Abstract
The current study focused on the effect of creatine supplementation with/without exercise on the expression of genes controlling mitochondrial biogenesis in skeletal and cardiac muscles, as well as its safety profile on the liver and kidney. A total of 40 male Wister rats were included in the present study. Two unexercised groups: The control sedentary group and the sedentary creatine-treated group (n=10) were treated daily with oral creatine (0.5 g/kg per day). Two exercised groups performed swimming exercise training 5 days/week for a period of 5 weeks; The Exercise training group, and exercise training and creatine (0.5 g/kg per day) treated group. After sacrifice, blood samples, cardiac and soleus muscles were collected for assessment of mtDNA copy number, gene expression analysis and nuclear extraction for the assay of PGC-1α. The results of the current study demonstrated that, physical activity with short-term creatine supplementation increased all factors of mitochondrial biogenesis, an effect that is devoid of any kidney or liver adverse effects. Further studies are still required to explore the potential of creatine supplementation in ameliorating mitochondrial diseases, including epilepsy, skeletal and cardiac myopathies, hepatopathies and nephropathies.
Keywords: creatine supplementation, γ coactivator-1α, mito-chondrial biogenesis, exercise physiology, soleus muscle, cardiac muscle, swimming exercise training, nutritional supplement
Introduction
The liver, kidney and pancreas are the sites where creatine is naturally synthesized from amino acids, including methionine, arginine and glycine (1). Creatine is a product of the arginine biosynthesis pathway in vivo, is stored in skeletal muscles and metabolizes into creatinine (2).
Creatine is as a dietary supplement that can increase phosphocreatine in the muscles and enhances performance during high-intensity, short extent activities or during sessions of high-intensity exercise with short rest periods, including sprinting, jumping and strength training (3).
ATP stores in the body are sufficient to provide maximal energy for one-two sec (4), meaning ATP is subsequently required, which is not available through the blood. The metabolism of phosphocreatine in muscles rapidly produces ATP for an additional 5 to 8 sec of maximal effort (4). Therefore, ATP and phosphocreatine provide energy for <10 sec of maximal activity. Lower energy output for sustained periods of time can be conserved by aerobic oxidation of glycogen within the mitochondria (4). Therefore, aerobic metabolism regulates the production of ATP during continuous exercise, highlighting the importance of mitochondria for overall metabolic homeostasis (4).
Mitochondria contains a circular DNA genome that is called mitochondrial DNA (mtDNA), and it is well-known that mitochondrial biogenesis increases in muscle cells upon exercise or in response to an electrically stimulated contraction (5). Mitochondrial biogenesis is a complex process that initiates the replication of mtDNA and the expression of mitochondrial proteins that are encoded by nuclear and mitochondrial genomes. Therefore, mitochondrial biogenesis serves a pivotal role in optimizing cellular mitochondrial function (6). The transcriptional coactivator γ coactivator-1alpha (PGC-1α) regulates genes that are associated with energy metabolism. PGC-1α interacts with the nuclear peroxisome proliferator-activated receptor (PPAR) that permits the interaction of PGC-1α with a number of transcription factors. PPAR and PGC-1α are considered master regulators of mitochondrial biogenesis as they activate nuclear respiratory factor 1 (NRF-1) which in turn, activates mtDNA transcription factor A (TFAM) that controls mtDNA transcription and replication (7). PGC-1α is a good sensor for the response of the cells to free radicals (8), and this indicates that free radicals generated in exercise may be signals of increased mitochondrial biogenesis (9).
Pyruvate and lactate are critical fuel substrates for muscles during exercise (10). Pyruvate is generated by glycolysis and can serve as a substrate for the mitochondrial TCA cycle to catabolize glucose producing maximal ATP, or is used to produce lactate via the less efficient ATP generation pathway (10). Additionally, the oxidation of lactase is a substantial source of pyruvate, where exercise increases lactate oxidation in skeletal muscle (10). Lactate dehydrogenase (LDH) is a key enzyme that catalyzes the conversion of pyruvate and lactate, and regulates cellular pyruvate and lactate homeostasis (10).
The aim of the current study was to investigate the effects of creatine supplementation alone or combined with exercise on the expression of genes controlling the pathway of mitochondrial biogenesis, including PGC-1α, NRF1, TFAM and mtDNA copy number, in skeletal and cardiac muscles.
Materials and methods
Experimental animals
In the current study, a total of 40 male Wister rats (weight, 120–150 g; age, 3 months) were used. Rats were obtained from the animal house of the Medical Research Institute, Alexandria University (Alexandria, Egypt). Rats were maintained in air-conditioned rooms (temperature, 23±1°C; humidity, 50–55%), with a 12 h light-dark cycle, 5 rats were placed in each cage and were fed with a standard diet (Table I) and tap water ad libitum. The animal procedures were approved by the Institutional Animal Care and Use Committee at the Medical Research Institute at Alexandria University. All procedures comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (11) (NIH Publications no. 8023; revised 1985) and regulations of Egypt's Guide for the Care and Use of Laboratory Animals (12). The current study adheres to the ARRIVE Guidelines for reporting in vivo experiments (13). All efforts were made to curtail the suffering of rats during the experimental period.
Table I.
Experimental diet composition fed to rats.
| Ingredients | Standard diet (g/kg) |
|---|---|
| Protein | 220 |
| Fat | 43 |
| Carbohydrates | 631 |
| Cellulose | 54 |
| Vitamin mix | 10 |
| Mineral mix | 40 |
| Total energy (kcal/g diet) | 3.8 |
Carbohydrates, Dextrose, Corn starch and Sucrose; Vitamin mix; Vitamin A, B1, B2, B6, E and K.
