Abstract
Over a period of 208 days a randomized, double-blind clinical trial was conducted to assess plaque and calculus accumulation in dogs provided with a xylitol-based drinking water additive. A crossover design was utilized allowing each dog to participate in each 90-day treatment and control phase. Inclusion of a xylitol drinking water additive resulted in a 5.1% decrease in mean tooth plaque score and a 14.9% decrease in mean calculus score. Daily administration of a palatable, xylitol drinking water additive that required little time and effort reduced plaque and calculus accumulation in dogs.
Résumé
Étude pilote sur l’efficacité d’un additif à base de xylitol à l’eau de boisson a pour réduire la plaque et l’accumulation de tartre chez les chiens. Pendant une période de 208 jours, un essai clinique randomisé à double-insu a été mené pour évaluer l’accumulation de plaque et de tartre chez des chiens supplémentés avec un additif à base de xylitol à l’eau de boisson. Une étude croisée fut utilisée permettant ainsi à chaque chien de participer dans chaque période de 90 jours au groupe traité et au groupe témoin. L’inclusion d’un additif à base de xylitol à l’eau de boisson a résulté en une diminution de 5,1 % du pointage de plaque dentaire et de 14,9 % du pointage moyen de tartre dentaire. L’administration quotidienne d’un additif palatable à base de xylitol dans l’eau de boisson ne requérant que peu de temps et d’efforts a permis de réduire l’accumulation de plaque et de tartre.
(Traduit par Dr Serge Messier)
Introduction
Periodontal disease is the most frequently diagnosed disease in dogs in all age groups (1). Periodontitis is the inflammation of the periodontium which includes the periodontal ligament, alveolar bone, cementum, and gingiva. Bacterial by-products and host response to plaque accumulation cause inflammation resulting in gingival recession, alveolar bone resorption, and periodontal pocket formation. Untreated periodontal disease may eventually progress to tooth mobility, loss, and/or endodontic disease (2). Oral pain is difficult to assess in veterinary patients and clients often do not recognize problems until periodontal disease has progressed to moderate or severe stages (3). The initial development and severity of periodontal disease are dependent on factors such as health status, breed, age, and diet (3–6). As well as causing significant oral pain, bone and tooth loss in dogs, an association between periodontal disease and systemic disease has been reported in the literature (7–10). Within the periodontal tissues, bacteria, bacterial by-products, and inflammatory mediators may be released, resulting in the development of systemic disease.
Plaque is the collection of bacteria, glycoproteins, epithelial and inflammatory cells, and extracellular polysaccharides that adhere to the tooth surface (2,11). Within seconds of a routine dental prophylaxis a thin pellicle accumulates on the tooth surfaces. Bacterial recolonization is reported to occur within 3 min following the placement of sterile enamel into the oral cavity. As the oral bacteria become firmly attached to the tooth surface, the plaque matures, and an organized structure or biofilm develops. The biofilm is composed of organic and inorganic materials that function to distribute nutrients throughout the plaque matrix (11). The bacteria that accumulate within the gingival sulcus are responsible for the development of gingivitis. Untreated gingivitis may progress to periodontitis. Within 2 to12 d following its development plaque mineralizes resulting in calculus formation on the tooth surfaces. Dental calculus is primarily composed of non-viable plaque microorganisms and calcium phosphate mineral salts provided by salivary and gingival crevicular fluids. A non-mineralized layer of dental plaque containing viable bacteria adheres to the surface of calculus deposits (11).
Plaque accumulation must be controlled in order to prevent the development or progression of periodontal disease (11). Mechanical or chemical anti-plaque agents can control the development of plaque and its progression to calculus. Multiple products have been developed in each category to decrease plaque accumulation (12). Frequent brushing is the most effective method of mechanically removing plaque in humans and dogs (13). In a recent study that compared brushing, daily dental chew and dental diet, dogs in the daily tooth brushing group had a statistically significant lower mean mouth score (14).
The goal of routine oral home care is to improve a patient’s general oral health in order to minimize the need for more invasive treatments. Decreased client compliance has been reported with respect to mechanical anti-plaque and anti-calculus methods (i.e., tooth brushing). Research by Miller and Harvey (15) revealed that 6 mo after periodontal treatment and home care education, only 53% of clients complied with homecare recommendations by brushing their dogs’ teeth several times a week. This lack of compliance is thought to primarily be due to the amount of time required and patient tolerance. Increased client compliance may be seen with chemical anti-plaque, anti-calculus agents due to the ease of administration and decreased time requirement. Xylitol is utilized for its plaque-reducing effect in humans and animals (16,17). The purpose of this randomized, double-blind clinical trial was to assess plaque and calculus accumulation in dogs following the use of a commercially available xylitol containing water additive.
