Abstract
Single moderate-to-severe traumatic brain injuries (TBIs) may increase subsequent risk for neurodegenerative disease by facilitating β-amyloid (Aβ) deposition. However, the chronic effects on Aβ pathogenesis of repetitive mild TBIs (rTBI), which are common in adolescents and young adults, remain uncertain. We examined the effects of rTBI sustained during adolescence on subsequent deposition of Aβ pathology in a transgenic APP/PS1 rat model. Transgenic rats received sham or four individual mild TBIs (rTBIs) separated by either 24- or 72-h intervals at post-natal day 35 (before Aβ plaque deposition). Animals were euthanized at 12 months of age and underwent immunohistochemical analyses of Aβ plaque deposition. Significantly greater hippocampal Aβ plaque deposition was observed after rTBI separated by 24 h relative to rTBI separated by 72 h or sham injuries. These increases in hippocampal Aβ plaque load were driven by increases in both plaque number and size. Similar, though less-pronounced, effects were observed in extrahippocampal regions. Increases in Aβ plaque deposition were observed both ipsilaterally and contralaterally to the injury site and in both males and females. rTBIs sustained in adolescence can increase subsequent deposition of Aβ pathology, and these effects are critically dependent on interinjury interval.
Keywords: : adolescence, Alzheimer's disease, amyloid, mild traumatic brain injury, transgenic rat
Introduction
Traumatic brain injury (TBI) is one of the strongest environmental risk factors for Alzheimer's disease (AD). Epidemiological studies suggest that TBI is associated with increased risk for AD1–3 and/or accelerated disease onset,4 particularly among men.2 Increased β-amyloid (Aβ) plaque formation is observed both acutely and chronically after severe TBI,5–7 providing a potential pathophysiological basis for TBI as a risk factor for AD. Likewise, post-mortem examinations of patients with a history of a single moderate-to-severe TBI indicate that they are significantly more likely to exhibit Aβ plaques and neurofibrillary tangles than controls with no previous TBI exposure.8 Past work has also suggested that injury severity plays an important role in AD risk, which is more clearly elevated after moderate and severe TBI than after mild TBI (mTBI),9 but a more recent meta-analysis concludes that mTBI may still be associated with elevated risk for neurodegenerative disease when a broader spectrum of disorders is assessed.3 More recently, a study of veterans 19–58 years of age showed that even mTBI can interact with genetic risk to increase risk for neurodegenerative diseases like AD.
Although the chronic effects of single mTBIs on AD and/or other neurodegenerative disorders remain uncertain, studies of retired professional football players, who are more likely to sustain repeated mTBIs than the general population, suggest that they are also more likely to be diagnosed with AD at time of death10 and have an earlier age of AD onset.11 Higher AD prevalence is observed among individuals who played positions characterized by higher mTBI frequency,10 and a greater risk of late-life cognitive impairment is observed among those reporting greater numbers of past mTBIs.11 Although more rigorous data investigating the potential relationship between repeated mTBI and AD in clinical populations remain sparse, such injuries have also been associated with increased Aβ deposition, particularly when post-mortem examinations are performed at older ages.12,13
To better define the relationship between repeat mTBI and Aβ pathology, researchers have turned to experimental animal models. Single and repeated experimental TBI of different severities have demonstrated acute and subacute elevations of soluble Aβ in both wild-type and transgenic animal models.14 However, mTBI in wild-type rodents has not consistently resulted in significant long-term accumulation of Aβ plaques.15 Therefore, investigators have explored the chronic effects of TBI in transgenic models that overexpress autosomal-dominant human AD mutations in amyloid precursor protein (APP) and/or presenilin-1 (PS1) and develop age-associated Aβ plaque pathology. Such studies have produced divergent results, depending on the type and severity of experimental TBI in conjunction with the duration of follow-up. Nagakawa and colleagues conducted two studies of controlled cortical impact (CCI) injuries in PDAPP mice, which resulted in marked cell death and atrophy as well as concomitant decreased Aβ plaque deposition (potentially attributed to decreased Aβ production arising from cell loss) at 4–8 months after this more-severe TBI.16,17 However, when APP/PS1 mice were examined 6 weeks after CCI, such injuries increased Aβ plaque deposition over this shorter interval.18 In contrast, Uryu and colleagues demonstrated that multiple (but not single) mTBIs sustained by adult Tg2576 mice at 9 months of age (when Aβ plaques are already beginning to accumulate) accelerated subsequent chronic Aβ plaque deposition over a 4-month interval.19 This result suggests that milder injuries (i.e., without significant cell loss) administered repetitively in adult animals may exacerbate, rather than reduce, existing Aβ pathophysiology.
The interval between repeat mTBIs may also play a significant role in increasing the risk of AD. A single mTBI results in a brief period of cerebral vulnerability where the brain is sensitized to a subsequent injury, resulting in cumulative and increased damage. Although clinical data are lacking in this area, Prins and colleagues have shown that interinjury interval modulates the duration of cerebral glucose metabolic depression after repeat mTBIs in adolescent male rats.20 Metabolic deficits after a single mTBI recover within 3 days. However, a second mTBI sustained within this initial recovery period results in prolonged cerebral hypometabolism, which is not observed with longer interinjury intervals. These data may explain the heterogeneity in subsequent risk for neurodegenerative disease among individuals who have sustained repeat mTBI.
Analyses of the risk of AD after TBI have typically focused on adult injuries, although children, specifically adolescents and young adults, are exposed to high numbers of TBIs. However, the potential effects on Aβ pathogenesis of repeat mTBIs sustained by the developing brain have yet to be comprehensively addressed. These questions are of particular importance given the epidemiology of TBI. The incidence of TBI peaks during adolescence and young adulthood, with the majority of mTBIs resulting from sports and recreational activities.21 Further, the majority of professional athletes who are now being evaluated for neurodegenerative disease typically began advanced play during this age and many accumulate concussive injuries starting during adolescence and continuing throughout their careers. Among the 3.5 million annual new TBI cases is a growing subpopulation of children and young adults who experience repeat mTBIs, which represent up to 35% of such cases.22 The risk for subsequent TBIs increases with both age and the number of previous TBIs; the incidence of a second TBI is 2-fold greater among children 14 years and younger and 3-fold greater for young adults between 15–24 years of age.23
Given that most repeat mTBI are sustained by adolescents and young adults, we sought to determine whether repetitive experimental mTBIs administered to adolescent transgenic APP/PS1 rats (preceding the deposition of Aβ plaques) would increase the subsequent production of age-associated accumulation of Aβ pathology. Further, we explored whether this risk is modified by sex, given past work suggesting that TBI might be a risk factor for AD in men but not women.2 We also incorporated interinjury interval into our design to determine the cumulative effects of injury on Aβ pathology. We hypothesized that greater potentiation of chronic Aβ pathology by repetitive mTBI (rTBI) in adolescence would be observed with shorter interinjury intervals relative to longer interinjury intervals.
Methods
Experimental animals
Our experiments utilized an APP/PS1 transgenic rat model that homozygously expresses three human gene constructs: 1) APP 695 with the K670N/M671L mutation (rat synapsin-1 promoter); 2) APP minigene with the K670N/M671L and V717F mutations (platelet-derived growth factor β promoter); and 3) PS1 with the M146V mutation (rat synapsin-1 promoter) on a Sprague–Dawley background.24 The neuropathological characterization of these animals has previously been described in detail.24–26 Sparse parenchymal Aβ plaques begin to appear between 7 and 9 months of age, and plaque density progressively increases with age in cortical and hippocampal regions. The Aβ plaques in this model are primarily diffuse in nature, with a much smaller proportion of fibrillar plaques identified through thioflavin-S staining.25,26 Animals obtained from Cephalon Inc. (West Chester, PA) were bred and aged at the UCLA School of Medicine vivarium facility. Both male and female rats were used in this study (Table 1). All animals were housed under a 12-h light/dark cycle and had access to standard laboratory rat chow ad libitum. The UCLA Chancellor's Animal Research Committee approved this study, and all animal experiments were conducted in compliance with its guidelines.
Table 1.