Exercise protocol
The exercise protocol was swimming for 1 h with a metal ring, which was customized for each individual rat to be 3% of the rat's body weight and enclosed to the torso to avoid the innate ability of rats to float on the water surface. This exercise protocol has been indicated to characterize moderate intensity exercise (14).
Experimental design
The animals were separated into four groups that were monitored for 5 weeks. Two unexercised groups consisting of the control sedentary group (CS; n=10) which exhibited only spontaneous movement in cages, and the sedentary creatine-treated group (SC; n=10), which included rats treated daily with oral creatine (0.5 g/kg per day) (15) and exhibited only spontaneous movement in cages. Two exercised groups performed swimming exercise training 5 days/week for five weeks and included the exercise training group (ET; n=10) and the exercise training and creatine treated (0.5 g/kg per day) group (ETC; n=10).
A period of 24 h after the last treatment and exercise training the rats were weighed and sacrificed by cervical dislocation under deep anaesthesia using ketamine/xylazine at a dose of 100/10 mg/kg. Blood samples were collected centrifuged at 1,000 × g for 20 min at 4°C to obtain the serum; and the cardiac and soleus muscle were removed and divided into three sections. The first section was used for DNA extraction for the assessment of mtDNA copy number, the second section was used for RNA extraction to analyze gene expression and the third section was used for nuclear extraction for the assay of PGC-1α.
Serum parameters
Serum lactate was determined using colorimetric L-lactate Assay kit (cat. no. ab65331; Abcam) and pyruvate were assayed using colorimetric Pyruvate Assay Kit (cat. no. ab65342; Abcam) according to the manufacturer's protocols. Serum urea (cat. no. UR3825), creatinine (cat. no. CR510), aspartate transaminase (AST; cat. no. AS7938) and alanine transaminase (ALT; cat. no. AL7930) activity were assayed using a Randox kit (Randox Laboratories Ltd.) according to the manufacturer's protocol.
Reverse transcription-quantitative PCR (RT-qPCR)
Cardiac and soleus muscle tissues were used for total RNA extraction using the RNeasy mini kit (Qiagen GmbH) and DNA extraction using DNeasy Blood and Tissue kit (Qiagen GmbH) according to manufacturer's protocols. Reverse transcription of muscular RNA was performed using a miScript II RT kit (Qiagen GmbH) according to the manufacturer protocol. The miScript II RT kit was used to perform a one-step, single-tube reverse transcription reaction. miScript HiFlex buffer was used to promote the conversion of all RNA species into cDNA.
The cDNA was used to quantify the gene expression of NRF1 and Tfam using Rotor-Gene Q qPCR (Qiagen, Inc.), which was performed using QuantiTect SYBR Green PCR Master Mix (Qiagen GmbH). qPCR amplification conditions started with an initial denaturation for 10 min at 55°C, followed by amplification by 40 cycles of PCR as follows: Denaturation at 95°C for 5 sec, annealing at 55°C for 15 sec and extension at 60°C for 15 sec. The housekeeping gene GAPDH was used as a reference gene for normalization. Primers used for rat genes were as follows: NRF1 (16) forward, 5′-TTACTCTGCTGTGGCTGATGG-3′ and reverse, 5′-CCTCTGATGCTTGCGTCGTCT-3′; TFAM (17) forward, 5′-GCTTCCAGGAGGCTAAGGAT-3′ and reverse, 5′-CCCAATCCCAATGACAACTC-3′; GAPDH forward, 5′-GGGTGTGAACCACGAGAAATA-3′ and reverse, 5′-AGTTGTCATGGATGACCTTGG-3′. The values of threshold cycle (Ct) were determined using Rotor-Gene Q-Pure Detection version 2.1.0 (build 9; Qiagen, Inc.). For each gene, the relative change in mRNA in samples was determined using the 2−ΔΔCq method (18) and normalized to the housekeeping gene (GAPDH).
Nuclear extraction and PGC-1α assessment
Immediately after the collection of blood, muscles were excised, washed with ice-cold saline and preserved at −80°C until subsequent assay. The nuclear extract of muscle tissues was obtained using Nuclear Extraction Kit (cat. no. ab113474; Abcam) according to manufacturer's protocol, which were then used for the determination of muscular PGC-1α contents. Total protein concentration was measured using Lowry method (19).
Mitochondrial DNA copy number
Total DNA was extracted from muscle tissues using RNeasy kit (Qiagen GmbH). Using the extracted total DNA, the mitochondrial DNA (mtDNA) content was assessed relative to the nuclear DNA specific gene (PGC1α) using RT-qPCR (20). The primers used were as follows: mtDNA forward, 5′-ACACCAAAAGGACGAACCTG-3′ and reverse, 5′-ATGGGGAAGAAGCCCTAGAA-3 and PGC1α forward, 5′-ATGAATGCAGCGGTCTTAGC-3′ and reverse, 5′-AACAATGGCAGGGTTTGTTC-3′. Reactions were carried out using SYBR Green PCR Master Mix (Applied Biosystems; Thermo Fisher Scientific, Inc.), 0.5 µM forward and reverse primer, and 50 ng genomic DNA were used with the following conditions: 95°C for 10 min followed by 40 cycles of 95°C for 15 sec, 60°C for 30 sec and 72°C for 30 sec. The relative mtDNA copy number was calculated using the 2−ΔΔCq method (18) as described previously (21).
