Abstract
Background
Ustilago cynodontis ranks among the relatively unknown itaconate production organisms. In comparison to the well-known and established organisms like Aspergillus terreus and Ustilago maydis, genetic engineering and first optimizations for itaconate production were only recently developed for U. cynodontis, enabling metabolic and morphological engineering of this acid-tolerant organism for efficient itaconate production. These engineered strains were so far mostly characterized in small scale shaken cultures.
Results
In pH-controlled fed-batch experiments an optimum pH of 3.6 could be determined for itaconate production in the morphology-engineered U. cynodontis Δfuz7. With U. cynodontis ∆fuz7r ∆cyp3r PetefmttA Pria1ria1, optimized for itaconate production through the deletion of an itaconate oxidase and overexpression of rate-limiting production steps, titers up to 82.9 ± 0.8 g L−1 were reached in a high-density pulsed fed-batch fermentation at this pH. The use of a constant glucose feed controlled by in-line glucose analysis increased the yield in the production phase to 0.61 gITA g−1GLC, which is 84% of the maximum theoretical pathway yield. Productivity could be improved to a maximum of 1.44 g L−1 h−1 and cell recycling was achieved by repeated-batch application.
Conclusions
Here, we characterize engineered U. cynodontis strains in controlled bioreactors and optimize the fermentation process for itaconate production. The results obtained are discussed in a biotechnological context and show the great potential of U. cynodontis as an itaconate producing host.
Keywords: Fermentation, pH control, Ustilago cynodontis, Process optimization, Product toxicity, Itaconic acid
Background
Itaconic acid is an unsaturated dicarboxylic acid with two pKa values at 3.8 and 5.5 Depending on the pH value, the undissociated form H2ITA, the single dissociated form HITA− and the double dissociated form ITA2− can exist [1, 2]. Further it contains a methylene group, and its functional groups are especially interesting for the polymer industry. Depending on the groups chosen for polymerization, products with different properties can be synthesized and used for different applications [3, 4]. In addition, itaconate is also gaining increasing visibility in the pharmaceutical sector [5]. Aspergillus terreus, Ustilago maydis and Ustilago cynodontis are known as good itaconate producing organisms [6–8]. The biochemical pathways and underlying gene clusters responsible for itaconate production in these organisms are well-studied [6, 9–12]. Since over 60 years A. terreus is used for itaconate production by surface or stirred tank fermentation [3, 13]. What exactly triggers itaconate production in A. terreus, and especially why it produces itaconate, is still unknown [8]. In general production is initiated at low pH-values [7, 14, 15 ]. After initiating efficient itaconate production, it could be shown that increasing pH-value can enhance itaconate titers, whereby the timing of the pH increase is important [2, 16]. By controlling the pH at 3.4 after the itaconate initiating phase, product titers up to 160 g L−1 could be achieved [2]. Further productivity could be increased by media optimization and pH-shift experiment to 1.15 g L−1 h−1 [16] and the highest reported yield with 0.72 gITA g−1GLC was reached by optimizing oxygen transfer [17]. Following submerged fermentation with A. terreus, the itaconic acid is typically purified by repeated crystallization in industrial settings [18]. Although A. terreus is a highly efficient itaconate producer, some drawbacks exist for this host. One feature that causes high costs is the ability to grow as pellet or mycelia, respectively [19]. While pellet sizes between 0.1 to 0.5 mm resulted in the highest itaconate productivity [20], growing in mycelial form leads to a stop of itaconate production [2, 19]. This morphology is strongly influenced by media compositions. Currently molasses is used as carbon source to reduce costs. Concentrations of > 5 µg/L of impurities like manganese are known to induce mycelium formation, and this impure substrate must therefore be pretreated by ion exchange chromatography or ferrocyanide treatment [7, 21]. Alternatively, these morphology issues might also be suppressed by the addition of short-chain alcohols or copper, which are used in citric acid production processes [22]. These additional steps or medium components, however, make medium preparation more costly and control of the fermentation process more complex [22]. Also, the biochemical basis for their beneficial effect is not fully elucidated and further investigations are necessary [7]. Other factors such as pH- and shear stress can also induce mycelial growth and reduce itaconate production in A. terreus [8]. Beyond the manganese sensitivity, the morphology issue and the peculiarities of Aspergillus in general drastically reduce the process window, including the applicable pH range, the presence of solids and the tolerance towards other medium impurities. Thus, in order to achieve a breakthrough in this very mature process, we investigate the Ustilaginaceae as alternative unicellular hosts that avoid these morphological and process-related drawbacks [23].
Besides A. terreus many Ustilaginaceae are known to produce itaconate naturally [24, 25]. The most well studied member of this family is U. maydis. In wildtype U. maydis, itaconate production is initiated by nitrogen limitation [26] and production takes place above pH-values of 5.5 [24], although engineered strains can produce itaconate at lower pH [27]. While its yeast-like growth behavior is a benefit especially for production in a bioreactor, current values for titer, yield and productivity on glucose are far away from that what is published for A. terreus [6, 9]. U. cynodontis is another promising Ustilaginaceae which, however, displayed strong filamentous growth [24, 28]. Unlike U. maydis, U. cynodontis has a high tolerance towards low pH, which poses major benefits for itaconate production. Recently we could overcome the strong filamentous growth behavior under biotechnologically relevant conditions through the deletion of fuz7, encoding a MAPK protein involved in the regulation of tube formation and filamentous growth [11]. Further it was possible to increase itaconate production up to 6.5-fold compared to the wildtype by metabolic engineering, involving the deletion of P450 monooxygenase-encoding cyp3, the overexpression of ria1 encoding the itaconate cluster regulator, and heterologous expression of the mitochondrial tricarboxylate transporter MttA from A. terreus [11]. In this study, we apply this optimized U. cynodontis strain in controlled bioreactors. The optimal pH value for itaconate production is determined, followed by process optimization to enhance itaconate production by different glucose feeding strategies and by repeated batch. By this means, we demonstrate the potential of U. cynodontis as alternative acid-tolerant itaconate producer with a stable yeast-like morphology.
Results and discussion
Influence of pH and yeast extract on itaconate production by engineered U. cynodontis
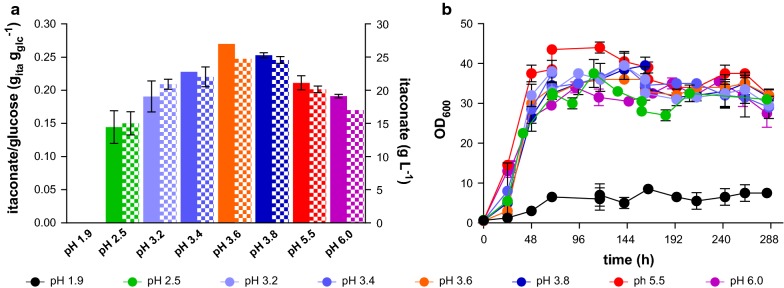
Previously we reported that by deletion of fuz7 the strong filamentous morphology of Ustilago cynodontis was switched to stable yeast like growth, resulting in a better production of itaconate. Shake flask experiments in different buffered media indicated that U. cynodontis has high acid tolerance, but the optimum for itaconate production could not be determined in this setup [11]. Since it is known that the pH is a key factor in itaconate production with considerable influence on later downstream processes, and that protonated itaconate leads to weak acid uncoupling [1, 2, 8, 16], we determined the optimal pH for itaconate production in U. cynodontis ∆fuz7 by pH-controlled pulsed fed-batch fermentations (Fig. 1). Cultures were performed at pH values of 1.9, 2.5, 3.2, 3.4, 3.6, 3.8, 5.5 and 6.0, set from the beginning of inoculation by manual addition of HCl and controlled afterwards with NaOH. The stirrer was set to 1000 rpm in batch medium without yeast extract, 0.8 g L−1 NH4Cl and 50 g L−1 glucose, after which 50 ml of a 500 g/l glucose stock solution was pulsed twice when the concentration reached ± 30 g L−1. Corresponding titers, yields and OD600 are depicted in Fig. 1.