Means and Standard Deviations for Age and Body Weight at Time of First and Last Sham or mTBI Injuries
| Sham | rTBI24 | rTBI72 | ||||
|---|---|---|---|---|---|---|
| M | F | M | F | M | F | |
| N | 5 | 5 | 8 | 6 | 6 | 7 |
| TBI.1 | ||||||
| Age (d) | — | — | 39.4 ± 3.6 | 39.2 ± 3.4 | 35.7 ± 1.0 | 36.1 ± 1.1 |
| Wt (g) | — | — | 171.9 ± 39.7 | 138.7 ± 34.1 | 150.5 ± 11.1 | 125.1 ± 22.1 |
| TBI.4 | ||||||
| Age (d) | 42.8 ± 3.0 | 40.4 ± 3.3 | 42.4 ± 3.6 | 42.2 ± 3.4 | 44.7 ± 1.0 | 45.1 ± 1.1 |
| Wt (g) | 167.8 ± 47.5 | 147.0 ± 15.9 | 185.1 ± 39.1 | 143.8 ± 38.4 | 200.8 ± 33.8 | 167.0 ± 30.9 |
mTBI, mild traumatic brain injury; Wt, weight; rTBI, repetitive mild TBI.
Closed-head injury model
The injury model used in this study was designed to reflect clinical aspects of mTBI and has also previously been described in detail.27 Briefly, under 2% isoflurane, each animal's head was shaven and a mask was used to mark the center of impact (−3 mm anterior/posterior and −4 mm medial/lateral relative to bregma28). The animal was then placed on a surface against a vertical board. A CCI tip (5 mm in diameter) was positioned at the skin surface at the center of impact and programmed to advance 8 mm at 36 pounds per square inch to displace the head. This injury produces a brief apnea, delayed toe pinch response, and increased righting times without mortality or skull fractures.20,27,29,30
We sought to model the chronic effects of rTBI in adolescent APP/PS1 rats, because the greatest incidence of both single TBI and rTBI in clinical populations occurs in adolescents.31 Although there are no exact guidelines for determining interspecies age equivalents for adolescence, we administered experimental mTBIs between post-natal day (PND) 35 and PND46 based on previously published developmental profiles. Sexual maturity is achieved between PND45 and PND60,32 and cerebral glucose metabolism reaches 90% of adult values at PND35.33 The molecular composition of PND34–44 rat synapses reflects synaptic changes observed in human teenagers,34 as do the increased risk-taking behaviors observed in PND28–45 rats.35
APP/PS1 rats from individual litters were randomly assigned to three groups. Group sizes of 10–15 animals each were determined by power analyses of preliminary immunohistochemical (IHC) data. The rTBI24 group received four repeat experimental mTBIs separated by 24-h intervals, the rTBI72 group received four repeat experimental mTBIs separated by 72-h intervals, and the sham group underwent a single induction of isoflurane anesthesia and positioning in the stereotaxic apparatus, but did not sustain any experimental mTBIs. The duration of each anesthesia exposure for injury induction and sham was 3 min. Previous work from our group has shown that single and repeated isoflurane exposures of such brief durations in sham animals result in similar levels of regional glucose metabolism20 and hormonal expression.29,30 Studies that document cerebral consequences of isoflurane exposure have involved prolonged periods (≥2–4 h) of anesthesia.36 No mortality associated with the sham or mTBI injuries was observed in this study.
Immunohistochemistry
At 12 months of age, animals were injected with a lethal dose of pentobarbital (100 mg/kg, intraperitoneal) and perfused transcardially with a 10% sucrose solution, followed by 4% paraformaldehyde. Brains were post-fixed overnight and cryoprotected in 20% sucrose before being frozen in methylbutane. Coronal sections (40 μm) were blocked with 0.5% H2O2, then blocked with 2% normal goat serum (Vector Laboratories, Burlingame, CA) and permeabilized with 0.3% Triton X-100 before incubation in DAE antibody (rabbit polyclonal anti-Aβ1-1337; 1:600 dilution; gift from Gregory M. Cole) at 4°C overnight. Sections were subsequently incubated for 1.5 h in 1:500 biotinylated goat antirabbit secondary antibody (BA1000; Vector Laboratories), and developed with ABC and DAB reaction kits (Vector Laboratories). Sections were mounted, dehydrated, and cover-slipped before quantitative analysis. DAE labels both Aβ40 and Aβ42.37 In order to differentiate between Aβ species, IHC was also performed using monoclonal mouse anti-Aβ40 (MM32-13.1.1, 1:400; Anaspec, Fremont, CA) and anti-Aβ42 (MM26-2.1.3, 1:5000; gift from Gregory M. Cole and Todd Golde) antibodies. Additional sections were also stained with thioflavin-S to label dense core Aβ plaques.
Quantification of β-amyloid plaque pathology
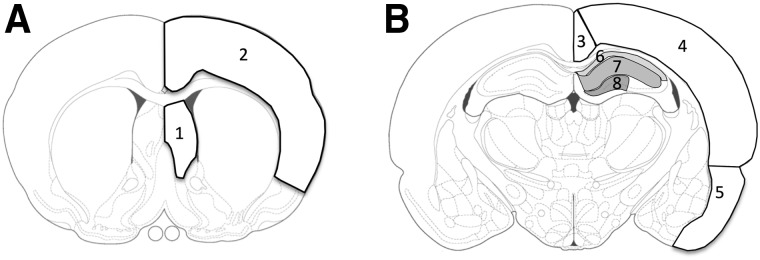
Examination of Aβ plaque deposition focused on two coronal planes: an anterior section (0.2 mm anterior of bregma) and a posterior section underlying the injury site (3.3 mm posterior of bregma). Images of these sections were captured at 5× magnification. A blinded rater used ImageJ (National Institutes of Health, Bethesda MD) to manually delineate Aβ plaques in multiple regions of interest (ROIs) in the anterior (septal nucleus and frontal motor/sensory cortex) and posterior (retrosplenial cortex, parietal auditory/sensory cortex, entorhinal cortex, and hippocampus) sections (Fig. 1). The hippocampus was further subdivided into the CA1 stratum oriens (CA1-SO), CA1 dendritic fields (CA1-DF: stratum pyramidale, stratum radiatum, and stratum lacunosum-moleculare layers), and the dentate gyrus (DG). Plaque density measured as: 1) Aβ staining area as a percentage of total ROI area; 2) number of total plaques per mm2; and 3) number of large plaques (>0.1 mm2) per mm2 was quantified and compared by injury group and sex.
FIG. 1.
Regions of interest in the coronal plane assessed for Aβ plaque burden. (A) Anterior section (0.2 mm anterior to bregma): 1) septal nucleus and 2) frontal motor/sensory cortex. (B) Posterior section (3.3 mm posterior to bregma): 3) retrosplenial cortex, 4) parietal auditory/sensory cortex, 5) entorhinal cortex, and hippocampus (subdivided into 6) CA1 stratum oriens, 7), CA1 dendritic layers, and 8) dentate gyrus). Figures adapted from Paxinos and Watson.25 Aβ, β-amyloid.
Technical issues (i.e., tissue damage, staining artifacts, etc.) limited our ability to quantify Aβ plaque pathology in individual ROIs from some animals in the rTBI24 and rTBI72 groups, resulting in different sample sizes for statistical comparisons of different regions. For the rTBI24 group, we were unable to obtain reliable bilateral measurements from selected animals for the entorhinal (n = 1), retrosplenial (n = 2), and parietal (n = 1) cortices. For the rTBI72 group, we were unable to obtain reliable bilateral measurements from selected animals for the hippocampus (n = 1), septum (n = 1), and entorhinal (n = 1), retrosplenial (n = 1), and frontal (n = 1) cortices.
Statistical analysis
All statistical analyses were performed using SPSS 22 for Macintosh (IBM, Armonk, NY). Physiological and IHC endpoints were compared between injury groups using analysis of variance (ANOVA) or analysis of covariance (ANCOVA), with Bonferroni correction for multiple comparisons. Intra-animal comparisons of Aβ pathology between hemispheres were conducted with paired t-tests. Data are expressed as means ± standard error, and comparisons for which p < 0.05 were considered statistically significant.
Results
Body weight
Mean age and body weights at the time of the first and last experimental mTBI are shown in Table 1. All sham and mTBI injuries were sustained between PND35 and PND46, with the ages of injuries staggered across groups to ensure overlapping distributions. Therefore, in the rTBI72 group, the initial injury was administered at a younger age, relative to the other groups, and the last injury was administered at an older age relative to the other groups. Total body weights at the time of the final injury were analyzed by ANCOVA, which revealed significant effects of age (F(1,29) = 42.36; p < 0.001) and sex (F(1,29) = 11.00; p = 0.002), but no effect of group (F(2,29) = 0.24; p = 0.79) and no group × sex interaction (F(2,29) = 2.27; p = 0.12).
