr-old cv. Sierra; (D) commercial VO from Haiti.
Figure 3.
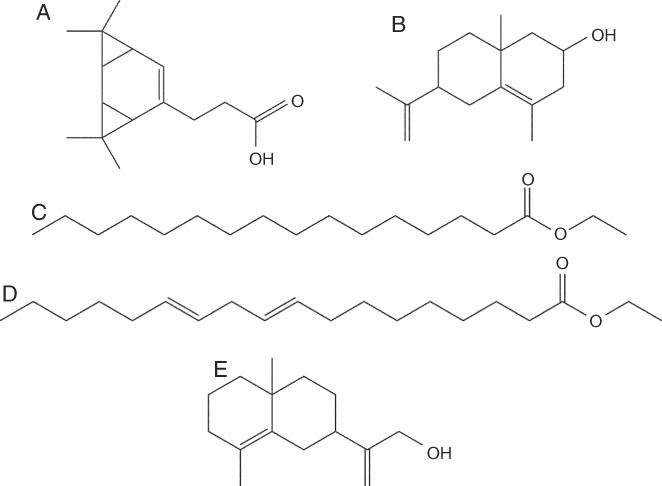
Chemical structures of the major constituents in vetiver root crude extracts and commercial vetiver oil from Vetiveria zizanioides, determined by GC/MS analysis: (A) 3,3,8,8-tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid; (B) 6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol; (C) ethyl hexadecanoate; (D) ethyl 9,12-octadecadienoate; (E) 2-(4a,8-dimethyl-1,2,3,4,4a,5,6,7-octahydro-naphthalen-2-yl)-prop-2-en-1-ol.
Discussion
This study demonstrated that vetiver produces compounds that are repellent and lethal to M. incognita J2. Aqueous root and shoot extracts killed up to 70% of immersed J2, while the highest tested concentration of ethanol root extracts resulted in ca. 40% nonviable J2. Although the dry weights of the aqueous root extracts were not determined for microwell assays, the lowest concentration was estimated to be 1.24 mg/ml (based on the weights from the samples used for J2 chemotaxis tests). This treatment resulted in J2 mortality of 44%, which was analogous to the mortality in a similar concentration of 0.95 mg/ml VRE extract. Vetiver aqueous extracts and ethanol extracts repelled J2 at 0.1 g/ml. Unlike the vetiver extracts, vetiver oil at the tested concentrations had no effect on J2 mortality, and did not attract or repel J2.
In a previous study with vetiver and M. javanica, root extract concentrations of 25%, 50%, 75%, and 100% resulted in J2 immobility ranging from 53% to 94% after 94 hr, compared with 35% immobile J2 in a tap water control (Ahuja et al., 2014). These results were comparable to those obtained with aqueous extracts in our research. However, Ahuja et al. (2014) did not report whether the nematodes recovered after extract treatment. They did observe that the root exudates also caused J2 immobility, but the nematodes recovered after removal from the exudates.
Our results with ethanol extracts differ from those reported by Wiratno et al. (2009), who tested the nematotoxicity of ethanol vetiver root extracts prepared from plants grown in an experimental garden in Indonesia. In that investigation, the plant parts were dried in the sun prior to ethanol extraction, and the vetiver extracts were dissolved in a solvent of DMSO:Tween 80:acetone. After 24 hr in 5 mg extract/ml medium, M. incognita J2 mortality was only 4% (Wiratno et al., 2009). This mortality was similar to the control and is considered nontoxic; the authors estimated an LC50 for vetiver root extracts of more than 19.2 mg/ml. In contrast, the vetiver root extracts in our study exhibited nematode antagonism at a much lower concentration. Approximately 25% to 30% J2 were inactive in 0.95 mg/ml ethanol root extracts after 24 hr, and about 38% to 42% J2 were dead on Day 3 after the water rinse. It is possible that dissimilarities between the nematode cultures or the extracts used for each study resulted in different J2 sensitivities. The final composition and activity of vetiver extract constituents can be variable, affected by plant genetics and environmental influences on the growing vetiver plants. Further differences may occur during the drying and chopping processes. For example, cutting before drying activates many enzymes, while lyophilizing before chopping minimizes enzymatic alteration of root contents. Extraction methods, and solvents used to dissolve the extracts, also influence the extract composition. However, our study with ethanol root extracts was conducted with vetiver plants from two cultivars, grown in differing soils/potting mixes and environmental conditions (field and two separate greenhouse bays), and for varying times, ranging from 2 mon to 4 yr. Even the extraction methods differed somewhat between the U.S. and Thailand. It is notable that, despite these variations, the effects on J2 were not highly variable among the different treatments. This would indicate that there were similarities in the active compounds produced by, and extracted from, the vetiver plants used in our research.
