Abstract
Background
Interpretation of mediastinal biopsy is often challenging even for experienced pathologists especially when a hematolymphoid neoplasm is suspected. Primary mediastinal large B-cell lymphoma (PMLBCL) and classic Hodgkin lymphoma (CHL) represent two major types of mature B-cell lymphomas of the mediastinum. Although PMLBCL and mediastinal CHL share many clinicopathologic characteristics, their treatment strategies and responses are remarkably different. We therefore aimed to find distinctive histologic or protein markers to better differentiate these two lesions.
Methods
Search for primary mediastinal B-cell lymphomas found 52 consecutive cases from 3 university hospitals of Korea during 2005 to 2012. Among them, 32 cases that were available for additional immunohistochemistry (IHC) assessment were enrolled in this study. These cases consisted of the following: CHL (N = 13), PMLBCL (N = 16), and B-cell lymphoma unclassifiable, with features intermediate between diffuse large B-cell lymphoma and CHL (gray zone lymphoma, N = 3). Along with the clinicopathologic findings, the expression of p63, GATA3 and cyclin E was investigated by IHC in the three categorized lesions mentioned above.
Results
Most clinical features overlapped between PMLBCL and CHL except for the increased disease progression and mortality found in PMLBCL. In the pathologic review, the presence of epithelioid granuloma favored a diagnosis of CHL, whereas reticulated or alveolar patterns of fibrosis were characteristic of PMLBCL. For protein markers, p63 was predominantly positive in PMLBCL (15/16) compared with CHL (2/13), which indicates that p63 is a marker of the highest diagnostic accuracy when calculated by the area under the ROC curve. GATA3 was expressed in the majority of CHL cases (10/13) compared with PMLBCL (0/16), while the expression of cyclin E was only rarely present in a minor population of PMLBCL.
Conclusions
P63 expression in tumor cells, even focal expression, and no GATA3 is the most helpful feature in distinguishing PMLBCL from mediastinal CHL.
Keywords: Primary mediastinal large B-cell lymphoma, Classic Hodgkin lymphoma, p63, GATA3, Cyclin E, Immunohistochemistry
Background
Malignant lymphoma is one of the most common causes of bulky anterior mediastinal masses in young patients [1]. Lymphomas of the mediastinum can originate either from the thymus or the lymph nodes, and thymic lymphomas show unique pathologic characteristics distinct from common nodal lymphomas [2]. Two primary representative T- and B-cell lymphomas arise in the thymus: precursor T-lymphoblastic lymphoma/leukemia (T-LBL) and primary mediastinal large B-cell lymphoma (PMLBCL). In T-LBL, prompt pathologic diagnosis is critical because the tumor cells are highly proliferative and frequently give rise to acute airway compression. However, T-LBL can be relatively easily excluded because of its uniform histologic features and by confirmation of the pathognomonic marker TdT by immunohistochemistry (IHC). On the contrary, the differential diagnosis of B-cell lymphomas is much more complicated. PMLBCL and classic Hodgkin lymphoma (CHL) constitute two major lymphomas of mediastinal B-cell origin, although other types of B-cell lymphomas such as marginal zone lymphoma do occur at a low incidence. PMLBCL and CHL share a considerable degree of similarity in terms of histomorphology, immunoprofiles and even gene expression patterns [3, 4]. The intrinsic nature of thymic B-cells and their microenvironment are responsible for the clinicopathologic features of these two lesions. Nevertheless, differentiation of PMLBCL from CHL or other B-cell lymphomas is crucial even in small specimens, because they each have different therapeutic strategies [5] and outcomes. However, histomorphologic features are frequently obscured by crush artifacts, which are almost inevitable in mediastinal biopsy due to limitations of the surgical approach. Therefore, recognition of the salient histologic patterns and careful selection of protein markers to minimize tissue loss should be pivotal in the pathologic diagnosis. This study aimed to find histopathologic parameters and immunohistochemical markers that will best characterize PMLBCL and CHL.
Methods
Case selection
Cases of B-cell lymphomas arising in the mediastinum from 2005 to 2012 were retrospectively reviewed from the archives of three university hospitals in Korea. Cases were selected when patients had solely mediastinal masses without lymphadenopathy of the other sites or when patients had a predominantly mediastinal presentation if accompanied by other nodal lesions. Histopathologic and immunohistochemical findings were reviewed at a consensus meeting of five board-certified pathologists (HJK, JEK, GSP, HKK, SKM) who agreed to use the diagnostic criteria of the 2017 WHO classification tumours of haematopoietic and lymphoid tissues [6]. Patients’ demographic data and other clinical characteristics along with follow-up data were retrieved from the electronic medical records.
