Abstract
Neuropathic pain develops in 40–70% of spinal cord injury (SCI) patients and markedly compromises quality of life. We examined plasma from SCI patients for autoantibodies to glial fibrillary acidic protein (GFAP) and collapsin response mediator protein-2 (CRMP2) and evaluated their relationship to the development of neuropathic pain. In study 1, plasma samples and clinical data from 80 chronic SCI patients (1–41 years post-SCI) were collected and screened for GFAP autoantibodies (GFAPab). Results from study 1 indicated that GFAPab were present in 34 of 80 (42.5%) patients, but circulating levels did not correlate with the occurrence of neuropathic pain. In study 2, longitudinal plasma samples and clinical data were collected from 38 acute SCI patients. The level of GFAPab measured at 16 ± 7 days post-SCI was found to be significantly higher in patients that subsequently developed neuropathic pain (within 6 months post-SCI) than patients who did not (T = 219; p = 0.02). In study 3, we identified CRMP2 as an autoantibody target (CRMP2ab) in 23% of acute SCI patients. The presence of GFAPab and/or CRMP2ab increased the odds of subsequently developing neuropathic pain within 6 months of injury by 9.5 times (p = 0.006). Our results suggest that if a causal link can be established between these autoantibodies and the development of neuropathic pain, strategies aimed at reducing the circulating levels of these autoantibodies may have therapeutic value.
Keywords: : autoantibodies, CRMP2, GFAP, neuropathic pain, spinal cord injury
Introduction
Neuropathic pain is an adverse consequence of traumatic spinal cord injury (SCI) that occurs in approximately 40–70% of patients.1–4 The International Association for the Study of Pain (IASP) defines neuropathic pain as “pain caused by a lesion or disease of the somatosensory system.”5–9 Neuropathic pain is typically described as “pins & needles,” “burning,” or “pain evoked by light touch,” which, in some cases, can be refractory to conventional treatments.10 Unmitigated pain limits physical activity, negatively impacts rehabilitation, infringes on work and social activities, and reduces quality of life. Experimental studies using rodent models of SCI have identified multiple mechanisms that may contribute to the development of neuropathic pain. For instance, damage to nociceptive primary afferents, chronic disruption of the blood–spinal cord barrier, and enhanced excitability of nociceptors have all been proposed as potential mechanisms.11–15 Although these studies are beginning to provide a cellular and molecular basis for neuropathic pain, there is currently no distinguishing characteristic or biomarker available to identify SCI patients that are at elevated risk for developing this condition.
Autoantibodies to myelin basic protein, myelin-associated glycoprotein, ganglioside GM1, and myelin proteolipid protein, as well as antinuclear antibodies, have been reported to be enhanced after experimental and clinical SCI.16–20 For example, anti-GM1 immunoglobulin G (IgG) levels (measured approximately 10 years after injury) have been found to be elevated in the serum of SCI patients, with the highest levels observed in persons with neuropathic pain.17 Recently, it has been reported that approximately 20–40% of SCI patients have autoantibodies to glial fibrillary acidic protein (GFAP) that can be detected in plasma as early as 2 weeks after the injury.21 However, whether these antibodies have prognostic value for predicting the subsequent development of neuropathic pain has yet to be examined.
In the present study, we examined the incidence and relative abundance of GFAP autoantibodies (GFAPab) in acute and chronic SCI patients with and without neuropathic pain. We find that acute increases in plasma levels of this autoantibody have fair value (area under the curve [AUC] = 0.71) in identifying patients who will subsequently develop neuropathic pain within 6 months of injury. Further, using two-dimensional (2D) gel western blot and liquid chromatography/tandem mass spectrometry (LC-MS/MS), we identified autoantibodies to collapsin response mediator protein-2 (CRMP2; CRMP2ab) in 23% of SCI patients. The presence of GFAPab and/or CRMP2ab in the acute stages of injury increased the odds of developing neuropathic pain within 6 months of injury by 9.5 times.
Methods
Reagents
BD vacutainer K2EDTA (dipotassium ethylenediaminetetraacetic acid) tubes (BD, Franklin Lakes, NJ) were used for blood collection. Affinity-purified rabbit anti-GFAP (antibody to GFAP) antiserum was purchased from Bethyl Laboratories (Montgomery, TX). Rabbit anti-CRMP2 was purchased from Sigma-Aldrich (St. Louis, MO). Alkaline phosphatase (ALP)-conjugated secondary antibodies were obtained from Vector Laboratories (Burlingame, CA). Secondary antibodies coupled to Alexa Fluors were purchased from Thermo Fisher Scientific (Rockford, IL). Purified recombinant GFAP (TP304548) and CRMP2 (TP309080) proteins were obtained from OriGene (Rockville, MD).
Human subjects
The protocol for the use of adult human subjects was reviewed and approved by the University of Texas Committee for the Protection of Human Subjects. All SCI subjects enrolled were over 18 years of age and had a traumatic, nonpenetrating SCI with deficit. Acute SCI subjects were enrolled within 5 days of SCI. Chronic SCI subjects were enrolled at greater than 1 year post-injury. Exclusion criteria included a known medical condition that accounted for neuropathic pain (e.g., diabetic neuropathy, renal insufficiency, human immunodeficiency virus–associated, and ethanol-associated neuropathy) or diagnosis of cancer within the previous 5 years. In addition, chronic SCI subjects were excluded if they had a known infection at the time of, or within 30 days preceding, blood sampling. Blood samples were obtained with informed consent and were de-identified to provide confidentiality.
Neuropathic pain classification
Patients' neurological levels of injury were classified according to the International Standards for Neurological Classification of SCI (ISNCSCI)/American Spinal Injury Association Impairment Scale (AIS).22 The scale ranges from A (complete injury, no sensory or motor function preserved below the neurological level of injury or in the sacral segments S4–S5) to E (normal sensory and motor function). The definition of neuropathic pain was based on a clinical diagnosis and documentation of neuropathic pain. In order for a subject to be classified as having neuropathic pain, there must have been documentation of pain that included the descriptor “neuropathic.” The IASP has proposed a uniform classification system to define pain as nociceptive (musculoskeletal and visceral) or neuropathic (at level and below level of injury).23 In accord with the proposed IASP classification and other recommendations, pain documented as “acute pain due to trauma” was not considered neuropathic pain, nor was nociceptive pain described as musculoskeletal, visceral, or headache.9,24 Treatment with gabapentin or pregabalin, commonly used medications for neuropathic pain, was used as confirmation of the neuropathic categorization.10,25 Additionally, chronic SCI subjects and 18 of the 38 acute subjects completed the Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS) pain scale and the Short-Form McGill Pain Questionnaire as additional validation of their pain classification.26–28 The S-LANSS scale has been validated against clinical judgement.27 The S-LANSS self-assessment form was administered by trained personnel who instructed subjects to consider their pain within the last week and to focus on the most severe pain. The instrument includes a numeric visual analog scale for rating pain intensity and a diagram on which the subject indicates where the pain exists. Descriptors on the S-LANSS and in the McGill Pain Questionnaire include words commonly associated with neuropathic pain (e.g., pins and needles, electric shock, and burning). All subjects with an S-LANSS score of 12 or greater had clinical diagnoses of neuropathic pain.