Materials and methods
A clinical trial was conducted at the University of Saskatchewan in accordance with the requirements of the Animal Research Ethics Board. Informed written consent was provided by the clients of study participants. Patients were admitted into the clinical trial based on the following criteria; age 2 to 6 y, medium to large breed dogs, mesaticephalic head type, and availability on the scheduled dates. Dogs were excluded from the trial for any of the following reasons: concurrent medications, systemic diseases, malocclusion, missing dentition required for scoring purposes, and periodontal disease. No form of compensation was given, but all participants were provided with the same diet throughout the entire trial period. No additional chemical or mechanical anti-plaque or anti-calculus agents were permitted during the trial period.
Eight client-owned dogs were entered into the clinical trial based on the inclusion and exclusion criteria. This pilot study sample size was chosen based on the early recommendations of the Veterinary Oral Health Council’s to include at least 5 subjects for a crossover clinical trial design. Oral examinations were performed on staff and faculty members’ dogs until the 8 patients were identified. Three male and five female dogs were studied with ages and weights ranging from 2 to 6 y (mean: 3.75 y) and 8.2 to 40.1 kg (mean: 28.9 kg), respectively. Two of the three male participants were neutered. Each participant had mild to moderate plaque and calculus accumulation prior to entering the study. Physical examination and laboratory testing including complete blood (cell) count (CBC), serum chemistry, and urinalysis performed before commencement of the study confirmed participants were healthy.
Participants were housed in their regular environment and received ad libitum untreated tap water and Association of American Feed Control Officials (AAFCO) approved adult kibble (Pro Plan Adult Chicken and Rice; Purina, St. Louis, Missouri, USA) for the first 14 d of the trial. During this period no mechanical or chemical anti-plaque products were given. On day 15 each patient was anesthetized for an oral examination and dental prophylaxis procedure in order to provide each participant with a mean mouth plaque and calculus score of zero before initiation of the study. Each participant was fasted 12 h before the commencement of general anesthesia. General anesthesia was performed for all scoring and dental prophylaxis procedures using the following protocols; placement of a cephalic IV catheter, induction with propofol (Rapinovet; Schering-Plough, Pointe Claire, Quebec), 6 mg/kg body weight (BW), IV, thiopental (Thiotal; Vétoquinol, Lavaltrie, Quebec), 10 mg/kg BW, IV, or ketamine (Vetalar; Bioniche, Belleville, Ontario), 5 mg/kg BW, IV, and diazepam (Diazepam; Sandoz, Boucherville, Quebec), 0.5 mg/kg BW, IV, to effect, intubation (Rusch Endotracheal Tube Murphy Eye High Volume Low Pressure Cuff; Teleflex Medical, Triangle Park, North Carolina, USA) and maintenance of general anesthesia via a rebreathing circle system with 1.5% to 2.0% isoflurane (IsoFlo; Abbott, Saint-Laurent, Quebec) in 3 L/min of oxygen. Fluid support (Normosol-R; Hospira, Montreal, Quebec) at a rate of 10 mL/kg BW per hour and body temperature were maintained using a warm fluid-circulating mat (T/Pump; Gaymar Industries, Orchard Park, New York, USA) during general anesthesia. Throughout the procedure the heart rate, respiratory rate, peripheral oxygen saturation, and indirect systolic blood pressure were measured and recorded every 5 min. Dental prophylaxis included supra- and sub-gingival scaling using the same sterilized piezoelectric and hand-scaling instruments for each patient. The teeth were polished using a low speed hand piece and single use rubber prophy cup (Prophylaxis polishing cups; Dentamerica, Industry, California, USA) and fine non-fluorinated prophy paste (Glitter professional prophylaxis paste; Premier, Philadelphia, Pennsylvania, USA). No chlorhexidine, fluoride or antibiotic treatments (anti-plaque agents) were administered. All dental prophylaxis procedures for the entire study were performed by the same second-year dental resident.
The study was divided into 2 phases allowing each participant to function as its own control. The investigator, second-year resident, and clients were blinded to which treatment the participant was receiving. To ensure blinding, the placebo was prepared to have similar physical characteristics as the treatment solution. The placebo included the following components; distilled water (500 mL), a coloring agent [F.D. & C Powder (Brilliant Blue); Professional Compounding Centers of America, Houston, Texas, USA] (0.05 mL) and an emulsifier (Tween 80 NF; Wiler Fine Chemicals Ltd., London, Ontario) (0.05 mL). The treatment and placebo solution were placed in similar containers and marked A and B, and the contents were not revealed to the investigators until completion of the study. In phase 1 each participant was blindly randomized using a computerized randomization program by the same pharmacist who prepared the placebo to receive either the treatment or control. Treatment group participants were offered the xylitol-treated water (Breathalyser Plus; ImRex, St. Joseph, Missouri, USA) at the concentration recommended by the manufacturer (10 mL of water additive per liter of untreated water) every 24 h for 90 d. Control group participants were given the placebo at the same concentration for the same time period.