Expression of protein markers and detection of EBV
Representative tissue sections that were 4 μm in thickness were used for IHC and EBV-encoded RNA (EBER) in situ hybridization (ISH). The primary antibodies used for routine pathologic examination are: CD3, CD5, CD10, CD15, CD20, CD23, CD30, CD79a, BCL6, Pax5, and IRF4/MUM-1. Additionally, IHC for GATA3 (Biocare Medical, Concord, CA), p63 (Lab Vision, Fremont, CA) and cyclin E (Santacruz Biotechnology, Santa Cruz, CA) was performed to determine their usefulness in the differential diagnosis. Expression of p63, GATA3 and cyclin E was considered positive if the percentage of positive cells was equal to or higher than 5%. To elucidate whether those three proteins are site specific marker, 88 cases of non-mediastinal DLBCL and 56 non-mediastinal CHL were investigated using tissue microarray blocks. All IHC and EBER ISH assays were conducted using the Bench Mark automated immunostainer (Ventana Medical Systems, Tucson AZ) according to validated protocols.
Statistical analysis
Nonparametric Mann-Whitney U-test and Fisher’s Exact Test were performed to compare IHC and EBER ISH results between each group of mediastinal B-cell lymphoma. Spearman’s rho was used to assess the correlation between protein expression scores. Overall survival (OS) was calculated from the date of diagnosis to the date of death and was compared using univariable Kaplan-Meier survival functions with log rank tests. The area under the receiver operating characteristics curve tested by the DeLong test was applied to determine the most discriminative diagnostic marker [7]. The results were considered statistically significant if a two-tailed P-value was < 0.05. All data were analyzed with SPSS software version 20.0 (SPSS Inc. IBM, NY, USA).
Results
Patient profile
Initially, 52 cases of mediastinal lymphomas of B-cell origin were reviewed and biopsy materials were obtained by transthoracic needle biopsy (N = 32), video-assisted thoracoscopic surgery (N = 12) or open surgical biopsy (N = 8). Half of the needle biopsy specimens (16 of 32) were removed from the study largely due to shortfall of samples either by quality- or quantity-based standards, and 4 cases of other type of B-cell lymphomas were also excluded because they were outside the scope of the study. Therefore, 32 cases were finally selected for this study: PMLBCL (N = 16), CHL (N = 13), B-cell lymphoma, unclassifiable, with features intermediate between DLBCL and classical Hodgkin lymphoma (gray-zone lymphoma) (N = 3). The detailed demographic data and survival plot of the patients are shown in Table 1 and Fig. 1. Cases were predominantly female, and the patients’ ages ranged from 15 to 86 years. Most PMLBCL patients were treated with rituximab-based chemotherapy such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) (13 of 16, 81.3%), while most CHL patients (12 of 13, 92.3%) were managed by ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine). Three patients with gray zone lymphoma were treated with the R-CHOP regimen because all had been previously diagnosed with DLBCL; however, the diagnosis was revised to gray zone lymphoma after review at the consensus meeting. The median follow-up was 72 months (range 1–116 months) for CHL, and 13 months (range 1–57 months) for PMLBCL. Cancer-specific death occurred in 5 patients, four of whom were diagnosed with PMLBCL and one of whom was diagnosed with gray zone lymphoma. Compared with patients with CHL, patients with PMLBCL showed significantly higher incidences of disease progression (p = 0.017) and a tendency for shorter OS (p = 0.08) (Fig. 1).
Table 1.