This premise was supported by the GC-MS analysis of the major compounds from the three ethanol root extracts and one vetiver oil. Although oil production and extraction with ethanol are different processes, there were similarities among all the root-derived samples. Ethyl palmitate, ethyl linoleate, and γ-costol (the latter was previously reported from vetiver oil; Lim, 2016) were not found in all the samples, but two of the main constituents were common to all four of the samples. These compounds, sesquiterpene acid 3,3,8,8-tetramethyltricyclo[5.1.0.0(2,4)]oct-5-ene-5-propanoic acid and sesquiterpene alcohol 6-isopropenyl-4,8a-dimethyl-1,2,3,5,6,7,8,8a-octahydronaphthalen-2-ol, have both been reported from vetiver oil (Chou et al., 2012), and were among the 25 active compounds from V. zizanioides to be tested for antivirus properties by Lavanya et al. (2016). The GC-MS analysis from previous research (Chou et al., 2012) indicated that the percentage areas under the peaks for the two compounds were 4.82 and 1.97, respectively. All of the samples in our study and the sample from Chou et al. (2012) differed in values for these areas. In particular, the sesquiterpene acid was less abundant than the sesquiterpene alcohol in the vetiver oil used in our study (percentage areas under the peaks from oil were 0.7 and 17.1, respectively). Consequently, the acid to alcohol ratio, as indicated by the percentage area under the peaks, was high in the extracts (1.4 to 8.7) and low in the vetiver oil (0.04).
It is possible that this difference in plant chemistry played a role in the nematotoxicity of the vetiver extracts vs. the vetiver oil in our study. The higher amounts of the acid in the root extracts than in the root oil may have resulted in repellency and death of the M. incognita J2. This is comparable to the research conducted with insects: vetiver oil is 65% sesquiterpenes, but the sesquiterpenol component is considered less active against insects than other sesquiterpenes (Chauhan and Raina, 2006). While it is possible that the nematicidal activity of our extracts was primarily due to the sesquiterpene acid, it must be recalled that nematotoxicity of plant extracts can be determined by the combined activity of multiple constituents. As summarized by Ntalli et al. (2011), nematotoxicity of essential oils “is not linearly dependent on the content of their main constituents,” and there can be synergistic or antagonistic interactions among the primary compounds, as well as activity from untested constituents. The isolated sesquiterpene acid would need to be tested to ascertain if it is a major factor affecting nematode viability.
Vetiver oil was not nematotoxic or repellent to M. incognita J2 in our assays. In a previous study, Haitian vetiver oil (10.0 mg/ml) was also not active against Bursaphelenchus xylophilus (Kong et al., 2006). In comparison, a range of results have been reported from nematode studies with compounds that are constituents of vetiver oil and other essential oils. For example, β-caryophyllene (a sesquiterpene) and linalool (a monoterpene alcohol) were attractants to some entomopathogenic nematodes (Būda and Čepulytė-Rakauskienė, 2011; Laznik and Trdan, 2013). Caryophyllene showed little or no toxicity at 5.0 mg/ml or less to M. incognita, M. javanica, or Heterodera avenae (Ntalli et al., 2010; Andrés et al., 2012; Bai et al., 2013; Li et al., 2015). Linalool was nontoxic and an attractant to the potato cyst nematodes Globodera pallida and G. rostochiensis (Būda and Čepulytė-Rakauskienė, 2011). Conversely, linalool was nematotoxic to the plant-parasitic nematodes Anguina tritici, Bursaphelenchus xylophilus, H. cajani, M. arenaria, M. incognita, M. javanica, and Tylenchulus semipenetrans (Sangwan et al., 1990; Echeverrigaray et al., 2010; Ntalli et al., 2010; Būda and Čepulytė-Rakauskienė, 2011). Meloidogyne incognita hatch and J2 mobility were only 24.2% and 19.8%, respectively, in 0.5 mg/ml linalool (Echeverrigaray et al., 2010). The EC50 value of linalool against M. incognita J2 was calculated as 0.284 mg/ml after 96 hr (Ntalli et al., 2010). In contrast, linalool was nontoxic to M. javanica at 1.0 mg/ml (Andrés et al., 2012). Linalool at 1,500 mg/kg soil reduced galling on tomato from M. arenaria, but not galling caused by M. incognita (Walker and Melin, 1996).