Physiological effects of repeat mild traumatic brain injury
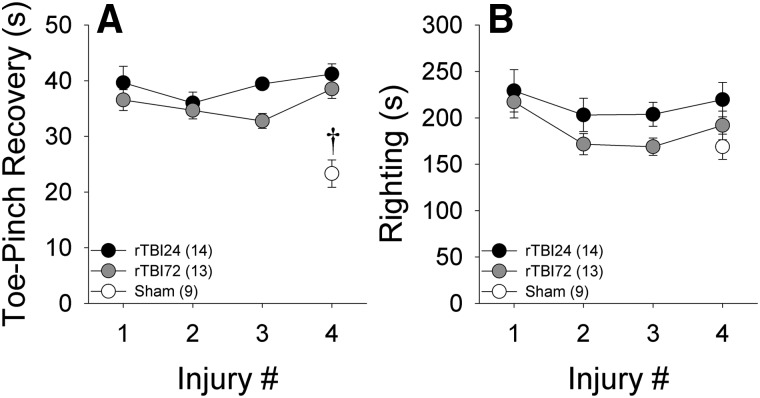
The time for post-mTBI recovery of toe-pinch reflexes is shown in Figure 2A. In the rTBI24 and rTBI72 groups, repeated-measures ANOVA indicated that there was no overall effect of injury number (F(3,69) = 2.45; p = 0.08), but that across injuries, the rTBI24 group had significantly longer toe-pinch recovery times than the rTBI72 group (F(1,23) = 5.67; p = 0.026). However, there was no group × injury number interaction (F(3,69) = 0.71; p = 0.55). Toe-pinch recovery times after the final injury exhibited a significant effect of group (F(2,30) = 21.42; p < 0.001), with shorter recovery times in the sham group, relative to the two rTBI groups (both ps < 0.001). Post-mTBI righting time is shown in Figure 2B. There were no significant overall effects of injury number (F(3,69) = 2.48; p = 0.07), group (F(1,23) = 2.92; p = 0.10), or group × injury number interaction (F(3,69) = 0.26; p = 0.85). When righting time was compared between the sham and rTBI groups (after the last injury), there was likewise no effect of group (F(2,30) = 1.75; p = 0.19). Sex was included as a variable in all analyses of post-mTBI physiological indices, but did not exert significant main effects or interactions (all ps > 0.10). As such, results in Figure 2 are presented in combined-sex groups. Overall, the relatively low variability observed in these physiological indices supports the consistency of the mTBI injuries induced by this model.
FIG. 2.
Physiological variables after each experimental injury. †p < 0.05 versus rTBI24 and rTBI72 groups. Data represented are means ± standard error. rTBI, repetitive mild TBI.
Hippocampal β-amyloid plaque deposition at 12 months of age
Representative hippocampal sections labeled with DAE at 12 months of age (approximately 10.5 months after the sham or final mTBI injuries were sustained) from each group are shown in Figure 3. Aβ plaque staining comprised similar percentages of hippocampal area both ipsilateral and contralateral to the side of impact in the sham (t(9) = 0.11; p = 0.92), rTBI24 (t(13) = 1.35; p = 0.20), and rTBI72 (t(11) = −0.74; p = 0.47) groups (Supplementary Fig. 1) (see online supplementary material at http://www.liebertpub.com). Therefore, subsequent analyses were performed using Aβ plaque indices combined across both ipsilateral and contralateral hippocampi.
FIG. 3.
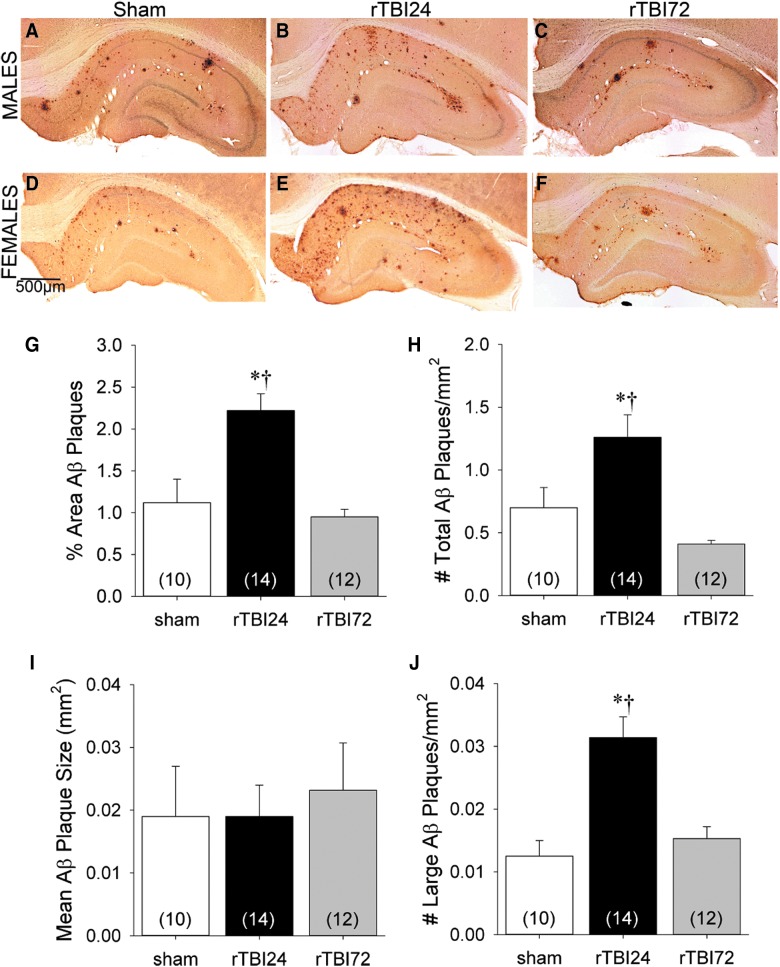
Representative hippocampal sections labeled with the anti-Aβ antibody, DAE, from male (A–C) and female (D–F) APP/PS1 rats at 12 months of age in the sham (A and D), rTBI24 (B and E), and rTBI72 (C and F) groups. Data represented are means ± standard error. Quantification of hippocampal Aβ plaque load across groups: Aβ plaque density expressed as percentage of hippocampal area (G) and total number of Aβ plaques per mm2 (H); mean size of Aβ plaques (I); and total number of large (>0.1 mm2) Aβ plaques per mm2 (J). *p < 0.05 versus sham group; †p < 0.05 versus rTBI72 group. Data represented are means ± standard error. Aβ, β-amyloid; APP, amyloid precursor protein; PS1, presenilin-1; rTBI, repetitive mild TBI.
Figure 3G illustrates Aβ plaque density expressed as a percentage of total hippocampal area in each group. Two-way ANOVA revealed a significant effect of group (F(2,30) = 13.28; p < 0.001), but no effect of sex (F(1,30) = 0.03; p = 0.86) or group × sex interaction (F(2,30) = 0.67; p = 0.52). Bonferroni-corrected post-hoc analyses indicated significantly greater hippocampal Aβ plaque staining in the rTBI24 group, relative to both the sham (p = 0.002) and rTBI72 (p < 0.001) groups, which did not differ from each other.
Figure 3H illustrates the total numbers of hippocampal Aβ plaques per mm2 in each group. There was a significant effect of group (F(2,30) = 11.92; p < 0.001). Although Aβ plaque density using this measure was numerically higher in female relative to male rats, this difference failed to reach statistical significance (F(1,30) = 3.05; p = 0.09) and there was no group × sex interaction (F(2,30) = 1.56; p = 0.23). Bonferroni-corrected post-hoc analyses indicated that there were significantly more Aβ plaques in the rTBI24 group relative to both the sham (p = 0.022) and rTBI72 (p < 0.001) groups, which did not differ from each other.
Figure 3I illustrates the average size for individual hippocampal Aβ plaques in each group. There was no effect of group (F(2,30) = 1.87; p = 0.17), but the average Aβ plaque size in males (mean = 0.024 mm2; standard deviation [SD] = 0.007) was significantly larger than in females (mean = 0.017 mm2; SD = 0.005; F(1,30) = 11.20; p = 0.002). There was no group × sex interaction (F(2,30) = 0.56; p = 0.58). Data demonstrating the absence of sex differences in plaque area are included in Table 2.
Table 2.
