Abstract
A rise in new HIV diagnoses among older adults is characterized by poor prognosis and reduced survival times. Although heterosexual transmission remains the main route of infection in women, little is known regarding immune functions in the genital tract of postmenopausal women, especially those who are HIV positive. Furthermore, effects of hormone replacement therapy (HRT) on the genital tract immune system are unclear. Using the Women's Interagency HIV Study repository, we obtained cervical–vaginal lavage (CVL) samples from premenopausal and postmenopausal HIV-positive and HIV-negative women, some of whom were on HRT. Samples were assayed for interleukin (IL)-6, IL-8, tumor necrosis factor (TNF)-α, secretory leukocyte protease inhibitor (SLPI), Elafin, human beta defensin-2 (HBD2), and macrophage inflammatory protein (MIP)-3α using ELISA. Anti-HIV activity in CVL was measured using TZM-bl indicator cells. Among HIV-positive women, the plasma viral load was significantly higher and CD4 count was significantly lower in postmenopausal compared with premenopausal women. Postmenopausal women, irrespective of HIV status, had significantly lower levels of HBD2 compared with premenopausal women. Among the HIV-negative individuals, postmenopausal women had significantly lower levels of MIP-3α, IL-6, and SLPI compared with premenopausal women. In contrast, HIV-positive postmenopausal women had significantly higher levels of TNF-α compared with HIV-positive premenopausal women. In most cases, HRT groups resembled the postmenopausal groups. No significant differences in anti-HIV activity by menopausal or by HIV status were noted. Our findings indicate that the female genital tract immune microenvironment is distinct by menopausal status and HIV status. Further studies are needed to assess the risk of HIV acquisition/transmission in this population.
Keywords: cervical-vaginal lavage, female genital tract, HIV, hormone replacement therapy, menopause, soluble immune mediators
Introduction
Rates of new HIV diagnoses in the >50 age group are on the rise, characterized by poorer prognosis and shorter survival times compared with younger age groups.1 Although many older adults remain sexually active, condom use and testing for sexually transmitted infections are significantly lower in this population.2–5 Additionally, the number of sexually active, HIV-positive older adults is increasing in developed nations due to the success of highly active antiretroviral therapy (HAART).2,6
The HIV/AIDS epidemic affects women disproportionately.7 As HIV/AIDS is often perceived as a condition impacting reproductive-age women, most epidemiological studies do not focus on older women. Therefore, little is known regarding the immunological mechanisms of HIV susceptibility in postmenopausal women and even less so in the HIV-positive population.
It is known that aging is associated with a gradual weakening of immune responses, along with a systemic increase in inflammation (reviewed8–10). In postmenopausal women, chronic systemic inflammation, characterized by higher levels of proinflammatory cytokines and reduced ability to respond to pathogenic stimuli, has been described.11–13 Less is known regarding immune functions in the female genital tract (FGT), which are critical to consider to prevent sexual acquisition/transmission of HIV. Ex vivo infection studies using cervical hysterectomy or biopsy samples have reported greater inflammation/immune activation,14,15 along with higher production of HIV p24 upon infection.14,16 We and others have previously shown reduced levels of soluble immune mediators in genital secretions among postmenopausal women compared with premenopausal women.16–18 As many of these immune mediators are reported to be regulated by estradiol,19,20 reduction following menopause is not surprising. However, it is less clear as to what extent this reduction affects overall functional immunity in the FGT.
Little is known regarding the immune microenvironment of postmenopausal women who are HIV positive. We have previously demonstrated increased inflammatory response, decreased levels of endogenous antimicrobials, and decreased functional anti-HIV activity in the FGT of HIV-positive premenopausal women.21,22 Rodriguez-Garcia et al. reported increased CCR5+ expression in Th17 cells in the FGT of postmenopausal women, along with a trend toward higher HIV infectivity.23 Clinically, the data are conflicting on whether HIV-positive women undergo premature menopause and whether effectiveness of HAART is reduced in this population.24
Hormone replacement therapy (HRT) (either estrogen or estrogen/progestin combination) is currently used by women to relieve menopausal symptoms, mostly as a topical treatment.25 Studies have demonstrated that HRT can be useful in partially reversing the deleterious aging-associated effects on systemic immune responses (reviewed8). In particular, a recent longitudinal study where postmenopausal women used topical HRT (vaginal estradiol cream) demonstrated improvement in FGT immune parameters after 1 month of use.16 However, the impact of systemic HRT on FGT immune responses is unknown.
To address the gaps in knowledge described above, our study investigated levels of soluble immune mediators and functional anti-HIV activity in cervical–vaginal lavage (CVL) samples from HIV-negative and HIV-positive premenopausal, postmenopausal, and postmenopausal women on HRT. We hypothesized that postmenopausal women will have an altered FGT immune microenvironment, which will be further distinct in the HIV-positive group. Furthermore, we hypothesized that levels of some of the critical immune mediators will be similar among women in the premenopausal group and the postmenopausal women using HRT.
As the population of HIV-positive postmenopausal women and HIV-negative at-risk postmenopausal women is on the rise, understanding immune responses in the FGT is of critical importance so that specific prevention/intervention strategies can be designed for this emerging high-risk population.