Plasma preparation
For autoantibody screening, blood samples were collected in K2EDTA tubes (BD) from 38 adult, acute, traumatic SCI patients at three time points: 6.4 ± 1 days post-injury (n = 37), 16 ± 7 days post-injury (n = 38), and 96 ± 54 days post-injury (n = 13). The 80 chronic SCI subjects (>1 year post-SCI) and 20 healthy volunteers (HVs) provided a one-time blood sample at enrollment. Blood cells were removed by centrifugation (4°C, 800g for 10 min), and the plasma fraction collected and centrifuged again (4°C, 10,000g for 10 min) to prepare platelet-poor plasma. Samples were aliquoted and frozen at −80°C until assayed.
Two-dimensional gel electrophoresis and immunoblotting
Large-format 2D gels (20 × 22 cm) were prepared by Kendrick Labs (Madison, WI) using cadaver brain tissue as the sample source. Resolved proteins were transferred to nitrocellulose membranes for western analysis. To identify potential autoantibodies that appear in plasma after an SCI, pairs of identically prepared membranes were probed with plasma collected at <3 and 16 ± 7 days after SCI from representative study subjects with early post-injury samples (n = 2). Because the IgG response to a stimulus typically peaks around 10–30 days, novel immunopositive signals observed with the 16-day plasma were considered to be triggered by the injury.29,30
Membranes were blocked with 5% bovine serum albumin in Tris-buffered saline (TBS) overnight, incubated with plasma (1:1000 dilution) for 3 h at room temperature, then washed in TBS. Immunoreactivity was detected using anti-human IgG secondary antibodies coupled to ALP followed by chemiluminescence visualization. Immunoreactive spots detected on the paired blots were compared, and novel immunoreactive spots on the membranes probed with the 16-day plasma samples were identified and used to guide excision of the corresponding protein spots from a duplicate Coomassie-stained gel. Excised spots were sent for LC-MS/MS identification (Dr. Costel Darie Laboratory, Clarkson University Protein Core, Potsdam, NY).
Capillary westerns
Capillary westerns were carried out using a WES system, essentially as described by the vendor (ProteinSimple, San Jose, CA). Purified recombinant GFAP protein (160 ng/μL) was denatured in a lithium dodecyl sulfate–containing buffer including fluorescent standards to control for differences in separation across capillaries. Each assay included positive controls consisting of a commercial anti-GFAP antibody and a validated plasma sample containing GFAPab, and a negative control in which the primary antibody was not added. All plasma samples (1:50) for individual subjects were assayed simultaneously. Peak detection, AUC and the signal-to-noise ratio were calculated using Compass software (ProteinSimple, San Jose, CA). Measurements of AUC, in arbitrary units, were used for comparison across groups.
Immunodepletion of collapsin response mediator protein-2 from brain homogenate
An immunodepletion column was prepared by attaching a commercial CRMP2 antibody to a protein A agarose resin (Pierce, Rockford, IL) per the manufacturer's instructions. Cadaver brain was homogenized in radioimmunoprecipitation assay buffer (10 mM of Tris-Cl [pH 8.0], 1 mM of EDTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 140 mM of NaCl, 1 mM of phenylmethylsulfonyl fluoride, and 10 μg/mL of leupeptin). The extract (100 μg) was applied to the CRMP2/protein A column (containing 25 μL of anti-CRMP2 resin) overnight at 4°C in order to allow binding of CRMP2. The flow-thru was then collected and used for western analysis to establish the specificity of the CRMP2ab signal.
Binding competition studies
To verify autoantibody specificity, competition studies were performed. Plasma samples were incubated overnight at 4°C with 160 ng of target protein (e.g., GFAP for GFAP westerns), an equimolar amount of an unrelated protein (e.g., CRMP2 for GFAP westerns), or an equal volume of resuspension solution. After the overnight incubation, plasma samples were diluted (1:50) and used as primary antibodies for capillary-based western analysis as described above. To be considered autoantibody positive, plasma samples had to meet the following criteria: 1) having immunoreactivity above baseline; 2) immunoreactivity that decreased by >30% when pre-blocked with the target protein; and 3) immunoreactivity was not decreased when blocked with a competing protein.
Statistical analysis
Summary demographics for subject groups are presented as mean ± standard deviation (SD). Data were assessed for normality using the Kolmogoroff–Smirnov test. Data that were not normally distributed were compared using an appropriate nonparametric test. Two group comparisons at a single time point (e.g., SCI vs. healthy volunteers) were analyzed using the Mann–Whitney rank-sum test, and the data are presented as medians. A Spearman rank-order correlation was used to examine if the level of GFAPab correlated with the S-LANSS score for neuropathic pain. Chi-square test was performed to determine the relationship between the presence of GFAPab or GFAPab + CRMP2ab and the development of neuropathic pain. A two-by-two table of subjects with and without neuropathic pain at 6 months, by those positive or negative for autoantibody at 16 days, was used to calculate the odds of developing neuropathic pain. Multiple logistic regression was performed controlling for age, sex, body mass index (BMI), complete injury, and cervical injury level to evaluate the association between GFAPab and/or CRMP2ab and neuropathic pain. Significance was defined as p ≤ 0.05.
Results
Linearity of detection using capillary westerns
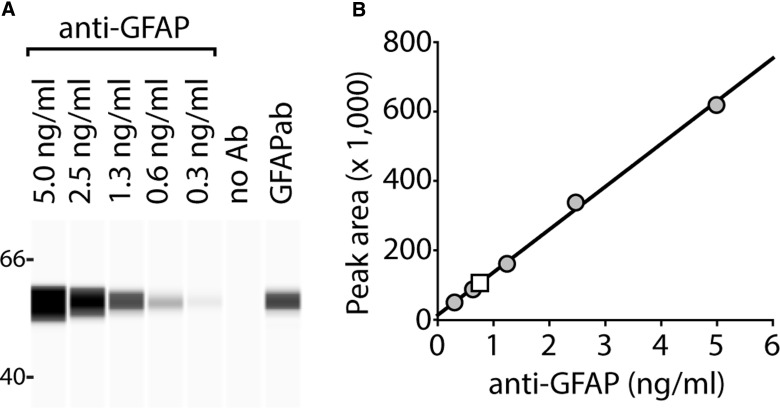
Before screening human subject plasma samples for the presence of autoantibodies, we first tested the ability of the capillary assay to detect increasing amounts of GFAP antibody. Purified recombinant GFAP protein (160 ng/μL) was separated by WES and probed with different concentrations of a commercial anti-GFAP antibody. The representative image shown in Figure 1A and the summary data presented in Figure 1B indicate that immunoreactive signal increases linearly with increasing concentration of the antibody, with a lower limit of detection of 0.3 ng/mL. When plasma of a representative SCI patient positive for GFAPab was used as the primary antibody (1:50 dilution), immunoreactivity within the linear range of the assay was detected (white square in Fig. 1B).
FIG. 1.
