Highlights
-
•
Flavorless e-liquid aerosol is less toxic than cinnamon aerosols in human osteoblasts.
-
•
Cinnamon-flavored e-liquids and aerosols induce oxidative stress.
-
•
Osteoblast collagen type I protein is unaltered by e-liquid aerosol exposure.
Keywords: Cytotoxicity, Collagen type I, Oxidative stress, Electronic cigarette liquid, MG-63 osteoblast-like cells, Bone
Abstract
As noncombustible nicotine delivery devices, electronic cigarettes (e-cigarettes) are the most popular tobacco product among youth. The widespread popularity of e-cigarettes combined with possible health consequences suggest a need to further research health hazards associated with e-cigarette use. Since conventional tobacco use is a risk factor for osteoporosis, this study investigates the impact of nicotine-free, cinnamon-flavored e-cigarette liquid (e-liquid) on bone-forming osteoblasts compared to flavorless e-liquid. Human tumor-derived osteoblast-like MG-63 cells were exposed for 24 h or 48 h to 0.0.4 %, 0.04 %, 0.4 % or 1 % of unvaped e-liquid or 0.0025 %, 0.025 %, 0.25 %, 1 % or 2.5 % of aerosol condensate in addition to a culture medium only control. Changes in cell viability were assessed by MTT assay, and the expression of a key bone protein, collagen type I, was analyzed by immunofluorescence. Production of reactive oxygen species (ROS) was detected by fluorometry to assess oxidative stress. Cell viability decreased in a dose-dependent manner, and ROS production increased, which was most pronounced with cinnamon-flavored e-liquids. There were no detectable changes in collagen type I protein following exposure to any of the aerosol condensates. This study demonstrates osteoblast-like cells are sensitive to both e-liquids and aerosol condensates and suggests the cytotoxicity of cinnamon-flavored e-liquids might be associated with oxidative stress rather than changes in collagen type I protein expression. This in vitro study provides insight into the potential impacts of e-cigarette use on bone cells.
1. Introduction
Since their debut on the American market in 2007, electronic cigarettes (e-cigarettes) have quickly gained worldwide popularity, becoming a multi-billion dollar industry (U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, 2016). E-cigarettes are noncombustible nicotine delivery devices that were first marketed as a smoking cessation tool and healthier alternative to conventional cigarettes [1]. An e-cigarette is composed of a battery that activates a heating element, which vaporizes a liquid in a chamber into an aerosol. The aerosol generated is then inhaled by the user in a process called vaping. The liquid in the e-cigarette, often called an e-liquid, is typically composed of varying concentrations of nicotine, flavoring agents and humectants such as propylene glycol (PG) and vegetable glycerin (VG).
The rapid rise in popularity of e-cigarettes among adult and youth smokers and never-smokers raises concerns as the effects of e-cigarette use on human health remain under investigated. In the US alone, it is estimated that 20.8 % of high school students and 4.9 % of middle school students were current e-cigarette users in 2018, which represents a 78 % increase in use among high school students and a 48 % increase among middle school students during the 2017–2018 period [2]. Studies suggest the rise in e-cigarette use among youth is linked to the availability of appealing flavors and the recent popularity of discreet e-cigarette models shaped like a USB flash drive [2,3]. While the adverse consequences of conventional cigarette use are well established, the potential health risks of e-cigarette use require further research.
Several reports associate e-cigarette use with respiratory, gastrointestinal and cardiovascular complications including pulmonary damage [4], relapse of ulcerative colitis [5] and disrupted endothelial function [6]. While many studies focus on the respiratory and cardiovascular systems, the impact of e-cigarette use on the skeletal system is still unknown. Adolescence is a critical period for skeletal development as more than half the skeleton is laid down during teenage years [7] with the peak bone mass being reached during the mid-thirties [8]. Therefore, young e-cigarette users are potentially impairing their bone health if vaping disrupts their bone development. Since the amount of bone acquired at the end of skeletal development is considered to be a significant factor of lifelong skeletal health [9], teenage e-cigarette users could be increasing their risk of developing osteoporosis later on. Osteoporosis is a disease characterized by fragile bones due to low bone mass and deteriorated bone structure [10]. As osteoporosis is the most common chronic metabolic bone disease and is becoming a global epidemic [10], it is necessary to determine risk factors that affect the prevalence of this disease.
Contrary to the belief that e-cigarette aerosol is safe, many studies report the presence of harmful chemicals other than nicotine. Some chemicals present in e-cigarette aerosol include carcinogens such as formaldehyde and acrolein [11], heavy metals like nickel and lead [1], and flavoring agents such as diacetyl which causes bronchiolitis obliterans [12]. With over 8000 different flavors on the market, the broad and rapid development of e-cigarette products outpaces scientific research. Despite being classified as “generally recognized as safe” for ingestion by the Flavor Extracts Manufacturers Association [1], the health effects of these flavoring agents when inhaled remain to be characterized. In particular, cinnamon-flavored e-liquids have gained special attention with in vitro exposure studies showing high cytotoxicity [[13], [14], [15], [16], [17]], increased inflammatory response [[18], [19], [20]] and impaired neutrophil phagocytotic function [18]. Additionally, cinnamaldehyde itself, the main chemical agent providing a cinnamon flavor, is highly cytotoxic in in vitro studies [[20], [21], [22]] and impairs bronchial epithelial cell ciliary motility [23]. One study found cinnamaldehyde in 20 out of 39 e-liquids tested with concentrations ranging from 1.7 × 10−5 to 1.1 M [21]. The high concentrations of flavoring agents found in e-liquids [21,22] and the adverse effects observed in these studies suggest the need to further investigate the effects of cinnamon-flavored e-liquids on human health, specifically in bone.
