Abstract
The aim of the present study was to explore the possible role of microRNA-144-5p (miR-144-5p) in rheumatoid arthritis (RA) and the associated mechanism. Following the induction of THP-1 cell differentiation into macrophages by phorbol ester (100 ng/ml) treatment, an in vitro inflammatory model of RA was established by treating the THP-1 macrophages with 1 µg/ml lipopolysaccharide (LPS). The level of miR-144-5p was subsequently measured using reverse transcription-quantitative PCR, which found that the expression of miR-144-5p was significantly reduced in LPS-treated THP-1 macrophages. Bioinformatics analysis and a dual-luciferase reporter assay were used to predict and confirm TLR2 as a direct target of miR-144-5p, respectively. Toll-like receptor 2 (TLR2) was then validated as a target gene of miR-144-5p. The effects of miR-144-5p upregulation and TLR2 silencing on LPS-treated THP-1 macrophages were then determined by transfection with miR-144-5p mimic and TLR2-siRNA, respectively. Cell viability was subsequently measured using a Cell Counting Kit-8 assay, whilst the expression of tumor necrosis factor-α (TNF-α), interleukin (IL)-6 and IL-8 secreted by THP-1 macrophages was measured using ELISA. Western blotting was performed to measure p65 phosphorylation (p-p65) in the NK-κB signaling pathway. It was found that miR-144-5p overexpression reduced macrophage cell viability, reduced the expression of TNF-α, IL-6 and IL-8, and reduced the expression of TLR2 and p-p65 compared with the control group. Likewise, TLR2 silencing also reduced macrophage cell viability and reduced the expression of TNF-α, IL-6 and IL-8 in THP-1 macrophages. In conclusion, the data from the present study suggested that miR-144-5p overexpression reduced THP-1 macrophage cell viability and inhibited the expression of TNF-α, IL-6 and IL-8 in cells, possibly by inhibiting the expression of TLR2 and suppressing the activation of NK-κB signaling. Therefore, miR-144-5p may serve as a novel therapeutic target for the treatment of RA.
Keywords: microRNA-144, rheumatoid arthritis, macrophages
Introduction
Rheumatoid arthritis (RA) is an autoimmune disease caused by systemic inflammation affecting bones and joints. The pathogenesis of RA involves a number of different mechanistic pathways in both the innate and adaptive immune systems (1).
Macrophages are a class of heterogeneous, plastic immune cells (2). To effectively adapt to the microenvironment, macrophages form sub-populations with different morphological functions by altering their phenotype, namely classically activated (also known as M1 macrophages) and alternatively activated macrophages (also known as M2 macrophages). M1 macrophages mainly express and secrete proinflammatory cytokines, including interleukin (IL)-6 and tumor necrosis factor-α (TNF-α), whereas M2 macrophages are characterized by the production of anti-inflammatory cytokines such as IL-10, which serve a role in promoting angiogenesis and tissue repair (3,4). It has been previously reported that macrophages serve a key role in the development of inflammation and joint damage in patients with RA (5), where the number of macrophages in the synovial layer and substratum has been reported to associate with disease progression (6). Additionally, proinflammatory factors secreted by M1 macrophages and/or anti-inflammatory cytokines produced by M2 macrophages have been reported to serve important roles in RA development (7).
Lipopolysaccharide (LPS) is a major constituent of the gram-negative bacterial cell wall. LPS is also one of the ligands of toll-like receptor 4 (TLR4), the triggering of which can lead to downstream signal transduction (8). Activation of this pathway results in NF-κB activation, in turn activating the extensive transcription of genes associated with inflammation and finally promoting the production of factors associated with the inflammatory response (9,10). LPS has been widely implicated in the development of various inflammatory diseases, such that its application to cell cultures in vitro has often been used as a model for simulating inflammatory responses.
MicroRNAs (miRNAs) are endogenous, single-stranded RNA molecules that are ~22 nucleotides in length and regulate the expression of target genes by binding to the 3′-untranslated regions (3′-UTR) of target mRNAs (11). Previous studies have demonstrated that miRNAs are involved in the regulation of a large number of physiological and biochemical processes, including cell proliferation, differentiation and apoptosis (12,13). In particular, miR-144-5p has been reported to serve important roles in cancer development and chronic periodontitis (14–16). However, the role of miR-144-5p in RA has not been previously studied. Therefore, in the present study, LPS-treated THP-1 macrophages was used as the model cell line to investigate the role of miR-144-5p in the pathophysiology of RA and associated mechanism.
Materials and methods
Induction of THP-1 cells into macrophages
The THP-1 cells used for the present study were obtained from the Type Culture Collection of the Chinese Academy of Sciences. THP-1 cells were cultured in RPMI 1640 medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 mg/ml streptomycin (Gibco; Thermo Fisher Scientific, Inc.) at 37°C incubator under 5% CO2.