Capillary electrophoresis assay to measure GFAP antibodies. (A) Image of a representative capillary western showing the relative immunoreactivities of different concentrations of a commercial anti-GFAP antibody against a fixed amount (160 ng) of purified recombinant GFAP protein. No immunoreactivity was observed when the antibody was eliminated from the incubation mixture. Positive immunoreactivity was observed with the plasma of an SCI subject used as the primary antibody. (B) Summary data showing the relationship between immunoreactivity and concentration of the antibody, indicating the linearity of the detection. When the plasma (1:50 dilution) of an SCI study subject was used as the primary antibody, immunoreactivity within the linear range of the assay was observed (white square). Ab, antibody; anti-GFAP, antibody to GFAP; GFAP, glial fibrillary acidic protein; GFAPab, autoantibodies to GFAP; SCI, spinal cord injury.
Study 1. Presence of plasma glial fibrillary acidic protein autoantibodies in the chronic stage of spinal cord injury does not correlate with neuropathic pain
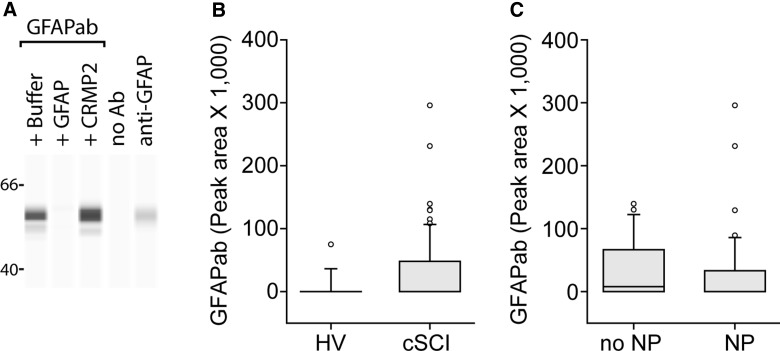
To assess GFAPab specificity in the patients' plasma, samples were pre-incubated with either GFAP or an unrelated (CRMP2) protein, then used as the source of primary antibodies in capillary westerns. As shown in Figure 2A, pre-incubation with purified GFAP (lane 2) significantly reduced immunoreactivity to purified GFAP separated by WES. In contrast, pre-incubation with CRMP2 (lane 3) had no demonstrable effect on immunoreactivity. Elimination of the plasma as the primary antibody (negative control, lane 4) resulted in no signal. In contrast, addition of the anti-GFAP antibody (positive control, lane 5) gave rise to a strong immunoreactive band. Plasma samples were screened using this binding competition assay to identify those containing GFAPab.
FIG. 2.
Circulating levels of GFAPab in chronic SCI. (A) Representative image of capillary western showing the specificity of the GFAPab immunoreactivity. Plasma (1:50 dilution) was pre-incubated with either 160 ng/uL of GFAP or an equimolar amount of CRMP2. GFAPab immunoreactivity was abolished after pre-incubation of plasma with recombinant GFAP, but not recombinant CRMP2. No immunoreactivity was detected when the primary antibody was eliminated (lane 4, negative control). The commercial anti-GFAP antibody (lane 5) is shown as a positive control. (B) Box plot (25th and 75th percentile) showing the relative levels of GFAPab in healthy volunteers (HV) and chronic SCI subjects (cSCI). (C) Box plot showing the relative levels of GFAPab in chronic SCI patients with neuropathic pain (NP) and those without neuropathic pain (No NP). Error bars are the 90th and 10th percentile. Outliers are represented as white circles. Horizontal line indicates the median. Ab, antibody; anti-GFAP, antibody to GFAP; CRMP2, collapsin response mediator protein-2; GFAP, glial fibrillary acidic protein; GFAPab, autoantibodies to GFAP; SCI, spinal cord injury.
To determine whether the presence of GFAPab in the chronic stage of SCI was related to neuropathic pain, a total of 80 chronic SCI subjects were consented and enrolled for their participation in this study. Demographics of these subjects are shown in Table 1. On average, these patients were 15 years from injury (1–41 years post-injury; mean, 15.3). The primary cause of injury in the majority of these patients was motor vehicle accidents (MVAs) and included primarily cervical injuries, with >50% of subjects classified as ISNCSCI/AIS A or B impairment level (A, complete loss of function; B, sensory, but no motor function, was preserved below the level of the injury). Of the 80 chronic SCI patients assayed, 34 (42.5%) were found to be immunopositive for GFAPab. By comparison, only 4 of 19 (21%) HVs were positive for GFAPab (Fig. 2B; 42.5% vs. 21%; p = 0.052; 1 healthy volunteer was found to be an outlier using the Grubbs test and was dropped [Z = 3.05; p < 0.05]).
Table 1.
Demographics of Study Subjects
| Demographic | Healthy volunteers (n = 20) | Study 1: chronic SCI subjects (n = 80) | Study 2: acute SCI subjects (n = 38) |
|---|---|---|---|
| Sex, count (%) | |||
| Male | 16 (80) | 59 (74) | 34 (89) |
| Female | 4 (20) | 21 (26) | 4 (11) |
| Age, average, years (SD) | 35 (13.2) | 44.1 (14.0) | 43.5 (17.7) |
| Age, range, years | 20–62 | 19–76 | 18–82 |
| Race, count (%) | |||
| White | 11 (55) | 66 (82) | 32 (84) |
| Asian | 6 (30) | 3 (4) | 0 (0) |
| Black | 3 (15) | 11 (14) | 5 (13) |
| Other | 0 (0) | 0 (0) | 1 (3) |
| Ethnicity | |||
| Hispanic | 0 (0) | 19 (24) | 6 (16) |
| Not Hispanic | 20 (100) | 61 (76) | 32 (84) |
| Mechanism of Injury, count (%) | |||
| MVA | 47 (59) | 23 (60) | |
| Fall | 19 (24) | 10 (26) | |
| Sports-related | 10 (13) | 4 (11) | |
| Assault | 4 (5) | 1 (3) | |
| Time post-SCI at enrollment | |||
| Average (SD) | 15.3 (12.3) years | 1.2 days | |
| Range | 1–41 years | 0.1–2.7 days | |
| Level of lesion | |||
| Cervical | 47 (60) | 31 (82) | |
| Thoracic | 22 (28) | 6 (16) | |
| Lumbar | 4 (5) | 1 (2) | |
| Unknown | 7 (9) | ||
| ISNCSCI/AIS Impairment Scale | |||
| A | 28 (35) | 23 (60) | |
| B | 21 (26) | 3 (8) | |
| C | 11 (14) | 8 (21) | |
| D | 12 (15) | 4 (11) | |
| Not classified | 8 (10) | ||
| Neuropathic pain | 46 (57.5) | 23 (60.5) | |
SD, standard deviation; MVA, motor vehicle accident; SCI, spinal cord injury; ISNCSCI/AIS, International Standards for Neurological Classification of SCI/American Spinal Injury Association Impairment Scale.