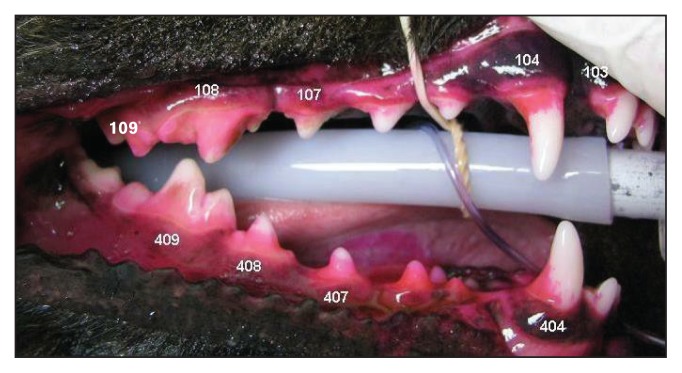
Following phase 1 (90 d) the participants were placed under general anesthesia as previously described for their first plaque and calculus assessment. All plaque and calculus evaluations throughout phases 1 and 2 of the study period were performed by the same blinded, second-year dental resident. Each participant’s plaque deposition was visually assessed and evaluated using a modified Quigley and Hein (Turesky) plaque index reported by Logan and Boyce (18). The following target teeth were scored in each participant: maxillary third incisors (103/203), maxillary canines (104/204), maxillary third and fourth premolars (107/207 and 108/208), maxillary first molars (109/209), mandibular canines (304/404), mandibular third and fourth premolars (307/407 and 308/408), and mandibular first molar (309/409). A 2% eosin plaque disclosing solution (Trace disclosing solution; JorVet, Loveland, Colorado, USA) was applied to the buccal and labial surface of the crown of the target teeth. The solution was immediately rinsed from teeth using an air water syringe held 15 cm from the tooth surface. Each tooth was divided horizontally into gingival and coronal halves and each half was assigned a numerical score. Scores were given for percentage of plaque coverage and thickness of plaque deposition based on intensity of staining of the plaque. Grading criteria for plaque coverage: 0 = no plaque detected, 1 = 1% to 24%, 2 = 25% to 49%, 3 = 50% to 74%, 4 = 75% to 100%. Grading criteria for plaque thickness: 1 = Light (pink to light red), 2 = Medium (red) (Figure 1). Each half of tooth (gingival and occlusal) received a score by multiplying the coverage and thickness numerical scores and the gingival and occlusal scores were added to provide a total tooth score. Participants mean mouth plaque scores were calculated by averaging the 18 total tooth scores.
Figure 1.
Evaluation of plaque on right maxillary 103, 104, 107, 108, 109, and right mandibular 404, 407, 408, and 409 target teeth. Application of plaque disclosing solution to the buccal and labial surfaces of the crown of the target teeth.
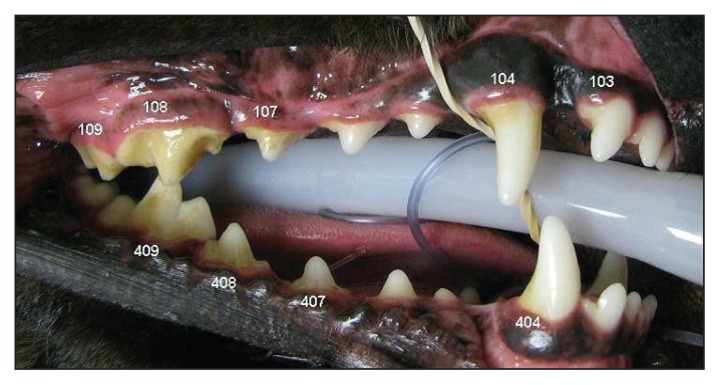
Calculus deposition was visually assessed and evaluated using a modified Schiff calculus index reported by Logan and Boyce (18). Eighteen target teeth; maxillary third incisors (103/203), maxillary canines (104/204), maxillary third and fourth premolars (107/207 and 108/208), maxillary first molars (109/209), mandibular canines (304/404), mandibular third and fourth premolars (307/407 and 308/408), and mandibular first molar (309/409) were scored in each participant. The plaque was removed by brushing the teeth with a standard soft bristle toothbrush and the teeth were air dried before scoring. Each target tooth was divided vertically into mesial, buccal, and distal thirds and each third was assigned a numerical score. Scores were given based on percentage of calculus coverage. Grading criteria for calculus coverage: 0 = no calculus detected, 1 = 1% to 24%, 2 = 25% to 49%, 3 = 50% to 74%, 4 = 75% to 100% (Figure 2). The numerical scores for the mesial, buccal, and distal aspect of the tooth were added to provide a total tooth score. The mean mouth score was calculated by averaging the 18 total tooth scores for each participant.
