Highlights
-
•
Dementia with Lewy bodies patients often have Alzheimer's disease co-pathology.
-
•
123I-FP-CIT dopamine transporter binding in pure and mixed DLB seems comparable.
-
•
123I-FP-CIT serotonin transporter binding may be more compromised in mixed pathology.
Keywords: Dementia with Lewy bodies, Alzheime’s disease, Dopamine transporter, Serotonin transporter, 123I-FP-CIT SPECT
Abstract
Purpose
To study the influence of concomitant Alzheimer's disease (AD) pathology in dementia with Lewy bodies (DLB) on dopamine transporter (DAT) and serotonin transporter (SERT) availability, using 123I-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (123I-FP-CIT) single photon emission computed tomography (SPECT).
Methods
Based on their cerebrospinal fluid biomarker profile, fifty-two patients with probable DLB were divided in a group with (DLB/AD+, N = 15) and without concomitant AD-pathology (DLB/AD-, N = 37). We conducted atrophy-corrected region of interest (ROI) analyses comparing binding ratios (BRs) in the DAT-rich striatal and SERT-rich extrastriatal brain areas (amygdala, hippocampus, thalamus, midbrain and pons).
Results
DLB/AD+ patients had significantly lower 123I-FP-CIT BRs in the left amygdala, and a trend was seen in the right hippocampus. Groups did not differ significantly in striatal 123I-FP-CIT BRs, neuropsychiatric or motor symptoms. Motor symptoms correlated negatively with striatal DAT BRs.
Conclusions
DLB/AD+ patients may have lower SERT binding in limbic brain regions than DLB/AD- patients, possibly indicating faster neurodegeneration in mixed pathology.
1. Introduction
After Alzheimer's disease (AD), dementia with Lewy bodies (DLB) is a relatively common neurodegenerative dementia, with a high frequency of neuropsychiatric symptoms and extrapyramidal signs. (McKeith et al., 2017) The pathological hallmark of DLB is the presence of cerebral Lewy bodies that contain alpha-synuclein. In addition, up to 50% of DLB-patients have concomitant AD-pathology (amyloid plaques and neurofibrillary tangles). This pathological overlap complicates the differential diagnosis of AD versus DLB. Patients with mixed pathology may be less likely to express core DLB symptoms (Lemstra et al., 2016; Merdes et al., 2003) and abnormal AD-biomarkers (such as cerebrospinal fluid (CSF) or amyloid positron emission tomography (PET)) may lead to a misdiagnosis of AD. Furthermore, DLB patients with mixed pathology seem to have a worse prognosis, and possibly more neuropsychiatric symptoms, such as hallucinations. (Lemstra et al., 2016)
123I-N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl)nortropane (123I-FP-CIT) single photon emission computed tomography (SPECT) studies have demonstrated reduced striatal dopamine transporter (DAT) binding in DLB and Parkinson's disease (PD)—an in-vivo proxy of nigrostriatal degeneration— but not in AD. 123I-FP-CIT SPECT has shown good diagnostic accuracy for DLB and is included in the diagnostic criteria as an indicative biomarker. (McKeith et al., 2017)
In addition to its high affinity to the DAT, 123I-FP-CIT binds also with modest affinity to the serotonin transporter (SERT). (Joling et al., 2018) Serotonin is a neurotransmitter that regulates the neuroendocrine system as well as cognitive functions and mood. Serotonergic deficits have been related to symptoms of depression and psychosis in both DLB and AD. (Vermeiren et al., 2015) DAT and SERT display a different distribution in subcortical structures, which makes it possible to simultaneously assess the striatal dopaminergic and extrastriatal serotonergic system with one 123I-FP-CIT SPECT scan. (Joling et al., 2018)
The influence of concomitant AD-pathology on 123I-FP-CIT binding is still unknown. We aimed to study if concomitant AD-pathology influences 123I-FP-CIT binding (both striatal DAT and extrastriatal SERT). We compared DAT and SERT binding between DLB with concomitant AD-pathology (DLB/AD+) and ‘pure DLB’ (DLB/AD-). Based on previously described differences in clinical symptoms (Lemstra et al., 2016; Merdes et al., 2003) we hypothesized that DLB/AD+ patients may have lower SERT binding and DLB/AD- patients may have lower DAT binding. We aimed to relate the findings to motor, cognitive and psychiatric symptoms.