Conventional tobacco cigarette use is associated with the development of osteoporosis as it can disrupt calcium homeostasis and impair osteoblast function, thereby reducing bone mineral density [[24], [25], [26], [27], [28]]. First, smoking conventional tobacco cigarettes decreases intestinal calcium absorption and alters the blood concentration of adrenal cortical hormones that act as precursors of estrogen and testosterone [27]. Tobacco smoke also induces an increase in production of parathyroid hormone and cortisol or a reduction in vitamin D metabolism, indirectly affecting the bone remodeling process [24,28]. Finally, tobacco smoke can directly target osteoblasts, disrupting their proliferation, differentiation and matrix deposition [26]. Osteoblasts are bone-forming cells which are essential in the mineralization of bone and production of collagen [29] such as collagen type I, the major structural organic component of the extracellular matrix [30]. Our previous report shows that a mango-flavored e-liquid upregulates collagen type I protein expression, suggesting a disruption in osteoblast function [16]. This study further explores the impact of cinnamon-flavored e-liquids on collagen type I protein expression to determine how e-cigarette could affect bone health. Conventional tobacco cigarette is also known to induce oxidative stress both in in vitro and in vivo experiments, increasing production of reactive oxygen species (ROS) in ovarian cells [31], elevating intracellular oxidative stress in corneal epithelial cells [32], causing oxidative lipid damage in rats [33], and inducing cardiovascular mitochondrial oxidative stress in mouse models [34,35].
A possible mechanism behind the observed cytotoxicity of e-liquids and aerosols is the induction of oxidative stress, which results from an imbalance between the antioxidant defenses of cells and the production of ROS. E-liquids and flavoring agents increase the levels of ROS in cell-free systems [19,20], and the levels of ROS produced is linked to the flavoring chemicals present in the e-liquids [19,36]. Additionally, e-cigarette aerosols are shown to induce ROS production in bronchial cells, keratinocyte [37] and vascular endothelial cells [38]. Exposure to e-cigarette aerosol also decreases the antioxidant power of bronchial cells and keratinocyte [37] in addition to reducing the levels of glutathione, an important antioxidant protein, in oral keratinocytes [39]. In vivo exposure to e-cigarette aerosols induces a reduction in the ferric reducing antioxidant power in a rat lung model [40]. Furthermore, a recent vaping human study reports increased low-density lipoprotein oxidizability when comparing e-cigarette users with nonuser control participants [41]. Taken together, these studies propose a potential mechanism through which e-cigarette use could affect tissues, justifying the current research which examines the impact of in vitro exposure to cinnamon-flavored e-liquids and aerosols on oxidative stress in bone-forming osteoblasts.
In vitro studies report similar effects of unvaped and aerosolized e-liquids, showing the potential of unvaped e-liquid exposure as a first-pass screening method [42,17,43]. In our previous report, we used a variety of flavored unvaped e-liquids and identified cinnamon-flavored e-liquids as the most cytotoxic in osteoblasts-like cells [16]. In order to simulate more closely the experience of an e-cigarette user, this follow-up study includes aerosolized e-liquid exposure. Additionally, it is important to note that multiple studies demonstrate the effects of flavored e-liquids can be independent of the presence of nicotine [13,16,17,44,45]. This is further supported by studies showing that flavoring agents alone can disrupt cellular function [20,23,46]. These findings provide a compelling rationale for the present study which focuses on the effects of nicotine-free e-liquids and aerosols.
As conventional tobacco use is a well-known risk factor for osteoporosis, we hypothesize that e-cigarette use impairs bone function by targeting bone-forming osteoblasts. In this study, human osteoblast-like cells MG-63 were exposed to commercially available, nicotine-free e-liquids and aerosol condensates in order to assess their effects on cell viability, collagen type I protein expression and ROS production. Since our previous research indicates e-liquid cytotoxicity is flavor-dependent [16], this study focuses on flavorless and cinnamon-flavored e-liquids to develop our understanding of the possible health risks associated with e-cigarette use.
2. Materials and methods
2.1. Cell culture
The human osteosarcoma cell line MG-63 was purchased from American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained using established culture conditions [16,47]. Briefly, MG-63 cells were cultured in minimal Eagles medium (EMEM) supplemented with 10 % fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 2 mM l-glutamine, 100 IU/mL penicillin and 100 μg/mL streptomycin (Sigma–Aldrich, St. Louis, MO, USA). Cells were cultured at 37 °C in air containing 5 % CO2. For routine maintenance, medium was changed every 3–4 days and cells were subcultured weekly.
2.2. Sources of e-liquids
Three commercially available e-liquids were purchased online for this study from two different brands, namely Mister-E-liquid (https://www.mister-e-liquid.com/) and Vape Dudes (https://www.vapedudes.com). Each e-liquid was in a sealed bottle labeled by the manufacturer as containing 0 mg/mL nicotine. Two cinnamon-flavored e-liquids were chosen for this study, namely Cinn Candy from Vape Dudes and Napalm from Mister-E-liquid. One flavorless e-liquid was selected as a control, Clear from Mister-E-liquid. The PG/VG ratio for each e-liquid was 50/50. PG and VG are used in e-liquids as humectants to keep flavorings in suspension and facilitate vaporization when heated.
2.3. E-liquid aerosol condensate preparation
An aerosol condensate of each e-liquid was generated using a vacuum to pull vaped e-liquid aerosol into a three-necked round bottom flask placed in ice. The e-cigarette used was a tank-model SMOK alien 220 W kit set to a wattage of 60 W. A new atomizer was used for each aerosol condensate preparation. The e-cigarette was connected to the flask via a plastic tube that was cleaned after every 5 puffs to ensure proper flow of the aerosol. A total of 40 puffs was collected for each aerosol condensate, with each puff lasting 3 s separated by a 27-seconds waiting time. The flow rate was set at 1 L/min. E-liquid aerosol condensates were generated in a method similar to previous aerosolized exposure studies [18,48].