Materials and Methods
Ethical statement
The Women's Interagency HIV Study (WIHS) protocol and this study were conducted according to the principles expressed in the Declaration of Helsinki. After approval by the participating institution's review board, study staff obtained written informed consent for collection and use of data and specimens from each research participant. George Washington University only had access to deidentified information.
Cohort characteristics and demographics
WIHS is an ongoing, prospective, observational cohort study of HIV-infected and sociodemographically similar uninfected women in the United States. Study methods, baseline cohort characteristics, and long-term retention have been previously described.26–28 Briefly, semiannual visits included an interview for collection of demographic, behavioral, and clinical factors and a physical and gynecologic examination with specimen collection for the repository. For this cross-sectional study, samples were provided by the Washington DC site. In the premenopausal category, we identified 30 HIV-negative and 30 HIV-positive women who were <45 years of age at visit and experiencing regular menstrual cycles. The cycle stage was determined based on self-reported timing of the last menstrual period (LMP). In the postmenopausal category, we identified 29 women in both HIV-negative and HIV-positive groups who were >45 years of age at visit and had no menstrual cycle for at least 1 year. Women on HRT (5 HIV-negative and 7 HIV-positive women) were on estrogen, progesterone, or combination therapy for at least the past 6 months. To characterize FGT immune condition in the absence of antiretroviral therapy (which can act as a confounder), we selected only those HIV-positive women who were not using any antiretroviral drugs for at least the past 6 months. Women were excluded if currently pregnant, breastfeeding, or lacking a cervix as a result of hysterectomy. Information on race/ethnicity, menstrual cycle staging, birth control usage (for premenopausal women), presence of reproductive tract infections (RTIs) or dysbiosis (Trichomonas vaginalis, herpes simplex virus, bacterial vaginosis, Candida albicans, Neisseria gonorrhea, or Chlamydia trachomatis), plasma viral loads (PVLs), and CD4 counts was obtained for all women from the WIHS database. CVL viral load data were also collected, but as almost 100% of the women had undetectable values, this parameter was not used in any analyses.
CVL processing
Previously frozen whole CVL was shipped on dry ice from the WIHS repository, thawed on ice, and processed immediately. The CVL was spun at 1,500 × g for 10 min, supernatant removed, and respun at 2,200 × g for 10 min. Supernatants were aliquoted and frozen at −80°C.
HIV viral stocks
HIV strains, IIIB (CXCR4 tropic) and BaL (CCR5 tropic), were obtained from Dr. P. Gupta (University of Pittsburgh, PA). Virus stocks were propagated in phytohemagglutinin (PHA)-stimulated human peripheral blood mononuclear cells (PBMCs) and stored frozen at −80°C. Virus titers were determined on TZM-bl cells.
Measurement of cytokines, chemokines, and antimicrobials in CVL
CVL supernatants were stored at −80°C until assayed for tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, macrophage inflammatory protein (MIP)-3α, secretory leukocyte protease inhibitor (SLPI), Elafin ELISA (R&D Systems, Minneapolis, MN), and HBD2 ELISA (PeproTech, Rocky Hill, NJ). Quantification was based on standard curves obtained using a microplate reader (Biotek, Winooski, VT). Concentrations below the lower limit of detection were set to the midpoint between 0 and the lowest detected value for that particular target and adjusted for dilution.
Determination of total protein concentration in CVL
Total protein concentration in each CVL sample was determined using the Pierce BCA Protein Assay kit (Thermo Fisher Scientific), according to the manufacturer's instructions. Concentrations of immune mediators were normalized to total protein content of the CVL.
Measurement of anti-HIV activity in CVL
Anti-HIV activity in the CVL was determined using the TZM-bl indicator cell line (AIDS Reagent Repository, NIH) essentially as previously described.18,29 Samples (tested in triplicates) were incubated with HIV IIIB and BaL at 250 tissue culture infectious dose (TCID50) for 1 h at 37°C and added to TZM-bl cells. Luciferase activity was measured in 48 h upon application of substrate beta-Glo (Promega, Madison, WI).
Viability of cells upon treatment with CVL was quantified using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega), as previously described.18
Statistical analyses
Possible covariates that can affect immune biomarkers in the context of our study of interest were compared across the six groups, separately by HIV status. The median values of each biomarker were compared by group using Kruskal–Wallis tests. For biomarkers with significant differences in medians, graphs were created using GraphPad Prism with Mann–Whitney U tests comparing each group with the control.
Heat maps for each group were constructed to assess the direction and strength of associations between individual biomarkers and other functional and clinical variables such as HIV inhibition, CD4, and PVL data by performing Spearman's rank-order correlation tests using R Studio, version 1.0.136.