For the differentiation of THP-1 cells into adherent growing macrophages, THP-1 cells were first incubated for 72 h in 100 ng/ml phorbol ester dissolved in serum-free RPMI-1640 medium. Following confirmation of successful differentiation by measuring the expression of markers CD14 and CD11 using flow cytometry assay (17), fresh medium was replaced 6 h before each experiment and the cell density was then adjusted to 1×106 cells/ml before subsequent experiments were performed.
LPS-induced inflammation model
Macrophages were seeded in 24-well plates at 1×106 cells/well. The cells were then divided into two groups, an LPS (1 µg/ml, Sigma-Aldrich; Merck KGaA) treatment and control group, following which they were either treated with LPS (1 µg/ml) or an equal amount of ultrapure water for 24 h. Each experiment was performed in triplicate and repeated three times.
Cell transfection
In total, 100 nM miR-144-5p mimics or the negative control of miR-144-5p mimics (NC) (Guangzhou RiboBio Co., Ltd.), 50 nM control-siRNA (sense, 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′) or TLR2-siRNA (sense, 5′-GGAACAGAGUGGCAACAGUTT-3′ and antisense, 5′-ACUGUUGCCACUCUGUUCCTT-3′) (Shanghai GenePharma Co., Ltd.). were transfected into THP-1 macrophages using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Transfection efficiency was subsequently evaluated using reverse transcription-quantitative PCR (RT-qPCR) 48 h after transfection.
Cell Counting Kit-8 (CCK-8) assay
THP-1 macrophages were first transfected with miR-144-5p mimics, NC, control-siRNA or TLR2-siRNA for 48 h, following which they were treated with LPS (1 µg/ml) for a further 24 h before cell viability was measured using the CCK-8 kit. The cell concentration was adjusted to 1×104/ml, and 100 µl cell suspension were added to each well of the 96-well plates then cultured for 12, 24 or 48 h. Subsequently, 10 µl CCK-8 reagent (Sigma-Aldrich; Merck KGaA) was added to each well followed by a further 2 h incubation. Absorbance values at the wavelength of 450 nm was then measured for each well using an ELISA plate reader (Thermo Fisher Scientific, Inc.). Each experiment was repeated three times.
ELISA
THP-1 macrophages (1×106 cells/well) were cultured in a 12-well culture plates and transfected with miR-144-5p mimics, NC, control-siRNA or TLR2-siRNA for 48 h before the cells were treated with LPS (1 µg/ml) for 24 h. Subsequently, the levels of TNF-α, IL-6 and IL-8 in the culture supernatant was analyzed using single ELISA kits (Qiagen GmbH), according to the manufacturer's protocols.
Western blot analysis
THP-1 macrophages were transfected with miR-144-5p mimics or NC for 48 h before the cells were treated with LPS (1 µg/ml) for 24 h. Cells were then washed twice with PBS and lysed in RIPA buffer (Gibco; Thermo Fisher Scientific, Inc.). Bicinchoninic Acid Protein Assay kit (Thermo Fisher Scientific, Inc.) was used to quantify protein concentrations. The protein samples (20 µg per lane) were separated using 10% SDS-PAGE and transferred to PVDF membranes (Bio-Rad laboratories, Inc.). After blocking nonspecific binding with TBS containing 0.1% Tween-20 supplemented with 5% non-fat milk for 1 h at room temperature, the membranes were then incubated with the respective primary antibodies at 4°C overnight. The next day, the membranes were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody for 2 h at room temperature. The protein bands were detected and visualized using ECL Western Blotting Detection Reagent (EMD Millipore). The bands were semi-quantitively analyzed using Image J (version 4.0; National Institutes of Health). All primary and secondary antibodies, including toll-like receptor 2 (TLR2; cat. no. 12276; 1:1,000), phosphorylated (p-)p65 (cat. no. 3033; 1:1,000), p65 (cat. no. 8242; 1:1,000), GAPDH (cat. no. 5174; 1:1,000) and HRP-conjugated goat anti-rabbit secondary antibody (cat. no. 7074; 1:1,000) were purchased from Cell Signaling Technology, Inc.
RT-qPCR
Total RNA was isolated from THP-1 macrophages using TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RNA was reverse transcribed into cDNA using a Transcriptor First Strand cDNA Synthesis kit (Roche Molecular Systems, Inc.), according to manufacturer's protocol. The temperature protocol for reverse transcription was 5 min at 25°C followed by 60 min at 42°C. qPCR was performed in a LightCycler® 480 system (Roche Molecular Systems, Inc.) using Fast SYBR® Green Master Mix (Thermo Fisher Scientific, Inc.), according to the manufacturer's protocols. The thermocycling conditions were as follows: Initial denaturation at 95°C for 5 min, followed by 38 cycles of denaturation at 95°C for 15 sec and annealing/elongation at 60°C for 30 sec. GAPDH or U6 was used as the internal control for mRNA and miRNA expression, respectively. Relative gene expression was quantified using the 2−ΔΔCq method (18). Primer sequences used for RT-qPCR were as follows: U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse, 5′-CGCTTCACGAATTTGCGTGTCAT-3′; GAPDH forward, 5′-CTTTGGTATCGTGGAAGGACTC-3′ and reverse, 5′-GTAGAGGCAGGGATGATGTTCT-3′; and TLR2 forward, 5′-AGCTTCATTGTTCCCTGTGTTAC-3′ and reverse, 5′-AGTTCACAGGAGCAGATGAAATG-3′.