2.4. Cell treatment
Cells were plated at different densities according to the assay. After 24 h, the culture medium was changed, and treatment was initiated in the serum-free and phenol-red free Opti-MEM medium (Invitrogen, Carlsbad, CA, USA). Treatments were prepared by diluting unvaped e-liquid or e-liquid aerosol condensate in Opti-MEM serum-free medium. Cells were exposed for either 24 h or 48 h to 0.0.4 %, 0.04 %, 0.4 % or 1.0 % of unvaped, nicotine-free e-liquids or to 0.0025 %, 0.025 %, 0.25 %, 1.0 % or 2.5 % of nicotine-free aerosol condensate. Volumes for the treatment dilutions were chosen based on previously reported cell culture conditions for e-liquids and aerosol condensates [15,18,48]. All experiments included an additional control whereby cells were treated with Opti-MEM serum-free medium only.
2.5. Cell viability assay
MG-63 cells were plated at a density of 6 × 104 cells/well in a 96-well plate. After 48 -h treatment, cells were washed with phosphate buffered saline (PBS) and incubated at 37 °C with 10 μg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium-bromide (MTT; ATCC, Manassas, VA, USA) for 4 h. The conversion of tetrazolium salt MTT to a purple-colored formazan by mitochondrial dehydrogenase was used to assess cell viability. After the supernatant was removed, 100 μL of dimethyl sulfoxide were added to each well and absorbance was read at 570 nm.
2.6. Immunofluorescence for collagen type I protein
MG-63 cells were plated at 7 × 104 cells/well in poly-d-lysine/laminin 8-well culture chamber slides (BD BioSciences, Bedford, MA, USA). After treatment, cells were washed with EMEM serum-free medium, fixed with 3.7 % formaldehyde, rinsed with PBS and permeabilized with cold methanol before being blocked for 1 h with 2 % bovine serum albumin + 0.1 % Triton X in PBS. Cells were incubated with a primary antibody to collagen type I (AbCam, Cambridge, MA, USA) for 90 min, washed twice with 0.1 % Triton X in PBS, and then incubated for 1 h with a secondary antibody conjugated to Alexa Fluor 488. Cells were then washed three times with 0.1 % Triton X in PBS. All incubations were performed at 37 °C. Collagen type I was visualized at 20X using a Nikon Epifluorescence microscope, and digital images were captured using ImagePro software by media Cybergenetics (Silver Spring, MD, USA) using a common exposure time and filter setting for all images.
2.7. Reactive oxygen species (ROS) assay
Cells were plated at a density of 6 × 104 cells/well in a 96-well plate. After 24 -h treatment, cells were washed with PBS and incubated at 37 °C with 5 μM Cell ROX™Green reagent (Invitrogen, Carlbad, CA, USA) for 30 min. Cell ROX™Green reagent is a dye which binds to DNA upon oxidation. Cells were washed once with PBS and then covered with clean PBS before measuring fluorescence using a BioTek Synergy HT fluorometer. Fluorescence intensity was measured at an excitation/emission of 485/528 and a gain of 120. Results were normalized to total number of cells per well.
2.8. Statistical analysis
For all experiments, the mean ± SEM values represent at least three independent experiments. MTT data and ROS data were analyzed using a one-way analysis of variance followed by a Student-Newman-Keuls for pairwise comparisons. EC50 value for each aerosol condensate was calculated with a linear model of the compiled cell viability data. Collagen type I immunofluorescence staining was quantified using Image J software (National Institute of Health, Bethesda, MD, USA), and the amount of staining was expressed as percentage of the total area of the captured image. Immunofluorescence results were analyzed using a t-test. P < 0.05 was considered statistically significant. Groups with different letters are significantly different from each other. All statistical analyses were performed using the software program SigmaPlot 13.0.
3. Results
3.1. Cinnamon-flavored aerosol condensate induces greater cytotoxicity than flavorless aerosol condensate
Our previous study showed that the degree of cytotoxicity of unvaped e-liquids is flavor-dependent [16]. Specifically, cinnamon-flavored e-liquids were found to be the most cytotoxic e-liquids and flavorless e-liquids were the least cytotoxic among a series of 23 e-liquids tested [16]. Two cinnamon-flavored and one flavorless e-liquids from two different brands were selected for further cytotoxicity screening using vaped e-liquids. Each experiment included a culture medium only control.
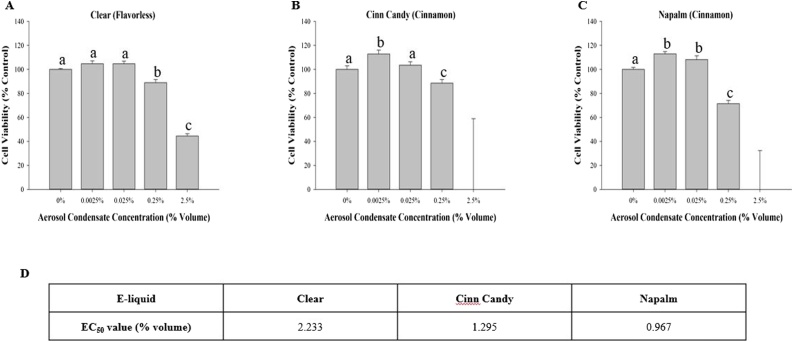
Dose-response MTT experiments using MG-63 cells exposed to selected flavorless and cinnamon-flavored aerosol condensates are shown in Fig. 1. A decrease in cell viability was detected after a 48 -h exposure to all aerosol condensates tested compared to culture medium only. It is important to note that the effect was more pronounced with both cinnamon-flavored aerosol condensates (Fig. 1B and C) compared to the flavorless aerosol condensate (Fig. 1A). Based on the EC50 values, Napalm appeared to be more cytotoxic than Cinn Candy with a value of 0.967 compared to 1.295, respectively (Fig. 1D). Both Cinn Candy and Napalm aerosol condensates were overly cytotoxic at 2.5 % volume, contributing to high data variability.
Fig. 1.