Results
Higher PVL and lower CD4 counts in postmenopausal women
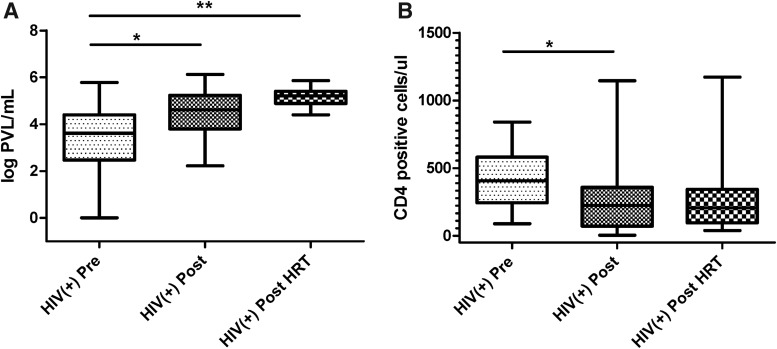
Study participants did not differ significantly in terms of race, RTIs, contraceptive use, and menstrual cycle stage (Table 1). However, HIV-positive postmenopausal women and postmenopausal women on HRT had significantly higher PVLs compared with HIV-positive premenopausal women (Fig. 1A and Table 1). Postmenopausal women also had significantly lower CD4 counts compared with premenopausal women (Fig. 1B and Table 1).
Table 1.
Participant Characteristics Among HIV-Negative and HIV-Positive Premenopausal, Postmenopausal, and Postmenopausal Women on Hormone Replacement Therapy
| Groups | HIV-negative Pre | HIV-negative Post | HIV-negative Post+HRT | HIV-positive Pre | HIV-positive Post | HIV-positive Post+HRT | |
|---|---|---|---|---|---|---|---|
| N | 30 | 29 | 5 | 30 | 29 | 7 | p |
| Participant characteristics | |||||||
| Agea | 37.7 ± 1 | 55.3 ± 1.6 | 49.4 ± 3 | 39.9 ± 1 | 52.2 ± 0.7 | 49.4 ± 2.3 | <.001 |
| Raceb | ns | ||||||
| White | 6 (20) | 6 (20.8) | 0 (0) | 5 (16.7) | 3 (10.3) | 1 (14.3) | |
| Black | 19 (63.3) | 17 (58.7) | 4 (80) | 24 (80) | 24 (82.8) | 6 (85.8) | |
| Hispanic | 1 (3.3) | 4 (13.8) | 1 (20) | 1 (5.3) | 1 (3.5) | 0 (0) | |
| Other | 4 (13.3) | 2 (7) | 0 (0) | 0 (0) | 1 (3.5) | 0 (0) | |
| Reproductive tract infectionsb | ns | ||||||
| Trichomonas | 2 (6) | 0 (0) | 0 (0) | 2 (6) | 0 (0) | 0 (0) | |
| Candida | 3 (10) | 0 (0) | 1 (20) | 2 (6) | 2 (6.9) | 0 (0) | |
| Bacterial vaginosis | 0 (0) | 0 (0) | 1 (20) | 1 (3.3) | 1 (3.4) | 0 (0) | |
| Contraceptive useb,c | 0 (0) | N/A | N/A | 4 (13.3) | N/A | N/A | N/A |
| Menstrual cycle stageb | |||||||
| Proliferative | 14 (48.3) | N/A | N/A | 9 (36) | N/A | N/A | ns |
| Secretory | 13 (44.8) | N/A | N/A | 12 (48) | N/A | N/A | |
| Perimenopausal | 1 (3.4) | N/A | N/A | 4 (16) | N/A | N/A | |
| Clinical variables | |||||||
| CD4 count (cells/μL)a | N/A | N/A | N/A | 412 ± 40.4 | 290.1 ± 56 | 333.3 ± 146.6 | .001 |
| Plasma viral load (log copies/mL)a | N/A | N/A | N/A | 4.79 ± 4.43 | 5.24 ± 4.8 | 5.34 ± 4.95 | .02 |
All values are “at visit.” None of the HIV-positive women were on ARV for at least the past 6 months.
Data presented as mean ± SEM.
Data presented as N (%).
Hormonal contraceptive in past 6 months.
ARV, antiretroviral; N/A, not applicable; ns, nonsignificant; Pre, premenopausal; Post, postmenopausal; Post+HRT, postmenopausal women on hormone replacement therapy.
FIG. 1.
Higher PVLs and lower CD4 counts in HIV-positive postmenopausal women. Data on PVLs (A) and CD4 counts (B) for all participants were obtained from the WIHS database and analyzed by menopausal status. Bars depict medians and interquartile range. p values are indicated as **p < .001, and *p < .05. PVL, plasma viral load; WIHS, Women's Interagency HIV Study.
Distinct levels of soluble immune mediators in CVL by menopausal status and HIV status
To determine whether levels of immune mediators were distinct in genital tract secretions by menopausal or by HIV status, we analyzed CVL samples for proinflammatory mediators IL-6, IL-8, and TNF-α and anti-HIV/protective mediators MIP-3α, SLPI, Elafin, and human beta defensin-2 (HBD2).