Clinical profile of patients with B-cell lymphoma of the mediastinum
| CHL, NS (N = 13) | PMLBCL (N = 16) | p value | Gray zone (N = 3) | ||
|---|---|---|---|---|---|
| Age | Median (Range) | 26(15–73) | 30 (19–51) | 0.175 | 28 (25–52) |
| Sex | Male: Female | 4(30.8%): 9(69.2%) | 6(37.5%): 10(62.5%) | 1.000 | 2: 1 |
| Methods of biopsy | Needle: VATS: Open | 6: 3: 4 | 10: 4: 2 | 0: 0: 3 | |
| IPI score | High risk (> 3) or low | 7(87.5%): 1(12.5%) | 13(86.7%): 2(13.3%) | 1.000 | 1: 2 |
| Ann Arbor stage | I-II: III-IV | 8(80.0%): 2(20.0%) | 10(71.4%): 4(28.5%) | 1.000 | 1: 2 |
| LDH | Elevated or not | 4(57.1%): 3(42.9%) | 12(80.0%): 3(20.0%) | 0.334 | 0: 3 |
| ECOG score | ≥2 or less | 0: 12(100%) | 0: 14(100%) | 0: 3 | |
| Bone marrow | Involved or not | 1(9.1%): 10(90.9%) | 2(13.3%): 13(86.7%) | 1.000 | 0: 3 |
| Bulky disease | Yes or No | 6(54.5%): 5(45.5%) | 11(73.3%): 4(26.7%) | 0.419 | 1: 2 |
| B symptoms | Yes or No | 5(55.6%): 4(44.4%) | 4(28.6%): 10(71.4%) | 0.383 | 1: 2 |
| Follow-up (months) | Median (Range) | 72 (1–116) | 14(1–57) | 48 (14–84) | |
| Dead/Alive | 0: 12(100%) | 4(26.7%): 11(73.3%) | 0.106 | 1: 2 | |
| Progression | Yes or No | 0: 12(100%) | 6(42.9%): 8(57.1%) | 0.017 | 1: 2 |
CHL classic Hodgkin lymphoma, NS nodular sclerosis, PMLBCL primary mediastinal diffuse large B-cell lymphoma (DLBCL); Gray, B-cell lymphoma unclassifiable, with features intermediate between DLBCL and classic Hodgkin lymphoma; VATS video-assisted thoracoscopic surgery
Fig. 1.
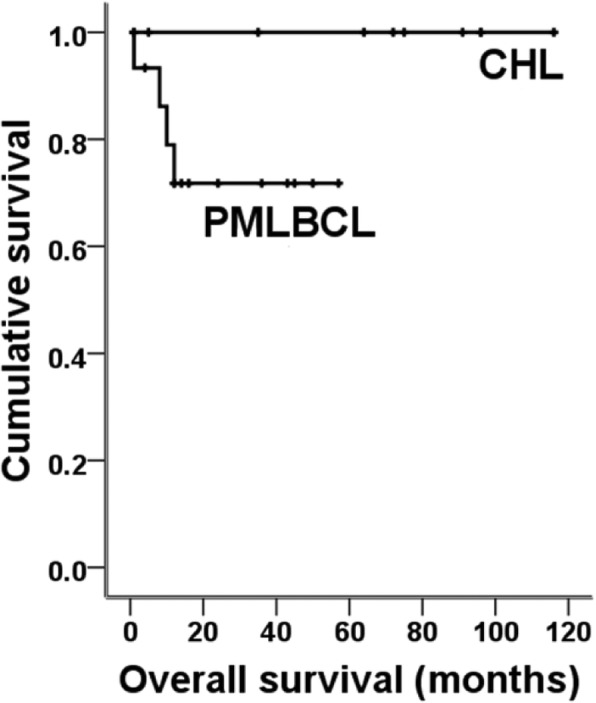
Overall survival of patients with primary mediastinal large B-cell lymphoma and classic Hodgkin lymphoma
Comparison of the pathologic features between PMLBCL and CHL
The review of the pathological findings (Table 2, Figs. 2 and 3) revealed significant differences between PMLBCL and CHL with respect to the presence of compartmentalizing alveolar pattern fibrosis (0 vs. 75%), dense collagenous bands (0 vs. 53%), diffuse sheet-like expression of CD20 (0 vs 62.5%) and CD30 (100% vs 37.5%), and positivity for CD23 (7.7% vs 37.5%), bcl-6 (0 vs 56.2%) and IRF4/MUM1 (100% vs 50%), p63 (15.4% vs 93.8%) and GATA3 (76.9% vs 0). Cyclin E was completely negative in all CHL cases, whereas a low frequency (two of 16 PMLBCL cases) of positive tumor cells was observed. An inflammatory background including granuloma or nodularity was not infrequently shared in the two disease groups. Among these pathologic parameters, expression of p63 or GATA3 and the presence of alveolar fibrosis were found to be extremely sensitive and specific markers for the differentiation of CHL from MLBCL after calculating the area under the receiver operating characteristics (Table 3). Additionally, expression of p63 and GATA3 were compared with non-mediastinal CHL and DLBCL (Additional file 1: Table S1 and Additional file 2: Table S2). GATA3 was preferentially present in tumor cells of mediastinal CHL rather than non-mediastinal origin (p = 0.000).
Table 2.