Fig. 1.
Controlled high-density pulsed fed-batch fermentation of U. cynodontis ∆fuz7 at different pH values. a Yield in gITA g−1GLC (filled bars) and itaconate concentration (patterned bars) and b OD600 during fermentation in a bioreactor containing batch medium without yeast extract with 50 g L−1 glucose, and 0.8 g L−1 NH4Cl, pulsed twice with 50 mL of a 50% glucose stock, controlled at different pH values titrated with NaOH. Error bars indicate the deviation from the mean (n = 2) with the exception of the fermentation at pH 3.6, which shows a single representative culture
Strong growth inhibition was observed at pH 1.9 compared to the other cultures. However, U. cynodontis Δfuz7 both grew and produced itaconate at the second lowest pH of 2.5, although the yield was 1.9-fold lower than at the optimal pH of 3.6 where a titer of 24.7 g L−1 and a yield of 0.27 gITA g−1GLC were reached. These differences in production are likely related to the higher level of weak acid stress. Below a pH value of 3.8 the protonated form (H2ITA) is predominant, which can diffuse through the plasma membrane and acidify the cytoplasm resulting in growth and/or product inhibition for the cell. In contrast, the dissociated forms HITA− and ITA2− cannot cross the membrane by diffusion due to their charge and stay in the fermentation broth [2, 29–31]. To determine the concentrations of each dissociation form of itaconic acid in this study CurTiPot was used [32, 33]. Between a pH value of 2.5 and 3.6 the concentration of protonated H2ITA at the end of the cultures was 14.6 ± 0.8 g L−1 (Table 1). This concentration is relatively constant, especially considering the much larger differences in total titer, indicating that this protonated product level is inhibitory for the cells. With further increasing pH H2ITA concentrations decrease and the relatively harmless dissociated forms become predominant, even though glucose was not fully consumed. Possibly, higher pH values change the regulation of the itaconate cluster genes. This was also observed in itaconic acid production in A. terreus where the optimum for production was determined at a pH of 3.4 [2]. However, with A. terreus a morphological change was the main reason for this decrease. Such a morphological change was excluded with U. cynodontis Δfuz7. Likely, the pH optimum for itaconate production is at least in part governed by regulatory mechanisms of the genes in the itaconate cluster.
Table 1.
Distribution of protonation states of itaconate in controlled high-density pulsed fed-batch fermentation of U. cynodontis ∆fuz7 at different pH values
| pH | H2ITA (g L−1) | HITA− (g L−1) | ITA2− (g L−1) | Total titer (g L−1) |
|---|---|---|---|---|
| 1.9 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 |
| 2.5 | 14.1 ± 1.2 | 0.9 ± 0.1 | 0.0 ± 0.0 | 15.0 ± 1.2 |
| 3.2 | 15.9 ± 0.4 | 5.0 ± 0.1 | 0.1 ± 0.0 | 20.9 ± 0.5 |
| 3.4 | 14.6 ± 0.7 | 7.2 ± 0.3 | 0.1 ± 0.0 | 22.0 ± 1.1 |
| 3.6 | 13.7 | 10.7 | 0.3 | 24.7 |
| 3.8 | 10.7 ± 0.2 | 13.3 ± 0.2 | 0.6 ± 0.0 | 24.6 ± 0.4 |
| 5.5 | 0.1 ± 0.0 | 6.2 ± 0.1 | 13.8 ± 0.2 | 20.1 ± 0.4 |
| 6 | 0.0 ± 0.0 | 2.1 ± 0.0 | 14.9 ± 0.0 | 17.0 ± 0.0 |
Errors indicate the deviation from the mean (n = 2) with the exception of the fermentation at pH 3.6, which shows a single representative culture
For all used pH values no filamentous growth was observed, in accordance with previous observations [11]. Differences in the color of the fermentation broth were observed. While at low pH the fermenter broth was yellowish or white, at higher pH values it became more pigmented. Low amounts of erythritol as side product were measured which did not show any particular trend. Another major side product was (S)-2-hydroxyparaconate. It has a lower pKa-value than itaconate [34] and in U. maydis, low pH values stimulate the conversion of itaconate to (S)-2-hydroxyparaconate, likely by enabling passive itaconate re-uptake [9]. Through the deletion of cyp3 (S)-2-hydroxyparaconate production could be abolished, leading to an increase in itaconate production. A further major increase was achieved by overexpression of ria1 and mttA. Possibly, these modifications affect the pH optimum at which U. cynodontis produces itaconate, which should be investigated in the future.
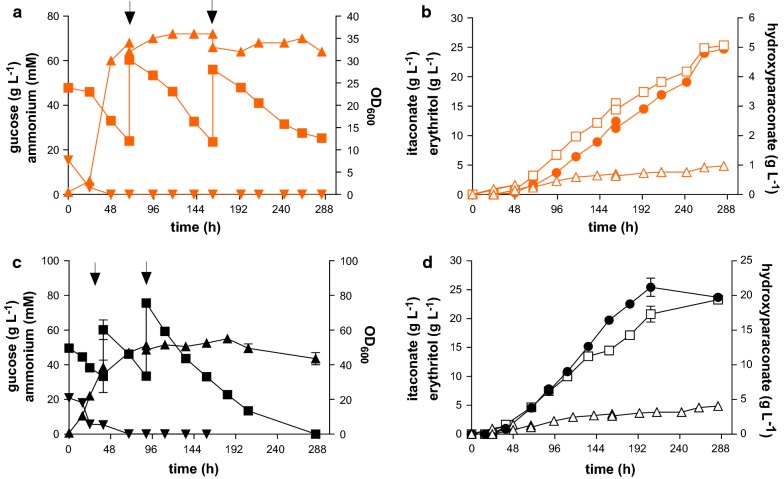
The abovementioned determination of the pH optimum was performed in a fully mineral medium. Previous fermentations with U. maydis were often performed with 1 g L−1 yeast extract added to the starting medium [24]. To see if this addition influences production, fermentations were repeated with the same conditions at the determined optimal pH of 3.6, with the exception that this time 1 g L−1 yeast extract was not omitted from the batch medium (Fig. 2). The maximum titer of 25.5 ± 1.1 g L−1 itaconate and yield of 0.25 ± 0.01 gITA g−1GLC were similar to those determined without yeast extract. In contrast maximum (S)-2-hydroxyparaconate production was increased by 3.5-fold to 17.3 ± 1.1 g L−1 and consequently the total acid concentration in batch medium with yeast extract was 1.4 fold higher. The key factor that could be improved in the case for itaconate was the productivity (Fig. 2b, d). Without yeast extract the total fermentation time was 288 h. Addition of yeast extract reduced this time to 206 h. This addition a complex medium component might increase the efforts for downstream purification, and the resulting rate gain should thus be considered in the context of the entire process [23]. For following experiments, full batch medium (which includes 1 g L−1 yeast extract) was used at a pH of 3.6.