Dual luciferase reporter assay
TargetScan bioinformatics software (www.targetscan.org/vert_71) (19) was used to predict the potential target genes of miR-144-5p, using which TLR2 was found. To confirm the prediction, the wild-type (WT-TLR2, 5′-UCAUAAGUCUAUUACUGAUAUCU-3′) and mutant (MUT-TLR2, 3′GAAUGUCAUAUACUACUAUAGG-5′) 3′UTR of TLR2, containing the miR-144-5p-binding elements, were generated by reverse transcription using a Transcriptor First Strand cDNA Synthesis Kit (temperature protocol, 5 min at 25°C followed by 60 min at 42°C; Roche Molecular Systems, Inc.) from total RNA preps extracted from THP-1 macrophages, and were cloned via BamHI and AscI sites of the pMIR-RB-Report™ dual luciferase reporter plasmid vector (Guangzhou RiboBio Co., Ltd.). THP-1 macrophages were then co-transfected with 500 ng each reporter construct (WT-TLR2 or MUT-TLR2) and miR-144-5p mimic or NC (100 nM) using Lipofectamine® 3000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. Luciferase activity was subsequently measured using a Dual-Luciferase® Reporter Assay system (Promega Corporation) 48 h after transfection, according to the manufacturer's protocol. All luciferase activities were normalized to that of Renilla luciferase.
Statistical analysis
Statistical analysis was performed using the SPSS 16.0 statistical package (SPSS, Inc.). Quantitative data area expressed as the mean ± SD. Student's t-test or one-way analysis of variance followed by Tukey's post-hoc test was performed for comparison. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-144-5p in THP-1 macrophages treated with LPS is significantly lower compared with that in the control group
THP-1 macrophages were treated with LPS or ultrapure water for 24 h, following which RT-qPCR was used to measure the expression of miR-144-5p. miR-144-5p expression was found to be significantly lower in the cells treated with LPS compared with water-treated cells (Fig. 1).
Figure 1.
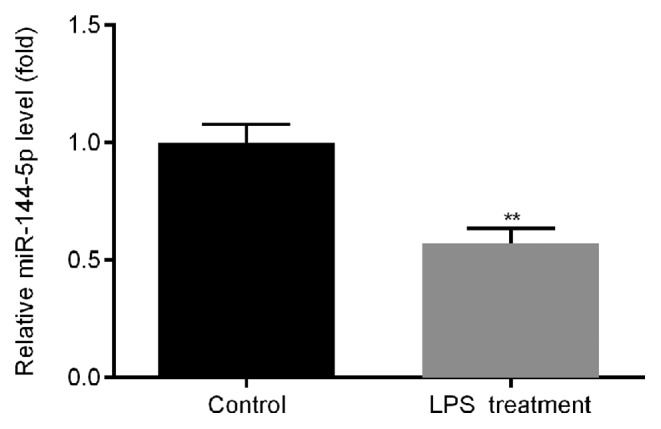
Expression of miR-144-5p in THP-1 macrophages treated with LPS. THP-1 macrophages were treated with LPS or ultrapure water for 24 h. Reverse transcription-quantitative PCR was subsequently used to detect the expression of miR-144-5p. Data are presented as the mean ± SD. **P<0.01 vs. Control. miR, microRNA; LPS, lipopolysaccharide.
Prediction of binding sites between TLR2 and miR-144-5p
Analysis via Targetscan revealed potential miR-144-5p binding sites in the TLR2 3′UTR (Fig. 2A). THP-1 macrophages were then transfected with miR-144-5p mimics or NC, followed by RT-qPCR analysis of miR-144-5p expression. Transfection with miR-144-5p mimics significantly increased miR-144-5p expression compared with the NC group (Fig. 2B).
Figure 2.