Other monoterpenes reported from essential oils, including vetiver oil, have been tested against Meloidogyne species. These studies clearly indicate that vetiver oil constituents are highly diverse in their nematicidal activities. Examples include borneol, bornyl acetate, eugenol, geraniol, limonene, linalyl acetate, myrcene, α-pinene, α-terpinene, terpinen-4-ol, and α-terpineol (Walker and Melin, 1996; Echeverrigaray et al., 2010; Ntalli et al., 2010). All but limonene, linalyl acetate, myrcene, and α-pinene suppressed hatching and J2 mobility of M. incognita at 0.25 mg/ml; these four monoterpenes were not as effective in reducing J2 mobility (Echeverrigaray et al., 2010; eugenol was not included in the study). Conversely, pinene (0.0005 mg/ml) killed 53% of M. incognita J2, but had no effect on the mortality of M. javanica J2 (Al-Banna et al., 2003). It is notable that the assay techniques differed from the Echeverrigaray et al. (2010) study. Borneol, geraniol, and α-terpineol mixed into soil at 100 and 250 mg/kg also significantly reduced M. incognita root galling on tomato (Echeverrigaray et al., 2010), but in another investigation geraniol at 1,500 mg/kg only suppressed galling caused by M. arenaria, and not by M. incognita (Walker and Melin, 1996). Limonene did not have a measurable EC50 against M. incognita J2, and there was no reduction in M. javanica J2 mobility at 0.5 ml/L (Oka et al., 2000; Ntalli et al., 2010). Limonene also did not reduce M. javanica gall indices on cucumber or tomato (Oka et al., 2000). Exposure to eugenol at 1.0 mg/ml for 72 hr had little effect on M. javanica J2, with 13.1% mortality (Andrés et al., 2012), but 0.0005 mg/ml resulted in 25% mortality of M. incognita J2 and 30% mortality of M. javanica J2 after 72 hr in another assay (Al-Banna et al., 2003). The LC50 value for eugenol was calculated as 1.24 mg/ml for M. javanica (Sangwan et al., 1990), and the EC50 as <0.133 mg/ml for M. incognita (Ntalli et al., 2010). Eugenol did not significantly decrease galling caused by either species on tomato (Walker and Melin, 1996). Clove oil consisting of ca. 75% eugenol also did not suppress M. incognita population densities on cucumber, compared with a water control (Meyer et al., 2008).
Vetiver shoot extracts were also active against nematodes in our study. These extracts were aqueous, so the major components were not identified by GC-MS, and the identities of potential nematicidal compounds present in aqueous extracts are unknown at this time. However, some compounds in the aerial parts of vetiver plants were extracted with other solvents and identified in earlier studies. Examples of such constituents include cholesterol, 1,2-bis(4-hydroxy-3-methoxyphenyl)-propane-1,3-diol, 1-O-feruloylglycerol, 1-O-p-coumaroylglycerol, trans-p-hydroxycinnamic acid, vladinol E, vladinol F, tricin 4’-O-(erythro-β-guaiacylglyceryl) ether, and tricin 4’-O-(threo-β-guaiacylglyceryl) ether (Gao et al., 2012). The two main compounds reported by Huang et al. (2004) were the triterpene squalene and 9-octadecenamide (oleamide). Some of these compounds are nematicidal. For example, p-coumaric acid (synonym of trans-p-hydroxycinnamic acid), identified in Melia azedarach (chinaberry) fruit pulp aqueous extract, was active against M. incognita J2 (Aoudia et al., 2012). The EC50 of the p-coumaric acid was 0.84 mg/ml. Aqueous leaf extracts from Corymbia citriodora (= Eucalyptus citriodora; lemon-scented gum), which contain coumaric acid as a constituent, were lethal to M. incognita J2 (El-Rokiek and El-Nagdi, 2011). The extract also reduced numbers of galls and egg masses on sunflower (Helianthus annuus) and purslane (Portulaca oleracea) (El-Rokiek and El-Nagdi, 2011). The phenolic acids trans-cinnamic acid and p-coumaric acid were nematicidal to the plant-parasitic nematode Nacobbus aberrans, causing greater than 80% J2 mortality at a concentration of 1 mg/ml acid (López-Martínez et al., 2011).