2. Material and methods
2.1. Patient selection
From our memory clinic population, the Amsterdam Dementia Cohort, we selected 55 patients with probable DLB[1] with an available 123I-FP-CIT SPECT scan, a T1-weighted magnetic resonance imaging (MRI) scan and CSF. One patient was excluded due to use of a serotonin reuptake inhibitor (possible interference with 123I-FP-CIT SERT-binding) and two due to segmentation failures. Fifty-two patients were divided, based on their CSF tau/ aβ-42 ratio (Duits et al., 2014) into DLB/AD+ (tau/ aβ-42 ratio>0.52, n = 15) or DLB/AD- (tau/ aβ-42 ratio≤0.52 (n = 37)). All patients had given written informed consent to use their clinical and imaging data. All patients provided informed consent to enter their clinical and imaging data, obtained as part of routine patient care, in a pseudonymised database for research purposes. This procedure was approved by the local Medical Ethics Committee.
Clinical data were collected prospectively: Neuropsychiatric symptoms were assessed by the Neuropsychiatric Inventory (NPI). The relevant subscales were delusions, hallucinations, agitation/aggression, dysphoria/depression, anxiety, apathy, disinhibition, aberrant motor activity, and nighttime behavioral disturbances. Presence of symptoms was defined as a subscale score>0. In addition to the NPI, depression was assessed by the short form of the Geriatric Depression Scale (GDS-S), presence of depressive symptoms was defined as a score>4. Parkinsonism was assessed by a preformatted checklist based on neurological examination including the presence of bradykinesia, hypokinesia, tremor and/or rigidity.
2.2. 123I-FP-CIT SPECT analysis
We performed imaging according to the guidelines of the European Association of Nuclear Medicine: 123I-FP-CIT was administered intravenously in a dose of approximately 185 MBq (specific activity >185 MBq/nmol; radiochemical purity >99%; produced as DaTSCAN™ at GE Healthcare, Eindhoven, The Netherlands). After 3 h, static images were obtained for 30 min using a dual-head gamma camera (E.Cam; Siemens, Munich, Germany) with a fan-beam collimator. We used Chang's attenuation correction (Chang, 1978) with an attenuation coefficient of 0.15, and then reconstructed and reoriented the scans to the anterior-posterior commissural plane in Statistical Parametric Mapping 12 software (SPM 12; Wellcome Trust Centre for Neuroimaging, London, UK).
Regions of interest (ROIs) were defined using FreeSurfer 6.0 (Athinoula A. Martinos Center for Biomedical Imaging, Boston, MA, USA) with default settings on the T1-weighted MRI scans to individually segment the ROIs per subject. We obtained intracranial volumes in FreeSurfer to be able to correct individual ROI volumes. All ROIs were visually inspected for segmentation errors. The bilateral DAT-rich striatal ROIs consisted of the nucleus accumbens and caudate nucleus as a combined region (to avoid spill-over effects) and the posterior putamen. The bilateral SERT-rich extrastriatal ROIs were: amygdala, hippocampus, thalamus, midbrain and pons. The 123I-FP-CIT SPECT scans were co-registered with the individual T1-weighted MRI scans within SPM 12 according to the previously described method. (Joling et al., 2018) We calculated BRs with the cerebellum as the reference region (REF; WFU Pickatlas, AAL; bilateral Crus 2), since it is relatively free from DAT and SERT, using the following formula: [BR = (ROI – REF) / REF]. (Joling et al., 2018)
2.3. Statistical analysis
Clinical variables were compared with t-tests or Mann–Whitney U tests, depending on the distribution, dichotomous measures with χ2 or Fisher's exacts test. BRs in each ROI were compared using linear regression analysis, corrected for age and (intracranial volume corrected) ROI volume. Pearson or Spearman correlations between BRs (DAT and SERT) and clinical measures were calculated. Statistical significance was set at p < 0.05, with Bonferroni correction (p < 0.05/ number of tests) to correct for multiple testing.
3. Results
Table 1 shows the demographic and clinical characteristics of both groups. DLB/AD+ patients (n = 15, 28.8%) were older (p = 0.02) than DLB/AD- patients and showed more right hemispheric medial temporal lobe atrophy (p = 0.02). There were no differences in the presence of parkinsonism, depressive or other neuropsychiatric symptoms.
Table 1.