Figure 2.
Evaluation of calculus on right maxillary 103, 104, 107, 108, 109, and right mandibular 404, 407, 408, and 409 target teeth. Deposition of calculus before application of plaque disclosing agent.
After completion of phase 1, a 14-day rest period was given during which participants received ad libitum AAFCO approved adult kibble, untreated tap water, and no mechanical or chemical anti-plaque products. Prior to initiating phase 2 a mean mouth score of zero was achieved before the second phase of the study using the previously described dental prophylaxis procedure under general anesthesia performed by the same second-year dental resident. In phase 2 each participant was provided with the opposite treatment that they received in phase 1, xylitol treated water or placebo treated water, at a concentration of 0.05 mg/mL every 24 h for 90 d. Plaque and calculus evaluations were performed by the same examiner upon completion of the second, 90-day test phase.
Participant #8 was eliminated from the study during the first test phase as gastrointestinal irritation following ingestion of compost necessitated systemic antibiotic therapy and diet change. The statistical comparisons do not include data from this participant. The mean mouth dental plaque and calculus scores for the remaining 7 study participants were utilized. A statistical software program (Stata SE 10; Stata Corp, College Station, Texas, USA) was used to perform statistical analyses. Comparisons between participants mean mouth dental plaque and calculus scores with and without the xylitol drinking water additive were made using a 2-tailed Wilcoxon signed-rank test and paired Student’s t-test, respectively. A P-value < 0.05 was considered significant.
Results
No adverse effects related to the diet or water additive were reported throughout the study period. The water additive was considered palatable as there were no reports of partial or complete refusal to accept the treated drinking water.
The crossover study design allowed for the comparison of each participant’s mean mouth plaque and calculus scores with and without the xylitol drinking water additive. All participants except dog #7 had a decreased mean mouth plaque score during the xylitol drinking water additive phase of the study. The participants’ averaged total mean mouth plaque scores were 8.06 [standard deviation (SD): +/− 1.33] without the xylitol drinking water additive and 7.65 (SD: +/− 0.57) with the xylitol drinking water additive (Table 1). A 5.1% decrease in plaque accumulation was noted in participants treated with the xylitol drinking water additive (Table 2); however, the results were not statistically significant (P = 0.2367). An outlier in the dataset resulted in an abnormal distribution and because of this, nonparametric evaluation (Wilcoxon signed-rank test) was performed. In addition, the abnormal distribution resulted in a large standard deviation resulting in a study power of < 10%.
Table 1.
Individual participants mean tooth plaque scores with and without water additive treatment.
| Mean tooth plaque scores | ||
|---|---|---|
|
| ||
| Participant number | No water additive | Water additive |
| 1 | 10.11 | 7.50 |
| 2 | 8.50 | 7.67 |
| 3 | 7.72 | 7.39 |
| 4 | 7.33 | 7.22 |
| 5 | 8.28 | 7.61 |
| 6 | 8.67 | 7.28 |
| 7 | 5.78 | 8.89 |
| 8 | ND | ND |
ND — no data.
Table 2.
Total mean tooth plaque scores with and without water additive treatment.
| Plaque | |||
|---|---|---|---|
|
| |||
| Treatment | Total mean tooth score +/− SD | Percent reduction | Significance |
| No water additive | 8.06 +/− 1.33 | 5.1% | P = 0.2367 |
| Water additive | 7.65 +/− 0.57 | ||
A decreased mean mouth calculus score was observed during the xylitol drinking water additive phase in all participants except dog #4. The participants averaged total mean mouth calculus scores were 4.84 (+/− 0.89) without the xylitol drinking water additive and 4.12 (+/− 0.95) with the xylitol drinking water additive (Table 3). A 14.9% statistically significant decrease in calculus accumulation was observed in participants during the xylitol drinking water additive treatment phase (P = 0.0469, Table 4). A normal distribution was observed in the calculus dataset; therefore, parametric testing (paired Student’s t-test) was used.
Table 3.
Individual participant mean tooth calculus scores with and without water additive treatment.
| Mean tooth calculus scores | ||
|---|---|---|
|
| ||
| Participant number | No water additive | Water additive |
| 1 | 5.94 | 4.39 |
| 2 | 4.22 | 3.72 |
| 3 | 5.44 | 5.22 |
| 4 | 3.94 | 4.22 |
| 5 | 5.78 | 4.39 |
| 6 | 4.83 | 4.72 |
| 7 | 3.78 | 2.22 |
| 8 | ND | ND |
ND — no data.
Table 4.