Comparison of pathologic parameters
| CHL, NS (N = 13) | PMLBCL (N = 16) | p value | Gray (N = 3) |
||
|---|---|---|---|---|---|
| Granuloma | Present or absent | 3(23.1%):10(76.9%) | 0:16(100%) | 0.078 | 0:3 |
| Fibrosis | Diffuse/nodular/reticular/none | 8(61.5%):5(38.5%):0:0 | 1(6.2%):1(6.2%):12(75.0%):2(12.5%) | < 0.001 | 1:2 |
| CD20 | Negative/focal/diffuse | 11(84.6%):2(13.4%):0 | 0:6(37.5%):10(62.5%) | < 0.001 | 0:3:0 |
| CD23 | Positive (> 30%) or negative | 1(8.3%):11(91.7%)a | 6(37.5%):10(62.5%) | 0.024 | 2:1 |
| CD30 | Diffuse/ focal/negative | 13(100%):0:0 | 6(37.5%):6(37.5%):4(25.0%) | 0.001 | 2:1 |
| IRF4/MUM1 | Positive (> 30%) or negative | 13(100%):0(0%) | 8(50.0%):8(50.0%) | 0.003 | 2:1 |
| BCL6 | Positive (> 30%) or negative | 0:12(100%)a | 9(56.2%):7(43.8%) | 0.003 | 1:2 |
| CD10 | Positive (> 30%) or negative | 0:12(100%)a | 1(6.3%):15(93.7%) | 1.000 | 0:3 |
| COO | GC or non-GCB | 0:12(100%)a | 6(37.5%):10(62.5%) | 0.024 | 0:3 |
| EBV | Present or absent | 1(7.7%):12(92.3%) | 1(6.2%):15(93.8%) | 1.000 | 0:3 |
| P63 | Positive (> 5%) or negative | 2(15.4%):11(84.6%) | 15(93.8%):1(6.2%) | < 0.001 | 2:1 |
| GATA3 | Positive (> 5%) or negative | 10(76.9%):3(23.1%) | 0:16(100%) | < 0.001 | 2:1 |
| CyclinE | Positive (> 5%) or negative | 0:13(100%) | 2(12.5%):14(87.5%) | 0.492 | 0:3 |
CHL classic Hodgkin lymphoma, NS nodular sclerosis, PMLBCL primary mediastinal diffuse large B-cell lymphoma (DLBCL); Gray, B-cell lymphoma unclassifiable, with features intermediate between DLBCL and classic Hodgkin lymphoma
a: immunohistochemistry for some antibodies was not done in one case due to exhaustion of stored tissues
Fig. 2.
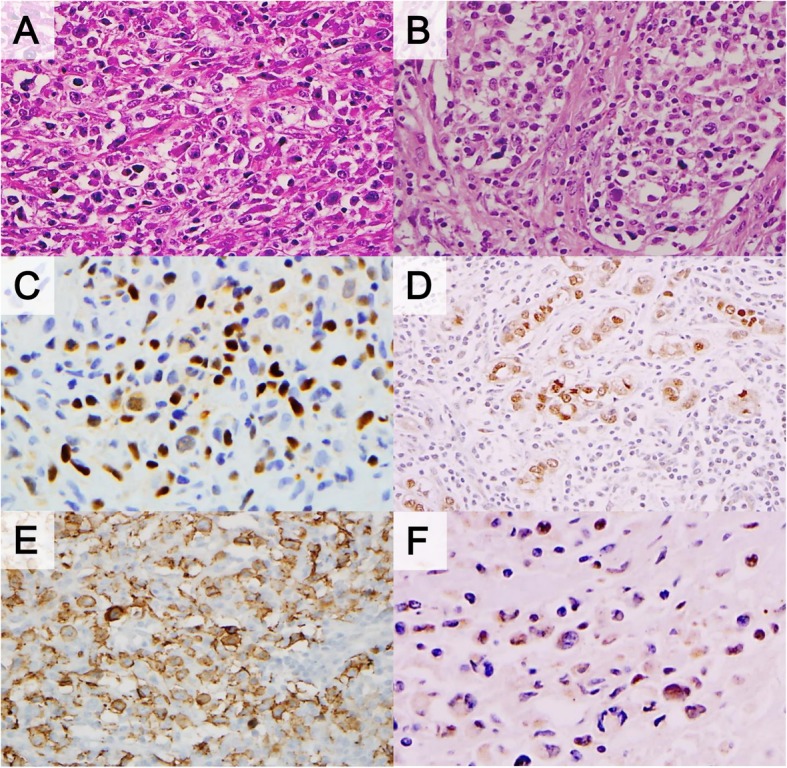
Pathologic findings of primary mediastinal large B-cell lymphoma. Reticular fibrosis (a) or alveolar type tumor aggregates (b) is characteristic. Expression of p63 (c), bcl-6 (d), and CD23 (e) were significantly higher than that of classic Hodgkin lymphoma. Minority of cases showed focal positivity for cyclin E (F)
Fig. 3.