French marigold is well known for activity against plant-parasitic nematodes, and is planted for suppression of multiple taxa, including M. incognita (Franzener et al., 2007; Wang et al. 2007a, 2007b; Krueger et al., 2016). Ethanol extracts from French marigold roots were therefore included in our study to compare with the effects of vetiver extracts. The French marigold root extract and the ethanol vetiver root extracts, tested at 0.1 g/ml, were repellent to J2. In microwell assays, the 0.95 mg/ml French marigold root extract was more nematotoxic than the vetiver root extract, resulting in 69% nonviable M. incognita J2. This was similar to the activity of an aqueous root extract from T. patula, which also caused 68% mortality of M. incognita J2 (Franzener et al., 2007). Unlike the vetiver extracts, the effect of French marigold extract on J2 mortality was highly dependent on the concentration of the extract in our study. Twice as many J2 died at the higher vs. the lower French marigold extract concentration, resulting in similar activity among the 0.095 mg/ml French marigold and vetiver extracts. The activity of French marigold is due to the production of allelopathic compounds, particularly alpha-terthienyl, although compounds such as linalool, limonene, and linalyl acetate are also constituents of this plant (Ibrahim et al., 2006; Wang et al., 2007a). In our study, the nematicidal compounds from French marigold were more strongly affected by concentration than were the primary active compounds from vetiver roots.
In conclusion, the nematicidal and nematode-repellent activity of vetiver root and shoot extracts indicate that the plant chemistry may contribute to the resistance of roots to M. incognita. The nematotoxicity also demonstrates that there is potential for shoots amended into the soil to adversely affect root-knot nematode populations. Further work on the compounds and on vetiver mulch will indicate whether vetiver soil amendments or chemical components from the plant might be candidates for field studies as alternative, environmentally safe products for minimizing the crop damage inflicted by this nematode. Extension of vetiver use as a nematode-suppressive mulch or as a source for nematotoxic compounds would increase the value of this commonly planted bunchgrass.
Acknowledgments
The authors extend their thanks to the Land Development Department, Thailand, for sample support, and to the Department of Plant Pathology, Faculty of Agriculture, Kasetsart University, and the Department of Phamacognosy, Faculty of Pharmacy, Mahidol University, Thailand. This work was partially supported by the Center for Advanced Studies for Agriculture and Food, Institute for Advanced Studies, Kasetsart University, under the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Ministry of Education, Thailand. The mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Literature Cited
- Aarthi, N., Murugan, K., Madhiyazhagan, P., Nataraj, T., Nareshkumar, A., Kalimuthu, K., Hwang, J.-S., Barnard, D.R., Wei, H., Chandrasekar, R., and Amsath, A.. 2014. Studies on the effect of Sida acuta and Vetiveria zizanioides against the malarial vector, Anopheles stephensi and malarial parasite, Plasmodium berghei. International Journal of Pure and Applied Zoology 2: 51-60. [Google Scholar]
- Adams, R.P., Nguyen, S., Johnston, D.A., Park, S., Provin, T.L., and Habte, M.. 2008. Comparison of vetiver root essential oils from cleansed (bacteria- and fungus-free) vs. non-cleansed (normal) vetiver plants. Biochemical Systematics and Ecology 36: 177-182. [Google Scholar]