Demographic and Clinical Characteristics*.
| Characteristic | DLB/AD+(N = 15) | DLB/AD-(N = 37) |
|---|---|---|
| Gender (f/m) | 2/13 | 5/32 |
| Age, mean (SD) | 69.9 (4.4) | 65.8 (6.0)* |
| CAMCOG, mean (SD) | 81.9 (8.3) | 79.3 (10.9) |
| CDR, median [IQR] | 1 [0.5–1] | 0.5 [0.5–1] |
| GDS, median [IQR] | 4 [3–6] | 3 [2–5.5] |
| GDS >4, n(%) | 4 (30.8%) | 12 (32.4%) |
| MMSE, median [IQR] | 24 [21–27] | 24 [22–26.5] |
| Symptom duration (yrs), mean (SD) | 3.9(2.6) | 4.0(3.1) |
| Parkinsonism | 66.7% | 62.2% |
| MTA_R, median [IQR] | 1 [0.75–2] | 1 [0–1]* |
| MTA_L, median [IQR] | 1 [0–2] | 1 [0–1] |
| NPI total score, median [IQR], N | 7[6–24], 9 | 10 [(5–15], 26 |
| NPI_delusion, n (%) | 1 (11.1%) | 0% |
| NPI_hallucination, n (%) | 4 (44.4%) | 6 (23.1%) |
| NPI_agitation/aggression, n (%) | 1 (11.1%) | 3 (11.5%) |
| NPI_depression, n (%) | 3 (33.3%) | 9 (34.6%) |
| NPI_anxiety, n (%) | 5 (55.6%) | 9 (34.6%) |
| NPI_apathy, n (%) | 8 (88.9%) | 21 (80.9%) |
| NPI_disinhibition, n (%) | 0% | 3 (11.5%) |
| NPI_aberrant motor activity, n (%) | 1 (11.1%) | 3 (11.5%) |
| NPI_sleep disturbances, n (%) | 4 (44.4%) | 9 (34.6%) |
Abbreviations: DLB/AD+ = Dementia with Lewy bodies and concomitant Alzheimer-pathology, DLB/AD- = Dementia with Lewy bodies without concomitant Alzheimer-pathology, CAMCOG = Cambridge Cognitive Examination (values >79 are considered normal), CDR = Clinical Dementia Rating (0 = Normal, 0.5 = Very Mild Dementia, 1 = Mild D., 2 = Moderate D., 3 = Severe D.), GDS = Geriatric Depression Scale (0–4 = not depressed, 5–10 = mildly depressed, 11–15 = severely depressed), MMSE = Mini Mental State Examination (values >24 are considered normal), Parkinsonism = presence of motor signs (bradykinesia, rigidity and tremor) registered dichotomously, MTA_R = medial temporal lobe atrophy (score) right hemisphere, MTA_L = medial temporal lobe atrophy (score) left hemisphere, NPI = Neuropsychiatric Inventory subscales.
p < 0.05.
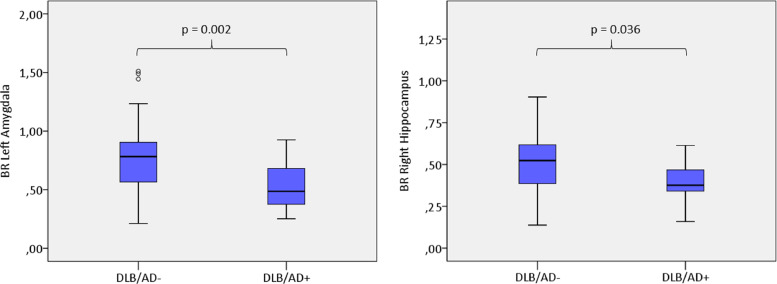
We did not find significant differences between DLB/AD+ and DLB/AD- in striatal 123I-FP-CIT BRs (Table 2). DLB/AD+ patients had statistically significant lower 123I-FP-CIT BRs than DLB/AD- patients for the left amygdala (p = 0.002) and the right hippocampus (p = 0.036) (Fig. 1). The difference for the left amygdala remained significant after Bonferroni correction (p < 0.008). For the other extrastriatal regions, group differences did not reach statistical significance.
Table 2.