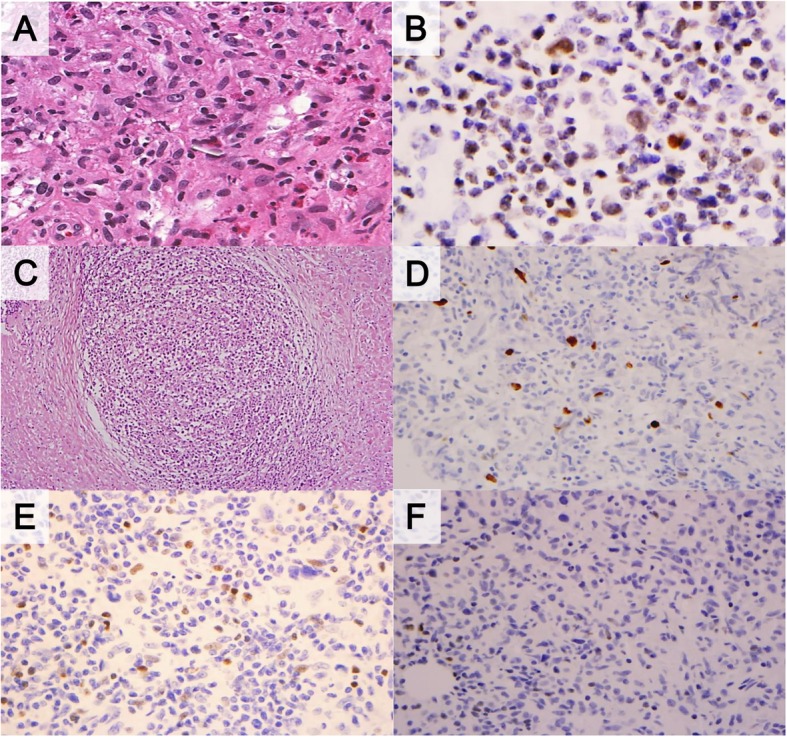
Classic Hodgkin lymphoma (a) showed significantly higher positivity for GATA3 (b). A case of gray zone lymphoma showed nodular fibrosis (c), focal positivity for MUM1 (d), p63 (e) and negativity for GATA3 (f)
Table 3.
Diagnostic utility of pathologic markers in mediastinal B-cell lymphomas
| Specificity (CHL, NS) |
Sensitivity (PMLBCL) | NPV (CHL, NS) |
PPV (PMLBCL) |
AUC (95% CI) | p value | DeLong test (p value) | |
|---|---|---|---|---|---|---|---|
| P63 | 84.6% | 93.8% | 91.7% | 88.2% | 0.892 (0.756, 1.000) | < 0.001 | reference |
| GATA3 | 75.0% | 100.0% | 100.0% | 84.2% | 0.875 (0.721, 1.000) | 0.001 | 0.918 |
| IRF4/MUM1 | 100.0% | 50.0% | 61.9% | 100.0% | 0.750 (0.570, 0.930) | 0.023 | 0.133 |
| BCL6 | 100.0% | 56.2% | 63.2% | 100.0% | 0.781 (0.609, 0.954) | 0.012 | 0.276 |
| CD30 | 100.0% | 62.5% | 68.4% | 100.0% | 0.813 (0.651, 0.974) | 0.004 | 0.386 |
| CD23 | 87.5% | 71.4% | 63.6% | 90.9% | 0.795 (0.595, 0.994) | 0.024 | 0.745 |
| EBV | 92.3% | 6.2% | 44.4% | 50.0% | 0.493 (0.278, 0.708) | 0.948 | |
| CyclinE | 100.0% | 12.5% | 46.2% | 100.0% | 0.563 (0.348, 0.777) | 0.577 | |
| Granuloma | 23.1% | 100.0% | 100.0% | 61.5% | 0.615 (0.402, 0.828) | 0.293 | |
| Alveolar fibrosis | 100.0% | 75.0% | 76.5% | 100.0% | 0.875 (0.739, 1.000) | 0.001 | 0.844 |
CHL classic Hodgkin lymphoma; NS, nodular sclerosis; PMLBCL; primary mediastinal diffuse large B-cell lymphoma; NPV negative predictive value; PPV positive predictive value; AUC area under the receiver operating characteristics
We performed the DeLong test to determine whether the addition of several important pathologic variables listed above could increase the diagnostic power for CHL and PMLBCL, but p63 was demonstrated to be the single most efficient marker of PMLBCL and was superior to various combinations (Table 3).