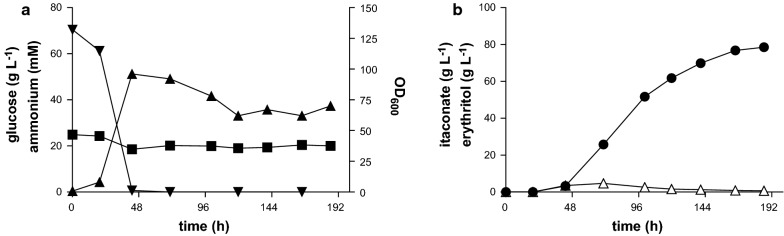
Fig. 2.
Controlled high-density pulsed fed-batch fermentation of U. cynodontis ∆fuz7. a, c OD600 (▲), glucose (■) and ammonium concentration (▼), b, d Concentration of itaconate (●), (S)-2-hydroxyparaconate (□) and erythritol (∆) during fermentation in a bioreactor containing batch medium without (a, b) or with yeast extract (c, d) with 50 g L−1 glucose, 0.8 g L−1 NH4Cl at pH 3.6 titrated with NaOH. Arrows indicate addition of 35 mL of a 50% glucose stock. Error bars indicate the deviation from the mean (n = 2) except for A and B, which show a single representative culture
Enhanced itaconate production with optimized U. cynodontis
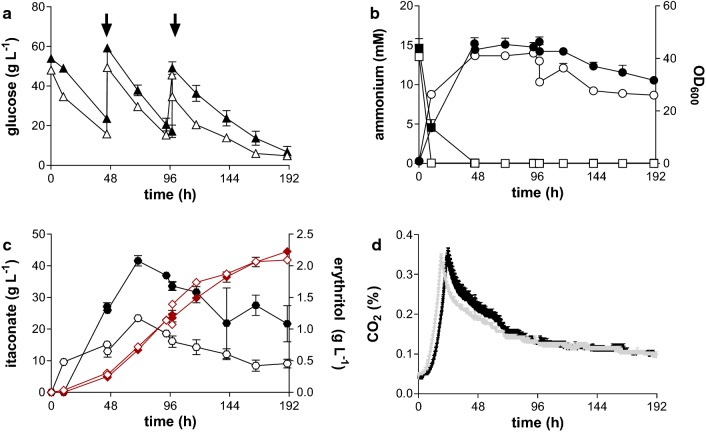
While pH optimum was determined with U. cynodontis ∆fuz7, the new hyperproducing strains described in Hosseinpour Tehrani et al. [11] were developed in parallel. The strain U. cynodontis ∆fuz7r ∆cyp3r PetefmttA Pria1ria1 produces 6.5-fold more itaconate in shake flasks compared to the wildtype. In order to assess the performance of this new strain in controlled fed-batch fermentation, it was cultured in batch medium at a constant pH of 3.6. Cultures of A. terreus are often started at a more neutral pH, letting it drop during growth after which pH control is switched on [2, 16]. This pH shift can have a positive impact in the growth phase by reducing low-pH stress, but it may also pose a higher risk of bacterial contamination at industrial scale. To test the effect of such a pH shift on the engineered U. cynodontis, another fermentation was started at pH 6.0, letting the pH drop to 3.6, after which it was controlled at this level with NaOH (Fig. 3 and Table 2). As expected, significantly more itaconate was produced in these fed-batch fermentations compared to shaken batch cultures with the optimized ∆fuz7r ∆cyp3r PetefmttA Pria1ria1 strain [11], and also compared to U. cynodontis ∆fuz7 in fed-batch cultures (Fig. 2).
Fig. 3.
Controlled high-density fed-batch fermentation of U. cynodontis NBRC 9727 ∆fuz7r ∆cyp3r PetefmttA Pria1ria1; a glucose concentration; b OD600 (circles) and ammonium (squares) concentration; c itaconate (diamonds, red) and erythritol (circles) concentration and d CO2 off-gas concentration during fermentation in a bioreactor containing batch medium with 50 g L−1 glucose, 0.8 g L−1 NH4Cl with a natural pH shift from 6 to 3.6 (filled symbols/continuous grey line) or a constant pH of 3.6 (empty symbols/continuous black line) controlled with NaOH. Arrows indicate addition of 35 mL of 50% glucose. Error bars indicate the standard error of the mean (n = 3)
Table 2.
Itaconate production parameter of various fermentation conditions with U. cynodontis NBRC 9727 ∆fuz7r ∆cyp3r PetefmttA Pria1ria1
| Constant pH 3.6 | pH shift 6–3.6 | High nitrogen | Constant glucose feed | |
|---|---|---|---|---|
| Titer (g L−1)a | 44.5 ± 1.6 | 41.8 ± 0.3 | 83.0 ± 0.8 | 78.6 |
| YP/S (gITA g−1GLC)b | 0.39 ± 0.0 | 0.39 ± 0.0 | 0.30 ± 0.0 | 0.45 |
| YMP/S (gITA g−1GLC)c | 0.47 ± 0.0 | 0.44 ± 0.0 | 0.36 ± 0.0 | 0.61 |
| rp (g L−1 h−1)d | 0.23 ± 0.0 | 0.21 ± 0.0 | 0.59 ± 0.0 | 0.42 |
| rMp (g L−1 h−1)e | 0.38 ± 0.0 | 0.34 ± 0.0 | 1.4 ± 0.0 | 0.85 |
Errors indicate the standard error from the mean (n = 3) while fermentation with a constant glucose feed is a single representative culture
aTiter: maximum itaconate concentration
bYP/S: overall yield of itaconate per consumed glucose
cYMP/S: maximum yield during the production phase
drp: overall production rate
erMp: maximum production rate
The growth phase (derived from offgas CO2 values) was approximately 5 h shorter when starting at pH 6, but in spite of this, the constant pH of 3.6 had no negative impact on production parameters. On the contrary, the maximum titer of 44.5 ± 1.6 g L−1 in the fermentation with a constant pH of 3.6 culture was slightly, but not significantly, higher than that of the fermentation with the pH shift with 41.8 ± 0.3 g L−1 (Fig. 3c, Table 2). Also similar values for biomass and CO2 formation were observed for both conditions (Table 3, Fig. 3b, d). Further, nitrogen limitation was achieved faster in fermentation with constant pH (Fig. 3b). For both conditions the same yield was observed (Table 2). The carbon balance of all tested conditions is closed to within 95% (Table 3). Some unidentified components such as ustilagic acid [24] may be produced, and should be investigated in the future.
Table 3.