Mean Ab Plaque Density Expressed as Percentage Area of Ab Immunostaining in Male and Female Rats in the Sham, rTBI24, and rTBI72 Groups
| Sham | rTBI24 | rTBI72 | ||||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Hippocampus | 1.12% (1.18) | 1.11% (0.59) | 2.06% (0.78) | 2.44% (0.68) | 1.07% (0.28) | 0.82% (0.31) |
| Septum | 0.18% (0.22) | 0.09% (0.10) | 0.15% (0.15) | 0.35% (0.40) | 0.02% (0.04) | 0.07% (0.07) |
| Frontal Ctx | 0.19% (0.17) | 0.04% (0.03) | 0.22% (0.22) | 0.08% (0.09) | 0.02% (0.02) | 0.06% (0.08) |
| Retrosplenial Ctx | 0.12% (0.13) | 0.06% (0.08) | 0.18% (0.10) | 0.45% (0.52) | 0.01% (0.03) | 0.01% (0.02) |
| Parietal Ctx | 0.10% (0.12) | 0.02% (0.01) | 0.15% (0.15) | 0.11% (0.10) | 0.01% (0.01) | 0.01% (0.01) |
| Entorhinal Ctx | 0.19% (0.18) | 0.10% (0.06) | 0.26% (0.23) | 0.26% (0.20) | 0.08% (0.09) | 0.15% (0.15) |
Parentheses denote standard deviations.
Ab, β-amyloid; rTBI, repetitive mild traumatic brain injury; Ctx, cortex.
Figure 3J illustrates the total numbers of large hippocampal Aβ plaques (>0.1 mm2) per mm2 in each group. There was a significant effect of group (F(2,30) = 14.02; p < 0.001), because there were significantly more large plaques in the rTBI24 group than in the sham and rTBI72 groups (both ps <0.001). There was no effect of sex (F(1,30) = 0.45; p = 0.51) or group × sex interaction (F(2,30) = 1.15; p = 0.33).
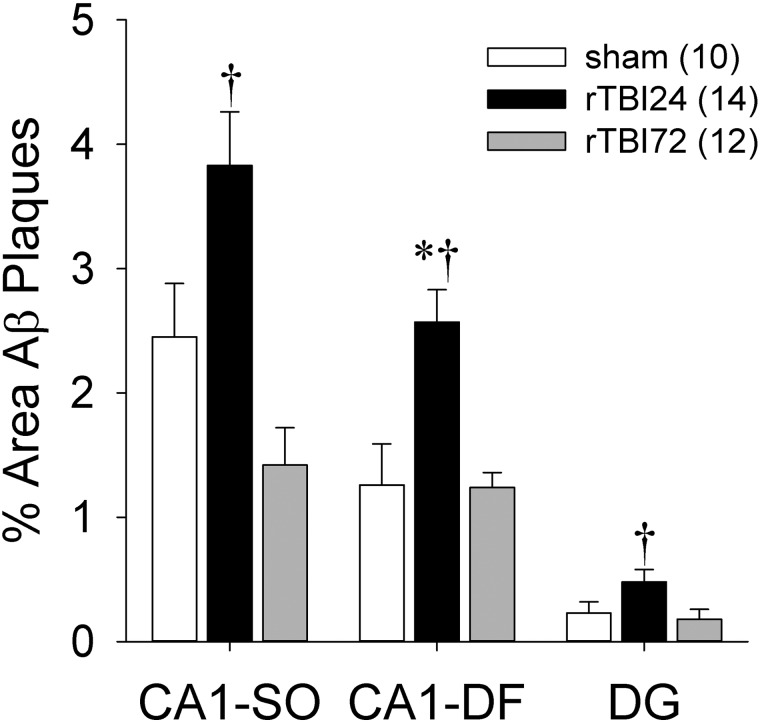
We subsequently investigated the distribution of increased Aβ plaque deposition across different hippocampal subregions relative to experimental group and sex (Fig. 4). Separate two-way ANOVAs demonstrated significant group effects in all three subregions (CA1-SO, F(2,30) = 9.71; p = 0.001; CA1-DF, F(2,30) = 10.96; p < 0.001; DG, F(2,30) = 4.39; p = 0.021). Bonferroni-corrected post-hoc comparisons indicated that total Aβ plaque area was significantly greater in the rTBI24 group, relative to the rTBI72 group, in all three subregions (CA1-SO, p < 0.001; CA1-DF, p = 0.001; DG, p = 0.046) and relative to the sham group in the CA1-DF region (p = 0.002). Similar total Aβ plaque areas were observed in the hippocampal subregions in the sham and rTBI72 groups, and there were no significant effects of sex or group × sex interactions (all ps > 0.05).
FIG. 4.
Aβ plaque density expressed as percentage area of Aβ immunostaining in hippocampal subregions. *p < 0.05 versus sham group; †p < 0.05 versus rTBI72 group. Data represented are means ± standard error. Aβ, β-amyloid; CA1-SO, CA1 stratum oriens; CA1-DF, CA1 dendritic fields; DG, dentate gyrus; rTBI, repetitive mild TBI.
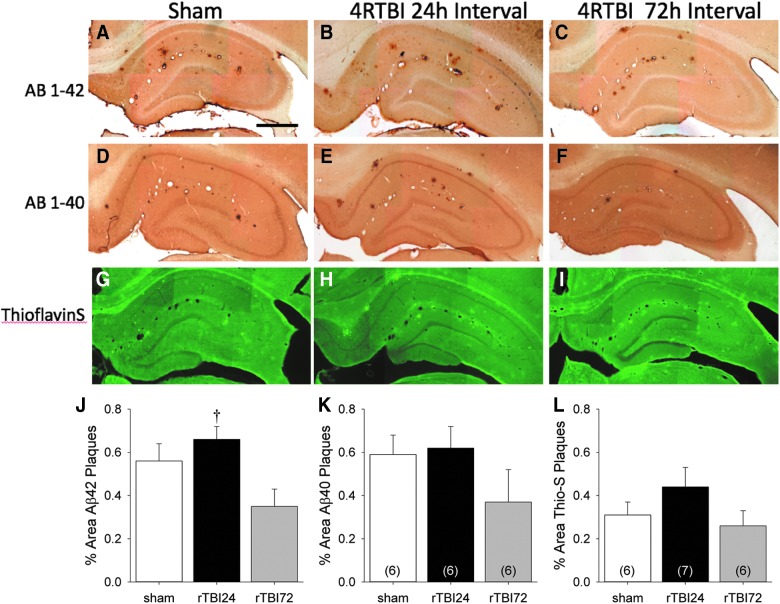
Whereas increased DAE labeling of Aβ plaques was observed after the rTBI24 group, this antibody does not differentiate between Aβ40 and Aβ42. Therefore, additional IHC was performed using anti-Aβ40 and anti-Aβ42 antibodies and thioflavin-S to further characterize Aβ plaque deposition in the three experimental groups (Fig. 5A–I). Plaque labeling for Aβ42, Aβ40, and thioflavin-S was quantified in male animals (Fig. 5J–L). There was a significant group effect in hippocampal Aβ42 labeling (Fig. 5J; F(2,16) = 4.95; p = 0.021), and Bonferroni-corrected post-hoc comparisons indicated greater Aβ42-positive plaque staining in the rTBI24 group relative to the rTBI72 group (p = 0.02). No group effects were observed with either Aβ40 (Fig. 6K; F(2,15) = 1.44; p = 0.27) or thioflavin-S (Fig. 6L; F(2,16) = 1.52; p = 0.25) labeling.
FIG. 5.
Representative photomicrographs of hippocampi from sham, RTBI at 24-h intervals, and 4RTBI at 72-h intervals. Sections are labeled with Aβ42 (A–C), Aβ40 (D–F), and thioflavin-S (G–I). Scale bar, 250 μm. Also shown are the quantification of plaque density measured by as Aβ42 (J), Aβ40 (K), and thioflavin-S (L). *p < 0.05 versus sham group; †p < 0.05 versus rTBI24 group. Data represented are means ± standard error. Aβ, β-amyloid; rTBI, repetitive mild TBI.
FIG. 6.
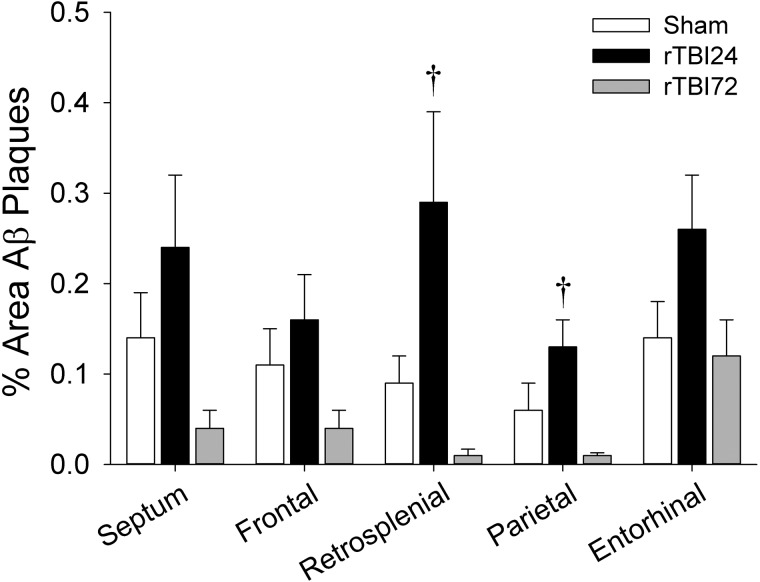
Aβ plaque density expressed as percentage area of Aβ immunostaining in the septum and the frontal, retrosplenial, parietal, and entorhinal cortices; †p < 0.05 versus rTBI72 group. Data represented are means ± standard error. Aβ, β-amyloid; rTBI, repetitive mild TBI.