Discussion
In this study, we evaluated the diagnostic efficacy of several pathologic parameters including a few relatively uncommon markers in the two most representative mediastinal B-cell lymphomas. As a result, alveolar or compartmental fibrosis was the most specific and authentic histologic feature supporting a PMLBCL diagnosis. Among the protein markers, p63 and GATA3 were the most useful diagnostic markers; p63 was almost exclusively present whereas GATA3 was completely negative in PMLBCL. However, because of the interpretation difficulty of GATA3 based on the extremely low proportion of positive tumor cells, the application of p63 IHC is more highly recommended since it is easily recognizable even in small biopsy materials.
Mediastinal CHL and PMLBCL are supposedly of the same cellular origin, which has been supported by the presence of common genetic alterations and gene expression profiles [3, 8, 9], and more recently by immune-oncologic signatures such as PD-L1 or PD-L2 expression [10, 11]. The cellular origin of both tumors is thought to be post-germinal center B-cells lacking surface immunoglobulin,7 and almost all the Hodgkin/Reed-Sternberg (HRS) cells of CHL and quite a few tumor cells in PMLBCL express CD30. However, these two lymphomas exhibit a spectrum of histopathologic and immunophenotypic diversity based on the plasticity of B-cells, which should undergo physiologic transformation in the thymus. As is well known, tumor cells of PMLBCL often reveal large atypical cells reminiscent of HRS or even lacunar cells, therefore pathologists often encounter diagnostic dilemmas. Our study demonstrated that presence of fine reticulated or alveolar fibrosis strongly favored PMLBCL, however, this pattern cannot be easily recognizable in small biopsy materials. Substantial efforts have been made to find a good immunohistochemical marker of the mediastinal lymphomas, and recently, p63, cyclin E and GATA3 [12–14] have emerged as new diagnostic markers. P63 is one of the best available markers of basal or myoepithelial differentiation, but little is known of these proteins in hematolymphoid tumors. Expression of p63 has been reported in anaplastic large cell lymphoma [15], PMLBCL [16] and gray zone lymphoma [11] but is almost uniformly absent in CHL. In our series, p63 was demonstrated to be the most specific marker of PMLBCL, whereas expression of GATA3, even in a single HRS-like cell supports a diagnosis of CHL. As in previous studies [11, 16], the frequency of either p63- or GATA3-positive tumor cells in each case is variable but generally very low. This is the practical reason why p63 is preferable to GATA3 because of a relatively higher proportion of positive tumor cells.
The molecular mechanisms of p63 expression in PMLBCL and GATA3 expression in CHL have not been clearly elucidated. Rearrangement or extra copies of the TP63 gene were demonstrated in a subset of anaplastic large cell lymphoma but not in PMLBCL. As a tumor suppressor that is closely linked to the regulation of proapoptotic activity by p53 in the germinal center, the coordinative or reciprocal relationship between p63 and B-cell signaling is unknown [17]. Likewise, the theoretical background of GATA3 expression in CHL is also uncertain. GATA3 is T-cell transcription factor required for early T-cell development and its expression in B-cell lymphoma including CHL is exceptional. Experimental studies have revealed that deregulation of NFκB and Notch-1 contributes to GATA3 expression in HRS cells, which consequently leads to the activation of multiple cytokines that control microenvironment of CHL [12]. Until now, no genetic alterations of GATA3 have been reported, and its prognostic significance is not very high in CHL. However, GATA3 positivity in even a few HRS-like cells strongly supports the diagnosis of CHL in the mediastinum, where GATA3 frequency is particularly higher than other sites. Based on the heterogeneity of GATA3 expression pattern, the aberrancy of GATA3 in HRS more likely relies on microenvironmental factors rather than genetic alteration.
We did not find p63, GATA3, or cyclin E to be helpful for further characterizing gray zone lymphoma because they showed intermediate pattern between CHL and PMLBCL in our three cases. Our results are also in agreement with the previous report performed by Eberle et al. [11], which suggested the presence of common immunophenotypic features and genetic alterations that link gray zone lymphoma, CHL and PMLBCL and that places gray zone lymphoma in a still ambiguous category.