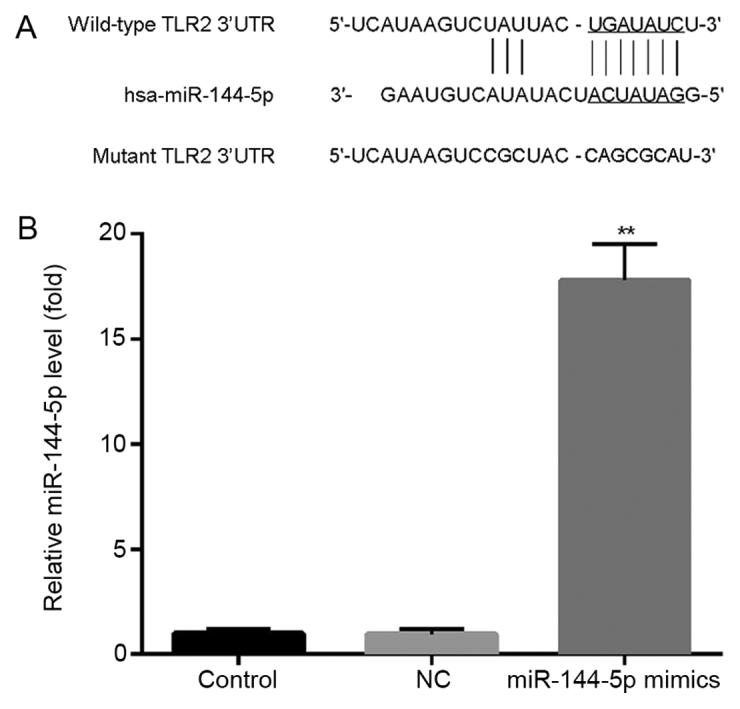
Binding sites between miR-144-5p and TLR2. (A) TargetScan was used to predict the binding sites between miR-144-5p and TLR2. (B) THP-1 macrophages were transfected with miR-144-5p mimics or mimic controls for 48 h. Reverse transcription-quantitative PCR was used to measure the expression of miR-144-5p in the Control, NC and miR-144-5p groups. Data are presented as the mean ± SD. **P<0.01 vs. NC group. miR, microRNA; LPS, lipopolysaccharide; NC, mimic negative control; TLR2, toll-like receptor 2; 3′UTR, 3′untranslated region.
Overexpression of miR-144-5p reduces the proliferation of macrophages
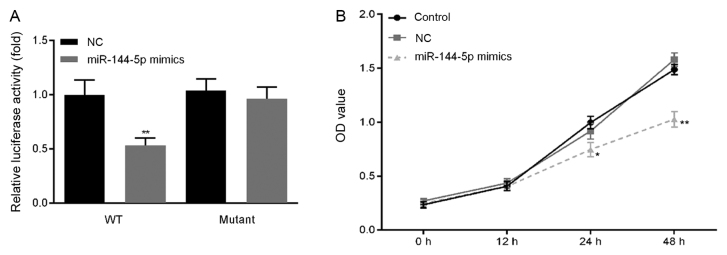
Previous studies have reported that miR-144 directly targets TLR2 (20,21). In the present study, a dual luciferase reporter assay was used to confirm the relationship between TLR2 and miR-144-5p in THP-1 macrophages. In cells transfected with the wt-TLR2 3′-UTR luciferase reporter vector, luciferase activity in the miR-144-5p mimics group was significantly lower compared with that in the NC group (Fig. 3A), whilst no significant difference was observed in the miR-144-5p mimics group transfected with the mut-TLR2 3′-UTR luciferase reporter vector.
Figure 3.
Effect of miR-144-5p overexpression on THP-1 macrophage viability. (A) A dual-luciferase reporter gene assay was used to confirm the interaction between miR-144-5p and TLR2. **P<0.01 vs. NC. (B) Cell Counting Kit-8 assay was used to measure the cell viability of THP-1 macrophages. *P<0.05 and **P<0.01 vs. NC group. Data are presented as the mean ± SD. miR, microRNA; LPS, lipopolysaccharide; NC, mimic negative control; OD, optical density; TLR2, toll-like receptor 2; WT, wild-type.
Cell viability was then measured by CCK-8 assay. miR-144-5p overexpression significantly reduced THP-1 macrophage viability compared with the NC group (Fig. 3B).
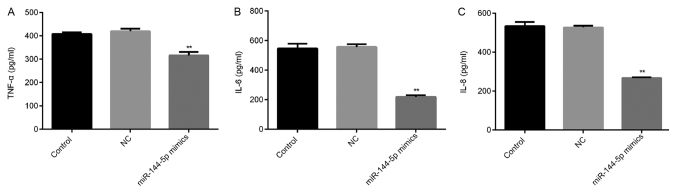
Overexpression of miR-144-5p reduces the secretion of TNF-α, IL-6 and IL-8
THP-1 macrophages were transfected with miR-144-5p mimics or NC for 48 h prior to treatment with LPS (1 µg/ml) for 24 h. The levels of TNF-α, IL-6 and IL-8 in the cell culture supernatant were subsequently measured using ELISA. miR-144-5p overexpression reduced the expression of TNF-α (Fig. 4A), IL-6 (Fig. 4B) and IL-8 (Fig. 4C) compared with the NC group.
Figure 4.