Carbon distribution of various fermentation conditions with U. cynodontis NBRC 9727 ∆fuz7r ∆cyp3r PetefmttA Pria1ria1 in batch medium with various glucose and NH4Cl concentrations
| Constant pH 3.6 | pH shift 6–3.6 | High nitrogen | |
|---|---|---|---|
| Itaconate (%) | 51.6 ± 0.7 | 50.7 ± 1.3 | 38.3 ± 0.7 |
| CO2 (%) | 25.4 ± 2.2 | 25.9 ± 1.9 | 28.8 ± 0.6 |
| CDW (%) | 19.9 ± 2.3 | 20.9 ± 0.8 | 26.0 ± 1.0 |
| Erythritol (%) | 1.0 ± 0.4 | 0.4 ± 0.1 | 2.2 ± 0.1 |
| Mass balance (%) | 97.8 ± 1.9 | 97.9 ± 2.2 | 95.3 ± 2.0 |
Errors indicate the standard error of the mean (n = 3)
Overall, the production parameters achieved with a constant pH of 3.6 were similar to those achieved with U. maydis at pH > 6 where a maximum titer of 54.8 ± 2.8 g L−1, productivity of 0.33 ± 0.02 g L−1 h−1 and a yield of 0.48 ± 0.02 gITA g−1GLC were reached [9]. Depending on the process setup, the low pH optimum of U. cynodontis can provide significant benefits for downstream processing [35, 36]. Also, the lower pH reduces the risk of contamination [1], possibly enabling auto-sterile conditions, although this is not given even for low pH processes [37]. Given these advantages and the fact that no differences in itaconate production were observed, further fermentations were performed at a constant pH of 3.6. However, it should be considered that the pH optimum for the production strain U. cynodontis ∆fuz7r ∆cyp3r PetefmttA Pria1ria1 may have shifted. The elimination of (S)-2-hydroxyparaconate, a monocarboxylate with a lower pKa value than itaconate [34], will alter acidification. Also, pH plays a role in the induction of itaconate production in A. terreus [2], making it plausible that the overexpression of the itaconate cluster regulator Ria1 affects induction of itaconate production in relation to pH in U. maydis. These putative effects will be subject to future study.
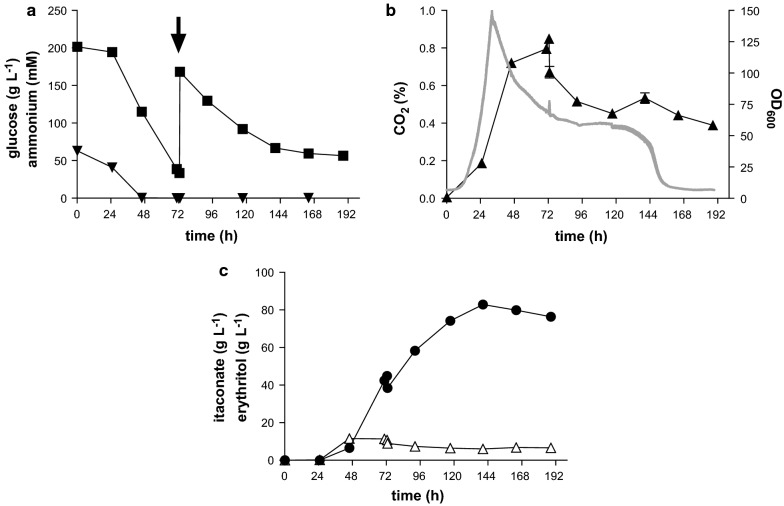
Previous high-density fermentations with U. maydis have resulted in higher titers and productivities [9, 38], potentially reducing process and investment costs in an industrial context [39, 40]. However, they often come at a cost of lower yields, although the relation between cell density and production yield, titer, and rate are often non-linear [41]. In order to investigate the effect of higher cell densities of U. cynodontis ∆fuz7r ∆cyp3r PetefmttA Pria1ria1, fermentations with 200 g L−1 glucose and 4 g L−1 NH4Cl in the batch medium were performed. With this change, however, it must also be taken into account that problems can arise such as limitation and/or inhibition of substrates, high evolution rates of CO2 and heat, high oxygen demand, and increased viscosity of the medium [42].
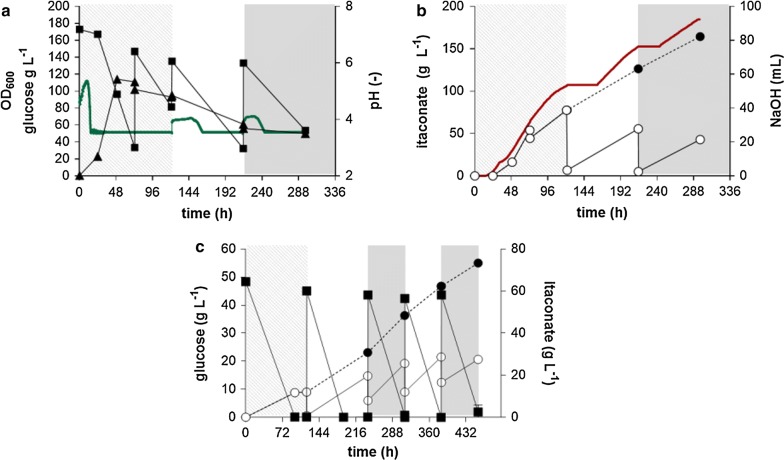
The fivefold increase in ammonium as growth-limiting nutrient resulted in a maximum titer of 82.9 ± 0.8 g L−1 itaconate after 140 h. This maximum was followed by a gradual decrease of itaconate, even though glucose was still present (Fig. 4). Simultaneously, the CO2 concentration in the exhaust gas dropped from 0.4% to 0.04% and glucose consumption stopped (Fig. 4a, b). A maximum productivity of 1.44 ± 0.02 g L−1 h−1 was reached between 46 and 73 h, which is 3.8-fold more compared to fermentation with low nitrogen content (Table 3).
Fig. 4.