Effect of e-liquid aerosol condensates on cell viability. MG-63 cells were exposed for 48 h to 0.0025 %, 0.025 %, 0.25 % or 2.5 % of nicotine-free aerosol condensate. Cell viability was determined using the MTT assay at an absorbance of 570 nm. Results are expressed as percentage cell viability. Each bar represents the mean ± SEM of at least three independent experiments. Groups with different letters are significantly different from each other (p < 0.05). The EC50 values are expressed as a percentage volume of the aerosol condensate, which was calculated using a linear model of the compiled cell viability data.
3.2. Collagen type I protein expression remains unchanged upon exposure to aerosol condensate
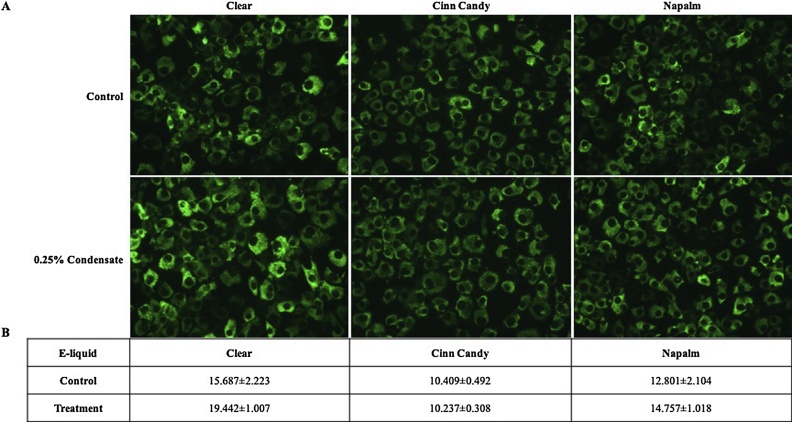
Next, we explored whether exposure to aerosol condensate alters the expression of collagen type I protein. Cells were treated for 48 h with a non-overly cytotoxic concentration of aerosol condensate and analyzed for cytosolic collagen type I expression with immunofluorescence. As shown in Fig. 2A, treatment with 0.25 % aerosol condensate resulted in no observable change in collagen type I protein for any of the aerosol condensates tested. Once the amount of staining was quantified, there was no statistically significant difference between the control and treatment (Fig. 2B).
Fig. 2.
Immunofluorescence detection of cytosolic collagen type I protein. MG-63 cells were exposed for 48 h to 0.25 % of nicotine-free aerosol condensate. Images were acquired at 20X magnification, then analyzed and quantified as the percentage area of the image within the intensity threshold using Image J software. Values presented are the mean ± SEM of at least three independent experiments.
3.3. Unvaped cinnamon-flavored e-liquids induce ROS production
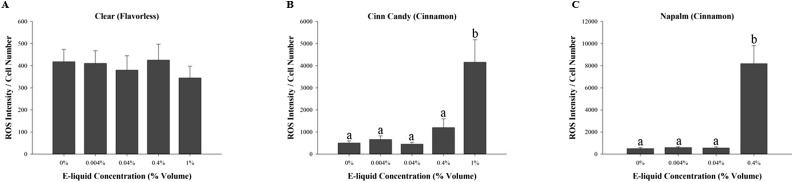
Since the e-liquid aerosol condensates did not induce a change in collagen type I protein, we explored whether the e-liquids and aerosol condensates induce oxidative stress through the production of ROS. First, we exposed the cells to unvaped e-liquids as was previously done for cytotoxicity and collagen type I immunofluorescence experiments [16]. Exposure to unvaped cinnamon-flavored e-liquids for 24 h significantly increased ROS production at concentration of 0.4 % volume for Napalm (Fig. 3C) and 1.0 % volume for Cinn Candy (Fig. 3B). The Napalm aerosol condensate was only tested up to a concentration of 0.4 % volume as the 1.0 % treatment was too cytotoxic. It is interesting to note that Napalm appears to have stronger effect on MG-63 cells than Cinn Candy because a lower concentration of e-liquids was required to induce a significant increase in ROS production. Cells exposed to the flavorless e-liquid Clear (Fig. 3A) did not show a significant change in ROS production at any concentration tested.
Fig. 3.
Effect of unvaped e-liquids on reactive oxygen species production. MG-63 cells were exposed for 24 h to 0.0.4 %, 0.04 %, 0.4 % or 1.0 % of unvaped, nicotine-free e-liquid. Results are expressed as fluorescence intensity per cell number. Each bar represents the mean ± SEM of at least six independent experiments. Groups with different letters are significantly different from each other (p < 0.05).
3.4. Cinnamon-flavored aerosol condensates induce ROS production
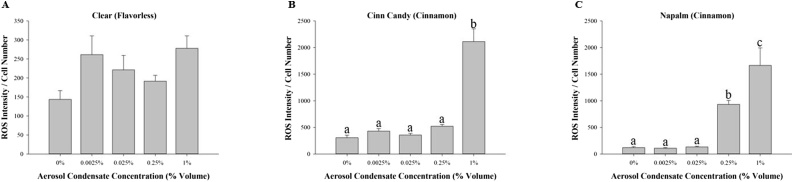
Since the cinnamon-flavored e-liquids appear to induce an increase in ROS production, we explored whether exposure to aerosol condensates would produce a similar response profile. Exposure to cinnamon-flavored aerosol condensates for 24 h significantly increased the production of ROS at concentration of 0.25 % volume for Napalm (Fig. 4C) and 1.0 % volume for Cinn Candy (Fig. 4B). Once again, MG-63 cells appear to be more sensitive to Napalm aerosol condensate compared to Cinn Candy based on the aerosol condensate concentration required to induce a significant increase in ROS production. Cells exposed to the flavorless e-liquid Clear (Fig. 4A) did not show a significant change in ROS production. Interestingly, based on the ROS intensity/cell number, the increase in ROS was much more pronounced for unvaped cinnamon-flavored e-liquids (Fig. 3B and C) in comparison to cinnamon-flavored aerosol condensates (Fig. 4B and C).
Fig. 4.