Statistical analysis
Values are expressed as mean ± standard deviation (n=10) and were analyzed using the GraphPad Prism v5.0 (GraphPad Software, Inc.). Multiple comparisons were performed using one-way ANOVA, followed by a Tukey post-hoc test. The correlation coefficients (r) between different assayed parameters were evaluated using Pearson's correlation coefficient. P<0.05 was considered to indicate a statistically significance difference.
Results
Effect of creatine supplementation on rats' weight
Rat weight was evaluated at the end of treatment period to compare between groups that were supplemented with creatine and rats not supplemented, and to record the difference between exercised and sedentary rats. As presented in Table II, although sedentary rats supplemented with creatine (SC) were indicated to exhibit an increase in weight, no significant difference (P>0.05) was observed in exercised rats supplemented with creatine (ETC) or any other group (Table II).
Table II.
Effect of creatine supplementation on rats' weight.
| Experimental groups | Weight (g) |
|---|---|
| Sedentary rats (CS) | 151.8±10.84 |
| Exercised rats (ET) | 148.8±13.96a |
| Sedentary rats supplemented with creatine (SC) | 157.3±12.87a,b |
| Exercised rats supplemented with creatine (ETC) | 146.3±9.85a–c |
Values are presented as mean ± standard deviation (n=10 rats/group). No statistical significance was observed between the four groups.
P=0.7459, ET vs. CS; P= 0.5375, SC vs. CS and P=0.4810, ETC vs. CS
P=0.4051, SC vs. ET and P=0.7796, ETC vs. ET
P=0.223, SC vs. ETC.
Effect of creatine supplementation on blood lactate/pyruvate level
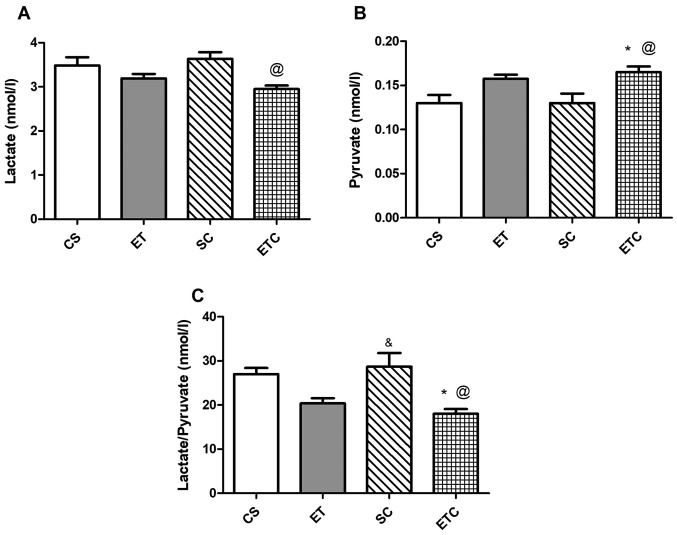
Supplementation of creatine upon 1 h of daily exercise (ETC rats) resulted in a significant decrease in blood lactate level compared with sedentary rats receiving creatine daily (SC), as presented in Fig. 1A (2.95±0.150 ETC vs. 3.64±0.298 SC). However, this decrease was not significantly different from rats that did not receive creatine, whether sedentary or exercised. However, blood pyruvate level in ETC rats exhibited a significant increase compared with sedentary rats, those receiving and not receiving creatine (Fig. 1B; 0.16±0.0129 ETC vs. 0.13±0.022 SC and vs. 0.13±0.018 CS). The lactate/pyruvate ratio was in accordance with the previously mentioned data demonstrating a significant decrease in ETC rats compared with sedentary rats receiving or not receiving creatine (Fig. 1C).
Figure 1.
Effect of creatine supplementation on blood lactate/pyruvate level. (A) Lactate, (B) pyruvate and (C) lactate/pyruvate ratio. Values are presented as mean ± standard deviation (n=10). @P<0.05 SC vs. ETC in lactate and pyruvate, @P<0.01 SC vs. ETC in lactate/pyruvate ratio. *P<0.05 CS vs. ETC in pyruvate and lactate/pyruvate ratio. &P<0.05 ET vs. SC in lactate/pyruvate ratio. CS, sedentary rats; SC, sedentary rats supplemented with creatine; ET, exercised rats; ETC, exercised rats supplemented with creatine.
Effect of creatine supplementation on gene expression in soleus muscle
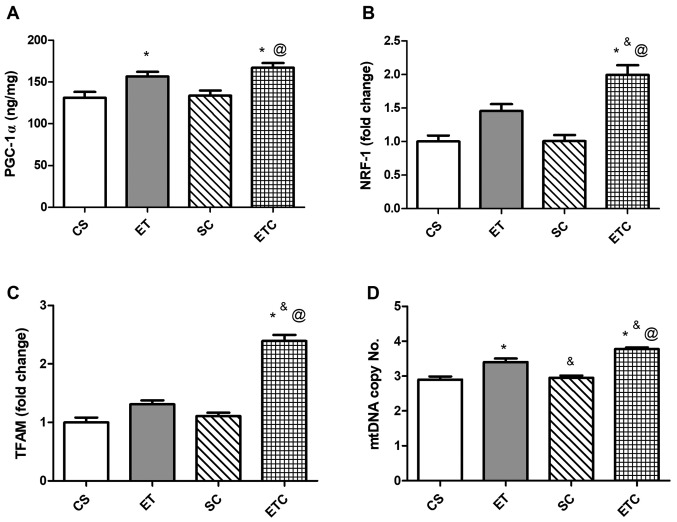
Training rats for 1 h per day exhibited a clear effect on the soleus muscle, where exercised rats were demonstrated to exhibit a significant increase in PGC-1α and mtDNA copy number compared with sedentary rats (Fig. 2A and D; 156.8±10.99 ET vs. 131.3±6.87 CS; 3.40±0.216 ET vs. 2.90±0.183 CS). Treatment with creatine in trained rats was indicated to increase gene expression of all measured parameters compared with all groups. When comparing ETC rats with SC rats, PGC-1α expression was increased by ~20% (Fig. 2A). NRF-1 and TFAM expression indicated a ~50 and ~53% increase, respectively (Fig. 2B and C), while mtDNA exhibited a significant increase of 21% (Fig. 2C).