Controlled high density fed-batch fermentation of U. cynodontis NBRC 9727 ∆fuz7r ∆cyp3r PetefmttA Pria1ria1. glucose (■) and ammonium (▼) concentration; b CO2 concentration (continuous line) and OD600 (▲); c itaconate (●) and erythritol (∆) concentration during fermentation in a bioreactor containing batch medium with 200 g L−1 glucose, 4.0 g L−1 NH4Cl at pH 3.6 titrated with NaOH. The arrow indicates addition of 100 mL of 50% glucose. Error bars indicate the standard error from the mean (n = 3)
Interestingly, although five times more nitrogen was used, the OD600 was only three times higher compared to the fermentation with 0.8 g L−1 NH4Cl, suggesting a possible limitation in other medium components like was observed in U. maydis [43]. Further Klement et al. [43] and Zambanini et al. [44] could show that inhibition by high NH4Cl concentrations affect biomass growth, which can be avoided by pulse-feeding the nitrogen source. A gradual decrease in productivity and biomass concentration is visible after 72 h indicating cell stress was initiated at this time point. The relatively sudden drop in the CO2 evolution rate at 140 h indicates that at this point a critical product concentration is reached at which the cells are unable to maintain their vigor, likely because at this point they are unable to counteract weak acids uncoupling due to the reduced substrate uptake rate. It is known that itaconate can inhibit isocitrate-lyase and fructose-6-phosphate 2-kinase, as well as substrate phosphorylation in mitochondria [45–47], which may further contribute to lowering the substrate uptake rate. With wildtype U. maydis external addition of 80 g L−1 itaconate fully inhibits its production [43]. Similar experiments should be performed with these engineered U. cynodontis strains to test the abovementioned hypothesis of product inhibition. In general, the high cell density cultures significantly increased the maximum titer and productivity compared to the low cell density cultures, at a relatively small cost to the product yield (Fig. 4 and Table 2). The pulsed feed in the abovementioned high-density culture coincides with a significant drop in the production rate, likely due to cumulative osmotic and weak acid stress. In addition, it is known for Ustilaginaceae that high a glucose concentration leads to slower growth and osmotic stress [9]. For these reasons, a fermentation with a constant glucose concentration of 20 g L−1 was performed whereby other parameters were equivalent to the fermentation with high nitrogen. In order to ensure a constant substrate concentration, an in-line system for the analysis of glucose from Trace Analytics (Braunschweig, Germany) was used. Cell-free in-line sampling was enabled by a dialysis probe. The glucose measurement itself is based on an enzymatic reaction with glucose oxidase [33]. The inline System of TraceAnalytics was connected to the BioFlo 120® system (Eppendorf, Jülich, Germany), enabling it to be coupled to a pump which regulates the glucose feed depending on the measured glucose concentration. Thus the glucose uptake rate could be determined by the rate of the pump. As an additional control, the consumption of the glucose stock solution was measured by weighing. Using this setup, nearly the same titer could be reached compared to the equivalent fermentation with pulsed glucose feeds, however, with a lower overall (0.42 g L−1 h−1) and maximum (0.84 g L−1 h−1) production rate (Fig. 5 and Table 2). In contrast, glucose consumption was reduced by 30% compared to the pulsed fed batch (Additional file 1: Fig. S1) leading to a much higher overall yield of 0.41 gITA g−1GLC. During the production phase between 43 and 186 h, a yield of 0.61 gITA g−1GLC was achieved, which is 84% of the theoretical maximum pathway yield. This much higher yield, along with the lower erythritol formation, strongly indicates that the cells suffer less from osmotic stress compared to the pulsed fed batch. This is also corroborated by the decrease in productivity upon the pulse in the fermentation with high nitrogen, where the combined stress of substrate and product concentrations is about fourfold higher. Despite the improvements achieved with the constant glucose concentration, the problem of product toxicity remains. Itaconate production and glucose uptake rates decreased above 50 g L−1, as also observed in the pulsed fed batch (Additional file 1: Fig. S1).
Fig. 5.
Controlled high density fed-batch fermentation of U. cynodontis NBRC 9727 ∆fuz7r ∆cyp3r PetefmttA Pria1ria1. a Glucose (■) and ammonium (▼) concentration and OD600 (▲); and b itaconate (●) and erythritol (∆) concentration during a single representative bioreactor cultivation in batch medium with constant glucose concentration, 4.0 g L−1 NH4Cl at pH 3.6 titrated with NaOH
Both fermentation approaches (pulsed fed batch and constant glucose concentration) show clear signs of product toxicity at around 80 g L−1 itaconate at a pH of 3.6 (Figs. 4, 5, 6 and Table 2). One way to overcome this would be in situ product removal by calcium salt precipitation as shown for U. maydis [48] and U. vetiveriae [44] or by reactive extraction methods [49]. Alternatively, a continuous or semi-continuous process with cell recycling can help to overcome product toxicity as well, by extending the productive time of the biomass [50].
Fig. 6.
Repeated batch with cell recycling of U. cynodontis NBRC 9727 ∆fuz7r ∆cyp3r PetefmttA Pria1ria1. a OD600 (▲) and pH (green line); b glucose (■) itaconate (●) and total itaconate (○, dashed line) concentration, and used NaOH (red line) during a single representative fermentation in a bioreactor containing batch medium with 200 g L−1 glucose, 4 g L−1 NH4Cl at pH 3.6 titrated with 10 M NaOH (a, b), or in shake flasks containing screening medium with 33 g L−1 CaCO3 and 50 g L−1 glucose (c). For the repeated batches (indicated by grey shading), culture broth was centrifuged and the biomass was subsequently re-suspended in 0.5 L fresh batch medium without nitrogen, 170 g L−1 glucose and 0.5 g L yeast extract (a, b), or culture broth was centrifuged and the biomass was subsequently re-suspended in screening medium without nitrogen, 50 g L−1 glucose and 25 g L−1 CaCO3 (c). Error bars indicate the standard error from the mean (n = 4)
Extension of productive time by repeated batch fermentation
In order to assess the stability of the biocatalyst under low pH, and to overcome product toxicity, a repeated batch approach with cell recycling was applied. The same conditions as in the pulsed fed-batch fermentation with 4.0 g L−1 NH4Cl were used, but after 120 h, cells were centrifuged and resuspended in fresh batch medium without NH4Cl. Yeast extract (0.5 g L−1) was added to the medium because this addition greatly improved cell recovery in initial pilot experiments. In the initial batch phase, 77.6 g L−1 itaconate was produced which corresponds to a yield of 0.4 gITA g−1GLC (Fig. 6). In the first repeated batch phase 49 g L−1 itaconate with a yield of 0.5 gITA g−1GLC and in the second repeated batch 38 g L−1 with a yield of 0.5 gITA g−1GLC were produced. Base totalizer revealed that in the first 39 h of the first repeated batch and 28 h of the second batch, a lag phase occurred in which no itaconate was produced (Fig. 6b). This lag phase can likely be attributed to the centrifugation steps used for the cell recycling, which deprive the cells of oxygen under low pH conditions. This lag phase was absent in shake flasks with CaCO3 at neutral pH (Fig. 6c) and in addition no yeast extract was necessary in shake flask to allow the cells to recover after the centrifuging step. The difference in the lag phases may be explained by different centrifugation times. While the first batch was centrifuged for 20 min, the second batch was only centrifuged for 5 min to minimize oxygen limitation. Cell density decreased with each repeated batch, which was reflected in the volumetric productivity. Overall, the cell recycling positively affected the product yield, which was stable across two repeated batches. However, significant lag phases and reductions in biomass and production rates indicate a high stress imposed by the centrifugation steps applied here for cell recycling. To overcome these issues a membrane-based cell retention system should be used [51].
Conclusions
This study demonstrates the applicability of the pH tolerant Ustilago cynodontis in controlled fed-batch cultivations, reaching high yield, titer and rate at a low pH value. High density fermentation, especially coupled with a continuous glucose feed, provided the overall best balance of production parameters, reaching high titers and yields with a minimal loss in productivity. Titers of up to 82.9 g L−1 were reached, which imposed significant product toxicity onto the cell, completely inhibiting the substrate uptake rate. Repeated-batch cultures indicated a high stability of the biomass, showing the potential to overcome product toxicity in a continuous itaconate production system with cell retention, especially if centrifugation steps can be avoided in the future. In all, this study demonstrates the possibilities enabled by the stable yeast-like morphology of the engineered U. cynodontis strain, while retaining the benefit of low pH fermentation for itaconic acid production.