Total mean tooth calculus scores with and without water additive treatment.
| Calculus | |||
|---|---|---|---|
|
| |||
| Treatment | Total mean tooth score +/− SD | Percent reduction | Significance |
| No water additive | 4.84 +/− 0.89 | 14.9% | P = 0.0469 |
| Water additive | 4.12 +/− 0.95 | ||
Discussion
Numerous successful plaque control methods have been documented in the literature, but client compliance is often unsatisfactory (15). A plaque-reducing drinking water additive may increase client compliance due to its ease of administration and palatability. The present study used xylitol as a drinking water additive to decrease the plaque and calculus accumulation in the study population. Xylitol is a natural polyalcohol that is commonly used as a low-calorie sugar substitute. Xylitol has been proposed to reduce the quantity of plaque, decrease the adhesion of plaque flora, be non-fermentable by plaque organisms, reduce Streptococcus mutans, participate in a futile metabolic cycle and result in an accumulation of xylitol-5-phosphate in some plaque streptococcal species (19). As discussed earlier, plaque is the collection of bacteria, salivary glycoproteins, and extracellular polysaccharides that adhere to the tooth surface. Plaque quantity is thought to be reduced by decreasing plaque adhesiveness. A reduced synthesis of insoluble polysaccharides was observed in S. mutans when exposed to xylitol in vitro (20). Since insoluble polysaccharides are required for proper plaque adhesion to the tooth surface it has been proposed that xylitol may affect the adhesiveness of plaque in vivo. Extensive research on xylitol metabolism has been performed on S. mutans because it constitutes a large proportion of the microbes found in human dental plaque. S. mutans is also one of the most significant contributors to the development of caries in humans. The anti-bacterial mechanism of xylitol on pathogenic oral bacteria involves cellular competition with sucrose as well as the direct toxic effects of xylitol metabolites. Although xylitol is readily absorbed by S. mutans, metabolism through fermentation does not occur. The inability to produce energy results in a net energy loss by the pathogenic oral bacteria known as a “futile cycle” and decreased bacterial growth. In addition, a metabolite of xylitol metabolism, xylitol-5-phosphate, is produced. Xylitol toxicity and death of pathogenic oral bacteria ensue following metabolism of xylitol-5-phosphate (21).
This clinical trial detected a significant decrease (14.9%) in calculus accumulation during the xylitol drinking water additive treatment phase. Greater decreases in calculus deposition (53.5%) were found in a similar study performed in 29 cats (17). The study designs were similar, however, species differences, number of participants, and blinding of the examiner may account for the differences in calculus accumulation seen between the 2 studies. Blinded, randomized, controlled clinical trials are required with a greater number of participants in order to establish the effectiveness of xylitol in dogs. Though not statistically significant, a decrease (5.1%) in plaque accumulation was detected in participants when provided with xylitol drinking water additive compared to untreated water. This is quite different from Clarke’s study in cats in which a 52.3% statistically significant decrease in plaque deposition was observed (17). Proposed hypotheses for the findings include; species differences, insufficient sample size, inadequate plaque scoring system, disruption of plaque deposition prior to scoring, and xylitol ineffectiveness.
Numerous plaque and calculus grading systems have been developed for humans and modified for veterinary patients. The precision, accuracy, and clinical relevance of these systems have been questioned in the literature (22–24). The method to index plaque and calculus for the current study was chosen based on research by Logan and Boyce (18). Recent plaque and calculus quantification methods state greater accuracy and reproducibility. A novel plaque quantification method proposed by Scherl et al (22) has been evaluated and accepted by the Veterinary Oral Health Council. A specialized instrument measures the lengths of the gingival margins and compares this measurement to the length of plaque on the gingival margin. Hennet et al (25) increased the accuracy and reproducibility of plaque quantification using shade scales and anatomic landmarks for division of the tooth. Perhaps utilizing these methods would have allowed the collection of data with greater accuracy and precision, resulting in greater difference between the treatment and control groups.
The mean mouth plaque score (5.78) for participant #7 was significantly decreased in comparison to the averaged mean mouth plaque score (8.06 +/− 1.33). There are many potential explanations for this outlier, our main concern was disruption of plaque deposition before scoring. Plaque disruption while unsupervised or during anesthesia may have affected the results, leading to our insignificant findings. Use of one of the previously discussed less invasive plaque evaluation techniques or by performing the research in a 24-hour controlled environment may have allowed collection of more accurate data (22).
A substantial amount of microbiological research and clinical trials on the effects of xylitol have been performed in humans. Streptococcus mutans has been the primary focus of microbiologic studies because it constitutes a large proportion of the microbes found in dental plaque and is a significant contributor to the development of caries in humans. In comparison to human microbiological flora, Streptococcus species are reportedly infrequent components of healthy subgingival flora in dogs (26). Thirty-eight to seventy-six percent of the microorganisms found in human plaque are comprised of Streptococcus species, whereas only 2.6% and 5.6% were reported in dogs (27). Further research into the effects of xylitol on common oral pathogenic bacteria in dogs is necessary.
