Abstract
Acinetobacter baumannii is an emerging opportunistic pathogen that has risen to become a serious global threat, prevalent in health care settings and the community, which results in high morbidity and mortality rates. Its alarming expansion of antibiotic resistance is one of the most problematic traits of A. baumannii and as so, this bacterium has been classified as a serious threat and high priority target by the CDC. The most common types of infections induced by this pathogen include pneumonia (both hospital and community acquired), bacteremia, skin and soft tissue, urinary tract infections, endocarditis, and meningitis. Nosocomial pneumonia is the most prevalent of these. This review summarizes the current state of the signaling and innate immune components activated in response to A. baumannii infection in the airway.
Keywords: Acinetobacter baumannii, airway, lung, host-pathogen, innate immunity
Acinetobacter baumannii Pathogenesis
Acinetobacter baumannii is a very versatile pathogen with an ability to quickly adapt to the environment. Its capacity to survive in dry and abiotic surfaces and sustain long periods of starvation (Chapartegui-Gonzalez and others 2018) provides the tools that allow this bacterium to thrive and persist in hospital environments. In addition, several virulence factors and an ability to acquire antibiotic resistance make this bacterium a leading cause of nosocomial infections. Antibiotic resistance is a severe worldwide health problem.
The crucial challenge in treating A. baumannii infections is its high antibiotic resistance. It is the only Gram-negative organism to have seen an increase in ICU-related pneumonias, both in the United States and globally (Gaynes and others 2005; Munoz-Price and Weinstein 2008). In fact, up to two-thirds of isolates are multidrug resistant, with antibiotic pan resistance associated with carbapenem resistance of increasing prevalence (Gottig and others 2014; Ozgur and others 2014; Li and others 2017; Wu and others 2018). In part due to this resistance, the WHO has it listed as “priority critical” and the number 1 ranked organism recommended for new research and development (Tacconelli and others 2018).
Although there is still much to uncover regarding A. baumannii pathogenesis some bacterial factors are already known to play an important role during infection. The polysaccharide capsule is one of those factors, which has been shown to protect A. baumannii against complement-mediated killing and phagocytosis (Russo and others 2010; Wang-Lin and others 2017). Also, overexpression of the capsule in a low virulence strain increases virulence (Geisinger and Isberg 2015). A recent report showed a role for the capsule in resistance to desiccation and disinfectants as well as survival in a mouse pneumonia model (Tipton and others 2018).
The outer membrane protein A (OmpA) is one of the most studied virulence factors in A. baumannii pathogenesis, since it can affect several processes such as cell adhesion, invasion, complement, and biofilm formation (Choi and others 2008; Gaddy and others 2009; Kim and others 2009; Schweppe and others 2015). Inactivation of ompA leads to increased survival in a mouse model of pneumonia (Schweppe and others 2015). Besides virulence factors, A. baumannii has significant genome plasticity, with natural genetic transformation facilitating the acquisition of genetic elements and adapting to antibiotic treatment (Gallagher and others 2015). Understanding how the host senses and responds to A. baumannii infection provides further information for the development of therapies to combat this pathogen.
Toll-Like Receptor Signaling
Toll-like receptors (TLRs) are key pathogen recognition receptors (PRRs) involved in initiating the innate host response. TLRs differ in cell localization as well as the ligand(s) they are able to sense (Kawai and Akira 2006; Kawasaki and Kawai 2014). Among those receptors, TLR4 has been extensively studied in response to Gram-negative bacterial infections since it senses lipopolysaccharide (LPS), found in the outer membrane of Gram-negative bacteria (Lu and others 2008). TLR4 and CD14, which help endocytose LPS, are both important host molecules for the clearance of A. baumannii from the airway. Deletion of Tlr4 or Cd14 from mice resulted in increased bacterial burden and dissemination compared with wild-type (WT) mice (Knapp and others 2006). Inactivation of these genes also leads to their inability to respond to LPS. A significant decrease in neutrophil influx was observed 4 h after infection in Tlr4−/− mice, as well as the production of the neutrophil chemoattractant MIP-2. This is consistent with a crucial role for early neutrophil recruitment in combating A. baumannii in the airway (van Faassen and others 2007; Qiu and others 2009a).
This contrasts to TLR2, known to recognize bacterial lipoproteins and peptidoglycan (Oliveira-Nascimento and others 2012) that when inactivated leads to variable levels of bacterial burden in the airway. With studies showing increased as well as decreased bacterial burden in the absence of TLR2 (Knapp and others 2006; Kim and others 2014), the use of different A. baumannii strains in these independent studies could contribute to the different phenotypes. In the case where deletion of Tlr2 resulted in increased bacterial clearance, an increase in neutrophil influx was also observed, although chemokine (C-X-C) ligand 1/keratinocyte-derived cytokine (CXCL1/KC) production, a known neutrophil chemoattractant, was found to be decreased (Knapp and others 2006). A separate study, using a human lung cell line demonstrated that A. baumannii infection led to interleukin-8 (IL-8) production, the human ortholog of CXCL1/KC, which was perturbed in the absence of TLR4 and TLR2 (March and others 2010).
These results suggest an important role for cell wall components in neutrophil lung recruitment. Infection with A. baumannii has been shown to increase TLR4 expression on alveolar macrophages and airway epithelial cells in a pneumonia model, as well as inducing IL-6 and tumor necrosis factor (TNF) production, which was impaired in immunocompromised rats. Immunocompromised rats had decreases in neutrophil influx and bacterial clearance was reduced (Wang and others 2016).