Methods
Strains and culture conditions
Controlled batch cultivations were performed with Ustilago cynodontis ∆fuz7 and U. cynodontis ∆fuz7r ∆cyp3r PetefmttA Pria1ria1 in a BioFlo® 115 bioreactor (Eppendorf, Germany) with a total volume of 1.3 L and a working volume of 0.5 L. The Eppendorf BioFlo® 120 bioprocess control station (Eppendorf, Germany) was used in combination with the online glucose measurement system from Trace Analytics (Trace Analytics, Germany) with a total volume of 2.0 L and a starting volume of 1.0 L. All cultivations were performed in batch medium according to Geiser et al. [9] containing 0.2 g L−1 MgSO4·7H2O, 0.01 g L−1 FeSO4·7H2O, 0.5 g L−1 KH2PO4, 1 g L−1 yeast extract (Merck Millipore, Germany) 1 mL L−1 vitamin solution, and 1 ml L−1 trace element solution and varying concentrations of glucose and NH4Cl as indicated. The vitamin solution contained (per liter) 0.05 g d-biotin, 1 g d-calcium panthotenate, 1 g nicotinic acid, 25 g myo-inositol, 1 g thiamine hydrochloride, 1 g pyridoxol hydrochloride, and 0.2 g para-aminobenzoic acid. The trace element solution contained (per liter) 15 g EDTA, 0.45 g of ZnSO4∙7H2O, 0.10 g of MnCl2∙4H2O, 0.03 g of CoCl2∙6H2O, 0.03 g of CuSO4∙5H2O, 0.04 g of Na2MoO4∙2H2O, 0.45 g of CaCl2∙2H2O, 0.3 g of FeSO4∙7H2O, 0.10 g of H3BO3, and 0.01 g of KI. During cultivation, pH 1.9, 2.5, 3.2, 3.4, 3.6, 3.8, 5.5 and 6.0 were maintained by automatic addition of 10 M NaOH and stirring rate was constant at 1000 rpm. For repeated-batch, the culture was centrifuged for 5 min to 20 min at 80 g and afterwards re-suspended in 0.5 L batch medium without NH4CL and 0.5 g L−1 yeast extract. The bioreactor was aerated with an aeration rate of 1 L min−1 (2 vvm) for working volume of 0.5 L or 2 L min−1 (1 vvm) for total volume of 2 L, while evaporation was limited by sparging the air through a water bottle. The bioreactor was inoculated to a final OD600 of 0.75 with cells from an overnight culture in 50 mL screening medium according to [24] containing 50 g L−1 glucose and 100 mM MES buffer. Repeated batch cultivation in shake flask cultivation was performed in screening medium according to Geiser et al. [24] containing 33 g L−1 CaCO3 and 50 g L−1 glucose. The cultures were centrifuged at 1473 g for 5 min at 30 °C with a Heraeus Megafuge 16R (Thermo Scientific) and a TX-400 rotor (Thermo Scietific). For subsequent cultivation, the cells were re-suspended in screening medium containing 25 g L−1 CaCO3 without NH4Cl and 50 g L−1 glucose.
Analytical methods
Cell densities were measured by determining the absorption at 600 nm with an Ultrospec 10 Cell Density Meter (Amersham Biosciences, Chalfont St Giles, UK).
For CDW determination 1 mL culture broth was centrifuged at maximum speed (Heraeus Megafuge 16R, TX-400 rotor, Thermo Scientific) and pellet was lyophilized (Scan Speed 40 lyophilizer, Labogene ApS) for 24 h at 38 °C and weighed afterwards.
Off-gas analysis for online monitoring of CO2 content were performed with BCpreFerm sensors (BlueSens gas sensor GmbH). The online CO2 signal (%) was converted into moles using a molar volume of 24 L mol−1. Mass balancing was achieved by subtracting the C-mol amount of biomass, off-gas and products (itaconate, erythritol), from the substrate glucose. For biomass, a carbon content of 57.9% (w/w) was assumed based on the biomass compositions of U. maydis under nitrogen limitation [43]. Differential interference contrast (DIC) microscopy was performed with a Leica DM500 light microscope (Leica Microsystems). Images were recorded with a Leica ICC50 digital microscope camera (Leica Microsystems). Images were taken at 630-fold magnification. The cell morphology was analyzed by microscopy at different time points in all cultivations.
The ammonium concentration in the culture supernatant was measured by a colorimetric method according to [52] using salicylate and nitroprusside.
Products in the supernatants were analyzed in a DIONEX UltiMate 3000 High Performance Liquid Chromatography System (Thermo Scientific, Germany) with an ISERA Metab AAC column 300 × 7.8 mm column (ISERA, Germany). As solvent 5 mM H2SO4 with a flow rate of 0.6 mL min−1 and a temperature of 40 °C was used. Samples were filtered with Rotilabo® (CA, 0.20 µm, Ø 15 mm) or Acrodisc® (GHP 0.20 µm, Ø 13 mm) syringe filters and afterwards diluted up to 1:30 with 5 mM H2SO4. Itaconate, (S)-2-hydroxyparaconate and erythritol, were determined with a DIONEX UltiMate 3000 Variable Wavelength Detector set to 210 nm, and glucose with a refractive index detector SHODEX RI-101 (Showa Denko Europe GmbH, Germany). Analytes were identified via retention time and UV/RI quotient compared to corresponding standards. Additionally, presence of glucose was verified with glucose test-stripes from Macherey–Nagel. For (S)-2-hydroxyparaconate standards, samples of previous studies were used, where (S)-2-hydroxyparaconate was synthesized and purified [9]. Since the purity (~ 70%) of these samples is not exactly known, indicated (S)-2-hydroxyparaconate values should be taken as rough estimates only.
All values are the arithmetic mean of at least two biological replicates otherwise it is indicated. Error bars indicate the deviation from the mean for n = 2, if n > 2 error bars indicate the standard error of the mean. Statistical significance was assessed by t test (two-tailed distribution, heteroscedastic, p ≤ 0.05).
Supplementary information
Additional file 1. Glucose consumption of fermentations in batch medium with pulsed or constant feed.
Acknowledgements
We thank Dr. Wolfgang Künnecke and Dr. Michael Hartlep (TRACE Analytics GmbH, Braunschweig Germany) for the provision and instruction of the glucose sensor and also Christoph van Eickels (Eppendorf AG, Germany) to implement the system into the BioFlo®120. We thank the Svenja Meyer (Institute of Applied Microbiology, RWTH Aachen University) for experimental support.
Authors’ contributions
All authors contributed significantly to the work. NW conceived and supervised the study. HHT designed and performed experiments and analyzed results with the help of NW and LMB. AT and KS performed fermentation experiments. HHT wrote the manuscript with help of NW and LMB. All authors read and approved the final manuscript.
Funding
This work was funded by the German Federal Ministry of Food and Agriculture (BMEL), through the Specialist agency renewable raw materials e. V. (FNR) as part of the ERA-IB project “TTRAFFIC” (FKZ 22030515). The laboratory of Lars M. Blank was partially funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy within the Cluster of Excellence 236 “TMFB” and 2186. The Fuel Science Center“.
Availability of data and materials
All data generated or analysed during this study are included in this published article and its Additional files.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12934-019-1266-y.
References
- 1.Klement T, Büchs J. Itaconic acid—a biotechnological process in change. Bioresour Technol. 2013;135:422–431. doi: 10.1016/j.biortech.2012.11.141. [DOI] [PubMed] [Google Scholar]
- 2.Krull S, Hevekerl A, Kuenz A, Pruss U. Process development of itaconic acid production by a natural wild type strain of Aspergillus terreus to reach industrially relevant final titers. Appl Microbiol Biotechnol. 2017;101:4063–4072. doi: 10.1007/s00253-017-8192-x. [DOI] [PubMed] [Google Scholar]
- 3.Okabe M, Lies D, Kanamasa S, Park EY. Biotechnological production of itaconic acid and its biosynthesis in Aspergillus terreus. Appl Microbiol Biotechnol. 2009;84:597–606. doi: 10.1007/s00253-009-2132-3. [DOI] [PubMed] [Google Scholar]
- 4.Steiger, M.G., Wierckx, N., Blank, L.M., Mattanovich, D., Sauer, M. 2016. Itaconic acid—an emerging building block, Wiley‐VCH Verlag GmbH & Co. KGaA. Industrial biotechnology: products and processes, pp. Chapter 15.