The presence or absence of neuropathic pain in chronic SCI subjects was determined according to the clinical criteria described in the Methods section. This diagnosis was confirmed using S-LANSS, which revealed that chronic SCI patients experiencing neuropathic pain had a significantly higher S-LANSS score than those without neuropathic pain (median, 9 vs. 0; T = 741; p < 0.001). Of the chronic SCI patients, 46 (57.5%) were classified as having neuropathic pain. Plasma GFAPab levels measured at the time of pain assessment were not significantly different between chronic SCI subjects without or with neuropathic pain (medians 7898.5 vs. 0; T = 1507; p = 0.16; Fig. 2C), nor was there a correlation between the severity of pain (using S-LANSS score) and intensity of GFAPab immunoreactivity (ρ = −0.04; p = 0.7).
Study 2. Presence of plasma glial fibrillary acidic protein autoantibodies in the acute stage of spinal cord injury predicts the development of neuropathic pain
We next examined whether plasma GFAPab in the acute stage of injury had prognostic value for identifying SCI patients who subsequently developed neuropathic pain within 6 months of injury. A total of 58 subjects (20 healthy volunteers and 38 acute SCI) were consented and enrolled for their participation in this study. Demographics of these subjects are shown in Table 1. On average, acute SCI patients had blood collected within 6.4 days post-injury and followed for 96 days. The primary cause of injury in the majority of acute SCI patients was MVAs, with >50% of these classified as ISNCSCI/AIS A or B impairment level.
Because IgG response peaks approximately 10–30 days after antigen presentation, we measured GFAPab in plasma samples collected 16 ± 7 days (n = 38) after SCI. A group of HVs was used as controls. At the 16-day time point, 21 of 38 (55%) acute SCI subjects were found to be immunopositive for GFAPab. Only 4 of 19 (21%) HVs were positive for GFAPab. Median GFAPab levels were found to be significantly increased in acute SCI subjects as compared to that measured in HVs (T = 401.5; medians, 26,377 vs. 0; p = 0.005; Fig. 3A).
FIG. 3.
Circulating levels of GFAPab in acute SCI predict the development of neuropathic pain. (A) Box plots showing the relative levels of GFAPab in plasma from healthy volunteers (HV) and acute SCI (aSCI) study subjects. Levels of GFAPab were significantly elevated in plasma of aSCI subjects compared to healthy volunteers. (B) Box plots showing relative levels of GFAPab in SCI subjects who subsequently developed neuropathic pain (NP) and those who did not (No NP). Acute SCI subjects who subsequently developed neuropathic pain had significantly higher GFAPab levels than those without neuropathic pain. Error bars are the 90th and 10th percentile. Outliers are represented as white circles. Horizontal line indicates the median. (C) Time course of GFAPab development for a subset of 13 SCI patients with and without neuropathic pain who had plasma samples available at four time points from 1.7 ± 0.7 to 96 ± 54 days. Longitudinal samples were not available for all of our study subjects. However, we did not identify any subjects with GFAPab-negative results at the 16- ± 7 d time point that were GFAPab positive at earlier or later time points. GFAP, glial fibrillary acidic protein; GFAPab, autoantibodies to GFAP; SCI, spinal cord injury.
Of the 38 acute SCI subjects, 23 (60.5%) subsequently developed neuropathic pain by 6 months after injury (Table 2). Age, BMI, sex, ISNCSCI/AIS, and cervical level did not differ between the pain classification groups. Using the plasma GFAPab levels at the 16-day time point, we found that acute SCI subjects with neuropathic pain had higher GFAPab levels than those without neuropathic pain (T = 219; p = 0.02; Fig. 3B). Presence of GFAPab was significantly associated with the development of neuropathic pain (χ2(1, N = 38) = 6.4; p = 0.01). Presence of GFAPab at 16 days after SCI increased the odds of developing neuropathic pain by 7.8 times (95% confidence interval [CI], 1.78–34.06; p = 0.01). Longitudinal samples from a subset of subjects (n = 13) assessed for development of GFAPab across time noted an increase of GFAPab at the 16-day time point (Fig. 3C). Age, BMI, sex, ISNCSCI/AIS, and cervical level were not significantly different between subjects with GFAPab-positive versus GFAPab-negative plasma measured at 16 days (Table 3).
Table 2.
Demographics of Acute SCI by Pain Group
| Demographic | Neuropathic pain (n = 23) | No neuropathic pain (n = 15) | p value |
|---|---|---|---|
| Age (years, median) | 36 | 47 | 0.69 |
| BMI (kg/m2, median) | 26.5 | 27.8 | 0.94 |
| Male sex | 19 | 15 | 0.14 |
| Complete (ISNCSCI A) | 14 | 9 | 1.0 |
| Cervical-level lesion | 17 | 14 | 0.2 |
SCI, spinal cord injury; BMI, body mass index; ISNCSCI, International Standards for Neurological Classification of SCI.
Table 3.
Demographics of Acute SCI by GFAPab Group
| Demographic | GFAPab positive (n = 21) | GFAPab negative (n = 17) | p value |
|---|---|---|---|
| Age (years, median) | 37 | 43 | 0.99 |
| BMI (kg/m2, median) | 26.3 | 27.8 | 0.5 |
| Male sex | 18 | 16 | 0.61 |
| Complete (ISNCSCI A) | 13 | 10 | 1.0 |
| Cervical-level lesion | 16 | 15 | 0.43 |
SCI, spinal cord injury; GFAPab, glial fibrillary acidic protein autoantibodies; BMI, body mass index; ISNCSCI, International Standards for Neurological Classification of SCI.
Study 3. Identification and prognostic accuracy of autoantibodies to collapsin response mediator protein-2
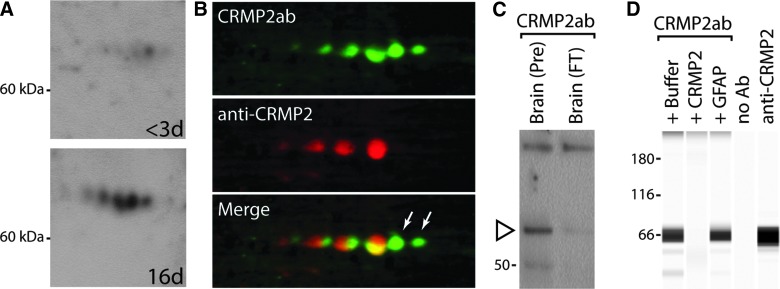
We next sought to identify additional autoantibodies and determine their prognostic utility alone or in combination with GFAPab. A total of 58 subjects (20 healthy volunteers and 38 acute SCI) were used in study 3. In preliminary screening using one-dimensional gels, we observed immunoreactivity in the 60- to 70-kDa range when probed with plasma samples collected at 16 ± 7 days, but not at <3 days, from the same SCI patient. To identify this putative autoantibody response, brain homogenates were separated by 2D gel electrophoresis and transferred to nitrocellulose membranes. Sections of the membranes containing the 60- to 70-kDa range were probed with <3- or 16-day post-SCI plasma samples. Figure 4A shows representative western blots indicating a series of novel immunopositive spots that were detected after incubation in the 16-day post-injury plasma. A total of 11 Coomassie-stained spots were excised from multiple gels and subjected to LC-MS/MS. All 11 spots were found to contain peptide fragments (9–109 unique peptides per spot) that mapped to CRMP2 (also known as dihydropyrimidinase-related protein 2, gil:4503377). CRMP2 has been reported to undergo post-translational modifications, such as phosphorylation, glycosylation, oxidation, SUMOylation, and proteolysis.31–40 These post-translational modifications can result in changes in a protein's migration on a 2D gel, and may account for the multiple CRMP2 spots we observed.