- Ahuja, P., Pretorius, M.S.A., and Fourie, H.. 2014. Potential of vetiver (Chrysopogon zizanioides) grass root exudates and extracts as a tool to manage Meloidogyne. Journal of Nematology 46: 133, (Abstr.). [Google Scholar]
- Al-Banna, L., Darwish, R.M., and Aburjai, T.. 2003. Effect of plant extracts and essential oils on root-knot nematode. Phytopathologia Mediterranea 42: 123-128. [Google Scholar]
- Andrés, M.F., González-Coloma, A., Sanz, J., Burillo, J., and Sainz, P.. 2012. Nematicidal activity of essential oils: a review. Phytochemistry Reviews 11: 371-390. [Google Scholar]
- Aoudia, H., Ntalli, N., Aissani, N., Yahiaoui-Zaidi, R., and Caboni, P.. 2012. Nematotoxic phenolic compounds from Melia azedarach against Meloidogyne incognita. Journal of Agricultural and Food Chemistry 60: 11675-11680. [DOI] [PubMed] [Google Scholar]
- Bai, P.H., Bai, C.Q., Liu, Q.Z., Du, S.S., and Liu, Z.L.. 2013. Nematicidal activity of the essential oil of Rhododendron anthopogonoides aerial parts and its constituent compounds against Meloidogyne incognita. Zeitschrift für Naturforschung 68c: 307-312. [PubMed] [Google Scholar]
- Belhassen, E., Filippi, J.-J., Brévard, H., Joulain, D., and Baldovini, N.. 2015. Volatile constituents of vetiver: a review. Flavour and Fragrance Journal 30: 26-82. [Google Scholar]
- Būda, V., and Čepulytė-Rakauskienė, R.. 2011. The effect of linalool on second-stage juveniles of the potato cyst nematodes Globodera rostochiensis and G. pallida. Journal of Nematology 43: 149-151. [PMC free article] [PubMed] [Google Scholar]
- Campos, R.N.S., Lima, C.B.N., Oliveira, A.P., Araújo, A.P.A., Blank, A.F., Alves, P.B., Lima, R.N., Araújo, V.A., Santana, A.S., and Bacci, L.. 2015. Acaricidal properties of vetiver essential oil from Chrysopogon zizanioides (Poaceae) against the tick species Amblyomma cajennense and Rhipicephalus (Boophilus) microplus (Acari: Ixodidae). Veterinary Parasitology 212: 324-330. [DOI] [PubMed] [Google Scholar]
- Champagnat, P., Figueredo, G., Calchat, J.-C., Carnat, A.-P., and Bessière, J.-M.. 2006. A study on the composition of commercial Vetiveria zizanioides oils from different geographic origins. Journal of Essential Oil Research 18: 416-422. [Google Scholar]
- Chauhan, K.R., and Raina, A.K.. 2006. Modified vetiver oil: economic biopesticide, in Rimando, A.M., and Duke, S.O. (Eds), Natural products for pest management, American Chemical Society Symposium Series 927, American Chemical Society, Washington DC, 210-218. [Google Scholar]
- Chomchalow, N.. 2001. The utilization of vetiver as medicinal and aromatic plants with special reference to Thailand. Technical Bulletin No. 2001/1. Office of the Royal Development Projects Board, Bangkok, Thailand.
- Chou, S.-T., Lai, C.-P., Lin, C.-C., and Shih, Y.. 2012. Study of the chemical composition, antioxidant activity and anti-inflammatory activity of essential oil from Vetiveria zizanioides. Food Chemistry 134: 262-268. [Google Scholar]
- Chou, S.-T., Shih, Y., and Lin, C.-C.. 2016. Vetiver grass (Vetiveria zizanioides) oils, in Preedy, V.R. (Ed.), Essential oils in food preservation, flavor and safety, Academic Press, New York, 843-848. [Google Scholar]
- Echeverrigaray, S., Zacaria, J., and Beltrão, R.. 2010. Nematicidal activity of monoterpenoids against the root-knot nematode Meloidogyne incognita. Phytopathology 100: 199-203. [DOI] [PubMed] [Google Scholar]
- El-Rokiek, K.G., and El-Nagdi, W.M.. 2011. Dual effects of leaf extracts of Eucalyptus citriodora on controlling purslane and root-knot nematode in sunflower. Journal of Plant Protection Research 51: 121-129. [Google Scholar]