Striatal (DAT) and extrastriatal (SERT) 123I-FP-CIT binding ratios.
| DLB/AD+(n = 15) | DLB/AD-(n = 37) | Mean difference/(SE)/p | |
|---|---|---|---|
| DAT binding ratios | |||
| Left Nucleus Accumbens/Caudate Nucleus combined | 1.64(0.38) | 1.95(0.50) | 0.22/(0.15)/0.16 |
| Right Nucleus Accumbens/Caudate Nucleus combined | 1.61(0.31) | 1.87(0.49) | 0.11/(0.14)/0.45 |
| Left Putamen posterior | 1.61(0.42) | 1.92(0.64) | 0.16/(0.18)/0.28 |
| Right Putamen posterior | 1.60(0.43) | 1.97(0.67) | 0.18/(0.18)/0.30 |
| SERT binding ratios | |||
| Left Amygdala | 0.55(0.20) | 0.78(0.30) | 0.30/(0.09)/0.002* |
| Right Amygdala | 0.59(0.28) | 0.69(0.28) | 0.05/(0.09)/0.61 |
| Left Hippocampus | 0.45(0.12) | 0.53(0.17) | 0.07/(0.05)/0.18 |
| Right Hippocampus | 0.39(0.12) | 0.51(0.18) | 0.12/(0.06)/0.036* |
| Left Thalamus | 0.56(0.16) | 0.60(0.17) | 0.019/(0.05)/0.73 |
| Right Thalamus | 0.56(0.13) | 0.60(0.16) | 0.013/(0.05)/0.79 |
| Pons | 0.36(0.11) | 0.42(0.11) | 0.052/(0.04)/0.16 |
| Midbrain | 0.53(0.11) | 0.59(0.13) | 0.037/(0.04)/0.37 |
Data are mean (SD) unless otherwise specified.
Abbreviations: DLB/AD+ = Dementia with Lewy bodies and concomitant Alzheimer-pathology, DLB/AD- = Dementia with Lewy bodies without concomitant Alzheimer-pathology, DAT = dopamine transporter, SERT = serotonin transporter, SE = standard error.
p < 0.05 corrected for age and ROI volume.
Fig.1.
Distribution of 123I-FP-CIT binding ratios (BRs) in the amygdala and hippocampus of DLB-patients with (DLB/AD+) and without (DLB/AD-) concomitant Alzheimer-pathology.
In the total sample, motor symptoms correlated negatively with DAT BRs in striatal ROIs: Spearman's rho left nucleus accumbens/caudate: r(df)=−0.27(50); p = 0.05, right nucleus accumbens/caudate r(df)=−0.33(50); p = 0.02, left putamen r(df)=−0.42(50); p = 0.002, right putamen r(df)=−0.43(50); p = 0.001).No significant correlations were found with MMSE or neuropsychiatric symptoms.
4. Discussion
This study is the first to explore the influence of concomitant AD pathology in DLB on neurotransmitter systems by studying both striatal and extrastriatal 123I-FP-CIT binding. The main findings were a lower 123I-FP-CIT SERT binding in the left amygdala of DLB/AD+-patients compared to DLB/AD-, and a trend towards a lower right hippocampal SERT binding in DLB/AD+. DAT binding was comparable between groups. In the overall sample, motor symptoms correlated negatively with striatal DAT binding as expected. (Del Sole et al., 2015) No correlations with neuropsychiatric symptoms were found.
Strikingly, all mean 123I-FP-CIT binding ratios were lower in the DLB/AD+ group than in the DLB/AD- group (Table 1). However, only the difference in the left amygdala reached statistical significance, after Bonferroni correction. The relatively small size of the DLB/AD+ group probably limited the study to detect other group differences. The overall lower BRs in the DLB/AD+ group, albeit not statistically significant, point in the direction of more extensive neurodegeneration, including the dopaminergic and serotonergic system, in mixed pathology, as has been shown by previous neuropathological and MRI research. (Howlett et al., 2015; Nedelska et al., 2015) Since we corrected for ROI volume, the low BRs in DLB/AD+ are unlikely to be caused only by atrophy. Our hypothesis that ‘pure DLB’ patients would have lower striatal binding than DLB/AD+ could not be confirmed.