FIG. 4.
Identification of CRMP2 autoantibodies. (A) Representative images of western blots from 2D gels showing immunoreactivity when <3-day and 16-day plasma from the same patient was used as the primary antibody (1:1000). A marked increase in immunoreactive spots can be seen when the membrane was probed with the 16-day plasma. (B) Image of a 2D gel membrane that was double-labeled with 16-day post-SCI plasma (top, CRMP2ab; green) and a commercially available CRMP2 antibody (middle, anti-CRMP2; red). Merged image (bottom) showing colocalization of the CRMP2ab and the commercial anti-CRMP2 antibody. White arrows point to CRMP2ab spots not detected by the commercial anti-CRMP2 antibody. (C) Representative western blot showing CRMP2ab immunoreactivity after depletion of CRMP2 from human brain homogenate sample. CRMP2ab immunoreactivity (open arrowhead, brain PRE) is markedly reduced after CRMP2 immunodepletion (brain FT). (D) Representative image of capillary western showing the specificity of the CRMP2ab immunoreactivity. Plasma (1:100 dilution) was pre-incubated with either 36 ng/uL of CRMP2 or an equimolar amount of GFAP. CRMP2ab immunoreactivity was markedly decreased after pre-incubation of plasma with recombinant CRMP2, but not recombinant GFAP. No immunoreactivity was detected when the primary antibody was eliminated (lane 4, negative control). A strong signal was observed with the commercial CRMP2 antibody (lane 5) used as the primary antibody. 2D, two-dimensional; Ab, antibody; anti-CRMP2, antibody to CRMP2; CRMP2, collapsin response mediator protein-2; CRMPab, autoantibodies to CRMP2; GFAP, glial fibrillary acidic protein; SCI, spinal cord injury.
To confirm the identification of the immunopositive spots as CRMP2, we double-immunostained a 2D gel membrane using 16-day post-SCI plasma (CRMP2ab; detected using anti-human Alexa-488) and a commercially available CRMP2 antibody (anti-CRMP2; detected using anti-rabbit Alexa-568). Figure 4B shows that the proteins detected by the CRMP2ab were also detected by the commercial CRMP2 antibody, though slight shifts in the immunostaining were observed. The commercial CRMP2 antibody did not label two spots (arrows) that were exclusively detected by the patient plasma containing CRMP2ab. It is unclear, at this time, why the commercial antibody did not label these spots.
Because our double-labeled western showed good, but not perfect, agreement between the commercial CRMP2 antibody and the patient plasma, we tested the effect of immunodepletion of CRMP2 on the immunoreactivity of the CRMP2ab. Human brain total protein homogenates were subjected to immunodepletion using the commercial CRMP2 antibody, separated by SDS/polyacrylamide gel electrophoresis, transferred to a polyvinylidene difluoride membrane, and probed with patient plasma that was CRMP2ab positive. Figure 4C shows that CRMP2ab plasma detected a band in the lane containing total human brain extract (brain PRE), but not in a lane containing CRMP2-immunodepleted brain extract (brain FT).
As described for GFAP, a plasma screening method using purified CRMP2 was developed. Figure 4D shows a representative capillary western indicating that 16-day plasma gives rise to an immunoreactive band that can be eliminated by pre-incubation of the plasma with purified recombinant CRMP2, but not with an unrelated protein (i.e., GFAP). No specific immunoreactivity was observed when the plasma sample was removed as the primary antibody. When the commercial CRMP2 antibody was used as the primary antibody, a strong immunoreactive band was observed. Using these positive and negative binding controls for screening, we classified 8 of 35 (23%) acute SCI subjects as having CRMP2ab plasma at the 16-day post-injury time point; 3 subjects did not meet classification criteria and were excluded. Levels of CRMP2ab measured at 16 days post-injury did not differ between SCI patients who subsequently developed neuropathic pain and those who did not (T = 231.000; p = 0.08). Of interest, 7 of the 8 immunopositive patients (87.5%) developed neuropathic pain, indicating that CRMP2ab is a specific, although not very sensitive, predictor of neuropathic pain.
Presence of autoantibodies to glial fibrillary acidic protein and/or collapsin response mediator protein-2 is an excellent predictor for the subsequent development of neuropathic pain
Because multiple autoantibodies may improve sensitivity and specificity to distinguish which subjects will develop neuropathic pain, we next tested whether the presence of one or both autoantibodies could be used to increase the prognostic value for identifying SCI subjects at risk for developing neuropathic pain.41 Our screening identified 24 of 38 (63%) SCI subjects that were positive for one or both autoantibodies. A significant relationship was found between the presence of autoantibodies and the development of neuropathic pain (χ2(1, N = 38) = 7.47; p = 0.006). The presence of GFAPab and/or CRMP2ab increased the odds of developing neuropathic pain within 6 months of injury by 9.5 times (95% CI, 2.08–43.50; p = 0.006). This remained significant after controlling for age, sex, BMI, complete injury, and cervical level.
Discussion
Neuropathic pain after SCI can profoundly diminish quality of life, and there is no objective method for predicting which patients will develop neuropathic pain. In the present study, we explored the relationship between plasma GFAPab, CRMP2ab, and neuropathic pain in SCI patients. Our results revealed three key findings: 1) The presence of plasma GFAPab (occurring in 42.5% of subjects) in the chronic stage of injury does not differentiate between subjects with and without neuropathic pain; 2) GFAPab is detectable in 55% of acute SCI subjects by 16 days post-injury, and its presence predicts the subsequent development of neuropathic pain within 6 months of injury; and 3) SCI subjects with detectable GFAPab and/or CRMP2ab at 16 days post-SCI were 9.5 times more likely to develop neuropathic pain within 6 months of injury than those that were negative for both autoantibodies. Our results suggest that the presence of these autoantibodies early after injury can be used as prognostic markers for the subsequent development of neuropathic pain.
At present, it is unclear why only a portion of SCI patients (63%) had autoantibodies (GFAPab and/or CRMP2ab) while others did not. The development of autoantibodies was not associated with ISNCSCI/AIS scores and lesion level of injury, indicating that injury severity and/or location are not indicators for autoantibody responses to these targets. It is plausible that in patients without these autoantibodies, insufficient amounts of GFAP or CRMP2 were released into circulation to elicit a B-cell response. After traumatic brain injury (TBI), it has been reported that GFAP can be detected in serum of patients within hours of the injury, returning to baseline by 3 days post-injury.42–47 Unfortunately, the kinetics and amount of GFAP and/or CRMP2 released into the circulation after human SCI have not yet been established.