- Flor-Weiler, L.B., Behle, R.W., and Stafford, K.C.. 2011. Susceptibility of four tick species, Amblyomma americanum, Dermacentor variabilis, Ixodes scapularis, and Rhipicephalus sanguineus (Acari: Ixodidae), to nootkatone from essential oil of grapefruit. Journal of Medical Entomology 48: 322-326. [DOI] [PubMed] [Google Scholar]
- Fourie, H., Leswifi, C., McDonald, A.H., and Waele, D.D.. 2007. Host suitability of vetiver grass to Meloidogyne incognita and M. javanica. Nematology 9: 49-52. [Google Scholar]
- Franzener, G., Martinez-Franzener, A.S., Stangarlin, J.R., Furlanetto, C., and Schwan-Estrada, K.R.F.. 2007. Protection of tomato plants by Tagetes patula aqueous extract against Meloidogyne incognita. Nematologia Brasileira 31: 27-36. [Google Scholar]
- Gao, G.-C., Lu, Z.-X., Xu, H.-X., Zheng, X.-S., and Yang, Y.-J.. 2012. Chemical constituents from the aerial parts of Vetiveria zizanioides. Chemistry of Natural Compounds 48: 128-129. [Google Scholar]
- Huang, J., Li, H., Yang, J., Chen, Y., Liu, Y., Li, N., and Nie, C.. 2004. Chemical components of Vetiveria zizanioides volatiles. Ying Yong Sheng Tai Xue Bao (The Journal of Applied Ecology) 15: 170-172. [PubMed] [Google Scholar]
- Ibrahim, S.A., Henderson, G., Zhu, B.C., Fei, H., and Laine, R.A.. 2004. Toxicity and behavioral effects of nootkatone, 1,10-dihydronootkatone, and tetrahydronootkatone to the formosan subterranean termite (Isoptera: Rhinotermitidae). Journal of Economic Entomology 97: 102-111. [DOI] [PubMed] [Google Scholar]
- Ibrahim, S.K., Traboulsi, A.F., and El-Haj, S.. 2006. Effect of essential oils and plant extracts on hatching, migration and mortality of Meloidogyne incognita. Phytopathologia Mediterranea 45: 238-246. [Google Scholar]
- Istianto, M., and Emilda, D.. 2011. Preliminary study of the activity of some essential oils against Fusarium oxysporum f. sp. cubense. Journal of Fruit and Ornamental Plant Research 19: 111-121. [Google Scholar]
- Joy, R.J.. 2009. ‘Sunshine’ vetivergrass Chrysopogon zizanioides (L.) Roberty. USDA, Natural Resources Conservation Service, Plant Guide. available at: www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_036941.pdf.
- Kong, J.-O., Lee, S.-M., Moon, Y.-S., Lee, S.-G., and Ahn, Y.-J.. 2006. Nematicidal activity of plant essential oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). Journal of Asia-Pacific Entomology 9: 173-178. [Google Scholar]
- Krishnaveni, V.. 2016. Analysis of chemical components and antimicrobial activity on vetiver extract for home textile applications. Journal of Textile Science and Engineering 6: 259-261. [Google Scholar]
- Krueger, R., Dover, K.E., McSorley, R., and Wang, K.-H.. 2016. Marigolds (Tagetes spp.) for nematode management. ENY-056 (NG045). Entomology and Nematology Department, University of Florida UF/IFAS Extension.
- Kumar, S.S., and Gayathri, K.. 2016. Chemical characterization of Vetiveria zizanioides Linn root. International Journal of Pharma and Bio Sciences 7B: 689-695. [Google Scholar]
- Laksanaphisut, S.. 2010. Control of green mold rot on citrus fruits cv. Sai-Numphaung Caused by Penicillium digitatum Sacc., with crude extracts of turmeric (Curcuma longa Linn.). MS. Thesis, Kasetsart University, Thailand. [Google Scholar]
- Lal, A., and Mathur, V.K.. 1982. Occurrence of Heterodera zeae on Vetiveria zizanioides. Indian Journal of Nematology 12: 405-407. [Google Scholar]
- Lavanya, P., Ramaiah, S., and Anbarasu, A.. 2016. Ethyl 4-(4-methylphenyl)-4-pentenoate from Vetiveria zizanioides inhibits dengue NS2B-NS3 protease and prevents viral assembly: a computational molecular dynamics and docking study. Cell Biochemistry and Biophysics 74: 337-351. [DOI] [PubMed] [Google Scholar]