Figure 2.
Effect of creatine supplementation on gene expression in soleus muscle. (A) PGC-1α expression. @P<0.01 SC vs. ETC, *P<0.01 CS vs. ETC and *P<0.05 CS vs. ET. (B) NRF-1 expression. @P<0.001 SC vs. ETC, *P<0.001 CS vs. ETC and &P<0.05 ET vs. ETC. (C) TFAM expression. @P<0.001 SC vs. ETC, *P<0.001 CS vs. ETC and &P<0.001 ET vs. ETC. (D) mtDNA copy number. @P<0.001 SC vs. ETC, *P<0.001 CS vs. ETC, *P<0.01 CS vs. ET, &P<0.05 ET vs. ETC and &P<0.01 ET vs. SC. Values are presented as mean ± standard deviation (n=10). PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-α; NRF-1, nod factor receptor 1; TFAM, mitochondrial transcription factor A; mtDNA, mitochondrial DNA; CS, sedentary rats; SC, sedentary rats supplemented with creatine; ET, exercised rats; ETC, exercised rats supplemented with creatine.
Effect of creatine supplementation on gene expression in cardiac muscle
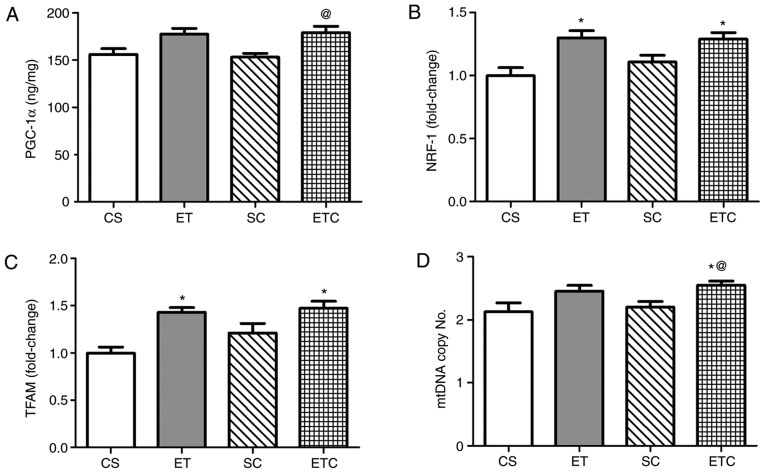
The clear effect of creatine on cardiac muscle was indicated in the soleus muscle. NRF-1 and TFAM genes (Fig. 3B and C) in exercised rats receiving (ETC) or not receiving (ET) creatine demonstrated a significant increase in expression compared with sedentary rats (CS). Supplementation of creatine in trained rats (ETC) was observed to increase PGC-1α expression by ~14.5% (Fig. 3A) and mtDNA by ~15% (Fig. 3D) compared with sedentary rats recieving creatine (SC).
Figure 3.
Effect of creatine supplementation on gene expression in cardiac muscle. (A) PGC-1α expression. @P<0.05 SC vs. ETC. (B) NRF-1 expression. *P<0.05 CS vs. ETC and *P<0.05 CS vs. ET. (C) TFAM expression. *P<0.01 CS vs. ETC and *P<0.01 CS vs. ET. (D) mtDNA copy number. @P<0.05 SC vs. ETC and *P<0.05 CS vs. ETC. Values are presented as mean ± standard deviation (n=10). PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1-α; NRF-1, nod factor receptor 1; TFAM, mitochondrial transcription factor A; mtDNA, mitochondrial DNA; CS, sedentary rats; SC, sedentary rats supplemented with creatine; ET, exercised rats; ETC, exercised rats supplemented with creatine.