The effect of e-liquid aerosol condensates on reactive oxygen species production. MG-63 cells were exposed for 24 h to 0.0025 %, 0.025 %, 0.25 % or 1.0 % of nicotine-free aerosol condensate. Results are expressed as fluorescence intensity per cell number. Each bar represents the mean ± SEM of at least six independent experiments. Groups with different letters are significantly different from each other (p < 0.05).
Collectively, these results suggest the higher cytotoxicity observed with cinnamon-flavored e-liquids might be associated with higher ROS production rather than to changes in the expression of collagen type I protein
4. Discussion
The rapid rise in popularity of e-cigarettes calls for further characterization of their impact on human health. Indeed, e-cigarettes are now the most commonly used tobacco products among youth [49]. This widespread popularity raises many concerns as the risks associated with e-cigarette use have not been well defined, especially long-term. While the effects of conventional tobacco products on the skeletal system have been extensively described, the effects of e-cigarettes on bone health remain unknown. Conventional tobacco cigarette use can disrupt the dynamic bone remodeling process, resulting in loss of bone mass and increased risk of osteoporosis [[24], [25], [26], [27], [28]]. The detrimental impact of conventional tobacco products on bone function stresses the need for a better understanding of the effects of e-cigarettes on the skeletal system. Previous in vitro studies show that the effects of e-cigarettes can occur independently of nicotine [13,16,17,44]. However, the effects appear to be dependent on the flavorings [13,15,16,42], with cinnamon-flavored e-liquids being the most cytotoxic [16,[20], [21], [22]]. Hence, the present study investigates the effects of nicotine-free, cinnamon-flavored e-liquids and aerosols on human tumor-derived osteoblast-like MG-63 cells by evaluating cytotoxicity, changes in collagen type I protein and ROS production.
A study involving 185 e-cigarette users reports that the median number of puffs per day is 132, with most users taking puffs in series [50]. On average, the puff lasts about 3.20 s with 13 s interval. In another study, Hua et al. [51] report an average puff lasting 4.3 s with the range of duration being from 1.9 to 8.3 s. The wide variation among e-cigarette users makes the development of standard exposure systems difficult, including the use of VITROCELL smoke exposure system modified for e-cigarette aerosol exposure [[52], [53], [54]]. This current study generates aerosol condensate from 40 puffs of 3 s with 27 s interval, which falls within the range of puff duration observed [51] and the method used by previous aerosolized exposure studies [15,18,42,46,48]. We observe a decrease in cell viability upon exposure to flavorless and cinnamon-flavored aerosol condensate. However, the effect is much more pronounced with cinnamon-flavored e-liquids, corroborating the results of previous in vitro studies that used human pulmonary fibroblasts, embryonic stem cells, lung epithelial cells and monocytes [13,14,20,22]. We reported a similar cytotoxicity profile in our previous study using unvaped e-liquids [16].
Cinnamaldehyde has been identified as the most potent chemical in cinnamon-flavored e-liquids
[14], and the levels of cinnamaldehyde used vary greatly between e-liquids, sometimes reaching very high concentrations [21,22]. Interestingly, cinnamaldehyde is detected in a wide variety of e-liquids other than cinnamon-flavored e-liquids such as cherry-flavored, espresso-flavored and caramel-flavored e-liquids [21]. Studies using cinnamaldehyde itself show that this flavoring agent is cytotoxic [21,42,20,22,43] and can induce proinflammatory responses and oxidative stress in human lung epithelial cells, fibroblasts and monocytotic cell lines [20,46]. Cinnamaldehyde also has immunosuppressive effects on respiratory innate immune cells [18]. Interestingly, our study reveals differences between brands, with the Napalm e-liquid from Mister-E-Liquids causing greater cytotoxicity than Cinn Candy from Vape Dudes. Preliminary studies suggest this might be due to cinnamaldehyde. Indeed, using quantitative ATR FTIR spectroscopy to measure the cinnamaldehyde concentrations in our unvaped e-liquid we observe higher cinnamaldehyde levels in Napalm compared to Cinn Candy (unreported data). The broad range of flavorings concentrations in e-liquids and brand-specific toxicological effects suggest a need for better regulation and limitation in the use of flavoring agents in the production of e-liquids.
Collagen type I is critical for a healthy microarchitecture of the skeletal system as this protein composes the main organic compounds in bones. The integrity of bone collagen is essential for the strength of bones and their resistance against fracture [55], which suggests that disrupting the collagen-depositing function of osteoblasts could increase the risk of osteoporosis. Our study reports no significant changes in the cytosolic levels of collagen type I protein when exposed to aerosol condensate compared to untreated cells. This suggests that collagen type I production is not a target for the cytotoxicity of the aerosol condensates tested in this study. Nonetheless, our previous study shows that unvaped mango-flavored e-liquid induces an increase in the expression collagen type I protein [16]. The differential responses might be due to different exposure methods and flavor as this study uses aerosolized cinnamon-flavored e-liquids instead of unvaped fruity-flavored e-liquids. Interestingly, a recent study reports impairment of normal mesenchymal stem cell (MSC) differentiation to osteoblasts upon exposure to strawberry-flavored aerosolized e-liquid in part through the inhibition of collagen type I mRNA [56]. The variability in collagen type I mRNA or protein expression in these studies points to the need for further research on potential flavor-dependent alteration in this important osteoblast protein.