After SCI, the disruption of the blood–spinal cord barrier releases central nervous system (CNS) antigens that may enter the systemic circulation and stimulate an immune response resulting in autoantibody formation. This immune response is likely to have both protective and destructive potential.48 The nonspecific innate immune response initiated immediately after SCI can cause vasodilation, edema, cytokine, and chemokine production and an influx of leukocytes and polymorphonuclear cells as the body works to repair damaged tissue. The adaptive immune response subsequently generates antibodies through B lymphocytes to assist with removal of cellular debris and neutralize unknown foreign substances. If mechanisms of self-tolerance fail, autoantibodies targeting a self-antigen may cause damage to tissues. However, presently the consequences of autoantibodies to GFAP and/or CRMP2 in the injured spinal cord tissue on secondary pathology or outcome are not known.
Experimental studies have shown that SCI causes a breakdown of the blood–spinal cord barrier that allows for not only the infiltration of circulating cells and molecules into the injured cord, but also allows the efflux of CNS proteins.49,50 Consistent with this, it has been previously reported that GFAP, as well as other CNS proteins, can be detected in plasma of SCI patients.51–53 Once in the bloodstream, proteins released from the CNS can serve as antigens to trigger a B-cell response and generation of immunoglobulins (i.e., IgGs). Alternatively, the infiltration of immune cells into the damaged cord may allow these cells to contact locally released GFAP, thereby eliciting an immune response.54
At present, the mechanism by which autoantibodies lead to the development of neuropathic pain after SCI is not known. Both central and peripheral mechanisms are thought to contribute to the development of neuropathic pain, including increased excitability of the primary pain sensory neurons in the dorsal root ganglion, chronic opening of the blood–spinal cord barrier leading to persistent inflammation, and sprouting of afferent and efferent neurons located in the spinal cord and/or the brain.12,14,55,56 Given that the function of the spinal cord endothelium is intimately linked to astrocytes and astrocytic endfeet, it is plausible that autoantibodies to GFAP may contribute to increased and/or prolonged blood–spinal cord barrier permeability leading to a state of persistent inflammation in the injured cord.14
Of the five members of the CRMP family, CRMP2 is the most highly expressed in the adult CNS.34 Autoantibodies to CRMP2 have been identified in autoimmune retinopathy and cancer-associated retinopathy (melanoma, breast cancer and lymphoma).57 None of the subjects had diagnoses of retinopathy or cancer. CRMP2 is localized in neurites and axonal growth cones, where it mediates growth-cone collapse.58 Thus, autoantibodies to CRMP2 may result in sprouting of neurites and axonal growth cones in neurons mediating pain, thereby enhancing pain signals. However, it is unknown whether CRMP2 levels are decreased, or its function is neutralized, in patients with CRMP2ab. Further, because both GFAP and CRMP2 are cytosolic proteins, whether autoantibodies to these proteins are endocytosed to block the function of these proteins remains to be demonstrated. Future experiments will be required to determine whether the presence of autoantibodies to GFAP or CRMP2 are causally related to the development of neuropathic pain.
Several weakness of the present study must be acknowledged. First, our samples were collected within pre-specified time frames and therefore may not have captured the full extent of GFAPab and CRMP2ab production for all the participants, given that individual immune responses can vary. Second, our classification of patients' neuropathic pain was based on a clinical diagnosis of the condition. Many SCI patients are on medications to control their pain, possibly leading to the misclassification of their pain status/levels. Although inaccurate diagnoses are possible, the affective descriptors (tiring-exhausting, sickening, fearful, and punishing-cruel) on the McGill Pain Score were significantly higher in patients with a clinical diagnoses of neuropathic pain compared to those without (mean, 3.5 ± 3.2 vs. 0.2 ± 0.4; p < 0.04). Third, although significant differences were detected, the sample size of this study was small, thereby limiting our ability to determine whether the autoimmune response differed between the sexes, or levels of injury (e.g., cervical vs thoracic) or ISNCSCI/AIS grades and the development of neuropathic pain. In preliminary experiments, we have determined that a titer of 1:128 can be used to differentiate between patients who will develop neuropathic pain and those who will not. However, a clinically useful titer is likely to be dependent on the technique utilized, given that each method has its own sensitivity and background that needs to be considered. For this testing to be applicable clinically, additional experiments would be required to develop a standardized, U.S. Food and Drug Administration–approved method for assessing autoantibodies after SCI. Further, it is likely that there are additional undiscovered autoantibodies. As more proteins are found to elicit an antigenic response after SCI, establishing a panel of autoantibodies will further refine the predictive value of these autoantibodies as biomarkers in order to detect those who are vulnerable to developing neuropathic pain with high sensitivity and specificity. With these caveats in mind, our study suggests that autoantibodies to GFAP or CRMP2 may play a role in the development of neuropathic pain. If it can be established that these autoantibodies contribute to the development of neuropathic pain, strategies aimed at reducing/preventing the immune response (e.g., immunosuppressant therapy), or reducing the circulating levels of the antigen and/or autoantibodies (e.g., plasmaphoresis), may have therapeutic value in managing neuropathic pain after SCI.
Acknowledgments
G.W.H. is grateful to Mission Connect/TIRR Foundation and the Vivian L. Smith Foundation for their support of this research. Research in the laboratory of P.K.D. is supported by grants from NINDS.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Andresen S.R., Biering-Sorensen F., Hagen E.M., Nielsen J.F., Bach F.W., and Finnerup N.B. (2016). Pain, spasticity and quality of life in individuals with traumatic spinal cord injury in Denmark. Spinal Cord 54, 973–979 [DOI] [PubMed] [Google Scholar]
- 2.Dijkers M., Bryce T., and Zanca J. (2009). Prevalence of chronic pain after traumatic spinal cord injury: a systematic review. J. Rehabil. Res. Dev. 46, 13–29 [PubMed] [Google Scholar]
- 3.Siddall P.J., McClelland J.M., Rutkowski S.B., and Cousins M.J. (2003). A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257 [DOI] [PubMed] [Google Scholar]