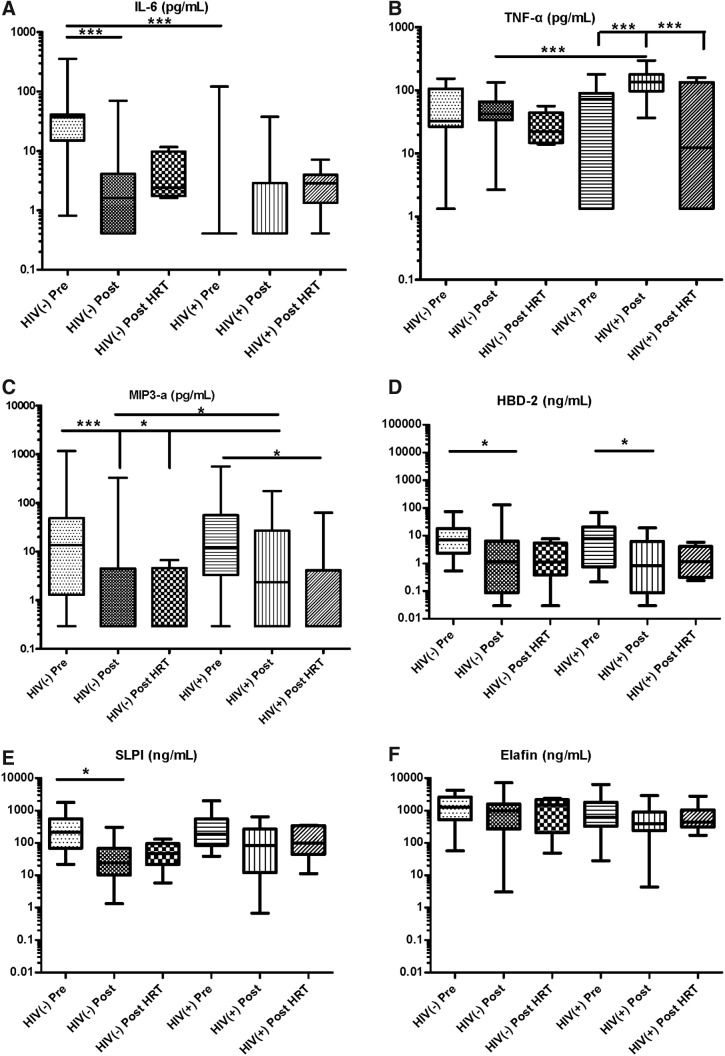
When analyzed by menopausal status, HIV-negative postmenopausal women had significantly lower levels of IL-6, MIP-3α, HBD2, and SLPI compared with HIV-negative premenopausal women (Fig. 2A, C–E and Table 2). For MIP-3α, postmenopausal women on HRT also had significantly lower levels compared with premenopausal women. Within the HIV-positive groups, postmenopausal women had significantly lower HBD2 compared with premenopausal women (Fig. 2D and Table 2) and postmenopausal women on HRT had significantly lower MIP-3α compared with premenopausal women (Fig. 2C and Table 2). In contrast, postmenopausal women had significantly higher levels of TNF-α compared with both premenopausal women and postmenopausal women on HRT (Fig. 2B and Table 2).
FIG. 2.
Differences in cervico–vaginal immune mediators by menopausal status in HIV-negative and HIV-positive women. CVL from HIV-negative and HIV-positive premenopausal, postmenopausal, and postmenopausal women on HRT were tested for (A) IL-6, (B) TNF-α, (C) MIP-3α, (D) HBD2, (E) SLPI, and (F) Elafin by standard ELISA. Bars depict medians and interquartile range. p values are indicated as ***p < .0001, and *p < .05. CVL, cervical–vaginal lavage; HBD2, human beta defensin-2; HRT, hormone replacement therapy; IL, interleukin; MIP-3α, macrophage inflammatory protein-3 alpha; SLPI, secretory leukocyte protease inhibitor; TNF, tumor necrosis factor.
Table 2.
Immune Parameters in Cervical–Vaginal Lavage from HIV-Negative and HIV-Positive Premenopausal, Postmenopausal, and Postmenopausal Women on Hormone Replacement Therapy
| Groups | HIV-negative Pre | HIV-negative Post | HIV-negative Post+HRT | HIV-positive Pre | HIV-positive Post | HIV-positive Post+HRT | ||
|---|---|---|---|---|---|---|---|---|
| N | 30 | 29 | 5 | 30 | 29 | 7 | p value | |
| CVL immune mediators | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | Median [IQR] | p | adjusted |
| IL-8 (pg/mL) | 252.63 [121.83, 1091.91] | 440.27 [246.66, 1243.85] | 392.33 [190.49, 925.04] | 354.84 [175.13, 988.12] | 411.97 [123.66, 1315.95] | 146.81 [146.75, 262.36] | .767 | .32 |
| IL-6 (pg/mL) | 36.90 [18.92, 40.08] | 1.62 [0.41, 4.00] | 2.41 [1.89, 7.84] | 0.41 [0.41, 0.41] | 0.41 [0.41, 1.34] | 2.86 [1.99, 3.53] | <.001 | <.001 |
| TNF-α (pg/mL) | 32.83 [26.84, 101.84] | 42.66 [35.81, 66.17] | 22.29 [15.66, 32.42] | 72.55 [1.34, 88.70] | 136.02 [100.10, 177.21] | 12.36 [2.41, 75.76] | <.001 | .001 |
| MIP-3α (pg/mL) | 13.54 [1.57, 35.67] | 0.29 [0.29, 0.67] | 0.29 [0.29, 2.44] | 12.00 [4.30, 48.15] | 2.34 [0.29, 26.56] | 0.29 [0.29, 3.19] | .001 | .003 |
| HBD2 (ng/mL) | 7.02 [2.48, 16.34] | 1.11 [0.14, 3.82] | 1.12 [0.72, 3.12] | 7.77 [1.00, 1.90] | 0.82 [0.1, 6.11] | 2.07 [0.77, 3.00] | <.001 | .002 |
| Elafin (ng/mL) | 1267.71 [55.44, 2508.90] | 986.62 [270.56, 1433.03] | 1453.456 [369.00, 1986.23] | 630.18 [352.56, 1634.21] | 387.00 [263.28, 859.63] | 438.63 [327.69, 825.50] | .052 | .009 |
| SLPI (ng/mL) | 216.289 [88.62, 466.59] | 24.21 [10.91, 65.28] | 47.22 [37.12, 61.40] | 185.87 [85.69, 509.52] | 82.53 [14.62, 267.77] | 97.85 [51.91, 330.45] | <.001 | <.001 |
| % of inhibition IIIB | 60.39 [17.42, 96.64] | 56.60 [2.81, 79.44] | 4.92 [−16.46, 35.21] | 51.91 [43.62, 81.26] | 29.16 [10.42, 54.46] | 27.66 [−3.19, 58.82] | .471 | |
| % of Inhibition BaL | 30.64 [5.12, 52.07] | 44.34 [29.97, 60.20] | 22.71 [17.06, 26.70] | 18.34 [−1.68, 42.31] | 35.02 [0.85, 51.35] | 25.26 [14.45, 37.20] | .225 | |