In addition to impaired MSC differentiation, Shaito et al. [56] report high levels of ROS production following e-cigarette extract exposure and suggest oxidative stress is involved in the inhibition of osteogenic differentiation. A recent study also reports an increase in free radical production from cinnamon-flavored e-liquid aerosols [36]. Similarly, this study demonstrates the potential of cinnamon-flavored e-liquids and aerosols to induce oxidative stress. Indeed, both unvaped and aerosolized cinnamon-flavored e-liquids increase the production of ROS, whereas flavorless e-liquids did not significantly alter the levels of ROS. These results suggest a mechanism for the cytotoxicity of cinnamon-flavored e-liquids through the induction of oxidative stress. It is worth noting that the levels of ROS were much higher following exposure to unvaped e-liquids compared to the aerosol condensates, potentially indicating a higher concentration of active chemicals in unvaped e-liquids. Preliminary studies are currently analyzing the levels of cinnamaldehyde in unvaped e-liquids and in aerosol condensates to establish a potential relationship between the levels of cinnamaldehyde, cytotoxicity and ROS production associated with cinnamon-flavored e-liquids. Together, the study by Shaito et al. [56] and this present study propose a potential mechanism through which e-cigarette use could impair the function of osteoblast by inducing oxidative stress through increased production of ROS.
We conclude that the elevated cytotoxicity of cinnamon-flavored e-liquids in human osteoblast-like cells is potentially linked to the production of reactive oxygen species rather than to changes in collagen type I protein expression. This research is one of the first in vitro studies to demonstrate the potential adverse consequences of e-cigarette use on bone cells.
Authors statement
Conception or design of the work: Sara Heggland and Florence Wavreil contributed equally
Supervised the research: Sara Heggland
Funding Acquisition: Sara Heggland
Project Administration: Sara Heggland
Data collection: Florence Wavreil
Data analysis and interpretation: Florence Wavreil and Sara Heggland contributed equally
Drafting the article: Florence Wavreil
Critical revision of the article: Sara Heggland
Final approval of the version to be published: Sara Heggland and Florence Wavreil
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This publication was made possible by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under Grant #P20GM103408.
References
- 1.U.S. Department of Health and Human Services. Centers for Disease Control and Prevention, N. C. f. C. D. P. a. H. P., Office on Smoking and Health . U.S. Department of Health and Human Services; Atlanta, GA: 2016. E-Cigarette Use Among Youth and Young Adults: A Report of the Surgeon General—Executive Summary. [Google Scholar]
- 2.Cullen K.A., Ambrose B.K., Gentzke A.S., Apelberg B.J., Jamal A., King B.A. Notes from the field: use of electronic cigarettes and any tobacco product among middle and high school students - United States, 2011-2018. MMWR Morb. Mortal. Wkly. Rep. 2018;67(45):1276–1277. doi: 10.15585/mmwr.mm6745a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai J., Walton K., Coleman B.N., Sharapova S.R., Johnson S.E., Kennedy S.M., Caraballo R.S. Reasons for electronic cigarette use among middle and high school students - national youth tobacco survey, United States, 2016. MMWR Morb. Mortal. Wkly. Rep. 2018;67(6):196–200. doi: 10.15585/mmwr.mm6706a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thirion-Romero I., Perez-Padilla R., Zabert G., Barrientos-Gutierrez I. Respiratory impact of electronic cigarettes and “low-risk” tobacco. Rev. Invest. Clin. 2019;71(1):17–27. doi: 10.24875/RIC.18002616. [DOI] [PubMed] [Google Scholar]
- 5.Hua M., Talbot P. Potential health effects of electronic cigarettes: a systematic review of case reports. Prev. Med. Rep. 2016;4:169–178. doi: 10.1016/j.pmedr.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Skotsimara G., Antonopoulos A.S., Oikonomou E., Siasos G., Ioakeimidis N., Tsalamandris S. Cardiovascular effects of electronic cigarettes: a systematic review and meta-analysis. Eur. J. Prev. Cardiol. 2019 doi: 10.1177/2047487319832975. [DOI] [PubMed] [Google Scholar]
- 7.Gordon C.M., Zemel B.S., Wren T.A., Leonard M.B., Bachrach L.K., Rauch F. The determinants of peak bone mass. J. Pediatr. 2017;180:261–269. doi: 10.1016/j.jpeds.2016.09.056. [DOI] [PubMed] [Google Scholar]
- 8.Bonjour J.P., Theintz G., Law F., Slosman D., Rizzoli R. Peak bone mass. Osteoporos. Int. 1994;4(Suppl 1):7–13. doi: 10.1007/BF01623429. [DOI] [PubMed] [Google Scholar]
- 9.Zemel B. Bone mineral accretion and its relationship to growth, sexual maturation and body composition during childhood and adolescence. World Rev. Nutr. Diet. 2013;106:39–45. doi: 10.1159/000342601. [DOI] [PubMed] [Google Scholar]
- 10.Sozen T., Ozisik L., Basaran N.C. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017;4(1):46–56. doi: 10.5152/eurjrheum.2016.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goniewicz M.L., Knysak J., Gawron M., Kosmider L., Sobczak A., Kurek J. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob. Control. 2014;23(2):133–139. doi: 10.1136/tobaccocontrol-2012-050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen J.G., Flanigan S.S., LeBlanc M., Vallarino J., MacNaughton P., Stewart J.H., Christiani D.C. Flavoring chemicals in e-cigarettes: diacetyl, 2,3-pentanedione, and acetoin in a sample of 51 products, including fruit-, candy-, and cocktail-flavored e-cigarettes. Environ. Health Perspect. 2016;124(6):733–739. doi: 10.1289/ehp.1510185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bahl V., Lin S., Xu N., Davis B., Wang Y.H., Talbot P. Comparison of electronic cigarette refill fluid cytotoxicity using embryonic and adult models. Reprod. Toxicol. 