Effect of creatine supplementation on liver and kidney functions
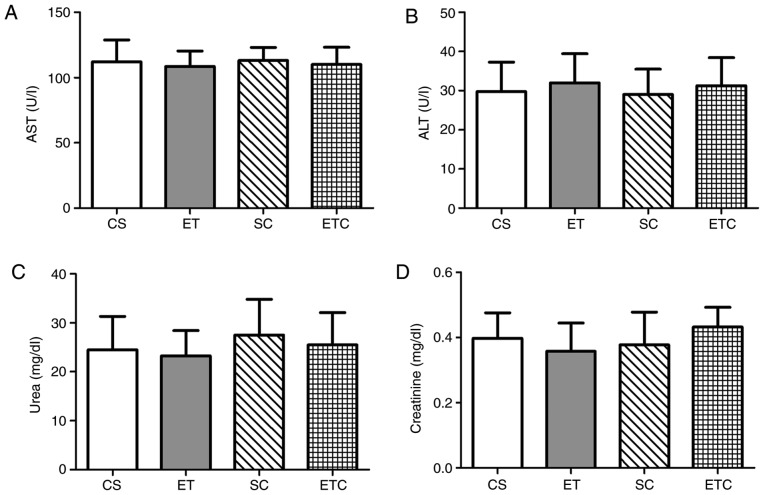
The normal concentrations of AST and ALT were determined upon the serum analysis of sedentary (CS) and exercised (ET) rats and are presented as means (112±16.89 U/l and 29.75±7.50 U/l; 108.5±11.90 U/l and 32±7.44 U/l, respectively). No significant elevations of AST (P=0.9224, CS vs. SC; P=0.8720, ET vs. ETC; Fig. 4A) and ALT (P=0.8847, CS vs. SC; P=0.8894, ET vs. ETC; Fig. 4B) serum levels were observed in creatine supplemented groups compared with the healthy controls, indicating that normal transaminases levels were neither affected by exercise nor creatine supplementation. Additionally, creatine supplementation did not induce significant effects on serum urea levels compared with healthy controls (P=0.5705, CS vs. SC; P=0.6115 ET vs. ETC; Fig. 4C). Similar observations were made on serum creatinine levels, which showed no significant differences between exercised and sedentary rats in a manner that was independent of whether creatine was supplemented (Fig. 4D; 0.43±0.06 ETC, 0.38±0.10 SC, 0.36±0.09 ET and 0.4±0.08 CS), suggesting normal kidney function.
Figure 4.
Effect of creatine supplementation on liver and kidney functions. (A) Serum Aspartate aminotransferase and (B) alanine aminotransferase activity, (C) serum urea and (D) creatinine levels. Values are presented as mean ± standard deviation (n=10). CS, sedentary rats; SC, sedentary rats supplemented with creatine; ET, exercised rats; ETC, exercised rats supplemented with creatine; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
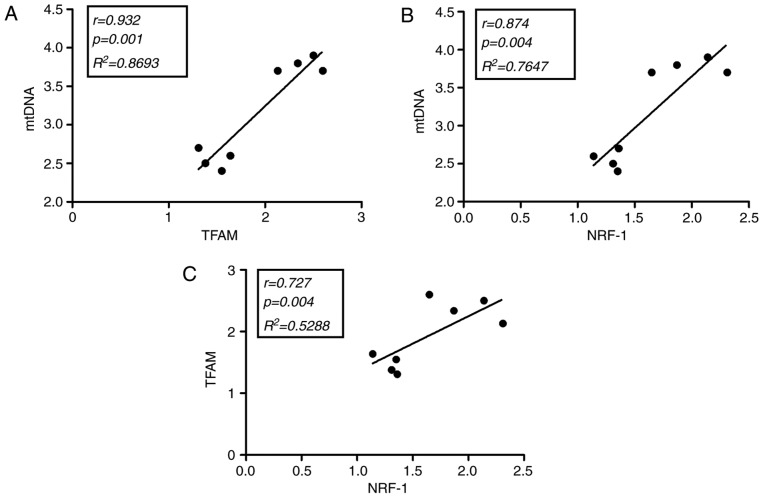
Correlation studies in soleus and cardiac muscles
Statistical analysis of the soleus and cardiac muscles in exercised rats supplemented with creatine indicated that mtDNA was positively correlated with TFAM (r=0.932; P=0.001; Fig. 5A) and NFR-1 (r=0.874; P=0.004; Fig. 5B). Additionally, TFAM was positively correlated with NFR-1 (r=0.727; P=0.004; Fig. 5C). Other correlations in ETC rats were not observed to be statistically significant (data not shown). All results exhibited a high degree of reproducibility.
Figure 5.
Correlations in soleus and cardiac muscles of exercised rats supplemented with creatine (ETC). NRF-1, nod factor receptor 1; TFAM, mitochondrial transcription factor A; mtDNA, mitochondrial DNA.
Discussion
Creatine supplementation is considered to be the most effective nutritional supplement and ergogenic aid to enhance anaerobic exercise performance in a number of sports (22). Additionally, research has focused on investigating the widespread application of this supplement within different target groups, where it can be beneficial for treating cancer, rheumatoid arthritis, type 2 diabetes and neurodegenerative disorders (23). To gain additional insight into the mechanistic pathway of creatine supplementation, the current study focused on the effect of Cr supplementation alone or combined with exercise on the expression of genes controlling the pathway of mitochondrial biogenesis in skeletal and cardiac muscles, and its effect on liver and kidney functions.
The results of the present study revealed that exercise utilizes endogenous creatine and increases the mitochondrial biogenesis which can be identified using increased expression of PGC-1α and mtDNA levels in soleus muscle, compared with non-exercised groups. Exogenous Cr supplementation increased biogenesis during exercise causing an increase of all key regulatory parameters of mitochondrial biogenesis that can be measured in soleus muscle (PGC-1α, NFR1, TFAM and mtDNA) and therefore, more ATP was available for muscle consumption.
These results are in accordance with the popular theory that creatine (Cr) is phosphorylated upon cellular uptake in the muscle, to further exist as free Cr (40%) and phophorylcreatine (PCr; 60%). Free Cr and phophorylcreatine serve a crucial role as ‘energy buffers’ at sites of high energy turnover, including in skeletal muscle and the heart, to operate if the required ATP quantity exceeds the normal rate produced by mitochondria, retaining the ATP homeostasis at these sites. When phosphocreatine level decreases due to the re-phosphorylation of ADP, the production of phosphofructokinase is accelerated to increase the speed of glycolysis (24), which is the major source of pyruvate.