- 4.Werhagen L., Budh C.N., Hultling C., and Molander C. (2004). Neuropathic pain after traumatic spinal cord injury—relations to gender, spinal level, completeness, and age at the time of injury. Spinal Cord 42, 665–673 [DOI] [PubMed] [Google Scholar]
- 5.Finnerup N.B., Haroutounian S., Kamerman P., Baron R., Bennett D.L., Bouhassira D., Cruccu G., Freeman R., Hansson P., Nurmikko T., Raja S.N., Rice A.S., Serra J., Smith B.H., Treede R.D., and Jensen T.S. (2016). Neuropathic pain: an updated grading system for research and clinical practice. Pain 157, 1599–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen T.S., Baron R., Haanpaa M., Kalso E., Loeser J.D., Rice A.S., and Treede R.D. (2011). A new definition of neuropathic pain. Pain 152, 2204–2205 [DOI] [PubMed] [Google Scholar]
- 7.Loeser J.D., and Treede R.D. (2008). The Kyoto protocol of IASP Basic Pain Terminology. Pain 137, 473–477 [DOI] [PubMed] [Google Scholar]
- 8.Treede R.D., Jensen T.S., Campbell J.N., Cruccu G., Dostrovsky J.O., Griffin J.W., Hansson P., Hughes R., Nurmikko T., and Serra J. (2008). Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology 70, 1630–1635 [DOI] [PubMed] [Google Scholar]
- 9.Treede R.D., Rief W., Barke A., Aziz Q., Bennett M.I., Benoliel R., Cohen M., Evers S., Finnerup N.B., First M.B., Giamberardino M.A., Kaasa S., Kosek E., Lavand'homme P., Nicholas M., Perrot S., Scholz J., Schug S., Smith B.H., Svensson P., Vlaeyen J.W., and Wang S.J. (2015). A classification of chronic pain for ICD-11. Pain 156, 1003–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guy S., Mehta S., Leff L., Teasell R., and Loh E. (2014). Anticonvulsant medication use for the management of pain following spinal cord injury: systematic review and effectiveness analysis. Spinal Cord 52, 89–96 [DOI] [PubMed] [Google Scholar]
- 11.Bedi S.S., Yang Q., Crook R.J., Du J., Wu Z., Fishman H.M., Grill R.J., Carlton S.M., and Walters E.T. (2010). Chronic spontaneous activity generated in the somata of primary nociceptors is associated with pain-related behavior after spinal cord injury. J. Neurosci. 30, 14870–14882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carlton S.M., Du J., Tan H.Y., Nesic O., Hargett G.L., Bopp A.C., Yamani A., Lin Q., Willis W.D., and Hulsebosch C.E. (2009). Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury. Pain 147, 265–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grau J.W., Huang Y.J., Turtle J.D., Strain M.M., Miranda R.C., Garraway S.M., and Hook M.A. (2017). When pain hurts: nociceptive stimulation induces a state of maladaptive plasticity and impairs recovery after spinal cord injury. J. Neurotrauma 34, 1873–1890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nesic O., Lee J., Johnson K.M., Ye Z., Xu G.Y., Unabia G.C., Wood T.G., McAdoo D.J., Westlund K.N., Hulsebosch C.E., and Regino Perez-Polo J. (2005). Transcriptional profiling of spinal cord injury-induced central neuropathic pain. J. Neurochem. 95, 998–1014 [DOI] [PubMed] [Google Scholar]
- 15.Yang Q., Wu Z., Hadden J.K., Odem M.A., Zuo Y., Crook R.J., Frost J.A., and Walters E.T. (2014). Persistent pain after spinal cord injury is maintained by primary afferent activity. J. Neurosci. 34, 10765–10769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ankeny D.P., Lucin K.M., Sanders V.M., McGaughy V.M., and Popovich P.G. (2006). Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 99, 1073–1087 [DOI] [PubMed] [Google Scholar]
- 17.Davies A.L., Hayes K.C., and Dekaban G.A. (2007). Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch. Phys. Med. Rehabil. 88, 1384–1393 [DOI] [PubMed] [Google Scholar]
- 18.Hayes K.C., Hull T.C., Delaney G.A., Potter P.J., Sequeira K.A., Campbell K., and Popovich P.G. (2002). Elevated serum titers of proinflammatory cytokines and CNS autoantibodies in patients with chronic spinal cord injury. J. Neurotrauma 19, 753–761 [DOI] [PubMed] [Google Scholar]
- 19.Petrova N.V., Ponomaryova A.M., Alyoshkin V.A., Eliseyev A.T., and Yumashev G.S. (1993). Serum rheumatoid factors in spinal cord injury patients. Paraplegia 31, 265–268 [DOI] [PubMed] [Google Scholar]
- 20.Zajarias-Fainsod D., Carrillo-Ruiz J., Mestre H., Grijalva I., Madrazo I., and Ibarra A. (2012). Autoreactivity against myelin basic protein in patients with chronic paraplegia. Eur. Spine J. 21, 964–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hergenroeder G.W., Moore A.N., Schmitt K.M., Redell J.B., and Dash P.K. (2016). Identification of autoantibodies to glial fibrillary acidic protein in spinal cord injury patients. Neuroreport 27, 90–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American Spinal Injury Association. (2008). American Spinal Injury Association: International Standards for Neurological Classification of Spinal Cord Injury. Revised 2000, Reprinted 2008. American Spinal Injury Association: Atlanta, GA [Google Scholar]
- 23.Siddall P.J., Yezierski R.P., and Loeser J.D. (2000). Pain following spinal cord injury: clinical features, prevalence, and taxonomy. International Association for the Study of Pain Newsletter 3, 3–7 [Google Scholar]
- 24.Siddall P.J., Taylor D.A., and Cousins M.J. (1997). Classification of pain following spinal cord injury. Spinal Cord 35, 69–75 [DOI] [PubMed] [Google Scholar]
- 25.Teasell R.W., Mehta S., Aubut J.A., Foulon B., Wolfe D.L., Hsieh J.T., Townson A.F., and Short C. (2010). A systematic review of pharmacologic treatments of pain after spinal cord injury. Arch. Phys. Med. Rehabil. 91, 816–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett M. (2001). The LANSS Pain Scale: the Leeds assessment of neuropathic symptoms and signs. Pain 92, 147–157 [DOI] [PubMed] [Google Scholar]
- 27.Bennett M.I., Smith B.H., Torrance N., and Potter J. (2005). The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J. Pain 6, 149–158 [DOI] [PubMed] [Google Scholar]
- 28.Melzack R. (1987). The short-form McGill Pain Questionnaire. Pain 30, 191–197 [DOI] [PubMed] [Google Scholar]
- 29.Hsueh P.R., Huang L.M., Chen P.J., Kao C.L., and Yang P.C. (2004). Chronological evolution of IgM, IgA, IgG and neutralisation antibodies after infection with SARS-associated coronavirus. Clin. Microbiol. Infect. 10, 1062–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Skoda D., Kranda K., Bojar M., Glosova L., Baurle J., Kenney J., Romportl D., Pelichovska M., and Cvachovec K. (2006). Antibody formation against beta-tubulin class III in response to brain trauma. Brain Res. Bull. 68, 213–216 [DOI] [PubMed] [Google Scholar]
- 31.Cole R.N., and Hart G.W. (2001). Cytosolic O-glycosylation is abundant in nerve terminals. J. Neurochem. 79, 1080–1089 [DOI] [PubMed] [Google Scholar]
- 32.Fujisawa H., Ohtani-Kaneko R., Naiki M., Okada T., Masuko K., Yudoh K., Suematsu N., Okamoto K., Nishioka K., and Kato T. (2008). Involvement of post-translational modification of neuronal plasticity-related proteins in hyperalgesia revealed by a proteomic analysis. Proteomics 8, 1706–1719 [DOI] [PubMed] [Google Scholar]
- 33.Gogel S., Lange S., Leung K.Y., Greene N.D., and Ferretti P. (2010). Post-translational regulation of Crmp in developing and regenerating chick spinal cord. Dev. Neurobiol. 70, 456–471 [DOI] [PubMed] [Google Scholar]