2012;34(4):529–537. doi: 10.1016/j.reprotox.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Behar R.Z., Davis B., Wang Y., Bahl V., Lin S., Talbot P. Identification of toxicants in cinnamon-flavored electronic cigarette refill fluids. Toxicol. In Vitro. 2014;28(2):198–208. doi: 10.1016/j.tiv.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Farsalinos K.E., Romagna G., Allifranchini E., Ripamonti E., Bocchietto E., Todeschi S. Comparison of the cytotoxic potential of cigarette smoke and electronic cigarette vapour extract on cultured myocardial cells. Int. J. Environ. Res. Public Health. 2013;10(10):5146–5162. doi: 10.3390/ijerph10105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Otero C.E., Noeker J.A., Brown M.M., Wavreil F.D.M., Harvey W.A., Mitchell K.A., Heggland S.J. Electronic cigarette liquid exposure induces flavor-dependent osteotoxicity and increases expression of a key bone marker, collagen type I. J. Appl. Toxicol. 2019;39(6):888–898. doi: 10.1002/jat.3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowell T.R., Reeber S.L., Lee S.L., Harris R.A., Nethery R.C., Herring A.H. Flavored e-cigarette liquids reduce proliferation and viability in the CALU3 airway epithelial cell line. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313(1):L52–l66. doi: 10.1152/ajplung.00392.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clapp P.W., Pawlak E.A., Lackey J.T., Keating J.E., Reeber S.L., Glish G.L., Jaspers I. Flavored e-cigarette liquids and cinnamaldehyde impair respiratory innate immune cell function. Am. J. Physiol. Lung Cell Mol. Physiol. 2017;313(2):L278–L292. doi: 10.1152/ajplung.00452.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lerner C.A., Sundar I.K., Yao H., Gerloff J., Ossip D.J., McIntosh S. Vapors produced by electronic cigarettes and e-juices with flavorings induce toxicity, oxidative stress, and inflammatory response in lung epithelial cells and in mouse lung. PLoS One. 2015;10(2) doi: 10.1371/journal.pone.0116732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muthumalage T., Prinz M., Ansah K.O., Gerloff J., Sundar I.K., Rahman I. Inflammatory and oxidative responses induced by exposure to commonly used e-cigarette flavoring chemicals and flavored e-liquids without nicotine. Front. Physiol. 2017;8:1130. doi: 10.3389/fphys.2017.01130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Behar R.Z., Luo W., Lin S.C., Wang Y., Valle J., Pankow J.F., Talbot P. Distribution, quantification and toxicity of cinnamaldehyde in electronic cigarette refill fluids and aerosols. Tob. Control. 2016;25(Suppl 2):ii94–ii102. doi: 10.1136/tobaccocontrol-2016-053224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omaiye E.E., McWhirter K.J., Luo W., Tierney P.A., Pankow J.F., Talbot P. High concentrations of flavor chemicals are present in electronic cigarette refill fluids. Sci. Rep. 2019;9(1):2468. doi: 10.1038/s41598-019-39550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clapp P.W., Lavrich K.S., van Heusden C.A., Lazarowski E.R., Carson J.L., Jaspers I. Cinnamaldehyde in flavored e-cigarette liquids temporarily suppresses bronchial epithelial cell ciliary motility by dysregulation of mitochondrial function. Am. J. Physiol. Lung Cell Mol. Physiol. 2019;316(3):L470–L486. doi: 10.1152/ajplung.00304.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abate M., Vanni D., Pantalone A., Salini V. Cigarette smoking and musculoskeletal disorders. Muscles Ligaments Tendons J. 2013;3(2):63–69. doi: 10.11138/mltj/2013.3.2.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanis J.A., Johnell O., Oden A., Johansson H., De Laet C., Eisman J.A. Smoking and fracture risk: a meta-analysis. Osteoporos. Int. 2005;16(2):155–162. doi: 10.1007/s00198-004-1640-3. [DOI] [PubMed] [Google Scholar]
- 26.Ko C.H., Chan R.L., Siu W.S., Shum W.T., Leung P.C., Zhang L., Cho C.H. Deteriorating effect on bone metabolism and microstructure by passive cigarette smoking through dual actions on osteoblast and osteoclast. Calcif. Tissue Int. 2015;96(5):389–400. doi: 10.1007/s00223-015-9966-8. [DOI] [PubMed] [Google Scholar]
- 27.Krall E.A., Dawson-Hughes B. Smoking increases bone loss and decreases intestinal calcium absorption. J. Bone Miner. Res. 1999;14(2):215–220. doi: 10.1359/jbmr.1999.14.2.215. [DOI] [PubMed] [Google Scholar]
- 28.Yoon V., Maalouf N.M., Sakhaee K. The effects of smoking on bone metabolism. Osteoporos. Int. 2012;23(8):2081–2092. doi: 10.1007/s00198-012-1940-y. [DOI] [PubMed] [Google Scholar]
- 29.Aubin J.E. Advances in the osteoblast lineage. Biochem. Cell Biol. 1998;76(6):899–910. [PubMed] [Google Scholar]
- 30.Shi S., Kirk M., Kahn A.J. The role of type I collagen in the regulation of the osteoblast phenotype. J. Bone Miner. Res. 1996;11(8):1139–1145. doi: 10.1002/jbmr.5650110813. [DOI] [PubMed] [Google Scholar]
- 31.Kim C.W., Go R.E., Hwang K.A., Bae O.N., Lee K., Choi K.C. Effects of cigarette smoke extracts on apoptosis and oxidative stress in two models of ovarian cancer in vitro. Toxicol. In Vitro. 2018;52:161–169. doi: 10.1016/j.tiv.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 32.Miao Q., Xu Y., Zhang H., Xu P., Ye J. Cigarette smoke induces ROS mediated autophagy impairment in human corneal epithelial cells. Environ. Pollut. 2019;245:389–397. doi: 10.1016/j.envpol.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 33.Lourenco M.A.M., Braz M.G., Aun A.G., Pereira B.L.B., Fernandes F.H., Kazmarek E.M. Lipid damage is the best marker of oxidative injury during the cardiac remodeling process induced by tobacco smoke. BMC Pharmacol. Toxicol. 2018;19(1):74. doi: 10.1186/s40360-018-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dikalov S., Itani H., Richmond B., Vergeade A., Rahman S.M.J., Boutaud O. Tobacco smoking induces cardiovascular mitochondrial oxidative stress, promotes endothelial dysfunction, and enhances hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019;316(3):H639–h646. doi: 10.1152/ajpheart.00595.