During exercise, pyruvate and lactate are essential fuel substrates for skeletal muscles, which produce ATP, and have been indicated to be correlated proportionally to exercise-induced PGC-1α signaling as stated by Liang et al (10). This relationship has been reflected in the current study, where increased PGC-1α expression in soleus muscle of exercised rats supplemented with Cr exhibited a significant increase in serum pyruvate level, but not the lactate. These results demonstrated that pyruvate serves directly as a substrate for the mitochondrial TCA cycle to catabolize glucose producing ATP, with no need for interconversion into lactate.
These findings are supported by correlation data, which indicated that mtDNA, NRF-1 and TFAM exhibit a statistically significant positive correlation in cardiac and soleus muscles of exercised rats supplemented with creatine, an effect that has not, to the best of our knowledge, yet been indicated in sedentary rats supplemented with creatine. The lack of immunohistochemical analysis to confirm the increased mitochondrial number is a limitation of the study and will be assessed in future work.
The PGC1-α gene is highly inducible in response to physiologic conditions that require an increased mitochondrial energy production (5). As demonstrated by Finck et al (25), PGC-1α expression is stimulated by exercise in skeletal muscle and by fasting in the cardiac muscle (25). Studies have also indicated that PGC-1-α is expressed preferentially in muscles rich in type I fibers, including in the soleus muscle (5,26).
As indicated by Head et al (27) and Murphy et al (28), Cr supplementation increased the sensitivity of contractile proteins to intracellular Ca2+, which enhances muscle performance and reduces muscle fatigue. This is attributed to the osmotic effect of Cr when entering the muscle, and its ability to re-synthesize ATP. This effect of creatine was further supported by an in vivo study in mice that demonstrated how Cr supplementation decreased skeletal muscle necrosis and improved mitochondrial respiration (29). Additional studies have demonstrated that increased signals of mitochondria biogenesis, including PGC-1α, NRF1 and TFAM expression, enhance mitochondrial protein synthesis, improve its respiration and improve its functional performance in a number of muscles types (30–32). As oxidants are regarded to negatively impact muscle fatigue and growth upon aerobic exercise, multiple studies have examined the effect of Cr supplementation on oxidative stress in muscles and proved a direct antioxidant activity of Cr affecting cell viability and DNA damage positively (24,33).
A similar effect has been previously observed in the cardiac muscle, with a decreased effect (26). Creatine supplementation revealed a tendency to increase mitochondrial biogenesis compared with sedentary rats, as indicated by the transcription levels of NRF-1 and TFAM and supported by the correlation data. There was no clear difference to exercised rats that were not supplemented with creatine, indicating that this was an exercise effect and not an effect of creatine supplementation. Transcriptional coactivator PGC-1α is the major regulator of mitochondrial biogenesis (5), and this is indicated by the increased expression of NFR-1, TFAM and mtDNA in the skeletal muscles of exercised rats supplemented with Cr, where an increase in the amount of PGC-1α and mtDNA can be observed. However, their levels fail to exhibit the same prominent increase as indicated in the soleus muscle, which was assessed in the current study. This may be due to the fact that PGC-1α expression is activated in the heart upon fasting, as identified by Lehman et al (34). Fasting is a physiologic stimulus that markedly increases the reliance of the heart on mitochondrial fat oxidation for ATP production (35).
This phenomenon has also been demonstrated by a study by Arany et al (26), where hearts of mice lacking PGC-1α exhibited reduced mitochondrial enzymatic activity, decreased ATP level and a decreased ability to increase the work output upon electrical or chemical stimulation (26). PGC-1α is known to coactivate PPARα and ERRα, and nuclear receptors (NRs) that regulate genes associated with cardiac fatty acid oxidation and mitochondrial respiratory function (36).
In the current study, sedentary rats that were supplemented with creatine (SC) demonstrated an increase in rat weight, since Cr is an osmotically active substance, and any increase in muscle Cr content can result in increased muscle water retention and weight gain (37). However, no significant differences in body weight were observed between exercised rats supplemented with creatine (ETC) or any other group. According to Becque et al (38), this increase in body mass due to Cr supplementation was the consequence of increased fat-free mass. While Deldicque et al (39) and Safdar et al (40) have reported that the administration of Cr may encourage overexpression of genes and proteins associated with abnormal enlargement of body parts or organs. These alterations would affect the translational process, triggering an increase in lean mass chronically. Chrusch et al demonstrated a significant increase in body mass, and this increase was identified to be lean body weight rather than water retention or fat (41). Another study also indicated significant increases in fat-free mass following only two resistance training sessions per week.
The results of the current study demonstrated that creatine supplementation for 5 weeks did not disrupt liver and kidney functions when compared with sedentary and exercised rats. An increase in creatinine level is a normal metabolic pathway that does not disrupt the normal functions of the kidney. This outcome is supported by previous studies that indicated no effect of short-, medium- and long-term Cr supplementation on kidney function (42), nominating creatine as a supplement with a high safety profile. These results strengthen the possibility of using Cr supplementation for individuals that are susceptible to impaired kidney function, including the elderly and patients with type 2 diabetes (42).