Effect of miR-144-5p overexpression on TNF-α, IL-6 and IL-8 levels in the THP-1 macrophage cell culture supernatant. (A) Cells transfected with miR144-5p mimic or mimic control were treated with LPS for 24 h, following which ELISA was used to measure the secretion of TNF-α, (B) IL-6 and (C) IL-8 in THP-1 macrophages. Data are presented as the mean ± SD. **P<0.01 vs. NC group. miR, microRNA; IL, interleukin; TNF-α, tumor necrosis factor-α; NC, mimic negative control; LPS, lipopolysaccharide.
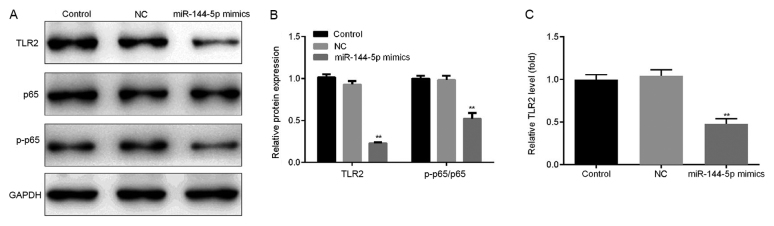
Effect of miR-144-5p overexpression on the NF-κB signaling pathway
The effect of miR-144-5p overexpression on the NF-κB pathway was investigated. THP-1 macrophages were first transfected with miR-144-5p mimics or NC for 48 h before the cells were stimulated with LPS (1 µg/ml) for a further 24 h. It was found that the overexpression of miR-144-5p reduced the protein (Fig. 5A and B) and mRNA (Fig. 5C) expression of TLR2 in addition to p65 phosphorylation compared with the NC group (Fig. 5A and B).
Figure 5.
Effect of miR-144-5p overexpression on NK-κB activation. Cells transfected with miR144-5p mimic or mimic control were stimulated with LPS for 24 h, following which western blotting was performed to measure the protein levels of TLR2, p-p65, and p65. (A) Representative western blot images of TLR2 expression and p65 phosphorylation. (B) Quantified densitometry results. (C) The mRNA level of TLR2 was detected using reverse transcription-quantitative PCR. Data are presented as the mean ± SD. **P<0.01 vs. NC group. miR, microRNA; LPS, lipopolysaccharide; NC, mimic negative control; TLR2, toll-like receptor 2; p-p65, phosphorylated p65.
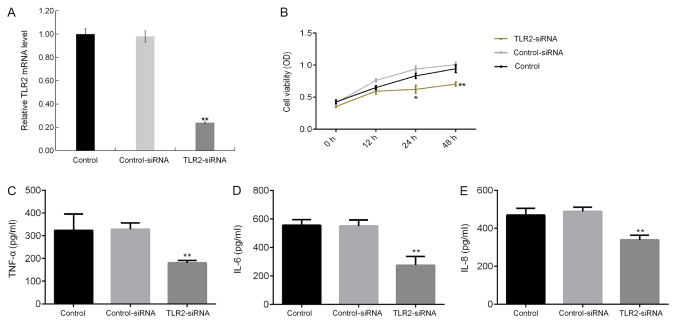
Effect of TLR2 silencing on LPS-induced macrophages
The effect of TLR2 knockdown on LPS-treated THP-1 macrophages was next determined. TLR2-siRNA transfection significantly reduced TLR2 mRNA expression in THP-1 macrophages (Fig. 6A). TLR2 knockdown significantly reduced THP-1 macrophage cell viability (Fig. 6B), in addition to significantly reducing the levels of TNF-α (Fig. 6C), IL-6 (Fig. 6D) and IL-8 (Fig. 6E) in the culture supernatant of THP-1 macrophages.
Figure 6.
Effect of TLR2 silencing on THP-1 macrophages. (A) THP-1 macrophages were transfected with control-siRNA or TLR2-siRNA for 48 h, following which the mRNA levels of TLR2 in THP-1 macrophages were measured using reverse transcription-quantitative PCR. THP-1 macrophages were transfected with control-siRNA or TLR2-siRNA for 48 h before treatment with LPS for a further 24 h. (B) A Cell Counting Kit-8 assay was subsequently used to measure the cell viability of THP-1 macrophages. (C) ELISA was performed to measure the secretion of TNF-α, (D) IL-6 and (E) IL-8 in the THP-1 macrophage culture supernatant. Data are presented as the mean ± SD. *P<0.05, **P<0.01 vs. Control-siRNA group. miR, microRNA; IL, interleukin; TNF-α, tumor necrosis factor-α; LPS, lipopolysaccharide; siRNA, small interfering RNA; TLR2, toll-like receptor 2.