β-amyloid plaque deposition at 12 months of age in extrahippocampal regions
Total Aβ plaque areas measured from ROIs in the anterior section and in the extrahippocampal regions of the posterior section are shown in Figure 6. In the anterior section, there were no significant differences in Aβ plaque density between groups in either the septal nucleus (F(2,29) = 3.12; p = 0.06) or the frontal motor/sensory cortex (F(2,32) = 1.86; p = 0.17). In the posterior section, there were significant effects of group on Aβ plaque density in the retrosplenial cortex (F(2,28) = 6.44; p = 0.005) and the parietal auditory/sensory cortex (F(2,29) = 4.98; p = 0.014), but not in the entorhinal cortex (F(2,29) = 2.46; p = 0.10). Bonferroni-corrected post-hoc comparisons indicated that Aβ plaque density was significantly greater in the rTBI24 group, relative to the rTBI72 group, in both the retrosplenial (p = 0.007) and parietal auditory/sensory (p = 0.009) cortices. There were no differences in extrahippocampal Aβ plaque densities between the sham and rTBI72 groups (all ps > 0.05). Likewise, there were no significant effects of sex or group × sex interactions on Aβ plaque density in any of the extrahippocampal regions (all ps > 0.05).
Discussion
Chronic effects of early-life traumatic brain injury
Our data indicate that four repetitive experimental mTBIs separated by 24-h intervals in adolescent transgenic APP/PS1 rats increased subsequent hippocampal and cortical Aβ plaque deposition by 12 months of age. These results support the hypothesis that TBI can augment underlying neurodegenerative disease38 and suggest that such injuries, when they occur in close succession, may have profound long-term consequences, even when sustained at a relatively young age.
Recent epidemiological work suggests that mTBI may have a stronger link with subsequent dementia when it is sustained at older ages,39 which is consistent with a past animal study indicating that experimental repetitive mTBIs at 9 months of age in Tg2576 mice, when behavioral and Aβ plaque pathology are already emerging, increased chronic Aβ plaque accumulation and cognitive deficits.19 Such reports raise the possibility that TBI may simply exacerbate pre-existing Aβ plaque pathology. However, our findings demonstrate that even repeat mTBI that occur before the normal onset of Aβ plaque deposition or behavioral impairments can also increase subsequent Aβ pathophysiology. These results parallel earlier work showing that more-severe CCI injuries delivered to presymptomatic APP/PS1 mice also increased subsequent Aβ plaque deposition.18 Nevertheless, comparisons of the relative magnitude of increases in Aβ plaque deposition after rTBI suggest that injuries at older ages may result in greater Aβ pathology (∼8-fold increase)19 than injuries in adolescence (∼2-fold increase). Although Aβ plaques appear to deposit more rapidly after past rTBI, it remains uncertain whether plaque accumulation actually begins at an earlier age, given that our pilot experiments did not detect increased plaque density when APP/PS1 rats injured at adolescence were euthanized at 7 or 8 months of age (data not shown).
Effects of interinjury interval
Our results further extend past work by Uryu and colleagues19 by demonstrating that both the number of mTBIs and the interinjury interval can affect Aβ plaque deposition. rTBI sustained at 24-h intervals increased diffuse plaques that were predominantly comprised of Aβ42 (Fig. 6), which has greater neurotoxicity and propensity for aggregation. Injuries at 72-h intervals yielded Aβ plaque indices that were not statistically different from those observed in sham-injured animals. However, in some extrahippocampal regions, the rTBI72 group did have numerically lower Aβ plaque densities relative to the sham group. This observation may suggest a pre-conditioning effect among the animals with longer interinjury intervals, but our experiments may not have had sufficient statistical power to detect this more-subtle effect. Although evidence of pre-conditioning after mTBI has been reported,40,41 the evidence for neuroprotection in rTBI has not been addressed in the literature.
Although it has been demonstrated that repeat concussions have cumulative effects,42–47 the effects of injury interval have not been well documented. Recent studies have described a window of vulnerability to cerebral hypometabolism, mitochondrial dysfunction, and oxidative injury after concussive events, where cells that normally would tolerate a single injury are now compromised when exposed to a second injury.20,48–50 Reductions in interinjury intervals also increase axonal injury51,52 and worsen glial and microglial reactivity.53,54 These past reports raise the possibility that rTBI at shorter intervals produce greater and longer lasting metabolic derangements and inflammatory responses that may contribute to accelerated Aβ deposition in this model. Work from Prins and colleagues indicates that longer interinjury intervals may provide sufficient time for injury-induced changes in cerebral glucose metabolism to normalize.20 Similarly, in youth sports, there is higher risk for more-severe concussions if athletes are reinjured within a week of a past concussion.55,56 These data suggest that longer interinjury recovery periods might reduce cumulative injury that could lead to accelerated Aβ deposition or other neurodegenerative processes.
We have conducted preliminary studies investigating the effects of rTBI in P35 transgenic rats on the expression of reactive astrocytes and microglia at shorter post-injury intervals (data not shown). At 1 week, transgenic animals exposed to rTBIs with 24-h interinjury intervals (and, to a lesser extent, animals exposed to repeat mTBIs with 72-h intervals) demonstrated increased in both glial fibrillary acidic protein (GFAP) and ionized calcium-binding adaptor molecule 1 (Iba1) expression in the ipsilateral hippocampus, gray-white matter junction, and thalamus relative to sham controls. Increased GFAP and Iba1 expression in a more-diffuse pattern was still evident at 1 month post-injury in both injury groups. However, by 7 months post-injury, increased expression of both markers emerges, even in transgenic animals in the sham group, and injury-related patterns of chronic inflammation could no longer be differentiated from the natural disease course in this model.
Absence of sex effects
There were no effects of sex on Aβ plaque load either within or between injury groups. These findings contrast with epidemiological and neuropathological studies from clinical populations that suggest that AD is more common and more severe in women relative to men.57 Studies of transgenic mouse models of cerebral amyloidosis also suggest more-advanced Aβ pathology and behavioral deficits in females relative to age-matched males,58–61 although this effect has not yet been reported in analogous transgenic rat models.25,62 Such differences have been previously attributed to sexual dimorphism during early brain development that arises from sex-specific hormones.63
Although women may be at higher overall risk for AD, epidemiological studies have suggested that this risk may not be further modulated by past TBI exposure to the same extent as in men.2 Those findings could, in principle, be attributable to sex hormone effects, differences in mechanism/type/severity of TBI between men and women, and/or statistical power issues. In contrast, both single TBIs and rTBIs in Tg2576 mice appear to have resulted in greater Aβ plaque accumulation in females than males.19 As such, it remains uncertain whether TBI interacts with sex to modulate risk, and the current study, which failed to find any such interactions, may not be sufficiently powered to conclusively address this question.
Absence of lateralization/localization effects
Despite the unilateral nature of our experimental mTBI model, similar Aβ burdens were observed ipsilateral and contralateral to the site of impact. Past studies of unilateral rTBI in adult Tg2576 mice19 and unilateral CCI in presymptomatic adult APP/PS1 mice18 have also demonstrated bilateral increases in Aβ pathology. Whereas our model produces a lateral injury, previous work from our group indicates that both unilateral and bilateral effects can be observed. Axonal damage measured by APP accumulation in subcortical white matter is more prominent in the hemisphere ipsilateral to the repetitive injuries,27 but acute cerebral hypometabolism of similar magnitude is observed bilaterally.20 Therefore, the bilateral acceleration of Aβ plaque deposition observed in the current study would appear to support mechanistic models that stress the more-diffuse effects of TBI on Aβ pathophysiology. Past work has raised the possibility that chronic neuroinflammation64 or oxidative damage19 that emerges after TBI may hasten Aβ deposition, but those mechanisms were not examined in the experiments described here.