In conclusion, the results of the current study add to increasing number of studies investigating creatine mechanistic pathways in the mitochondria. It can be concluded that, activity coupled with short-term Cr supplementation increased all factors of mitochondrial biogenesis and improved skeletal and heart muscle functions, and this effect is unrelated to kidney or liver adverse effects. Further studies are required to explore the possibility of Cr supplementation in ameliorating mitochondrial diseases, including epilepsy, skeletal and cardiac myopathies, hepatopathies and nephropathies. In the current study, Cr supplementation with exercise enhanced PGC-1α expression, however, whether this effect can alter the muscle fiber types was not determined. The effect of creatine on different muscle fibers should be assessed in future studies.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- CS
control sedentary group
- ET
exercise training group
- ETC
exercise training and creatine treated group
- mtDNA
mitochondrial DNA
- NRF-1
nuclear respiratory factor 1
- PGC-1α
γ coactivator-1α
- SC
sedentary creatine-treated group
- TFAM
transcription factor A
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
MAK designed the experiments and contributed to the writing and revising of the paper. SAM and MAG extracted RNA and DNA, assayed the molecular parameters, analyzed the data, wrote and revised the manuscript. NAS and YES performed experimental design, rat training, wrote and revised the manuscript. All authors read and approved the final manuscript.
Ethical approval and consent to participate
The animal procedures were approved by the Institutional Animal Care and Use Committee at the Medical Research Institute, Alexandria University. All procedures comply with the National Institutes of Health guide for the care and use of Laboratory Animals (NIH Publications no. 8023, revised 1978), regulations of Egypt's guide for the care and use of laboratory animals (12) and the ARRIVE guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bemben MG, Witten M, Carter J, Eliot K, Knehans A, Bemben D. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J Nutr Health Aging. 2010;14:155–159. doi: 10.1007/s12603-009-0124-8. [DOI] [PubMed] [Google Scholar]
- 2.Brosnan JT, da Silva RP, Brosnan ME. The metabolic burden of creatine synthesis. Amino Acids. 2011;40:1325–1331. doi: 10.1007/s00726-011-0853-y. [DOI] [PubMed] [Google Scholar]
- 3.Karimian J, Esfahani PS. Supplement consumption in body builder athletes. J Res Med Sci. 2011;16:1347–1353. [PMC free article] [PubMed] [Google Scholar]
- 4.Holloway GP. Nutrition and training influences on the regulation of mitochondrial adenosine diphosphate sensitivity and bioenergetics. Sports Med. 2017;47(Suppl 1):S13–S21. doi: 10.1007/s40279-017-0693-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813:1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yin F, Cadenas E. Mitochondria: The cellular hub of the dynamic coordinated network. Antioxid Redox Signal. 2015;22:961–964. doi: 10.1089/ars.2015.6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang Y, Xia W, Yang J, Zhu Y, Chang H, Liu J, Huo W, Xu B, Chen X, Li Y, Xu S. BPA-induced DNA hypermethylation of the master mitochondrial gene PGC-1α contributes to cardiomyopathy in male rats. Toxicology. 2015;329:21–31. doi: 10.1016/j.tox.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Olsen RK, Cornelius N, Gregersen N. Redox signalling and mitochondrial stress responses; lessons from inborn errors of metabolism. J Inherit Metab Dis. 2015;38:703–719. doi: 10.1007/s10545-015-9861-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr. 2008;87:142–149. doi: 10.1093/ajcn/87.1.142. [DOI] [PubMed] [Google Scholar]
- 10.Liang X, Liu L, Fu T, Zhou Q, Zhou D, Xiao L, Liu J, Kong Y, Xie H, Yi F, et al. Exercise inducible lactate dehydrogenase B regulates mitochondrial function in skeletal muscle. J Biol Chem. 2016;291:25306–25318. doi: 10.1074/jbc.M116.749424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Care IoLARCo, Animals UoL, Resources NIoHDoR. Guide for the care and use of laboratory animals: National Academies. 1985 [Google Scholar]
- 12.Fahmy SR, Gaafar K. Establishing the first institutional animal care and use committee in Egypt. Philos Ethics Humanit Med. 2016;11:2. doi: 10.1186/s13010-016-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Araujo LC, de Souza IL, Vasconcelos LH, Brito Ade F, Queiroga FR, Silva AS, da Silva PM, Cavalcante Fde A, da Silva BA. Chronic aerobic swimming exercise promotes functional and morphological changes in rat ileum. Biosci Rep. 2015;35(pii):e00259. doi: 10.1042/BSR20150001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguiar AF, de Souza RW, Aguiar DH, Aguiar RC, Vechetti IJ, Jr, Dal-Pai-Silva M. Creatine does not promote hypertrophy in skeletal muscle in supplemented compared with nonsupplemented rats subjected to a similar workload. Nutr Res. 2011;31:652–657. doi: 10.1016/j.nutres.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Q, Wu Y, Sha H, Zhang P, Jia J, Hu Y, Zhu J. Early exercise affects mitochondrial transcription factors expression after cerebral ischemia in rats. Int J Mol Sci. 2012;13:1670–1679. doi: 10.3390/ijms13021670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Piantadosi CA, Suliman HB. Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem. 2006;281:324–333. doi: 10.1074/jbc.M508805200. [DOI] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Lee N, Shin S, Chung HJ, Kim DK, Lim JM, Park H, Oh HJ. Improved quantification of protein in vaccines containing aluminum hydroxide by simple modification of the Lowry method. Vaccine. 2015;33:5031–5034. doi: 10.1016/j.vaccine.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 20.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 21.Kamel MA, Helmy MH, Hanafi MY, Mahmoud SA, Elfetooh HA, Badr MS. Maternal obesity and malnutrition in rats differentially affect glucose sensing in the muscles and adipose tissues in the offspring. Int J Biochem Res Rev. 2014;4:440–469. doi: 10.9734/IJBCRR/2014/10649. [DOI] [Google Scholar]