Although compromised SERT binding in different brain regions (e.g., midbrain, thalamus and hypothalamus) has been shown in DLB, no consistent 123I-FP-CIT SERT binding-pattern has been demonstrated. (Joling et al., 2018; Roselli et al., 2010) In autopsy and animal studies, low concentrations of serotonin in amygdala and hippocampus, amongst other regions, have been shown in both DLB and AD. Degeneration of the raphe nucleus even seemed more pronounced in DLB- compared to in AD-patients with a depression. (Vermeiren et al., 2015) It is a possibility that DLB-pathology drives a major part of the serotonergic degeneration, but there may be a synergistic effect of the concomitant AD-pathology.
Serotonergic deficits may be associated with psychiatric symptoms in neurodegenerative disease. (Vermeiren et al., 2015) In this study, we could not demonstrate the association between these symptoms and 123I-FP-CIT SERT binding. Possible explanations can be the relatively low occurrence of neuropsychiatric symptoms in our sample and the small sample size.
This study provides grounds for future studies on the serotonergic system in DLB, and particularly in DLB/AD+, ideally in larger samples with higher prevalence of neuropsychiatric symptoms. A limitation of this study is that we used a non-selective radiotracer to study SERT, which hampers assessment of SERT binding in the striatum and cortex. Therefore, confirmation of our findings using a selective SERT tracer, such as 11C-3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile (11C-DASB), would be of interest. Insights in the role of serotonergic deficits could have implications for symptomatic therapy. Furthermore, although we corrected for atrophy, we cannot exclude that the trend for a lower 123I-FP-CIT binding in DLB/AD+ in the right hippocampus can be (partly) explained by partial volume effects. Finally, we did not assess parkinsonism with the widely used UPDRS motor score. However, extrapyramidal symptoms were assessed prospectively in a standardized manner in all patients.
In summary, this study provides a first exploration of the influence of mixed DLB and AD pathology on dopaminergic and serotonergic dysfunction, measured by 123I-FP-CIT SPECT. Our results advocate further research into the clinical significance of serotonergic deficits in DLB, and particularly in patients with concomitant AD-pathology.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Funding
This study was funded by ZonMW/Memorabel(grant number #733,050,509) and Stichting Dioraphte.
Declaration of Competing Interest
The authors declare that they have no conflicts of interest.
References
- Chang L.T. Method for attenuation correction in radionuclide computed tomography. IEEE Trans. Nucl. Sci. 1978;25:638–643. [Google Scholar]
- Del Sole A. Correlation between 123I-FP-CIT brain SPECT and parkinsonism in dementia with Lewy bodies: caveat for clinical use. Clin. Nucl. Med. 2015;40(1):32–35. doi: 10.1097/RLU.0000000000000602. [DOI] [PubMed] [Google Scholar]
- Duits F.H. The cerebrospinal fluid "Alzheimer profile": easily said, but what does it mean? Alzheimers Dement. 2014;10(6) doi: 10.1016/j.jalz.2013.12.023. 713–723 e2. [DOI] [PubMed] [Google Scholar]
- Howlett D.R. Regional multiple pathology scores are associated with cognitive decline in Lewy body dementias. Brain Pathol. 2015;25(4):401–408. doi: 10.1111/bpa.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joling M. Lower (123)I-FP-CIT binding to the striatal dopamine transporter, but not to the extrastriatal serotonin transporter, in Parkinson's disease compared with dementia with Lewy bodies. Neuroimage Clin. 2018;19:130–136. doi: 10.1016/j.nicl.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemstra A.W. Concomitant AD pathology affects clinical manifestation and survival in dementia with Lewy bodies. J. Neurol. Neurosurg. Psychiatry. 2016 doi: 10.1136/jnnp-2016-313775. [DOI] [PubMed] [Google Scholar]
- McKeith I.G. Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB consortium. Neurology. 2017;89(1):88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merdes A.R. Influence of alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60(10):1586–1590. doi: 10.1212/01.wnl.0000065889.42856.f2. [DOI] [PubMed] [Google Scholar]
- Nedelska Z. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiol. Aging. 2015;36(1):452–461. doi: 10.1016/j.neurobiolaging.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli F. Midbrain SERT in degenerative parkinsonisms: a 123I-FP-CIT SPECT study. Mov. Disord. 2010;25(12):1853–1859. doi: 10.1002/mds.23179. [DOI] [PubMed] [Google Scholar]
- Vermeiren Y. The monoaminergic footprint of depression and psychosis in dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Res. Ther. 2015;7(1):7. doi: 10.1186/s13195-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]