| Total protein concentration (μg/mL) | 261.04 [150.79, 389.72] | 141.08 [97.19, 265.25] | 180.70 [137.55, 204.88] | 316.53 [192.64, 521.23] | 289.03 [127.17, 396.86] | 181.92 [136.66, 280.07] | .012 |
Significant p-values are denoted in bold.
All values are “at visit.” None of the HIV-positive women were on ARVs for at least the past 6 months. p value adjusted: normalized to total protein levels for a given sample and a given mediator.
CVL, cervical–vaginal lavage; HBD2, human beta defensin-2; IL, interleukin; IQR, interquartile range; MIP-3α, macrophage inflammatory protein-3 alpha; SLPI, secretory leukocyte protease inhibitor; TNF, tumor necrosis factor.
When analyzed by HIV status, IL-6 levels were significantly higher in premenopausal HIV-negative women compared with premenopausal HIV-positive women (Fig. 2A and Table 2). However, TNF-α and MIP-3α were at significantly higher levels in postmenopausal HIV-positive women compared with postmenopausal HIV-negative women (Fig. 2B, C and Table 2).
IL-8 levels were measurable, but did not vary significantly among groups by menopausal or HIV status (Table 2).
Total protein concentration in CVL did change significantly between the groups with a trend toward higher values in HIV-positive samples (Table 1). All mediators that were significantly different among groups before protein adjustment remained so following protein adjustment. The one exception was Elafin, which was nonsignificant before adjustment, but became significant after adjustment, with significantly higher levels in samples from HIV-negative premenopausal and postmenopausal women compared with those from HIV-positive premenopausal and postmenopausal women (Table 2).
Anti-HIV activity in CVL
Intrinsic anti-HIV activity in CVL samples from HIV-positive and HIV-negative women has been previously demonstrated.21,30,31 To determine whether women have differential anti-HIV activity based on menopausal and/or HIV status, we tested the CVL against HIV-1 IIIB and BaL using the TZM-bl indicator cell line. All HIV-positive women were not on HAART for at least 6 months before sampling visit, thereby giving us the opportunity to characterize anti-HIV activity in the absence of drugs. A wide range of anti-HIV activities were detected in all groups, but no significant differences were present (Table 2).
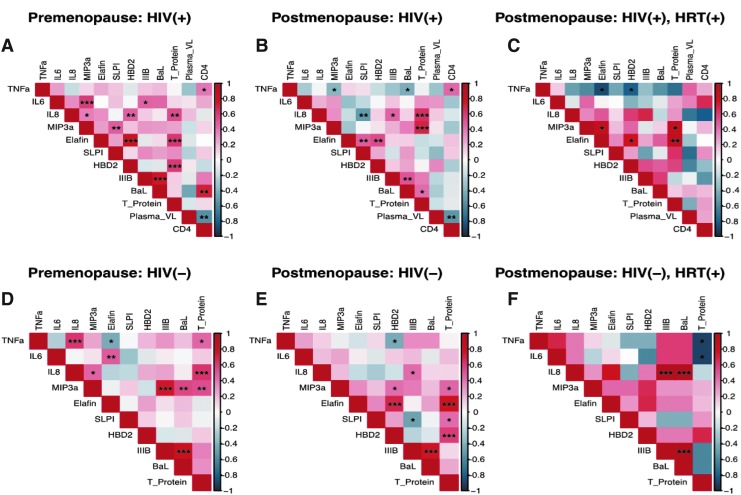
Correlations among immune mediators, anti-HIV activity, and clinical parameters
Whereas ELISA-based assays determine levels of mediators, associations between immune mediators and clinical parameters are also critical for understanding underlying mechanisms. Distinct associations and clustering of immune mediators have been described previously in the context of HIV infection and susceptibility.32 We and others have previously reported distinct immune clustering in adolescent cohorts as well as premenopausal versus postmenopausal women.18,33 In the current study, we observed distinct immune clustering patterns among the six groups (Fig. 3). In particular, HIV-positive groups showed greater numbers of significant interactions. CD4 counts showed significant positive interactions with the proinflammatory cytokine TNF-α in premenopausal and postmenopausal groups. However, this interaction was not observed in the HIV-positive HRT group, which is suggestive of a nonimmune-activated phenotype. In fact, in this group, TNF-α showed significant negative associations with anti-inflammatory mediators, Elafin and HBD2. HRT groups in both HIV-positive and HIV-negative categories showed the least number of significant interactions.