- 34.Hensley K., Venkova K., Christov A., Gunning W., and Park J. (2011). Collapsin response mediator protein-2: an emerging pathologic feature and therapeutic target for neurodisease indications. Mol. Neurobiol. 43, 180–191 [DOI] [PubMed] [Google Scholar]
- 35.Ju W., Li Q., Wilson S.M., Brittain J.M., Meroueh L., and Khanna R. (2013). SUMOylation alters CRMP2 regulation of calcium influx in sensory neurons. Channels (Austin.) 7, 153–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khanna R., Wilson S.M., Brittain J.M., Weimer J., Sultana R., Butterfield A., and Hensley K. (2012). Opening Pandora's jar: a primer on the putative roles of CRMP2 in a panoply of neurodegenerative, sensory and motor neuron, and central disorders. Future Neurol. 7, 749–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morinaka A., Yamada M., Itofusa R., Funato Y., Yoshimura Y., Nakamura F., Yoshimura T., Kaibuchi K., Goshima Y., Hoshino M., Kamiguchi H., and Miki H. (2011). Thioredoxin mediates oxidation-dependent phosphorylation of CRMP2 and growth cone collapse. Sci. Signal 4, 1–12 [DOI] [PubMed] [Google Scholar]
- 38.Nagai J., Owada K., Kitamura Y., Goshima Y., and Ohshima T. (2016). Inhibition of CRMP2 phosphorylation repairs CNS by regulating neurotrophic and inhibitory responses. Exp. Neurol. 277, 283–295 [DOI] [PubMed] [Google Scholar]
- 39.Taghian K., Lee J.Y., and Petratos S. (2012). Phosphorylation and cleavage of the family of collapsin response mediator proteins may play a central role in neurodegeneration after CNS trauma. J. Neurotrauma 29, 1728–1735 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Z., Ottens A.K., Sadasivan S., Kobeissy F.H., Fang T., Hayes R.L., and Wang K.K. (2007). Calpain-mediated collapsin response mediator protein-1, -2, and -4 proteolysis after neurotoxic and traumatic brain injury. J. Neurotrauma 24, 460–472 [DOI] [PubMed] [Google Scholar]
- 41.Dervieux T., Conklin J., Ligayon J.A., Wolover L., O'Malley T., Alexander R.V., Weinstein A., and Ibarra C.A. (2017). Validation of a multi-analyte panel with cell-bound complement activation products for systemic lupus erythematosus. J. Immunol. Methods 446, 54–59 [DOI] [PubMed] [Google Scholar]
- 42.Moghieb A., Bramlett H.M., Das J.H., Yang Z., Selig T., Yost R.A., Wang M.S., Dietrich W.D., and Wang K.K. (2016). Differential neuroproteomic and systems biology analysis of spinal cord injury. Mol. Cell Proteomics 15, 2379–2395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meier T.B., Nelson L.D., Huber D.L., Bazarian J.J., Hayes R.L., and McCrea M.A. (2017). Prospective assessment of acute blood markers of brain injury in sport-related concussion. J. Neurotrauma 34, 3134–3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Welch R.D., Ayaz S.I., Lewis L.M., Unden J., Chen J.Y., Mika V.H., Saville B., Tyndall J.A., Nash M., Buki A., Barzo P., Hack D., Tortella F.C., Schmid K., Hayes R.L., Vossough A., Sweriduk S.T., and Bazarian J.J. (2016). Ability of serum glial fibrillary acidic protein, ubiquitin C-terminal hydrolase-L1, and S100B to differentiate normal and abnormal head computed tomography findings in patients with suspected mild or moderate traumatic brain injury. J. Neurotrauma 33, 203–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mondello S., Kobeissy F., Vestri A., Hayes R.L., Kochanek P.M., and Berger R.P. (2016). Serum concentrations of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein after pediatric traumatic brain injury. Sci.Rep. 6, 28203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Z., Bramlett H.M., Moghieb A., Yu D., Wang P., Lin F., Bauer C., Selig T.M., Jaalouk E., Weissman A.S., Rathore D.S., Romo P., Zhang Z., Hayes R.L., Wang M.Y., Dalton D.W., and Wang K.K. (2018). Temporal profile and severity correlation of a panel of rat spinal cord injury protein biomarkers. Mol. Neurobiol. 55, 2174–2184 [DOI] [PubMed] [Google Scholar]
- 47.Papa L., Brophy G.M., Welch R.D., Lewis L.M., Braga C.F., Tan C.N., Ameli N.J., Lopez M.A., Haeussler C.A., Mendez Giordano D.I., Silvestri S., Giordano P., Weber K.D., Hill-Pryor C., and Hack D.C. (2016). Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 73, 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schwartz M. and Ziv Y. (2008). Immunity to self and self-maintenance: a unified theory of brain pathologies. Trends Immunol. 29, 211–219 [DOI] [PubMed] [Google Scholar]
- 49.Beck K.D., Nguyen H.X., Galvan M.D., Salazar D.L., Woodruff T.M., and Anderson A.J. (2010). Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain 133, 433–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raad M., Nohra E., Chams N., Itani M., Talih F., Mondello S., and Kobeissy F. (2014). Autoantibodies in traumatic brain injury and central nervous system trauma. Neuroscience 281, 16–23 [DOI] [PubMed] [Google Scholar]
- 51.Yokobori S., Zhang Z., Moghieb A., Mondello S., Gajavelli S., Dietrich W.D., Bramlett H., Hayes R.L., Wang M., Wang K.K., and Bullock M.R. (2015). Acute diagnostic biomarkers for spinal cord injury: review of the literature and preliminary research report. World Neurosurg. 83, 867–878 [DOI] [PubMed] [Google Scholar]
- 52.Kwon B.K., Stammers A.M., Belanger L.M., Bernardo A., Chan D., Bishop C.M., Slobogean G.P., Zhang H., Umedaly H., Giffin M., Street J., Boyd M.C., Paquette S.J., Fisher C.G., and Dvorak M.F. (2010). Cerebrospinal fluid inflammatory cytokines and biomarkers of injury severity in acute human spinal cord injury. J. Neurotrauma 27, 669–682 [DOI] [PubMed] [Google Scholar]
- 53.Ahadi R., Khodagholi F., Daneshi A., Vafaei A., Mafi A.A., and Jorjani M. (2015). Diagnostic value of serum levels of GFAP, pNF-H, and NSE compared with clinical findings in severity assessment of human traumatic spinal cord injury. Spine (Phila Pa 1976.) 40, E823–E830 [DOI] [PubMed] [Google Scholar]
- 54.Ankeny D.P., and Popovich P.G. (2010). B cells and autoantibodies: complex roles in CNS injury. Trends Immunol. 31, 332–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bedi S.S., Lago M.T., Masha L.I., Crook R.J., Grill R.J., and Walters E.T. (2012). Spinal cord injury triggers an intrinsic growth-promoting state in nociceptors. J. Neurotrauma 29, 925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gwak Y.S., and Hulsebosch C.E. (2011). Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury. Curr. Pain Headache Rep. 15, 215–222 [DOI] [PubMed] [Google Scholar]
- 57.Adamus G., Bonnah R., Brown L., and David L. (2013). Detection of autoantibodies against heat shock proteins and collapsin response mediator proteins in autoimmune retinopathy. BMC Ophthalmol. 13, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Charrier E., Reibel S., Rogemond V., Aguera M., Thomasset N., and Honnorat J. (2003). Collapsin response mediator proteins (CRMPs): involvement in nervous system development and adult neurodegenerative disorders. Mol. Neurobiol. 28, 51–64 [DOI] [PubMed] [Google Scholar]