Although the rTBI-mediated increases in Aβ plaque accumulation were observed bilaterally, the overall effects were more robust in the posterior ROIs (i.e., directly below the impact) than in the anterior ROIs. Although these findings could support a focal association between injury location and subsequent Aβ accumulation, an alternative explanation is that rTBIs merely exacerbate the normal pathological distribution of Aβ plaques in this transgenic APP/PS1 model. Past neuropathological studies in these rats have implicated the hippocampus (one of the posterior ROIs) as a region with earlier and more-pronounced Aβ plaque deposition.24–26 In contrast, Aβ deposition in the septum (one of the anterior ROIs) is relatively sparse in these animals.25 Likewise, within hippocampal subregions, the most robust increases in Aβ plaque deposition were observed in the CA1 dendritic fields, which also harbor the highest levels of intrahippocampal Aβ plaque pathology in uninjured animals.25
Potential mechanistic relationships between repetitive mild traumatic brain injury and β-amyloid accumulation
Aβ accumulation is one of the pathological hallmarks of AD, but the primacy of the amyloid hypothesis of AD pathogenesis remains controversial. Aβ appears necessary, but not sufficient, to produce the entire spectrum of AD pathophysiology.65 Therefore, Aβ accumulation may work in conjunction with bioenergetic changes to initiate a series of interacting cascades that increase oxidative stress, inflammation, synaptic dysfunction, glucose metabolic depression, and Aβ plaque deposition to facilitate neurodegenerative disease.65,66 As such, TBI may chronically exacerbate Aβ and other proteinopathies through such mechanisms, given that a number of the biochemical sequelae of TBI (i.e., decreased glucose metabolism, increased oxidative stress, and increased neuroinflammation) overlap with those observed in AD. These cascades may also affect the regulatory mechanisms of Aβ production. Acute TBI-associated increases in Aβ production may trigger the propagation and/or exacerbation of subsequent neurodegenerative changes at chronic time points. Further, TBI may also affect Aβ clearance through the glymphatic system.67 TBI reduces glymphatic function by up to 60% at both acute and chronic time points68 and thus may play a significant role in Aβ accumulation within the brain. These mechanisms, either alone or in combination, are likely to facilitate Aβ deposition and other aspects of neurodegeneration. However, further work will be needed to identify specific relationships between the initiation of such processes by rTBI sustained in early life and subsequent acceleration of Aβ pathogenesis.
Significance and limitations
The functional significance of increased Aβ plaque deposition after rTBI in this model remains somewhat uncertain, given that one of the main limitations of this study is the absence of behavioral endpoints. Transgenic mouse models of cerebral amyloidosis suggest that soluble Aβ oligomer levels may be more closely associated with cognitive deficits than Aβ plaque density.69–71 Whereas preliminary data from our laboratory (not shown) suggest that rTBI in the transgenic rat model used in this study results in acute increases in soluble Aβ oligomer levels in the hippocampus (similar to those observed after more-severe injuries in 3xTg-AD mice72), future studies across longer post-injury intervals will be needed to determine their significance and persistence. However, other studies of TBI in such models have shown parallel increases in Aβ plaque density and cognitive decline,18,19 lending credence to the hypothesis that our experimental repetitive mTBI model meaningfully increases Aβ pathogenesis.
Investigating the effects of TBI on AD pathogenesis in wild-type rodents remains challenging, given that they do not develop Aβ plaques with normal aging. Although our use of a transgenic APP/PS1 rat model facilitates the analyses of the chronic effects of rTBI on Aβ plaque deposition, it may not entirely reflect the association between rTBI and Aβ pathogenesis in human subjects with sporadic AD, who do not carry rare autosomal-dominant AD mutations. Although transgenic rodent models analogous to the APP/PS1 rats used in this study have greatly facilitated our understanding of AD pathophysiology, they do not completely replicate the breadth of findings observed in human AD.73 In such models, synapses in periplaques regions are particularly vulnerable, with significant changes in dendritic morphology74 and synaptic markers.75 Given the increased plaque densities observed in the RTBI24 animals, we would expect that the amount of synaptic dysfunction and changes in morphology would also increase in a commensurate fashion. Likewise, accelerated deposition of Aβ plaques in the rTBI24 group could activate and recruit additional microglia that further facilitate adjacent neuritic changes.76 Although these questions were not the focus of the current study, future investigations targeting these issues will be necessary to further unravel the interactions between repetitive mTBI and neurodegenerative disease.
Supplementary Material
Acknowledgments
This work was supported by the NIA (K08 AG34628 [jointly sponsored by the NIA, the American Federation for Aging Research, the John A. Hartford Foundation, the Atlantic Philanthropies, the Starr Foundation, and an anonymous donor] to E.T.) and the UCLA Brain Injury Research Center.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Mortimer J.A., van Duijn C.M., Chandra V., Fratiglioni L., Graves A.B., Heyman A., Jorm A.F., Kokmen E., Kondo K., Rocca W.A., Shalat S.L., Soininen H., and Hofman A. (1991). Head trauma as a risk factor for Alzheimer's disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. Int. J. Epidemiol. 20, Suppl. 2, S28–S35 [DOI] [PubMed] [Google Scholar]
- 2.Fleminger S., Oliver D.L., Lovestone S., Rabe-Hesketh S., and Giora A. (2003). Head injury as a risk factor for Alzheimer's disease: the evidence 10 years on; a partial replication. J. Neurol. Neurosurg. Psychiatry 74, 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perry D.C., Sturm V.E., Peterson M.J., Pieper C.F., Bullock T., Boeve B.F., Miller B.L., Guskiewicz K.M., Berger M.S., Kramer J.H., and Welsh-Bohmer K.A. (2016). Association of traumatic brain injury with subsequent neurological and psychiatric disease: a meta-analysis. J. Neurosurg. 124, 511–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nemetz P.N., Leibson C., Naessens J.M., Beard M., Kokmen E., Annegers J.F., and Kurland L.T. (1999). Traumatic brain injury and time to onset of Alzheimer's disease: a population-based study. Am. J. Epidemiol. 149, 32–40 [DOI] [PubMed] [Google Scholar]
- 5.Ikonomovic M.D., Uryu K., Abrahamson E.E., Ciallella J.R., Trojanowski J.Q., Lee V.M., Clark R.S., Marion D.W., Wisniewski S.R., and DeKosky S.T. (2004). Alzheimer's pathology in human temporal cortex surgically excised after severe brain injury. Exp. Neurol. 190, 192–203 [DOI] [PubMed] [Google Scholar]
- 6.Roberts G.W., Gentleman S.M., Lynch A., and Graham D.I. (1991). Beta A4 amyloid protein deposition in brain after head trauma. Lancet 338, 1422–1423 [DOI] [PubMed] [Google Scholar]
- 7.Roberts G.W., Gentleman S.M., Lynch A., Murray L., Landon M., and Graham D.I. (1994). Beta amyloid protein deposition in the brain after severe head injury: implications for the pathogenesis of Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 57, 419–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson V.E., Stewart W., and Smith D.H. (2012). Widespread tau and amyloid-beta pathology many years after a single traumatic brain injury in humans. Brain Pathol. 22, 142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plassman B.L., Havlik R.J., Steffens D.C., Helms M.J., Newman T.N., Drosdick D., Phillips C., Gau B.A., Welsh-Bohmer K.A., Burke J.R., Guralnik J.M., and Breitner J.C. (2000). Documented head injury in early adulthood and risk of Alzheimer's disease and other dementias. Neurology 55, 1158–1166 [DOI] [PubMed] [Google Scholar]
- 10.Lehman E.J., Hein M.J., Baron S.L., and Gersic C.M. (2012). Neurodegenerative causes of death among retired National Football League players. Neurology 79, 1970–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guskiewicz K.M., Marshall S.W., Bailes J., McCrea M., Cantu R.C., Randolph C., and Jordan B.D. (2005). Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery 57, 719–726 [DOI] [PubMed] [Google Scholar]