FIG. 3.
Heat map showing distinct correlations between cervico-vaginal immune mediators, clinical parameters, and anti-HIV activity against HIV IIIB and BaL for (A) HIV-positive premenopausal, (B) HIV-positive postmenopausal, (C) HIV-positive postmenopausal on HRT, (D) HIV-negative premenopausal, (E) HIV-negative postmenopausal, and (F) HIV-negative postmenopausal women on HRT. Distinct network and clustering patterns between immune mediators were observed among groups. Each cell of the heat map is color coded based on Spearman correlation coefficients. Cells highlighted in shades of red indicate a positive association with darker shades representing stronger associations. Similarly, cells highlighted in shades of blue indicate a negative association with darker shades representing stronger associations. Statistically significant associations are denoted by ***p < 0.0001, **p < 0.001, and *p < 0.05.
Discussion
Our study evaluated the presence of soluble immune mediators and anti-HIV activity in genital tract secretions of HIV-negative and HIV-positive premenopausal and postmenopausal women. Our results indicate significant alterations in immune mediators in CVL both by menopausal status and HIV status.
We and others have previously shown lower levels of several critical immune mediators in CVL samples from healthy, HIV-negative postmenopausal women compared with premenopausal women.16–18 Our current data extend these findings and confirm similar patterns for most mediators that we tested in HIV-positive women who have not been on antiretroviral therapy at least for the past 6 months. Two of these immune mediators, SLPI and HBD2, have in vitro anti-HIV functions.34–36 Therefore, their reduction can potentially result in decreased immune protection in the FGT. Another mediator, MIP-3α, has also been shown to have anti-HIV activity in vitro and therefore reduced levels in postmenopausal women might be detrimental.37 However, MIP-3α has also been shown to enhance HIV infection in genital mucosa by attracting target cells38 and its precise in vivo role in these women cannot be determined from the current study.
Our data showing significant reduction of IL-6 in CVL samples of HIV-negative postmenopausal women compared with premenopausal women confirm our findings in previous publications.18 In HIV-positive women, TNF-α levels were higher in postmenopausal compared with premenopausal women. This suggests that specific inflammatory pathways are upregulated in postmenopausal HIV-positive women not currently on antiretroviral therapy and this effect is not observed in premenopausal HIV-positive women. This observation, combined with significantly higher PVLs and lower CD4 counts in the HIV-positive postmenopausal group, indicates inflammatory immune dysregulation in the FGT.
HRT is used by postmenopausal women to relieve menopausal symptoms, both systemically and more frequently as a topical cream or gel. We hypothesized that postmenopausal women using HRT will have an improved outcome of FGT mucosal variables compared with those not using HRT. However, in our study, the HRT groups looked very similar to non-HRT postmenopausal groups in terms of levels of immune mediators and PVL/CD4 values. The exception was TNF-α for HIV-positive women, where levels in postmenopausal women were significantly higher compared with levels in premenopausal and postmenopausal women on HRT. Interestingly, by heat map analysis, we did see few significant associations between immune mediators and clinical parameters in the two HRT groups, with samples from HIV-negative women receiving HRT demonstrating the least number of associations. This pattern points toward an immune nonreactive phenotype. Thurman et al. recently reported16 improvement of mucosal outcomes upon treatment with vaginal estradiol. It is possible we did not see any effects because HRT usage in our cohort differed in terms of route (oral vs. topical) and regimen (estradiol, progesterone, or combination of both) and the sample sizes were particularly small.
Our data from this study showed significant differences in CVL protein concentration among groups. We have previously reported no significant changes in this parameter between premenopausal and postmenopausal HIV-negative women.18 Upon closer examination, it was revealed that CVL protein concentration was not significantly different within the HIV-negative groups, confirming our previous findings. The driver for the observed significant changes was the presence of higher levels of protein in HIV-positive postmenopausal samples compared with HIV-negative postmenopausal samples.