- Laznik, Ž., and Trdan, S.. 2013. An investigation on the chemotactic responses of different entomopathogenic nematode strains to mechanically damaged maize root volatile compounds. Experimental Parasitology 134: 349-355. [DOI] [PubMed] [Google Scholar]
- Leite, B.. 2012. Extraction of essential oils from vetiver (Vetiveria zizanioides) grass, M.S. thesis, University of KwaZulu- Natal, South Africa. [Google Scholar]
- Li, Y.C., Ji, H., and Li, H.T.. 2015. Gas chromatography-mass spectrometric analysis of nematicidal essential oil of Valeriana amurensis P Smirn ex Kom (Valerianaceae) roots and its activity against Heterodera avenae. Tropical Journal of Pharmaceutical Research 14: 1673-1678. [Google Scholar]
- Lim, T.K.. 2016. Edible medicinal and non-medicinal plants. vol.11, modified stems, roots, bulbs, Springer International Publishing, Switzerland. [Google Scholar]
- López-Martínez, N., Colinas-León, M.T., Peña-Valdivia, C.B., Salinas-Moreno, Y., Fuentes-Montiel, P., Biesaga, M., and Zavaleta-Mejía, E.. 2011. Alterations in peroxidase activity and phenylpropanoid metabolism induced by Nacobbus aberrans Thorne and Allen, 1944 in chilli (Capsicum annuum L.) CM334 resistant to Phytophthora capsici Leo. Plant and Soil 338: 399-409. [Google Scholar]
- Maffei, M.. 2002. Introduction to the genus Vetiveria, in Maffei, M. (Ed.), Vetiveria: The Genus Vetiveria, Taylor & Francis, New York, 1-18. [Google Scholar]
- Mao, L., Henderson, G., Bourgeois, W.J., Vaughn, J.A., and Laine, R.A.. 2006. Vetiver oil and nootkatone effects on the growth of pea and citrus. Industrial Crops and Products 23: 327-332. [Google Scholar]
- Martinez, J., Rosa, P.T.V., Menut, C., Leydet, A., Brat, P., Pallet, D., and Meireles, M.A.A.. 2004. Valorization of Brazilian vetiver (Vetiveria zizanioides (L.) Nash ex Small) oil. Journal of Agricultural and Food Chemistry 52: 6578-6584. [DOI] [PubMed] [Google Scholar]
- Meyer, S.L.F., Chauhan, K.R., and MacDonald, M.H.. 2016. Evaluation of roselle (Hibiscus sabdariffa) leaf and pomegranate (Punica granutum) fruit rind for activity against Meloidogyne incognita. Nematropica 46: 85-96. [Google Scholar]
- Meyer, S.L.F., Lakshman, D.K., Zasada, I.A., Vinyard, B.T., and Chitwood, D.J.. 2008. Phytotoxicity of clove oil to vegetable crop seedlings and nematotoxicity to root-knot nematodes. HortTechnology 18: 631-638. [Google Scholar]
- Meyer, S.L.F., Zasada, I.A., Roberts, D.P., Vinyard, B.T., Lakshman, D.K., Lee, J.-K., Chitwood, D.J., and Carta, L.K.. 2006. Plantago lanceolata and Plantago rugelii extracts are toxic to Meloidogyne incognita but not to certain microbes. Journal of Nematology 38: 333-338. [PMC free article] [PubMed] [Google Scholar]
- Ntalli, N.G., Ferrari, F., Giannakou, I., and Menkissoglu-Spiroudi, U.. 2010. Phytochemistry and nematicidal activity of the essential oils from 8 Greek Lamiaceae aromatic plants and 13 terpene components. Journal of Agricultural and Food Chemistry 58: 7856-7863. [DOI] [PubMed] [Google Scholar]
- Ntalli, N.G., Ferrari, F., Giannakou, I., and Menkissoglu-Spiroudi, U.. 2011. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Management Science 67: 341-351. [DOI] [PubMed] [Google Scholar]
- Oka, Y., Nacar, S., Putievsky, E., Ravid, U., Yaniv, Z., and Spiegel, Y.. 2000. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology 90: 710-715. [DOI] [PubMed] [Google Scholar]
- Panella, N.A., Dolan, M.C., Karchesy, J.J., Xiong, Y., Peralta-Cruz, J., Khasawneh, M., Montenieri, J.A., and Maupin, G.O.. 2005. Use of novel compounds for pest control: Insecticidal and acaricidal activity of essential oil components from heartwood of Alaska yellow cedar. Journal of Medical Entomology 42: 352-358. [DOI] [PubMed] [Google Scholar]