As shown in this study, thymic B-cell neoplasms are predominantly large cell types, but other types of B-cell lymphoma can occur [18–22]. Thymic B cells represent only 0.3% of total cellularity in the thymus, and the majority are mature B cells that would not undergo neoplastic transformation. However, in certain conditions such as myasthenia gravis or inflammation, migration of multipotent hematopoietic cells leads to B-cell proliferation and germinal center formation. In our series, two cases of CHL were found to have concomitant thymic cysts. The emergence of CHL in the preexisting thymic cysts has been reported. As in previous studies, our cases also support the idea that the pathogenesis of mediastinal CHL is associated with localized inflammation resulting in B-cell hyperplasia. The development of other types of B-cell lymphomas can be explained with a similar background.
Conclusions
The distinction of mediastinal CHL and PMLBCL is critical but challenging. We suggest that the presence of alveolar fibrosis and p63 expression strongly indicates PMLBCL, whereas GATA3 positivity favors CHL even when GATA3 is expressed in a very low proportion of tumor cells.
Supplementary information
Additional file 1: Table S1. Expression of p63 and GATA3 between mediastinal and non-mediastinal Hodgkin lymphoma.
Additional file 2: Table S2. Expression of p63 and GATA3 between mediastinal and non-mediastinal B-cell lymphoma.
Acknowledgements
Not applicable.
Abbreviations
- CHL
Classic Hodgkin lymphoma
- DLBCL
Diffuse large B-cell lymphoma
- EBER
EBV-encoded RNA
- Gray zone lymphoma
B-cell lymphoma unclassifiable, with features intermediate between diffuse large B-cell lymphoma and classic Hodgkin lymphoma
- IHC
Immunohistochemistry
- ISH
In situ hybridization; OS: Overall survival
- PMLBCL
Primary mediastinal large B-cell lymphoma
- T-LBL
T-lymphoblastic lymphoma/leukemia
Authors’ contributions
All authors have made substantial contributions to the paper. JEK designed the work. HJK, HJC, HKL, and SJC analyzed and interpreted the patient data. HJK, HKK, SKM, GSP, and SKM reviewed and analyzed the histopathological and immunohistochemical findings. HJK, HYN and JYC contributed to writing the manuscript and revising it. All authors read and approved the final manuscript.
Funding
This study was supported by a grant-in-aid for Scientific Research from Inje University Research Foundation (20180109) to Hyun-Jung Kim.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due the institutional review board restricts the use of the datasets to the current study only.
Ethics approval and consent to participate
Our research was performed in accordance with the Declaration of Helsinki. This study was approved by the institutional review board of Sanggye Paik Hospital (SGPAIK2016–08-033).
Consent for publication
Not applicable.
Competing interests
All authors declare any financial support or relationship that may pose a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13000-019-0918-x.
References
- 1.Carter BW, Okumura M, Detterbeck FC, et al. Approaching the patient with an anterior mediastinal mass: a guide for radiologists. J Thorac Oncol. 2014;9:S110–S118. doi: 10.1097/JTO.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 2.Cazals-Hatem D, Lepage E, Brice P, et al. Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA ("Groupe d'Etude des Lymphomes de l'Adulte") study. Am J Surg Pathol. 1996;20:877–888. doi: 10.1097/00000478-199607000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Savage KJ, Monti S, Kutok JL, et al. The molecular signature of mediastinal large B-cell lymphoma differs from that of other diffuse large B-cell lymphomas and shares features with classical Hodgkin lymphoma. Blood. 2003;102:3871–3879. doi: 10.1182/blood-2003-06-1841. [DOI] [PubMed] [Google Scholar]
- 4.Rosenwald A, Wright G, Leroy K, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198:851–862. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunleavy K, Wilson WH. Primary mediastinal B-cell lymphoma and mediastinal gray zone lymphoma: do they require a unique therapeutic approach? Blood. 2015;125:33–39. doi: 10.1182/blood-2014-05-575092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues, revised 4th ed: IARC Press Lyon; 2017.