Extensive research into the safety of xylitol in humans resulted in classification of the additive as generally recognized as safe (GRAS) and approval by the Food and Drug Administration. Toxic effects (i.e., osmotic diarrhea) are documented in humans at doses of 130 g/day (28). Toxicity, including acute liver necrosis, coagulopathies, and hypoglycemia have been reported in dogs receiving doses > 100 mg/kg body weight (BW) (29,30). Early research in dogs performed by Kuzuya et al (31) revealed a 2.5 times increase in insulin concentration following IV administration of 400 mg/kg BW of xylitol in comparison to IV administration of 400 mg/kg BW of glucose (31). Following oral xylitol administration 2 studies also observed a dose-related release of insulin and resultant hypoglycemia (32,33). Hypoglycemia following xylitol administration in dogs is thought to result from hypersecretion of endogenous insulin from the pancreas. A toxicity trial performed by Xia et al (33) showed that orally administered doses of 100 and 400 mg/kg BW of xylitol resulted in hyperinsulinemia and hypoglycemia as well as changes such as hypokalemia, hypophosphatemia, hyperbilirubinemia, and increased serum aspartate aminotransferase and alanine aminotransferase concentrations. Clinical signs in all dogs following xylitol administration included inactivity and depression. One dog exhibited mild to moderate dystaxia and mild tremors. All dogs recovered without treatment and blood glucose values returned to reference interval within 3 to 4 h following xylitol administration. The ASPCA Animal Poison Control Center considers that dogs that ingest > 100 mg/kg BW and 500 mg/kg BW of xylitol are at risk for developing hypoglycemia and hepatotoxicity, respectively. Gastrointestinal decontamination and blood glucose monitoring are recommended for toxicities > 50 mg/kg BW (29). The vast difference in outcomes following oral ingestion of xylitol is thought to be caused by the complete and rapid absorption of xylitol in dogs in comparison to humans (32).
Xylitol toxicity research in dogs is ongoing. A retrospective study of 199 dogs that ingested 3 to 3640 mg/kg BW of xylitol found that the clinical signs ranged from none to vomiting, lethargy, diarrhea, or seizures. This study showed that if veterinary care is sought following ingestion of xylitol-containing products, a good prognosis for full recovery is expected (34). A prospective study in which dogs received the xylitol drinking water additive at the dose recommended by the manufacturer and at 5 times the recommended dosage resulted in no clinical signs or statistically significant alterations in CBC or serum chemistry values (35). If prepared according to package instructions (0.05 mg/mL concentration), a 15-kg dog with an average daily water intake (60 mL/kg BW) of 900 mL of treated water would result in a total xylitol dose of 45 mg. The same 15 kg dog would need to ingest 1500 mg or 30 L of appropriately treated water/day in order to receive the reported toxic dose (100 mg/kg BW). Regardless of size the maximum daily dose recommended by the manufacturer is 50 mg; therefore, even a 2-kg dog would receive well under the reported toxic dose. If adequate instructions are given and client compliance is good, the product used in this clinical trial poses minimal acute health risk to patients. Despite this, there are no studies reporting the health risks of chronic, low dose administration of xylitol in dogs.
Clinical trials similar to those performed in humans are required in dogs in order to determine therapeutic doses of xylitol in dogs. If the therapeutic dose is extrapolated from data performed in humans, the therapeutic dose must be expressed as a dose per unit of body weight (mg/kg BW) due to the wide difference in average weight from humans to dogs. In order to reach therapeutic (anti-plaque) effects, the required daily dose of xylitol in humans is 5000 to 10 000 mg divided into 3 doses (16). An average human weight of 70 kg results in a recommendation of 71 to 143 mg/kg BW per day to reach plaque-reducing therapeutic levels of xylitol. As discussed earlier, the toxic effects reported in dogs are seen with doses > 100 mg/kg BW. At the maximum dose recommended by the manufacturer (50 mg) the maximum daily dose of xylitol for a 15-kg dog would be 3 mg/kg BW. Although this is well below the reported toxic dose, it is also well below the therapeutic range recommended for humans (71 to 143 mg/kg BW per day). Following microbiological and further safety trials, therapeutic levels of xylitol need to be determined in dogs.