- Prajna, J., Richa, J., and Dipjyoti, C.. 2013. HPLC quantification of phenolic acids from Vetiveria zizanioides (L.) Nash and its antioxidant and antimicrobial activity. Journal of Pharmaceutics 2013:Article ID 240472, 6 page. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos, J.-L.. 2016. Essential oils: what they are and how the terms are used and defined, in Preedy, V.R. (Ed.), Essential oils in food preservation, flavor and safety, Academic Press, New York, 3-10. [Google Scholar]
- Sangwan, N.K., Verma, B.S., Verma, K.K., and Dhindsa, K.S.. 1990. Nematicidal activity of some essential plant oils. Pesticide Science 28: 331-335. [Google Scholar]
- Soni, A., and Dahiya, P.. 2015. Screening of phytochemicals and antimicrobial potential of extracts of Vetiveria zizanioides and Phragmites karka against clinical isolates. International Journal of Applied Pharmaceutics 7: 22-24. [Google Scholar]
- Subhadradevi, V., Asokkumar, K., Umamaheswari, M., Sivashanmugam, A., and Sankaranand, R.. 2010. In vitro antioxidant activity of Vetiveria zizanioides root extract. Tanzania Journal of Health Research 12: 274-279. [PubMed] [Google Scholar]
- Sujatha, S.. 2010. Essential oil and its insecticidal activity of medicinal aromatic plant Vetiveria zizanioides (L.) against the red flour beetle Tribolium castaneum (Herbst). Asian Journal of Agricultural Sciences 2: 84-88. [Google Scholar]
- Truong, P.. 2000. Vetiver grass system: potential applications for soil and water conservation in California. Stiff Grass Technology Seminar, Yolo County Flood Control & Water Conservation District and Family Water Alliance, Woodland, CA.
- Truong, P.. 2002. Vetiver grass technology, in Maffei, M. (Ed.), Vetiveria: the genus Vetiveria, Taylor & Francis, New York, 114-132. [Google Scholar]
- Vázquez-Sánchez, D., Cabo, M.L., and Rodríguez-Herrera, J.J.. 2014. Antimicrobial activity of essential oils against Staphylococcus aureus biofilms. Food Science and Technology International 21: 559-570. [DOI] [PubMed] [Google Scholar]
- Walker, J.T., and Melin, J.B.. 1996. Mentha × piperita, Mentha spicata and effects of their essential oils on Meloidogyne in soil. Journal of Nematology 28 No. 4S, 629-635. [PMC free article] [PubMed] [Google Scholar]
- Wang, K.-H., Hooks, C.R., and Ploeg, A.. 2007a. Protecting Crops from nematode pests: using marigold as an alternative to chemical nematicides. PD-35, Plant Disease. Cooperative Extension Service, College of Tropical Agriculture and Human Resources, University of Hawai’i at Mānoa.
- Wang, Q., Li, Y., Handoo, Z., and Klassen, W.. 2007b. Influence of cover crops on populations of soil nematodes. Nematropica 37: 79-92. [Google Scholar]
- West, L., Sterling, G., and Truong, P.N.. 1996. Resistance of vetiver grass to infection by root-knot nematodes (Meloidogyne spp.). The Vetiver Network Newsletter 20: 20-22. [Google Scholar]
- Wiratno, Taniwiryono, D., Van den Berg, H., Riksen, J.A.G., Rietjens, I.M.C.M., Djiwanti, S.R., Kammenga, J.E., and Murk, A.J.. 2009. Nematicidal activity of plant extracts against the root-knot nematode, Meloidogyne incognita. The Open Natural Products Journal 2: 77-85. [Google Scholar]
- Zhu, B.C.R., Henderson, G., Chen, F., Maistrello, L., and Laine, R.A.. 2001a. Nootkatone is a repellent for Formosan subterranean termite (Coptotermes formosanus). Journal of Chemical Ecology 27: 523-531. [DOI] [PubMed] [Google Scholar]
- Zhu, B.C.R., Henderson, G., Chen, F., Fei, H., and Laine, R.A.. 2001b. Evaluation of vetiver oil and seven insect-active essential oils against the Formosan subterranean termite. Journal of Chemical Ecology 27: 1617-1625. [DOI] [PubMed] [Google Scholar]