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Engle M.L., Monk J.N., Jania C.M., Martin J.R., Gomez J.C., Dang H. Dynamic changes in lung responses after single and repeated exposures to cigarette smoke in mice. PLoS One. 2019;14(2) doi: 10.1371/journal.pone.0212866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bitzer Z.T., Goel R., Reilly S.M., Elias R.J., Silakov A., Foulds J. Effect of flavoring chemicals on free radical formation in electronic cigarette aerosols. Free Radic. Biol. Med. 2018;120:72–79. doi: 10.1016/j.freeradbiomed.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganapathy V., Manyanga J., Brame L., McGuire D., Sadhasivam B., Floyd E. Electronic cigarette aerosols suppress cellular antioxidant defenses and induce significant oxidative DNA damage. PLoS One. 2017;12(5) doi: 10.1371/journal.pone.0177780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson C., Majeste A., Hanus J., Wang S. E-cigarette aerosol exposure induces reactive oxygen species, DNA damage, and cell death in vascular endothelial cells. Toxicol. Sci. 2016;154(2):332–340. doi: 10.1093/toxsci/kfw166. [DOI] [PubMed] [Google Scholar]
- 39.Ji E.H., Sun B., Zhao T., Shu S., Chang C.H., Messadi D. Characterization of electronic cigarette aerosol and its induction of oxidative stress response in oral keratinocytes. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canistro D., Vivarelli F., Cirillo S., Babot Marquillas C., Buschini A., Lazzaretti M. E-cigarettes induce toxicological effects that can raise the cancer risk. Sci. Rep. 2017;7(1):2028. doi: 10.1038/s41598-017-02317-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moheimani R.S., Bhetraratana M., Yin F., Peters K.M., Gornbein J., Araujo J.A., Middlekauff H.R. Increased cardiac sympathetic activity and oxidative stress in habitual electronic cigarette users: implications for cardiovascular risk. JAMA Cardiol. 2017;2(3):278–284. doi: 10.1001/jamacardio.2016.5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behar R.Z., Luo W., McWhirter K.J., Pankow J.F., Talbot P. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci. Rep. 2018;8(1):8288. doi: 10.1038/s41598-018-25575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behar R.Z., Wang Y., Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob. Control. 2018;27(3):325–333. doi: 10.1136/tobaccocontrol-2016-053472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaur G., Muthumalage T., Rahman I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett. 2018;288:143–155. doi: 10.1016/j.toxlet.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leslie L.J., Vasanthi Bathrinarayanan P., Jackson P., Mabiala Ma Muanda J.A., Pallett R., Stillman C.J.P., Marshall L.J. A comparative study of electronic cigarette vapor extracts on airway-related cell lines in vitro. Inhal. Toxicol. 2017;29(3):126–136. doi: 10.1080/08958378.2017.1318193. [DOI] [PubMed] [Google Scholar]
- 46.Gerloff J., Sundar I.K., Freter R., Sekera E.R., Friedman A.E., Robinson R. Inflammatory response and barrier dysfunction by different e-cigarette flavoring chemicals identified by gas chromatography-mass spectrometry in e-liquids and e-vapors on human lung epithelial cells and fibroblasts. Appl. In Vitro Toxicol. 2017;3(1):28–40. doi: 10.1089/aivt.2016.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ha T.T., Burwell S.T., Goodwin M.L., Noeker J.A., Heggland S.J. Pleiotropic roles of Ca(+2)/calmodulin-dependent pathways in regulating cadmium-induced toxicity in human osteoblast-like cell lines. Toxicol. Lett. 2016;260:18–27. doi: 10.1016/j.toxlet.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olmedo P., Navas-Acien A., Hess C., Jarmul S., Rule A. A direct method for e-cigarette aerosol sample collection. Environ. Res. 2016;149:151–156. doi: 10.1016/j.envres.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jamal A., Gentzke A., Hu S.S., Cullen K.A., Apelberg B.J., Homa D.M., King B.A. Tobacco use among middle and high school students - United States, 2011-2016. MMWR Morb. Mortal. Wkly. Rep. 2017;66(23):597–603. doi: 10.15585/mmwr.mm6623a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dautzenberg B., Bricard D. Real-time characterization of e-cigarettes use: the 1 million puffs study. J. Addict. Res. Ther. 2015;6(2) [Google Scholar]
- 51.Hua M., Yip H., Talbot P. Mining data on usage of electronic nicotine delivery systems (ENDS) from YouTube videos. Tob. Control. 2013;22(2):103–106. doi: 10.1136/tobaccocontrol-2011-050226. [DOI] [PubMed] [Google Scholar]
- 52.Bishop E., Haswell L., Adamson J., Costigan S., Thorne D., Gaca M. An approach to testing undiluted e-cigarette aerosol in vitro using 3D reconstituted human airway epithelium. Toxicol. Vitr. 2019 doi: 10.1016/j.tiv.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Fowler K., Fields W., Hargreaves V., Reeve L., Bombick B. Development, qualification, validation and application of the Ames test using a VITROCELL((R)) VC10((R)) smoke exposure system. Toxicol. Rep. 2018;5:542–551. doi: 10.1016/j.toxrep.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Keyser B.M., Leverette R., Hollings M., Seymour A., Reeve L., Fields W. Investigation of multiple whole smoke dosimetry techniques using a vitrocell® VC10® smoke exposure system. Toxicol. Rep. 2019 doi: 10.1016/j.toxrep.2019.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willett T.L., Dapaah D.Y., Uppuganti S., Granke M., Nyman J.S. Bone collagen network integrity and transverse fracture toughness of human cortical bone. Bone. 2019;120:187–193. doi: 10.1016/j.bone.2018.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shaito A., Saliba J., Husari A., El-Harakeh M., Chhouri H., Hashem Y. Electronic cigarette smoke impairs normal mesenchymal stem cell differentiation. Sci. Rep. 2017;7(1):14281. doi: 10.1038/s41598-017-14634-z. [DOI] [PMC free article] [PubMed] [Google Scholar]