A product offering high client compliance with plaque and calculus reducing properties is ideal. Previous reports in cats have documented great success in the reduction of plaque (52.3%) and calculus (53.5%) using a xylitol drinking water additive (21). This study did not find similar results, although a 14.9% and 5.1% decrease in calculus and plaque accumulation was reported. While an overall decrease was seen in plaque and calculus development with use of a xylitol drinking water additive, in order to achieve an optimal oral health status it should be used in conjunction with additional anti-plaque and anti-calculus agents such as dental diets, treats, oral gels and rinses, tooth brushing, and routine oral examinations by a veterinarian. The current study shows potential results into the plaque and calculus reducing effects of xylitol in dogs. Because of this, further research is warranted to evaluate the effect of xylitol drinking water additives on plaque and calculus accumulation in dogs. Potential research may involve microbiological effects of xylitol on common subgingival plaque in dogs. Larger clinical trials using less invasive scoring methods are required to investigate the therapeutic levels of xylitol. CVJ
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Lund EM, Armstrong PJ, Kirk CA, Kolar LM, Klausner JS. Health status and population characteristics of dogs and cats examined at private veterinary practices in the United States. J Am Vet Med Assoc. 1999;214:1336–4131. [PubMed] [Google Scholar]
- 2.Wiggs RB, Lobprise HB. Veterinary Dentistry, Principles and Practice. Philadelphia, Pennsylvania: Lippincott Raven; 1997. Periodontology; pp. 186–231. [Google Scholar]
- 3.Harvey CE, Shofer SS, Laster L. Association of age and body weight with periodontal disease in North American Dogs. J Vet Dent. 1994;11:94–105. [PubMed] [Google Scholar]
- 4.Logan EI. Dietary influences on periodontal health in dogs and cats. Vet Clin North Am Small Anim Pract. 2006;36:1385–1401. doi: 10.1016/j.cvsm.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Jensen L, Logan EI, Finney O, et al. Reduction in accumulation of plaque, stain, and calculus in dogs by dietary means. J Vet Dent. 1995;12:161–163. [PubMed] [Google Scholar]
- 6.Hennet P, Servet E, Soulard Y, Biourge V. Effect of pellet food size and polyphosphates in preventing calculus accumulation in dogs. J Vet Dent. 2007;24:236–239. doi: 10.1177/089875640702400405. [DOI] [PubMed] [Google Scholar]
- 7.Pavlica Z, Petelin M, Juntes P, Erzen D, Crossley DA, Skaleric U. Periodontal disease burden and pathological changes in organs of dogs. J Vet Dent. 2008;25:97–105. doi: 10.1177/089875640802500210. [DOI] [PubMed] [Google Scholar]
- 8.Glickman LT, Glickman NW, Moore GE, Goldstein GS, Lewis HB. Evaluation of the risk of endocarditis and other cardiovascular events on the basis of the severity of periodontal disease in dogs. J Am Vet Med Assoc. 2009;234:486–494. doi: 10.2460/javma.234.4.486. [DOI] [PubMed] [Google Scholar]
- 9.DeBowes LJ, Mosier D, Logan E, Harvey CE, Lowry S, Richardson DC. Association of periodontal disease and histologic lesions in multiple organs from 45 dogs. J Vet Dent. 1996;13:57–60. [PubMed] [Google Scholar]
- 10.Rawlinson JE, Goldstein RE, Reiter AM, Attwater DZ, Harvey CE. Association of periodontal disease with systemic health indices in dogs and the systemic response to treatment of periodontal disease. J Am Vet Med Assoc. 2011;238:601–609. doi: 10.2460/javma.238.5.601. [DOI] [PubMed] [Google Scholar]
- 11.Quirynen M, Teughels W, Jakubovics N. Carranza’s Clinical Periodontology. St. Louis, Missouri: WB Saunders; 2012. Periodontal microbiology; pp. 232–270. [Google Scholar]
- 12.Roudebush P, Logan E, Hale FA. A systematic review of homecare for prevention of periodontal disease in dogs and cats. J Vet Dent. 2005;22:6–14. doi: 10.1177/089875640502200101. [DOI] [PubMed] [Google Scholar]
- 13.Harvey C, Serfilippi L, Barnvos D. Effect of frequency of brushing teeth on plaque and calculus accumulation, and gingivitis in dogs. J Vet Dent. 2015;32:16–21. doi: 10.1177/089875641503200102. [DOI] [PubMed] [Google Scholar]
- 14.Allan RM, Adams VJ, Johnston NW. Prospective randomised blinded clinical trial assessing effectiveness of three dental plaque control methods in dogs. J Small Anim Pract. 2019;60:212–217. doi: 10.1111/jsap.12964. [DOI] [PubMed] [Google Scholar]