Discussion
Macrophages are inflammatory cells that can be activated by a large number of cytokines or intercellular interactions. Following activation, macrophages have the ability to produce high levels of cytokines and chemokines, including IL-1β, TNF-α, IL-8 and monocyte chemoattractant protein-1 (22), some of which can aggravate the progression of chronic inflammation. In particular, TNF-α serves a pivotal role in inflammation associated with RA (23). TNF-α is mainly produced by macrophages, neutrophils, natural killer cells and endothelial cells, whilst activated lymphocytes can also produce small levels of TNF-α (24). The presence of TNF-α can also induce the production of other proinflammatory factors, including IL-1, IL-8, IL-6 and matrix metalloproteinases (25). Previous studies have demonstrated that serum TNF-α expression is elevated in patients with RA, which is associated with inflammatory disease and joint damage (26). Of interest, a number of biological agents exploiting this concept have been applied for the treatment of RA clinically (27), where the use of anti-TNF-α antibodies or soluble pseudo receptors have been reported to successfully reduce the degree of inflammation in RA (28).
miRNA has attracted widespread attention over the past decade (11), due to reports of a large quantity of aberrantly expressed miRNAs associated with the pathogenesis of RA (29–32). Studying the abnormal expression of these miRNAs in RA can potentially facilitate the understanding of RA pathogenesis. In the present study, the relative expression levels of miR-144-5p were measured in LPS-treated THP-1 macrophages, which found that miR-144-5p expression was significantly reduced by LPS treatment, indicating the important role of miR-144-5p in RA.
To explore the role of miR-144-5p LPS-treated THP-1 macrophages further, the potential targets of miR-144-5p were predicted using the Targetscan software, the binding sites on the TLR2 3′UTR were identified. This interaction between TLR2 and miR-144-5p was subsequently confirmed using a dual-luciferase reporter assay. Previous studies have reported that TLR2 serves important roles in the inflammatory response during RA pathogenesis (33–35), wherein TLR2 activation induces migratory and invasive mechanisms (36). Therefore, it was hypothesized in the present study that miR-144-5p may be involved in the development and progression of Rheumatoid arthritis by regulating TLR2 expression. To test this, the effects of miR-144-5p on LPS-treated THP-1 macrophages were then investigated. Consistent with previous studies (37,38), LPS-treated THP-1 macrophages had high levels of inflammatory factors, including TNF-α, IL-6, IL-1β and IL-8. In addition, the overexpression of miR-144-5p reduced cell viability and reduced the levels of TNF-α, IL-6 and IL-8 in the cell culture supernatants. NF-κB is a predominant transcription factor in amplifying the inflammatory response, such that the NF-κB inflammatory cascade in RA highlights the crucial role of the inhibition of the activity of NF-κB in RA development (39–41). Therefore, in the present study, the effect of miR-144-5p overexpression on NF-κB signaling was examined in LPS-treated THP-1 macrophages, specifically by measuring the phosphorylation status of p65, a subunit of NF-κB. Overexpression of miR-144-5p significantly reduced p65 phosphorylation, suggesting suppression of the NK-κB pathway. Additionally, silencing of TLR2 expression by siRNA transfection significantly reduced the viability of LPS-treated THP-1 macrophages and reduced the levels of TNF-α, IL-6 and IL-8 in the cell culture supernatants.
However, it should be noted that the present study is a preliminary study of the role of miR-144-5p and TLR2 in RA. To verify these findings, further in-depth studies are required. For example, the expression levels of CD14 and TLR4, which may influence the extent of the LPS response (42), should be measured following miR-144-5p overexpression. In addition, the role of miR-144-5p and TLR2 in an in vivo model of RA and the correlation of miR-144-5p expression with the clinicopathological characteristics of patients with RA will also need to be studied in the future.
In conclusion, miR-144-5p overexpression inhibited the secretion of TNF-α, IL-6 and IL-8 in LPS-treated THP-1 macrophages by suppressing TLR2 expression, in turn reducing NK-κB activation whilst also reducing cell viability. This may serve as a potential mechanism through which the development of RA can be inhibited. Therefore, miR-144-5p may serve as an important target for the development of novel therapeutic interventions for RA.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