- 7.Lopez-Raton M, Cadarso-Suarez C, Rodriguez-Alvarez MX, et al. OptimalCutpoints: an R package for selecting optimal Cutpoints in diagnostic tests. J Stat Softw. 2014;61:1–36. doi: 10.18637/jss.v061.i08. [DOI] [Google Scholar]
- 8.Calaminici M, Piper K, Lee AM, et al. CD23 expression in mediastinal large B-cell lymphomas. Histopathology. 2004;45:619–624. doi: 10.1111/j.1365-2559.2004.01969.x. [DOI] [PubMed] [Google Scholar]
- 9.Dunleavy K, Grant C, Eberle FC, et al. Gray zone lymphoma: better treated like hodgkin lymphoma or mediastinal large B-cell lymphoma? Curr Hematol Malig Rep. 2012;7:241–247. doi: 10.1007/s11899-012-0130-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y, Maeshima AM, Nomoto J, et al. Expression pattern of PD-L1 and PD-L2 in classical Hodgkin lymphoma, primary mediastinal large B-cell lymphoma, and gray zone lymphoma. Eur J Haematol. 2018;100:511–517. doi: 10.1111/ejh.13033. [DOI] [PubMed] [Google Scholar]
- 11.Eberle FC, Salaverria I, Steidl C, et al. Gray zone lymphoma: chromosomal aberrations with immunophenotypic and clinical correlations. Mod Pathol. 2011;24:1586–1597. doi: 10.1038/modpathol.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stanelle J, Doring C, Hansmann ML, et al. Mechanisms of aberrant GATA3 expression in classical Hodgkin lymphoma and its consequences for the cytokine profile of Hodgkin and reed/Sternberg cells. Blood. 2010;116:4202–4211. doi: 10.1182/blood-2010-01-265827. [DOI] [PubMed] [Google Scholar]
- 13.Lees C, Keane C, Gandhi MK, et al. Biology and therapy of primary mediastinal B-cell lymphoma: current status and future directions. Br J Haematol. 2019;185:25–41. doi: 10.1111/bjh.15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tzankov A, Gschwendtner A, Augustin F, et al. Diffuse large B-cell lymphoma with overexpression of cyclin e substantiates poor standard treatment response and inferior outcome. Clin Cancer Res. 2006;12:2125–2132. doi: 10.1158/1078-0432.CCR-05-2135. [DOI] [PubMed] [Google Scholar]
- 15.Gualco G, Weiss LM, Bacchi CE. Expression of p63 in anaplastic large cell lymphoma but not in classical Hodgkin's lymphoma. Hum Pathol. 2008;39:1505–1510. doi: 10.1016/j.humpath.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoeller S, Zihler D, Zlobec I, et al. BOB.1, CD79a and cyclin E are the most appropriate markers to discriminate classical Hodgkin's lymphoma from primary mediastinal large B-cell lymphoma. Histopathology. 2010;56:217–228. doi: 10.1111/j.1365-2559.2009.03462.x. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Xu-Monette ZY, Ok CY, et al. Prognostic impact of c-Rel nuclear expression and REL amplification and crosstalk between c-Rel and the p53 pathway in diffuse large B-cell lymphoma. Oncotarget. 2015;6:23157–23180. doi: 10.18632/oncotarget.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavabre-Bertrand T, Donadio D, Fegueux N, et al. A study of 15 cases of primary mediastinal lymphoma of B-cell type. Cancer. 1992;69:2561–2566. doi: 10.1002/1097-0142(19920515)69:10<2561::AID-CNCR2820691028>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 19.Go H, Cho HJ, Paik JH, et al. Thymic extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue: a clinicopathological and genetic analysis of six cases. Leuk Lymphoma. 2011;52:2276–2283. doi: 10.3109/10428194.2011.596968. [DOI] [PubMed] [Google Scholar]
- 20.Yu G, Kong L, Qu G, et al. Composite lymphoma in the anterior mediastinum: a case report and review of the literature. Diagn Pathol. 2011;6:60. doi: 10.1186/1746-1596-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaari Z, Charfi S, Hentati A, et al. Primary Burkitt lymphoma in the posterior mediastinum. Asian Cardiovasc Thorac Ann. 2015;23:1110–1112. doi: 10.1177/0218492315587817. [DOI] [PubMed] [Google Scholar]
- 22.Cajozzo M, Palumbo VD, Buscemi S, et al. Mediastinal syndrome from plasmablastic lymphoma in human immunodeficiency virus and human herpes virus 8 negative patient with polycythemia vera: a case report. J Med Case Rep. 2017;11:75. doi: 10.1186/s13256-016-1183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Expression of p63 and GATA3 between mediastinal and non-mediastinal Hodgkin lymphoma.
Additional file 2: Table S2. Expression of p63 and GATA3 between mediastinal and non-mediastinal B-cell lymphoma.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due the institutional review board restricts the use of the datasets to the current study only.