- 15.Miller BR, Harvey CE. Compliance with oral hygiene recommendations following periodontal treatment of client owned dogs. J Vet Dent. 1994;11:18–19. [PubMed] [Google Scholar]
- 16.Ly KA, Milgrom P, Rothen M. The potential of dental-protective chewing gum in oral health interventions. J Am Dent Assoc. 2008;139:553–563. doi: 10.14219/jada.archive.2008.0215. [DOI] [PubMed] [Google Scholar]
- 17.Clarke DE. Drinking water additive decreases plaque and calculus accumulation in cats. J Vet Dent. 2006;23:79–82. doi: 10.1177/089875640602300203. [DOI] [PubMed] [Google Scholar]
- 18.Logan EI, Boyce EN. Oral health assessment in dogs: Parameters and methods. J Vet Dent. 1994;11:58–63. [PubMed] [Google Scholar]
- 19.Maguire A, Rugg-Gunn AJ. Xylitol and caries prevention — Is it a magic bullet? Br Dent J. 194:429–436. doi: 10.1038/sj.bdj.4810022. [DOI] [PubMed] [Google Scholar]
- 20.Söderling E, Alaräisänen L, Scheinin A, Mäkinen KK. Effect of xylitol and sorbitol on polysaccharide production by and adhesive properties of Streptococcus mutans. Caries Res. 1987;21:109–116. doi: 10.1159/000261011. [DOI] [PubMed] [Google Scholar]
- 21.Trahan L, Bareil M, Gauthier L, Vadeboncoeur C. Transport and phosphorylation of xylitol by a fructose phosphotransferase system in Streptococcus mutans. Caries Res. 1985;19:53–63. doi: 10.1159/000260829. [DOI] [PubMed] [Google Scholar]
- 22.Scherl DS, Coffman L, Van Cleave M, Lowry S. Validation of a new dental plaque quantification method in dogs. J Vet Dent. 2007;24:14–20. doi: 10.1177/089875640702400103. [DOI] [PubMed] [Google Scholar]
- 23.Hennet P. Review of studies assessing plaque accumulation and gingival inflammation in dogs. J Vet Dent. 1999;16:23–29. [PubMed] [Google Scholar]
- 24.Harvey CE. Shape and size of teeth of dogs and cats–Relevance to studies of plaque and calculus accumulation. J Vet Dent. 2002;19:186–95. doi: 10.1177/089875640201900401. [DOI] [PubMed] [Google Scholar]
- 25.Hennet P, Servet E, Salesse H, Soulard Y. Evaluation of the Logan & Boyce plaque index for the study of dental plaque accumulation in dogs. Res Vet Sci. 2006;80:175–180. doi: 10.1016/j.rvsc.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Loret J. These d’edoctorate en pharmacie. Université Paul Sabatier; Toulouse, France: Dec, 1990. Etude de l’efficacité in vivo in vitro de l’association spiramycine-metronidazole sur la flore paradontale après induction experimentale d’une paradontite chez le chien. [Google Scholar]
- 27.Wunder JA, Briner WW, Calkins GP. Identification of the cultivable bacteria in dental plaque from the beagle dog. J Dent Res. 1976;55:1097–1102. doi: 10.1177/00220345760550061601. [DOI] [PubMed] [Google Scholar]
- 28.Forster H, Quadbeck R, Gottstein U. Metabolic tolerance to high doses of oral xylitol in human volunteers not previously adapted to xylitol. Int J Vitam Nutr Res Suppl. 1982;22:67–88. [PubMed] [Google Scholar]
- 29.Dunayer EK. New findings on the effects of xylitol ingestion in dogs. Vet Med. 2006;12:791–796. [Google Scholar]
- 30.Dunayer EK, Qwaltney-Brant SM. Acute hepatic failure and coagulopathy associated with xylitol ingestion in eight dogs. J Am Vet Med Assoc. 2006;229:1113–1117. doi: 10.2460/javma.229.7.1113. [DOI] [PubMed] [Google Scholar]
- 31.Kuzuya T, Kanazawa Y, Kosaka K. Plasma insulin response to intravenously administered xylitol in dogs. Metabolism. 1966;15:1149–1152. doi: 10.1016/0026-0495(66)90105-3. [DOI] [PubMed] [Google Scholar]
- 32.Kuzuya T, Kanazawa Y, Kosaka K. Stimulation of insulin secretion by xylitol in dogs. Endocrinology. 1969;84:200–207. doi: 10.1210/endo-84-2-200. [DOI] [PubMed] [Google Scholar]
- 33.Xia Z, He Y, Yu J. Experimental acute toxicity of xylitol in dogs. J Vet Pharmacol Therap. 2009;32:465–469. doi: 10.1111/j.1365-2885.2009.01065.x. [DOI] [PubMed] [Google Scholar]
- 34.DuHadway MR, Sharp CR, Meyers KE, Koenigshof AM. Retrospective evaluation of xylitol ingestion in dogs: 192 cases (2007–2012) J Vet Emerg Crit Care. 2015;25:646–654. doi: 10.1111/vec.12350. [DOI] [PubMed] [Google Scholar]
- 35.Anthony JM, Weber LP, Alkemade S. Blood glucose and liver function in dogs administered a xylitol drinking water additive at zero, one and five times dosage rates. Vet Sci Develop. 2011;2:7–9. [Google Scholar]