- 5.Bambouskova M, Gorvel L, Lampropoulou V, Sergushichev A, Loginicheva E, Johnson K, Korenfeld D, Mathyer ME, Kim H, Huang LH, Duncan D, Bregman H, Keskin A, Santeford A, Apte RS, Sehgal R, Johnson B, Amarasinghe GK, Soares MP, Satoh T, Akira S, Hai T, de Guzman Strong C, Auclair K, Roddy TP, Biller SA, Jovanovic M, Klechevsky E, Stewart KM, Randolph GJ, Artyomov MN. Electrophilic properties of itaconate and derivatives regulate the IκBζ-ATF3 inflammatory axis. Nature. 2018;556:501–504. doi: 10.1038/s41586-018-0052-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geiser E, Tehrani H, Meyer S, Blank LM, Wierckx N. Evolutionary freedom in the regulation of the conserved itaconate cluster by Ria1 in related Ustilaginaceae. Fungal Biol Biotechnol. 2018;5:14. doi: 10.1186/s40694-018-0058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karaffa L, Kubicek CP. Citric acid and itaconic acid accumulation: variations of the same story? Appl Microbiol Biotechnol. 2019;103:2889–2902. doi: 10.1007/s00253-018-09607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuenz A, Krull S. Biotechnological production of itaconic acid-things you have to know. Appl Microbiol Biotechnol. 2018;102:3901–3914. doi: 10.1007/s00253-018-8895-7. [DOI] [PubMed] [Google Scholar]
- 9.Geiser E, Przybilla SK, Engel M, Kleineberg W, Buttner L, Sarikaya E, Den Hartog T, Klankermayer J, Leitner W, Bölker M, Blank LM, Wierckx N. Genetic and biochemical insights into the itaconate pathway of Ustilago maydis enable enhanced production. Metab Eng. 2016;38:427–435. doi: 10.1016/j.ymben.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Geiser E, Przybilla SK, Friedrich A, Buckel W, Wierckx N, Blank LM, Bolker M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb Biotechnol. 2015;9:116–126. doi: 10.1111/1751-7915.12329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosseinpour Tehrani H, Tharmasothirajan A, Track E, Blank LM, Wierckx N. Engineering the morphology and metabolism of pH tolerant Ustilago cynodontis for efficient itaconic acid production. Metab Eng. 2019;54:293–300. doi: 10.1016/j.ymben.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Li A, van Luijk N, ter Beek M, Caspers M, Punt P, van der Werf M. A clone-based transcriptomics approach for the identification of genes relevant for itaconic acid production in Aspergillus. Fungal Genet Biol. 2011;48:602–611. doi: 10.1016/j.fgb.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Willke T, Vorlop KD. Biotechnological production of itaconic acid. Appl Microbiol Biotechnol. 2001;56:289–295. doi: 10.1007/s002530100685. [DOI] [PubMed] [Google Scholar]
- 14.Eimhjellen KE, Larsen H. The mechanism of itaconic acid formation by Aspergillus terreus. 2. The effect of substrates and inhibitors. Biochem J. 1955;60:139–147. doi: 10.1042/bj0600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robert C Nubel, Ratajak, E.J.1962.Process for producing itaconic acid.
- 16.Hevekerl A, Kuenz A, Vorlop KD. Influence of the pH on the itaconic acid production with Aspergillus terreus. Appl Microbiol Biotechnol. 2014;98:10005–10012. doi: 10.1007/s00253-014-6047-2. [DOI] [PubMed] [Google Scholar]
- 17.Shin WS, Lee D, Kim S, Jeong YS, Chun GT. Application of scale-up criterion of constant oxygen mass transfer coefficient (kLa) for production of itaconic acid in a 50 L pilot-scale fermentor by fungal cells of Aspergillus terreus. J Microbiol Biotechnol. 2013;23:1445–1453. doi: 10.4014/jmb.1307.07084. [DOI] [PubMed] [Google Scholar]
- 18.Magalhaes AI, Jr, de Carvalho JC, Medina JDC, Soccol CR. Downstream process development in biotechnological itaconic acid manufacturing. Appl Microbiol Biotechnol. 2017;101:1–12. doi: 10.1007/s00253-016-7972-z. [DOI] [PubMed] [Google Scholar]
- 19.Gao Q, Liu J, Liu LM. Relationship between morphology and itaconic acid production by Aspergillus terreus. J Microbiol Biotechnol. 2014;24:168–176. doi: 10.4014/jmb.1303.03093. [DOI] [PubMed] [Google Scholar]
- 20.Gyamerah MH. Oxygen requirement and energy relations of itaconic acid fermentation by Aspergillus terreus NRRL 1960. Appl. Microbiol. Biot. 1995;44:20–26. doi: 10.1007/BF00164475. [DOI] [Google Scholar]
- 21.Karaffa L, Diaz R, Papp B, Fekete E, Sandor E, Kubicek CP. A deficiency of manganese ions in the presence of high sugar concentrations is the critical parameter for achieving high yields of itaconic acid by Aspergillus terreus. Appl Microbiol Biotechnol. 2015;99:7937–7944. doi: 10.1007/s00253-015-6735-6. [DOI] [PubMed] [Google Scholar]
- 22.Kubicek CP, Röhr M, Rehm HJ. Citric acid fermentation. Crit Rev Biotechnol. 1985;3:331–373. doi: 10.3109/07388558509150788. [DOI] [Google Scholar]
- 23.Regestein L, Klement T, Grande P, Kreyenschulte D, Heyman B, Maßmann T, Eggert A, Sengpiel R, Wang Y, Wierckx N, Blank LM, Spiess A, Leitner W, Bolm C, Wessling M, Jupke A, Rosenbaum M, Büchs J. From beech wood to itaconic acid: case study on biorefinery process integration. Biotechnol Biofuels. 2018;11:279. doi: 10.1186/s13068-018-1273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geiser E, Wiebach V, Wierckx N, Blank LM. Prospecting the biodiversity of the fungal family Ustilaginaceae for the production of value-added chemicals. Fungal Biol Biotechnol. 2014;1:2. doi: 10.1186/s40694-014-0002-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wierckx N, Agrimi G, Lübeck SP, Steiger MG, Mira PN, Punt PJ. Metabolic specialization in itaconic acid production: a tale of two fungi. Curr Opin Biotechnol. 2020;62:153–159. doi: 10.1016/j.copbio.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 26.Zambanini T, Hartmann SK, Schmitz LM, Büttner L, Hosseinpour Tehrani H, Geiser E, Beudels M, Venc D, Wandrey G, Büchs J, Schwarzländer M, Blank LM, Wierckx N. Promoters from the itaconate cluster of Ustilago maydis are induced by nitrogen depletion. Fungal Biol Biotechnol. 2017;4:11. doi: 10.1186/s40694-017-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hosseinpour Tehrani H, Geiser E, Engel M, Hartmann SK, Hossain AH, Punt PJ, Blank LM, Wierckx N. The interplay between transport and metabolism in fungal itaconic acid production. Fungal Genet Biol. 2019;125:45–52. doi: 10.1016/j.fgb.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 28.Guevarra ED, Tabuchi T. Accumulation of itaconic, 2-hydroxyparaconic, itatartaric, and malic-acids by strains of the genus Ustilago. Agric Biol Chem. 1990;54:2353–2358. [Google Scholar]
- 29.Casal M, Paiva S, Queiros O, Soares-Silva I. Transport of carboxylic acids in yeasts. FEMS Microbiol Rev. 2008;32:974–994. doi: 10.1111/j.1574-6976.2008.00128.x. [DOI] [PubMed] [Google Scholar]
- 30.Lambert RJ, Stratford M. Weak-acid preservatives: modelling microbial inhibition and response. J Appl Microbiol. 1999;86:157–164. doi: 10.1046/j.1365-2672.1999.00646.x. [DOI] [PubMed] [Google Scholar]
- 31.Plumridge A, Hesse SJ, Watson AJ, Lowe KC, Stratford M, Archer DB. The weak acid preservative sorbic acid inhibits conidial germination and mycelial growth of Aspergillus niger through intracellular acidification. Appl Environ Microbiol. 2004;70:3506–3511. doi: 10.1128/AEM.70.6.3506-3511.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CurTiPot-pH and acid-base titration curves: analysis and simulation freeware, version 4.2 Accesible. http://www.iq.usp.br/gutz/Curtipot_.html.