GZ wrote the manuscript, analyzed and interpreted the data. YL designed the study and revised the manuscript. JN analyzed and interpreted the data. PJ and ZB collected the experimental data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Abeles AM, Pillinger MH. The role of the synovial fibroblast in rheumatoid arthritis: Cartilage destruction and the regulation of matrix metalloproteinases. Bull NYU Hosp Jt Dis. 2006;64:20–24. [PubMed] [Google Scholar]
- 2.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeannin P, Paolini L, Adam C, Delneste Y. The roles of CSFs on the functional polarization of tumor-associated macrophages. FEBS J. 2018;285:680–699. doi: 10.1111/febs.14343. [DOI] [PubMed] [Google Scholar]
- 4.Zhou D, Chen L, Yang K, Jiang H, Xu W, Luan J. SOCS molecules: The growing players in macrophage polarization and function. Oncotarget. 2017;8:60710–60722. doi: 10.18632/oncotarget.19940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kokkonen H, Söderström I, Rocklöv J, Hallmans G, Lejon K, Rantapää Dahlqvist S. Up-regulation of cytokines and chemokines predates the onset of rheumatoid arthritis. Arthritis Rheum. 2010;62:383–391. doi: 10.1002/art.27186. [DOI] [PubMed] [Google Scholar]
- 6.Ha M, Kim VN. Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Han CC, Cui D, Li Y, Ma Y, Wei W. Is macrophage polarization important in rheumatoid arthritis? Int Immunopharmacol. 2017;50:345–352. doi: 10.1016/j.intimp.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 8.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/S1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 9.Park H, Park SG, Kim J, Ko YG, Kim S. Signaling pathways for TNF production induced by human aminoacyl-tRNA synthetase-associating factor, p43. Cytokine. 2002;20:148–153. doi: 10.1006/cyto.2002.1992. [DOI] [PubMed] [Google Scholar]
- 10.Elias JA, Reynolds MM, Kotloff RM, Kern JA. Fibroblast interleukin 1 beta: Synergistic stimulation by recombinant interleukin 1 and tumor necrosis factor and posttranscriptional regulation. Proc Natl Acad Sci USA. 1989;86:6171–6175. doi: 10.1073/pnas.86.16.6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Lee CG. MicroRNA and cancer-focus on apoptosis. J Cell Mol Med. 2009;13:12–23. doi: 10.1111/j.1582-4934.2008.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bueno MJ, Pérez de Castro I, Malumbres M. Control of cell proliferation pathways by microRNAs. Cell Cycle. 2008;7:3143–3148. doi: 10.4161/cc.7.20.6833. [DOI] [PubMed] [Google Scholar]
- 14.Yamada Y, Arai T, Kojima S, Sugawara S, Kato M, Okato A, Yamazaki K, Naya Y, Ichikawa T, Seki N. Regulation of antitumor miR-144-5p targets oncogenes: Direct regulation of syndecan-3 and its clinical significance. Cancer Sci. 2018;109:2919–2936. doi: 10.1111/cas.13722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song L, Peng L, Hua S, Li X, Ma L, Jie J, Chen D, Wang Y, Li D. miR-144-5p enhances the radiosensitivity of non-small-cell lung cancer cells via targeting ATF2. Biomed Res Int. 2018;2018:5109497. doi: 10.1155/2018/5109497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Wang R, Ge Y, Chen D, Wu B, Fang F. Assessment of microRNA-144-5p and its putative targets in inflamed gingiva from chronic periodontitis patients. J Periodontal Res. 2019;54:266–277. doi: 10.1111/jre.12627. [DOI] [PubMed] [Google Scholar]
- 17.Sokol RJ, Hudson G, James NT, Frost IJ, Wales J. Human macrophage development: A morphometric study. J Anat. 1987;151:27–35. [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Agarwal V, Bell GW, Nam J, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. Elife. 2015;4 doi: 10.7554/eLife.05005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li D, Wang X, Lan X, Li Y, Liu L, Yi J, Li J, Sun Q, Wang Y, Li H, et al. Down-regulation of miR-144 elicits proinflammatory cytokine production by targeting toll-like receptor 2 in nonalcoholic steatohepatitis of high-fat-diet-induced metabolic syndrome E3 rats. Mol Cell Endocrinol. 2015;402:1–12. doi: 10.1016/j.mce.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Lan X, Liu L, Yi J, Li J, Li Y, Wang M, Li J, Song LM, Li D. MicroRNA 144 negatively regulates Toll-like receptor 2 expression in rat macrophages. Nan Fang Yi Ke Da Xue Xue Bao. 2015;35:319–325. (In Chinese) [PubMed] [Google Scholar]
- 22.Ma Y, Pope RM. The role of macrophages in rheumatoid arthritis. Curr Pharm Des. 2005;11:569–580. doi: 10.2174/1381612053381927. [DOI] [PubMed] [Google Scholar]
- 23.Wei ST, Sun YH, Zong SH, Xiang YB. Serum levels of IL-6 and TNF-α may correlate with activity and severity of rheumatoid arthritis. Med Sci Monit. 2015;25:4030–4038. doi: 10.12659/MSM.895116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korani S, Kazemi B, Haghighi A, Nikpoor AR, Bandehpour M. The effect of human recombinant tumor necrosis factor receptor-2 on reducing inflammatory of collagen -induced arthritis in Balb/c Mice. Iran J Biotechnol. 2019;17:e2153. doi: 10.21859/ijb.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasanthi P, Nalini G, Rajasekhar G. Role of tumor necrosis factor-alpha in rheumatoid arthritis: A review. APLAR J Rheumatol. 2007;10:270–274. doi: 10.1111/j.1479-8077.2007.00305.x. [DOI] [Google Scholar]