We observed a range of anti-HIV activities in all samples, but no significant differences between groups. This is consistent with previous findings in HIV-negative premenopausal and postmenopausal women.16,18 One study39 did report lower CVL anti-HIV activity in postmenopausal women compared with premenopausal women. However, this differs from our findings likely because different cell lines and experimental conditions were used in that study. We have previously demonstrated a range of anti-HIV activities in CVL samples from HIV-positive women who were not on HAART.21
There were several limitations to our study. One was our inability to define menopause by measuring estradiol/progesterone levels in blood. However, we used clinical criteria for defining menopause: at least 1 year of no menses,40 and it is likely that our postmenopausal women were categorized correctly. However, from this study, we cannot be certain whether our findings in postmenopausal women are attributable to chronological aging or biological aging (i.e., menopause). Similarly, we were unable to accurately determine the cycle stage in premenopausal women and were limited by a rough analysis of self-reported LMP. Other critical mucosal parameters that can affect HIV acquisition/transmission risks are the presence of RTI and contraceptive usage.41 Our cohort had a very small number of women with RTIs in all the groups and only 4 (of total 60 premenopausal women) were on contraceptives (all on progestin-only Depo-provera regimen). Therefore, with such small numbers, we were unable to adjust for these parameters and decided to not exclude them so as to not reduce the sample size further. Future studies with larger sample sizes should take these factors into account.
To our knowledge, this is the first study to characterize FGT immune mediators in HIV-negative and HIV-positive premenopausal and postmenopausal women. Our data suggest that immune alterations occur in the FGT based on both menopausal status and HIV status. Additional studies with larger sample sizes are warranted to understand the mechanisms underlying these changes.
Acknowledgments
The authors would like to thank Dr. Charles Wira and his group at Geisel School of Medicine at Dartmouth for advice on data analysis and manuscript writing. Data in this article were collected by the Metropolitan Washington Women's Interagency HIV Study (WIHS) (Principal Investigator, Dr. Seble Kassaye), U01-AI-034994. The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). This research was funded, in part, by a microgrant award from the District of Columbia Center for AIDS Research, an NIH-funded program (AI117970), which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, NIGMS, NIDDK, and OAR. This study was funded by GWU start-up funds (M.G.) and a DC-CFAR microgrant (M.G.).
Preliminary data were reported at the HIV Research for Prevention Conference, Cape Town, South Africa, November 2014 (Poster).
Disclaimer
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Centers for Disease Control and Prevention: HIV Among Older Americans. Updated September 2018. Available at www.cdc.gov/hiv/group/age/olderamericans/index.html (2015). Last accessed January28, 2019
- 2. UNAIDS: Available at www.unaids.org/sites/default/files/media_asset/20131101_JC2563_hiv-and-aging_en_0.pdf (2013). Last accessed January28, 2019
- 3. Brooks JT, Buchacz K, Gebo KA, Mermin J: HIV infection and older Americans: The public health perspective. Am J Public Health 2012;102:1516–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Beaulaurier R, Fortuna K, Lind D, Emlet CA: Attitudes and stereotypes regarding older women and HIV risk. J Women Aging 2014;26:351–368 [DOI] [PubMed] [Google Scholar]
- 5. Pilowsky DJ, Wu LT: Sexual risk behaviors and HIV risk among Americans aged 50 years or older: A review. Subst Abus Rehabil 2015;6:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samji H, Cescon A, Hogg RS, et al. : Closing the gap: Increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One 2013;8:e81355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. amfAR: Statistics: Women and HIV/AIDS. Updated September 2018. Available at www.amfar.org/about-hiv-and-aids/facts-and-stats/statistics--women-and-hiv-aids/ (2015) Last accessed January28, 2019
- 8. Ghosh M, Rodriguez-Garcia M, Wira CR: The immune system in menopause: Pros and cons of hormone therapy. J Steroid Biochem Mol Biol 2014;142:171–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Larbi A, Fülöp T, Pawelec G: Immune receptor signaling, aging and autoimmunity. Adv Exp Med Biol 2008;640:312–324 [DOI] [PubMed] [Google Scholar]
- 10. Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T: Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin Immunol 2012;24:331–341 [DOI] [PubMed] [Google Scholar]
- 11. Gameiro CM, Romão F, Castelo-Branco C: Menopause and aging: Changes in the immune system—A review. Maturitas 2010;67:316–320 [DOI] [PubMed] [Google Scholar]
- 12. Abu-Taha M, Rius C, Hermenegildo C, et al. : Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. J Immunol 2009;183:1393–1402 [DOI] [PubMed] [Google Scholar]
- 13. Goetzl EJ, Huang MC, Kon J, et al. : Gender specificity of altered human immune cytokine profiles in aging. FASEB J 2010;24:3580–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rollenhagen C, Asin SN: Enhanced HIV-1 replication in ex vivo ectocervical tissues from post-menopausal women correlates with increased inflammatory responses. Mucosal Immunol 2011;4:671–681 [DOI] [PubMed] [Google Scholar]