- 22.Martinez N, Campbell B, Franek M, Buchanan L, Colquhoun R. The effect of acute pre-workout supplementation on power and strength performance. J Int Soc Sports Nutr. 2016;13:29. doi: 10.1186/s12970-016-0138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gualano B, Artioli GG, Poortmans JR, Lancha Junior AH. Exploring the therapeutic role of creatine supplementation. Amino Acids. 2010;38:31–44. doi: 10.1007/s00726-009-0263-6. [DOI] [PubMed] [Google Scholar]
- 24.Riesberg LA, Weed SA, McDonald TL, Eckerson JM, Drescher KM. Beyond muscles: The untapped potential of creatine. Int Immunopharmacol. 2016;37:31–42. doi: 10.1016/j.intimp.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finck BN, Kelly DP. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arany Z, He H, Lin J, Hoyer K, Handschin C, Toka O, Ahmad F, Matsui T, Chin S, Wu PH, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Head SI, Greenaway B, Chan S. Incubating isolated mouse EDL muscles with creatine improves force production and twitch kinetics in fatigue due to reduction in ionic strength. PLoS One. 2011;6:e22742. doi: 10.1371/journal.pone.0022742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy RM, Stephenson DG, Lamb GD. Effect of creatine on contractile force and sensitivity in mechanically skinned single fibers from rat skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1589–C1595. doi: 10.1152/ajpcell.00276.2004. [DOI] [PubMed] [Google Scholar]
- 29.Passaquin AC, Renard M, Kay L, Challet C, Mokhtarian A, Wallimann T, Ruegg UT. Creatine supplementation reduces skeletal muscle degeneration and enhances mitochondrial function in mdx mice. Neuromuscul Disord. 2002;12:174–182. doi: 10.1016/S0960-8966(01)00273-5. [DOI] [PubMed] [Google Scholar]
- 30.Li-Sha G, Yi-He C, Na-Dan Z, Teng Z, Yue-Chun L. Effects of carvedilol treatment on cardiac cAMP response element binding protein expression and phosphorylation in acute coxsackievirus B3-induced myocarditis. BMC Cardiovasc Disord. 2013;13:100. doi: 10.1186/1471-2261-13-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halling JF, Ringholm S, Olesen J, Prats C, Pilegaard H. Exercise training protects against aging-induced mitochondrial fragmentation in mouse skeletal muscle in a PGC-1α dependent manner. Exp Gerontol. 2017;96:1–6. doi: 10.1016/j.exger.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 32.Drake JC, Wilson RJ, Yan Z. Molecular mechanisms for mitochondrial adaptation to exercise training in skeletal muscle. FASEB J. 2016;30:13–22. doi: 10.1096/fj.15-276337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deminice R, Jordao AA. Creatine supplementation reduces oxidative stress biomarkers after acute exercise in rats. Amino Acids. 2012;43:709–715. doi: 10.1007/s00726-011-1121-x. [DOI] [PubMed] [Google Scholar]
- 34.Lehman JJ, Barger PM, Kovacs A, Saffitz JE, Medeiros DM, Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J Clin Invest. 2000;106:847–856. doi: 10.1172/JCI10268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sverdlov AL, Elezaby A, Behring JB, Bachschmid MM, Luptak I, Tu VH, Siwik DA, Miller EJ, Liesa M, Shirihai OS, et al. High fat, high sucrose diet causes cardiac mitochondrial dysfunction due in part to oxidative post-translational modification of mitochondrial complex II. J Mol Cell Cardiol. 2015;78:165–173. doi: 10.1016/j.yjmcc.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huss JM, Kelly DP. Nuclear receptor signaling and cardiac energetics. Circ Res. 2004;95:568–578. doi: 10.1161/01.RES.0000141774.29937.e3. [DOI] [PubMed] [Google Scholar]
- 37.Powers ME, Arnold BL, Weltman AL, Perrin DH, Mistry D, Kahler DM, Kraemer W, Volek J. Creatine supplementation increases total body water without altering fluid distribution. J Athl Train. 2003;38:44–50. [PMC free article] [PubMed] [Google Scholar]
- 38.Becque MD, Lochmann JD, Melrose DR. Effects of oral creatine supplementation on muscular strength and body composition. Med Sci Sports Exerc. 2000;32:654–658. doi: 10.1097/00005768-200003000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Deldicque L, Atherton P, Patel R, Theisen D, Nielens H, Rennie MJ, Francaux M. Effects of resistance exercise with and without creatine supplementation on gene expression and cell signaling in human skeletal muscle. J Appl Physiol (1985) 2008;104:371–378. doi: 10.1152/japplphysiol.00873.2007. [DOI] [PubMed] [Google Scholar]
- 40.Safdar A, Yardley NJ, Snow R, Melov S, Tarnopolsky MA. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol Genomics. 2008;32:219–228. doi: 10.1152/physiolgenomics.00157.2007. [DOI] [PubMed] [Google Scholar]
- 41.Chrusch MJ, Chilibeck PD, Chad KE, Davison KS, Burke DG. Creatine supplementation combined with resistance training in older men. Med Sci Sports Exerc. 2001;33:2111–2117. doi: 10.1097/00005768-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Neves M, Jr, Gualano B, Roschel H, Lima FR, Lúcia de Sá-Pinto A, Seguro AC, Shimizu MH, Sapienza MT, Fuller R, Lancha AH, Jr, Bonfá E. Effect of creatine supplementation on measured glomerular filtration rate in postmenopausal women. Appl Physiol Nutr Metab. 2011;36:419–422. doi: 10.1139/h11-014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.