- 26.Edrees AF, Misra SN, Abodou NI. Anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis: Correlation of TNF-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clin Exp Rheumatol. 2005;23:469–474. [PubMed] [Google Scholar]
- 27.Venkatesha SH, Dudics S, Acharya B, Moudgil KD. Cytokine-modulating strategies and newer cytokine targets for arthritis therapy. Int J Mol Sci. 2014;16:887–906. doi: 10.3390/ijms16010887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doyle MK, Rahman MU, Frederick B, Birbara CA, de Vries D, Toedter G, Wu X, Chen D, Ranganath VK, Westerman ME, Furst DE. Effects of subcutaneous and intravenous golimumab on inflammatory biomarkers in patients with rheumatoid arthritis: Results of a phase 1, randomized, open-label trial. Rheumatology (Oxford) 2013;52:1214–1219. doi: 10.1093/rheumatology/kes381. [DOI] [PubMed] [Google Scholar]
- 29.Huang RY, Wu JQ, Liu ZH, Sun SL. MicroRNAs in rheumatoid arthritis: What is the latest with regards to diagnostics? Expert Rev Mol Diagn. 2019;19:363–366. doi: 10.1080/14737159.2019.1599716. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Pan A, Chen X, Tu J, Xia X, Sun L. MiR-5571-3p and miR-135b-5p, derived from analyses of microRNA profile sequencing, correlate with increased disease risk and activity of rheumatoid arthritis. Clin Rheumatol. 2019;38:1753–1765. doi: 10.1007/s10067-018-04417-w. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Kong R, Zhou X, Ji L, Zhang J, Zhao D. Correction to: MiRNA-126 expression inhibits IL-23R mediated TNF-α or IFN-γ production in fibroblast-like synoviocytes in a mice model of collagen-induced rheumatoid arthritis. Apoptosis. 2019;24:382. doi: 10.1007/s10495-018-1503-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu FY, Xie CQ, Jiang CL, Sun JT, Feng HC, Li C, Huang XW. MiR-92a inhibits fibroblast-like synoviocyte proliferation and migration in rheumatoid arthritis by targeting AKT2. J Biosci. 2018;43:911–919. doi: 10.1007/s12038-018-9803-0. [DOI] [PubMed] [Google Scholar]
- 33.McGarry T, Biniecka M, Gao W, Cluxton D, Canavan M, Wade S, Wade S, Gallagher L, Orr C, Veale DJ, Fearon U. Resolution of TLR2-induced inflammation through manipulation of metabolic pathways in Rheumatoid Arthritis. Sci Rep. 2017;7:43165. doi: 10.1038/srep43165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arjumand S, Shahzad M, Shabbir A, Yousaf MZ. Thymoquinone attenuates rheumatoid arthritis by downregulating TLR2, TLR4, TNF-α, IL-1, and NFκB expression levels. Biomed Pharmacother. 2019;111:958–963. doi: 10.1016/j.biopha.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Quero L, Hanser E, Manigold T, Tiaden AN, Kyburz D. TLR2 stimulation impairs anti-inflammatory activity of M2-like macrophages, generating a chimeric M1/M2 phenotype. Arthritis Res Ther. 2017;19:245. doi: 10.1186/s13075-017-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McGarry T, Veale DJ, Gao W, Orr C, Fearon U, Connolly M. Toll-like receptor 2 (TLR2) induces migration and invasive mechanisms in rheumatoid arthritis. Arthritis Res Ther. 2015;17:153. doi: 10.1186/s13075-015-0664-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie J, Li Q, Zhu XH, Gao Y, Zhao WH. IGF2BP1 promotes LPS-induced NFκB activation and pro-inflammatory cytokines production in human macrophages and monocytes. Biochem Biophys Res Commun. 2019;513:820–826. doi: 10.1016/j.bbrc.2019.03.206. [DOI] [PubMed] [Google Scholar]
- 38.Robertson RC, Guihéneuf F, Bahar B, Schmid M, Stengel DB, Fitzgerald GF, Ross RP, Stanton C. The anti-inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar Drugs. 2015;13:5402–5424. doi: 10.3390/md13085402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roman-Blas JA, Jimenez SA. NF-kappaB as a potential therapeutic target in osteoarthritis and rheumatoid arthritis. Osteoarthritis Cartilage. 2006;14:839–848. doi: 10.1016/j.joca.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Aravilli RK, Vikram SL, Kohila V. Phytochemicals as potential antidotes for targeting NF-κB in rheumatoid arthritis. 3 Biotech. 2017;7:253. doi: 10.1007/s13205-017-0888-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang HJ, Wei QF, Wang SJ, Zhang HJ, Zhang XY, Geng Q, Cui YH, Wang XH. LncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-κB pathway. Int Immunopharmacol. 2017;50:283–290. doi: 10.1016/j.intimp.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 42.Luo X, Xiao B, Xiao Z. Anti-inflammatory activity of adenosine 5′-trisphosphate in lipopolysaccharide-stimulated human umbilical vein endothelial cells through negative regulation of toll-like receptor MyD88 signaling. DNA Cell Biol. 2019 doi: 10.1089/dna.2019.4773. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.