- 12.Stein T.D., Montenigro P.H., Alvarez V.E., Xia W., Crary J.F., Tripodis Y., Daneshvar D.H., Mez J., Solomon T., Meng G., Kubilus C.A., Cormier K.A., Meng S., Babcock K., Kiernan P., Murphy L., Nowinski C.J., Martin B., Dixon D., Stern R.A., Cantu R.C., Kowall N.W., and McKee A.C. (2015). Beta-amyloid deposition in chronic traumatic encephalopathy. Acta Neuropathol. 130, 21–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts G.W., Allsop D., and Bruton C. (1990). The occult aftermath of boxing. J. Neurol. Neurosurg. Psychiatry 53, 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bird S.M., Sohrabi H.R., Sutton T.A., Weinborn M., Rainey-Smith S.R., Brown B., Patterson L., Taddei K., Gupta V., Carruthers M., Lenzo N., Knuckey N., Bucks R.S., Verdile G., and Martins R.N. (2016). Cerebral amyloid-beta accumulation and deposition following traumatic brain injury—a narrative review and meta-analysis of animal studies. Neurosci. Biobehav. Rev. 64, 215–228 [DOI] [PubMed] [Google Scholar]
- 15.Mouzon B.C., Bachmeier C., Ferro A., Ojo J.O., Crynen G., Acker C.M., Davies P., Mullan M., Stewart W., and Crawford F. (2014). Chronic neuropathological and neurobehavioral changes in a repetitive mild traumatic brain injury model. Ann. Neurol. 75, 241–254 [DOI] [PubMed] [Google Scholar]
- 16.Nakagawa Y., Nakamura M., McIntosh T.K., Rodriguez A., Berlin J.A., Smith D.H., Saatman K.E., Raghupathi R., Clemens J., Saido T.C., Schmidt M.L., Lee V.M., and Trojanowski J.Q. (1999). Traumatic brain injury in young, amyloid-beta peptide overexpressing transgenic mice induces marked ipsilateral hippocampal atrophy and diminished Abeta deposition during aging. J. Comp. Neurol. 411, 390–398 [PubMed] [Google Scholar]
- 17.Nakagawa Y., Reed L., Nakamura M., McIntosh T.K., Smith D.H., Saatman K.E., Raghupathi R., Clemens J., Saido T.C., Lee V.M., and Trojanowski J.Q. (2000). Brain trauma in aged transgenic mice induces regression of established abeta deposits. Exp. Neurol. 163, 244–252 [DOI] [PubMed] [Google Scholar]
- 18.Tajiri N., Kellogg S.L., Shimizu T., Arendash G.W., and Borlongan C.V. (2013). Traumatic brain injury precipitates cognitive impairment and extracellular Abeta aggregation in Alzheimer's disease transgenic mice. PLoS One 8, e78851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uryu K., Laurer H., McIntosh T., Pratico D., Martinez D., Leight S., Lee V.M., and Trojanowski J.Q. (2002). Repetitive mild brain trauma accelerates Abeta deposition, lipid peroxidation, and cognitive impairment in a transgenic mouse model of Alzheimer amyloidosis. J. Neurosci. 22, 446–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prins M.L., Alexander D., Giza C.C., and Hovda D.A. (2013). Repeated mild traumatic brain injury: mechanisms of cerebral vulnerability. J. Neurotrauma 30, 30–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakhos L.L., Lockhart G.R., Myers R., and Linakis J.G. (2010). Emergency department visits for concussion in young child athletes. Pediatrics 126, e550–e556 [DOI] [PubMed] [Google Scholar]
- 22.Langburt W., Cohen B., Akhthar N., O'Neill K., and Lee J.C. (2001). Incidence of concussion in high school football players of Ohio and Pennsylvania. J. Child Neurol. 16, 83–85 [DOI] [PubMed] [Google Scholar]
- 23.Annegers J.F., Grabow J.D., Kurland L.T., and Laws E.R., Jr (1980). The incidence, causes, and secular trends of head trauma in Olmsted County, Minnesota, 1935–1974. Neurology 30, 912–919 [DOI] [PubMed] [Google Scholar]
- 24.Flood D.G., Lin Y.G., Lang D.M., Trusko S.P., Hirsch J.D., Savage M.J., Scott R.W., and Howland D.S. (2009). A transgenic rat model of Alzheimer's disease with extracellular Abeta deposition. Neurobiol. Aging 30, 1078–1090 [DOI] [PubMed] [Google Scholar]
- 25.Liu L., Orozco I.J., Planel E., Wen Y., Bretteville A., Krishnamurthy P., Wang L., Herman M., Figueroa H., Yu W.H., Arancio O., and Duff K. (2008). A transgenic rat that develops Alzheimer's disease-like amyloid pathology, deficits in synaptic plasticity and cognitive impairment. Neurobiol. Dis. 31, 46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teng E., Kepe V., Frautschy S.A., Liu J., Satyamurthy N., Yang F., Chen P.P., Cole G.B., Jones M.R., Huang S.C., Flood D.G., Trusko S.P., Small G.W., Cole G.M., and Barrio J.R. (2011). [F-18]FDDNP microPET imaging correlates with brain Abeta burden in a transgenic rat model of Alzheimer disease: effects of aging, in vivo blockade, and anti-Abeta antibody treatment. Neurobiol. Dis. 43, 565–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prins M.L., Hales A., Reger M., Giza C.C., and Hovda D.A. (2010). Repeat traumatic brain injury in the juvenile rat is associated with increased axonal injury and cognitive impairments. Dev. Neurosci. 32, 510–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G. and Watson C. (2005). The Rat Brain in Stereotaxic Coordinates, 5th ed. Academic: San Diego, CA [Google Scholar]
- 29.Greco T., Hovda D., and Prins M. (2013). The effects of repeat traumatic brain injury on the pituitary in adolescent rats. J. Neurotrauma 30, 1983–1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greco T., Hovda D.A., and Prins M.L. (2015). Adolescent TBI-induced hypopituitarism causes sexual dysfunction in adult male rats. Dev. Neurobiol. 75, 193–202 [DOI] [PubMed] [Google Scholar]
- 31.Centers for Disease Control and Prevention. (2011). Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤19 years—United States, 2001–2009. MMWR Morb. Mortal. Wkly. Rep. 60, 1337–1342 [PubMed] [Google Scholar]
- 32.Lewis E.M., Barnett J.F., Jr, Freshwater L., Hoberman A.M., and Christian M.S. (2002). Sexual maturation data for CRL Sprague-Dawley rats: criteria and confounding factors. Drug Chem. Toxicol. 25, 437–458 [DOI] [PubMed] [Google Scholar]
- 33.Nehlig A., de Vasconcelos A.P., and Boyet S. (1988). Quantitative autoradiographic measurement of local cerebral glucose utilization in freely moving rats during postnatal development. J. Neurosci. 8, 2321–2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Counotte D.S., Li K.W., Wortel J., Gouwenberg Y., Van Der Schors R.C., Smit A.B., and Spijker S. (2010). Changes in molecular composition of rat medial prefrontal cortex synapses during adolescent development. Eur. J. Neurosci. 32, 1452–1460 [DOI] [PubMed] [Google Scholar]
- 35.Spear L.P. (2000). The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 24, 417–463 [DOI] [PubMed] [Google Scholar]
- 36.Calloway J.K., Wood C., Jenkins T.S., Royse A.G., and Royse C.F. (2016). Isoflurane in the presence of absence of surgery increases hippocampal cytokines associated with memory deficits and responses to brain injury in rats. Behav. Brain Res. 303, 44–52 [DOI] [PubMed] [Google Scholar]
- 37.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., and Cole G.M. (2001). The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 21, 8370–8377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner R.C., and Yaffe K. (2014). Traumatic brain injury may increase risk of young onset dementia. Ann. Neurol. 75, 339–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gardner R.C., Burke J.F., Nettiksimmons J., Kaup A., Barnes D.E., and Yaffe K. (2014). Dementia risk after traumatic brain injury vs nonbrain trauma: the role of age and severity. JAMA Neurol. 71, 1490–1497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Di Pietro, V., Amorini A.M., Tavazzi B., Hovda D.A., Signoretti S., Giza C.C., Lazzarino G., Vagnozzi R., Lazzarino G., and Belli A. (2013). Potentially neuroprotective gene modulation in an in vitro model of mild traumatic brain injury. Mol. Cell. Biochem. 375, 185–198 [DOI] [PubMed] [Google Scholar]
- 41.Costa T., Constantino L.C., Mendonca B.P., Pereira J.G., Herculano B., Tasca C.I., and Boeck C.R. (2010). N-methyl-D-aspartate preconditioning improves short-term motor deficits outcome after mild traumatic brain injury in mice. J. Neurosci. Res. 88, 1329–1337 [DOI] [PubMed] [Google Scholar]
- 42.Collins M.W., Grindel S.H., Lovell M.R., Dede D.E., Moser D.J., Phalin B.R., Nogle S., Wasik M., Cordry D., Daugherty K.M., Sears S.F., Nicolette G., Indelicato P., and McKeag D.B. (1999). Relationship between concussion and neuropsychological performance in college football players. JAMA 282, 964–970 [DOI] [PubMed] [Google Scholar]