- 15. Meditz AL, Moreau KL, MaWhinney S, et al. : CCR5 expression is elevated on endocervical CD4+ T cells in healthy postmenopausal women. J Acquir Immune Defic Syndr 2012;59:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thurman AR, Yousefieh N, Chandra N, et al. : Comparison of mucosal markers of human immunodeficiency virus susceptibility in healthy premenopausal versus postmenopausal women. AIDS Res Hum Retroviruses 2017;33:807–819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jais M, Younes N, Chapman S, Cu-Uvin S, Ghosh M: Reduced levels and bioactivity of endogenous protease cathepsin D in genital tract secretions of postmenopausal women. AIDS Res Hum Retroviruses 2017;33:407–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jais M, Younes N, Chapman S, Cu-Uvin S, Ghosh M: Reduced levels of genital tract immune biomarkers in postmenopausal women: Implications for HIV acquisition. Am J Obstet Gynecol 2016;215:324.e1–324.e10 [DOI] [PubMed] [Google Scholar]
- 19. Wira CR, Ghosh M, Smith JM, et al. : Epithelial cell secretions from the human female reproductive tract inhibit sexually transmitted pathogens and Candida albicans but not Lactobacillus. Mucosal Immunol 2011;4:335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ghosh M: Secreted mucosal antimicrobials in the female reproductive tract that are important to consider for HIV prevention. Am J Reprod Immunol 2014;71:575–588 [DOI] [PubMed] [Google Scholar]
- 21. Lahey T, Ghosh M, Fahey JV, et al. : Selective impact of HIV disease progression on the innate immune system in the human female reproductive tract. PLoS One 2012;7:e38100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mukura LR, Ghosh M, Fahey JV, Cu-Uvin S, Wira CR: Genital tract viral load in hiv type 1-positive women correlates with specific cytokine levels in cervical-vaginal secretions but is not a determinant of infectious virus or anti-hiv activity. AIDS Res Hum Retroviruses 2012;28:1533–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodriguez-Garcia M, Barr FD, Crist SG, Fahey JV, Wira CR: Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal Immunol 2014;7:1375–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bull L, Tittle V, Rashid T, Nwokolo N: HIV and the menopause: A review. Post Reprod Health 2017; DOI: 10.1177/2053369117748794, PMID [DOI] [PubMed] [Google Scholar]
- 25. Lindahl SH: Reviewing the options for local estrogen treatment of vaginal atrophy. Int J Womens Health 2014;6:307–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barkan SE, Melnick SL, Preston-Martin S, et al. : The women's interagency HIV study. WIHS collaborative study group. Epidemiology 1998;9:117–125 [PubMed] [Google Scholar]
- 27. Bacon MC, von Wyl V, Alden C, et al. : The women's interagency HIV study: An observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005;12:1013–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hessol NA, Weber KM, Holman S, et al. : Retention and attendance of women enrolled in a large prospective study of HIV-1 in the United States. J Womens Health (Larchmt) 2009;18:1627–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Montefiori D.: Protocol for neutralizing antibody screening assay for HIV-1 in TZM-bl Cells. Updated November 2018. Available at www.hiv.lanl.gov/content/nab-reference-strains/html/Protocol-for-Neutralizing-Antibody-Assay-for-HIV-1-in-TZMbl-cells_Nov2018.pdf Last accessed January2019
- 30. Ghosh M, Fahey JV, Shen Z, et al. : Anti-HIV activity in cervical-vaginal secretions from HIV-positive and -negative women correlate with innate antimicrobial levels and IgG antibodies. PLoS One 2010;5:e11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keller MJ, Madan RP, Torres NM, et al. : A randomized trial to assess anti-HIV activity in female genital tract secretions and soluble mucosal immunity following application of 1% tenofovir gel. PLoS One 2011;6:e16475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lisco A, Introini A, Munawwar A, et al. : HIV-1 imposes rigidity on blood and semen cytokine networks. Am J Reprod Immunol 2012;68:515–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madan RP, Carpenter C, Fiedler T, et al. : Altered biomarkers of mucosal immunity and reduced vaginal Lactobacillus concentrations in sexually active female adolescents. PLoS One 2012;7:e40415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wahl SM, McNeely TB, Janoff EN, et al. : Secretory leukocyte protease inhibitor (SLPI) in mucosal fluids inhibits HIV-I. Oral Dis 1997;3 Suppl 1:S64–S69 [DOI] [PubMed] [Google Scholar]
- 35. Moreau T, Baranger K, Dadé S, Dallet-Choisy S, Guyot N, Zani ML: Multifaceted roles of human elafin and secretory leukocyte proteinase inhibitor (SLPI), two serine protease inhibitors of the chelonianin family. Biochimie 2008;90:284–295 [DOI] [PubMed] [Google Scholar]
- 36. Weinberg A, Quiñones-Mateu ME, Lederman MM: Role of human beta-defensins in HIV infection. Adv Dent Res 2006;19:42–48 [DOI] [PubMed] [Google Scholar]
- 37. Ghosh M, Shen Z, Schaefer TM, Fahey JV, Gupta P, Wira CR: CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am J Reprod Immunol 2009;62:60–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Q, Estes JD, Schlievert PM, et al. : Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009;458:1034–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chappell CA, Isaacs CE, Xu W, et al. : The effect of menopause on the innate antiviral activity of cervicovaginal lavage. Am J Obstet Gynecol 2015;213:204.e1–204.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harlow SD, Gass M, Hall JE, et al. : Executive summary of the stages of reproductive aging workshop +10: Addressing the unfinished agenda of staging reproductive aging. J Clin Endocrinol Metab 2012;97:1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Fichorova RN, Chen PL, Morrison CS, et al. : The contribution of cervicovaginal infections to the immunomodulatory effects of hormonal contraception. MBio 2015;6:e00221–e00215 [DOI] [PMC free article] [PubMed] [Google Scholar]