- 33.Application report: Glucose/Lactate. https://www.trace.de/fileadmin/secureDownload/Applikationsbeispiele/Application_report_Glucose_Lactate_Dialysis.pdf. 2015.
- 34.Guevarra ED, Tabuchi T. Production of 2-hydroxyparaconic and itatartaric acids by Ustilago cynodontis and simple recovery process of the acids. Agric Biol Chem. 1990;54:2359–2365. [Google Scholar]
- 35.Roa Engel CA, van Gulik WM, Marang L, van der Wielen LA, Straathof AJ. Development of a low pH fermentation strategy for fumaric acid production by Rhizopus oryzae. Enzyme Microb Technol. 2011;48:39–47. doi: 10.1016/j.enzmictec.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Sauer M, Porro D, Mattanovich D, Branduardi P. Microbial production of organic acids: expanding the markets. Trends Biotechnol. 2008;26:100–108. doi: 10.1016/j.tibtech.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 37.Straathof AJJ, Wahl SA, Benjamin KR, Takors R, Wierckx N, Noorman HJ. Grand research challenges for sustainable industrial biotechnology. Trends Biotechnol. 2019;37(10):1042–1050. doi: 10.1016/j.tibtech.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 38.Maassen N, Panakova M, Wierckx N, Geiser E, Zimmermann M, Bölker M, Klinner U, Blank LM. Influence of carbon and nitrogen concentration on itaconic acid production by the smut fungus Ustilago maydis. Eng Life Sci. 2014;14:129–134. doi: 10.1002/elsc.201300043. [DOI] [Google Scholar]
- 39.Roffler SR, Blanch HW, Wilke CR. In situ extractive fermentation of acetone and butanol. Biotechnol Bioeng. 1988;31:135–143. doi: 10.1002/bit.260310207. [DOI] [PubMed] [Google Scholar]
- 40.Werpy, T., Peterson, G. 2004. Top value added chemicals from biomass. 2004 US-DoE report PNNL-16983.
- 41.Zambanini T, Kleineberg W, Sarikaya E, Buescher JM, Meurer G, Wierckx N, Blank LM. Enhanced malic acid production from glycerol with high-cell density Ustilago trichophora TZ1 cultivations. Biotechnol Biofuels. 2016;9(1):135. doi: 10.1186/s13068-016-0553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riesenberg D, Guthke R. High-cell-density cultivation of microorganisms. Appl Microbiol Biotechnol. 1999;51:422–430. doi: 10.1007/s002530051412. [DOI] [PubMed] [Google Scholar]
- 43.Klement T, Milker S, Jager G, Grande PM, Dominguez de Maria P, Büchs J. Biomass pretreatment affects Ustilago maydis in producing itaconic acid. Microb Cell Fact. 2012;11:43. doi: 10.1186/1475-2859-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zambanini T, Hosseinpour Tehrani H, Geiser E, Merker D, Schleese S, Krabbe J, Buescher JM, Meurer G, Wierckx N, Blank LM. Efficient itaconic acid production from glycerol with Ustilago vetiveriae TZ1. Biotechnol Biofuels. 2017;10:131. doi: 10.1186/s13068-017-0809-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michelucci A, Cordes T, Ghelfi J, Pailot A, Reiling N, Goldmann O, Binz T, Wegner A, Tallam A, Rausell A, Buttini M, Linster CL, Medina E, Balling R, Hiller K. Immune-responsive gene 1 protein links metabolism to immunity by catalyzing itaconic acid production. Proc Natl Acad Sci. 2013;110:7820–7825. doi: 10.1073/pnas.1218599110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nemeth B, Doczi J, Csete D, Kacso G, Ravasz D, Adams D, Kiss G, Nagy AM, Horvath G, Tretter L, Mocsai A, Csepanyi-Komi R, Iordanov I, Adam-Vizi V, Chinopoulos C. Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage. Faseb J. 2016;30:286–300. doi: 10.1096/fj.15-279398. [DOI] [PubMed] [Google Scholar]
- 47.Sakai A, Kusumoto A, Kiso Y, Furuya E. Itaconate reduces visceral fat by inhibiting fructose 2,6-bisphosphate synthesis in rat liver. Nutrition. 2004;20:997–1002. doi: 10.1016/j.nut.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 48.Hosseinpour Tehrani H, Becker J, Bator I, Saur K, Meyer S, Rodrigues Lóia AC, Blank LM, JLMupke N. Integrated strain- and process design enable production of 220 g L−1 itaconic acid with Ustilago maydis. Biotechnol Biofuels. 2019;12:263. doi: 10.1186/s13068-019-1605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreyenschulte D, Heyman B, Eggert A, Maßmann T, Kalvelage C, Kossack R, Regestein L, Jupke A, Büchs J. In situ reactive extraction of itaconic acid during fermentation of Aspergillus terreus. Biochem Eng J. 2018;135:133–141. doi: 10.1016/j.bej.2018.04.014. [DOI] [Google Scholar]
- 50.Dashti MG, Abdeshahian P. Batch culture and repeated-batch culture of Cunninghamella bainieri 2A1 for lipid production as a comparative study. Saudi J Biol Sci. 2016;23:172–180. doi: 10.1016/j.sjbs.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burgé G, Chemarin F, Moussa M, Saulou-Bérion C, Allais F, Spinnler H-É, Athès V. Reactive extraction of bio-based 3-hydroxypropionic acid assisted by hollow-fiber membrane contactor using TOA and Aliquat 336 in n-decanol. J Chem Technol Biotechnol. 2016;91:2705–2712. doi: 10.1002/jctb.4878. [DOI] [Google Scholar]
- 52.Willis RB, Montgomery ME, Allen PR. Improved method for manual, colorimetric determination of total kjeldahl nitrogen using salicylate. J Agric Food Chem. 1996;44:1804–1807. doi: 10.1021/jf950522b. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Glucose consumption of fermentations in batch medium with pulsed or constant feed.
Data Availability Statement
All data generated or analysed during this study are included in this published article and its Additional files.