- 43.Gronwall D., and Wrightson P. (1975). Cumulative effect of concussion. Lancet 2, 995–997 [DOI] [PubMed] [Google Scholar]
- 44.Slobounov S., Slobounov E., Sebastianelli W., Cao C., and Newell K. (2007). Differential rate of recovery in athletes after first and second concussion episodes. Neurosurgery 61, 338–344 [DOI] [PubMed] [Google Scholar]
- 45.Gaetz M., Goodman D., and Weinberg H. (2000). Electrophysiological evidence for the cumulative effects of concussion. Brain Inj. 14, 1077–1088 [DOI] [PubMed] [Google Scholar]
- 46.Wall S.E., Williams W.H., Cartwright-Hatton S., Kelly T.P., Murray J., Murray M., Owen A., and Turner M. (2006). Neuropsychological dysfunction following repeat concussions in jockeys. J. Neurol. Neurosurg. Psychiatry 77, 518–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bijur P.E., Haslum M., and Golding J. (1996). Cognitive outcomes of multiple mild head injuries in children. J. Dev. Behav. Pediatr 17, 143–148 [PubMed] [Google Scholar]
- 48.Vagnozzi R., Signoretti S., Tavazzi B., Floris R., Ludovici A., Marziali S., Tarascio G., Amorini A.M., Di Pietro, V., Delfini R., and Lazzarino G. (2008). Temporal window of metabolic brain vulnerability to concussion: a pilot 1H-magnetic resonance spectroscopic study in concussed athletes—part III. Neurosurgery 62, 1286–1295 [DOI] [PubMed] [Google Scholar]
- 49.Vagnozzi R., Tavazzi B., Signoretti S., Amorini A.M., Belli A., Cimatti M., Delfini R., Di Pietro, V., Finocchiaro A., and Lazzarino G. (2007). Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment—part I. Neurosurgery 61, 379–388 [DOI] [PubMed] [Google Scholar]
- 50.Tavazzi B., Vagnozzi R., Signoretti S., Amorini A.M., Belli A., Cimatti M., Delfini R., Di Pietro, V., Finocchiaro A., and Lazzarino G. (2007). Temporal window of metabolic brain vulnerability to concussions: oxidative and nitrosative stresses—part II. Neurosurgery 61, 390–395 [DOI] [PubMed] [Google Scholar]
- 51.Fujita M., Wei E.P., and Povlishock J.T. (2012). Intensity- and interval-specific repetitive traumatic brain injury can evoke both axonal and microvascular damage. J. Neurotrauma 29, 2172–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weil Z.M., Gaier K.R., and Karelina K. (2014). Injury timing alters metabolic, inflammatory and functional outcomes following repeated mild traumatic brain injury. Neurobiol. Dis. 70, 108–116 [DOI] [PubMed] [Google Scholar]
- 53.Huang L., Coats J.S., Mohd-Yusof A., Yin Y., Assaad S., Muellner M.J., Kamper J.E., Hartman R.E., Dulcich M., Donovan V.M., Oyoyo U., and Obenaus A. (2013). Tissue vulnerability is increased following repetitive mild traumatic brain injury in the rat. Brain Res. 1499, 109–120 [DOI] [PubMed] [Google Scholar]
- 54.Bolton A.N., and Saatman K.E. (2014). Regional neurodegeneration and gliosis are amplified by mild traumatic brain injury repeated at 24-hour intervals. J. Neuropathol. Exp. Neurol. 73, 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guskiewicz K.M., McCrea M., Marshall S.W., Cantu R.C., Randolph C., Barr W., Onate J.A., and Kelly J.P. (2003). Cumulative effects associated with recurrent concussion in collegiate football players: the NCAA Concussion Study. JAMA 290, 2549–2555 [DOI] [PubMed] [Google Scholar]
- 56.Leddy J.J., Sandhu H., Sodhi V., Baker J.G., and Willer B. (2012). Rehabilitation of concussion and post-concussion syndrome. Sports Health 4, 147–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mielke M.M., Vemuri P., and Rocca W.A. (2014). Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin. Epidemiol. 6, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sturchler-Pierrat C. and Staufenbiel M. (2000). Pathogenic mechanisms of Alzheimer's disease analyzed in the APP23 transgenic mouse model. Ann. N. Y. Acad. Sci. 920, 134–139 [DOI] [PubMed] [Google Scholar]
- 59.Callahan M.J., Lipinski W.J., Bian F., Durham R.A., Pack A., and Walker L.C. (2001). Augmented senile plaque load in aged female beta-amyloid precursor protein-transgenic mice. Am. J. Pathol. 158, 1173–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hirata-Fukae C., Li H.F., Hoe H.S., Gray A.J., Minami S.S., Hamada K., Niikura T., Hua F., Tsukagoshi-Nagai H., Horikoshi-Sakuraba Y., Mughal M., Rebeck G.W., LaFerla F.M., Mattson M.P., Iwata N., Saido T.C., Klein W.L., Duff K.E., Aisen P.S., and Matsuoka Y. (2008). Females exhibit more extensive amyloid, but not tau, pathology in an Alzheimer transgenic model. Brain Res. 1216, 92–103 [DOI] [PubMed] [Google Scholar]
- 61.Wang J., Tanila H., Puolivali J., Kadish I., and van Groen T. (2003). Gender differences in the amount and deposition of amyloidbeta in APPswe and PS1 double transgenic mice. Neurobiol. Dis. 14, 318–327 [DOI] [PubMed] [Google Scholar]
- 62.Heggland I., Storkaas I.S., Soligard H.T., Kobro-Flatmoen A., and Witter M.P. (2015). Stereological estimation of neuron number and plaque load in the hippocampal region of a transgenic rat model of Alzheimer's disease. Eur. J. Neurosci. 41, 1245–1262 [DOI] [PubMed] [Google Scholar]
- 63.Carroll J.C., Rosario E.R., Kreimer S., Villamagna A., Gentzschein E., Stanczyk F.Z., and Pike C.J. (2010). Sex differences in beta-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain Res. 1366, 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Webster S.J., Van Eldik L.J., Watterson D.M., and Bachstetter A.D. (2015). Closed head injury in an age-related Alzheimer mouse model leads to an altered neuroinflammatory response and persistent cognitive impairment. J. Neurosci. 35, 6554–6569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Musiek E.S., and Holtzman D.M. (2015). Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat. Neurosci. 18, 800–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.VanItallie T.B. (2015). Biomarkers, ketone bodies, and the prevention of Alzheimer's disease. Metabolism 64, 3 Suppl. 1, S51–S57 [DOI] [PubMed] [Google Scholar]
- 67.Tarasoff-Conway J.M., Carare R.O., Osorio R.S., Glodzik L., Butler T., Fieremans E., Axel L., Rusinek H., Nicholson C., Zlokovic B.V., Frangione B., Blennow K., Menard J., Zetterberg H., Wisniewski T., and de Leon M.J. (2015). Clearance systems in the brain-implications for Alzheimer disease. Nat. Rev. Neurol. 11, 457–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iliff J.J., Chen M.J., Plog B.A., Zeppenfeld D.M., Soltero M., Yang L., Singh I., Deane R., and Nedergaard M. (2014). Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 34, 16180–16193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng I.H., Scearce-Levie K., Legleiter J., Palop J.J., Gerstein H., Bien-Ly N., Puolivali J., Lesne S., Ashe K.H., Muchowski P.J., and Mucke L. (2007). Accelerating amyloid-beta fibrillization reduces oligomer levels and functional deficits in Alzheimer disease mouse models. J. Biol. Chem. 282, 23818–23828 [DOI] [PubMed] [Google Scholar]
- 70.Lesne S., Kotilinek L., and Ashe K.H. (2008). Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience 151, 745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lesne S., Koh M.T., Kotilinek L., Kayed R., Glabe C.G., Yang A., Gallagher M., and Ashe K.H. (2006). A specific amyloid-beta protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 72.Washington P.M., Morffy N., Parsadanian M., Zapple D., and Burns M.P. (2014). Experimental traumatic brain injury induces rapid aggregation and oligomerization of amyloid-beta in an Alzheimer's disease mouse model. J. Neurotrauma 31, 125–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hall A.M. and Roberson E.D. (2012). Mouse models of Alzheimer's disease. Brain Res. Bull. 88, 3–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Spires T.L., Meyer-Luehmann M., Stern E.A., McLean P.J., Skoch J., Nguyen P.T., Bacskai B.J., and Hyman B.T. (2005). Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J. Neurosci. 25, 7278–7287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mitew S., Kirkcaldie M.T., Dickson T.C., and Vickers J.C. (2013). Altered synapses and gliotransmission in Alzheimer's disease and AD model mice. Neurobiol. Aging 34, 2341–2351 [DOI] [PubMed] [Google Scholar]
- 76.Meyer-Luehmann M., Spires-Jones T.L., Prada C., Garcia-Alloza M., de Calignon, A., Rozkalne A., Koenigsknecht-Talboo J., Holtzman D.M., Bacskai B.J., and Hyman B.T. (2008). Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer's disease. Nature 451, 720–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.