TLR9 has also been described as an important molecule in the host response to A. baumannii. Unlike the previously described TLRs, TLR9 is an intracellular receptor found in endosomes where it is activated by unmethylated CpG motifs present in DNA, a common feature of bacterial DNA (Bauer and others 2001; Blasius and Beutler 2010). In vivo work has shown that TLR9-deficient mice are significantly more susceptible to A. baumannii infection. In the absence of Tlr9 higher bacterial burdens, increased immune cell infiltration, loss of lung architecture, and alveolar wall necrosis resulted in severe lung pathology, compared with similarly infected WT mice (Noto and others 2015). The detrimental outcome in the lungs of Tlr9−/− mice also leads to increased dissemination of A. baumannii to the spleen. This poorer outcome was not due to a defect in immune cell recruitment, as cells such as neutrophils were in greater abundance (36 h postinfection), indicating the potential for TLR9 signaling in the influence of immune cell function.
The requirement for TLR9 appears to be specific to the airway as in a systemic model of infection, Tlr9−/− mice had significantly higher bacterial numbers in the lungs but not in the spleen or liver when compared with WT mice (Noto and others 2015). The important role of TLR9 in the lung could be due to the fact that TLR9 is highly expressed by several cell types, including alveolar macrophages and neutrophils (Schneberger and others 2013). It is clear that the role of these receptors is impacted by several factors such as bacterial strain, severity of infection, location of infection, and host fitness.
Nucleotide-Binding Oligomerization Domain Signaling
Nucleotide-binding oligomerization domain (NOD)-like proteins NOD1 and NOD2 are intracellular PRRs that sense specific cell wall components of bacteria, namely peptidoglycan, and trigger an antimicrobial response (Caruso and others 2014). Given that these receptors are found in the cytosol of host cells and that A. baumannii has been historically considered an extracellular pathogen (Garcia-Patino and others 2017), studies on the role of these receptors in A. baumannii infection have only surfaced recently, after reports describing the capacity of A. baumannii to invade cells (Choi and others 2008; Gaddy and others 2009).
Infection of a human lung epithelial cell line with A. baumannii led to a significant increase in transcription of the gene encoding the adaptor molecule RIP2 (Bist and others 2014). Silencing of RIP2 enhanced A. baumannii entry into epithelial cells as well as its replication, which was also confirmed using primary human bronchial epithelial cells. This work identified a crucial role for RIP2 in NF-κB activation, as an absence of RIP2 resulted in significant decreases in TNF and IL-8 production in response to A. baumannii (Bist and others 2014).
Since RIP2 is a molecular adaptor functioning as a signal transducer in response to several PRRs, including NOD1 and NOD2, the authors explored the roles of these molecules in response to infection. Silencing of NOD1 or NOD2 resulted in increased rates of epithelial cell invasion and intracellular bacterial burden. Furthermore, 48 h after infection, siRNA knockdown of NOD2 in lung epithelial cells led to 20-fold more bacteria than knockdown of NOD1, indicating a more predominate role for NOD2 (Bist and others 2014). Interestingly, there seems to be a cell-type specificity for the NOD1/NOD2/RIP2 axis in response to A. baumannii. No differences in bacterial numbers were observed when infecting Nod2−/− bone marrow-derived macrophages (BMDMs) as well as the human monocyte/macrophage THP-1 cell line, where NOD1, NOD2, and RIP2 were silenced. However, IL-8 and TNF levels were still decreased in these conditions (Bist and others 2014).
One of the antibacterial mechanisms of the NOD1/NOD2/RIP2 axis could be through production of β-defensins, which have been shown to be effective in killing A. baumannii (Bist and others 2014). Transcription of β-defensin 2 was significantly decreased in response to A. baumannii in the absence of these proteins (Bist and others 2014).
In vivo, NOD2 has been shown to be important at early stages of infection, as Nod2−/− mice had increased bacterial burden at 4 and 12 h postinfection when compared with WT. This phenotype was observed irrespective of the virulence of the A. baumannii isolate (Kale and others 2017). However, by 24 h postinfection bacterial burden was similar to WT mice. Increased neutrophil infiltration was observed in the lungs of Nod2−/− infected mice 4 h postinfection (Kale and others 2017). This was a surprising finding, since several reports have demonstrated how crucial early recruitment of neutrophils is to properly combat A. baumannii pneumonia (van Faassen and others 2007; Qiu and others 2009a). In addition to increased neutrophil infiltration in Nod2−/− mice, levels of IL-1β were also significantly increased in the bronchoalveolar lavage fluid (BALF). The increased IL-1β could help explain the poor outcome at early stages of infection, given the described negative role of IL-1β in A. baumannii pneumonia (Wu and others 2003b).
A direct link exists between NOD2 signaling and reactive oxygen species (ROS) production (Lipinski and others 2009). In the absence of NOD2, mice produce significantly less ROS and reactive nitrogen species when compared with WT mice (Kale and others 2017). This could contribute to the lack of protection in Nod2−/− mice, despite increased early neutrophil recruitment, as reduced ROS production by neutrophils led to insufficient killing of A. baumannii (Qiu and others 2009b). Activation of immune cells heavily relies on their capacity to sense the invading pathogens through PRRs. However, the degree at which these PRRs activate and induce signaling in response to A. baumannii is affected by different factors, such as cellular localization and strain virulence.
Inflammasome
A major outcome of innate signaling is inflammation, which helps the host cope with infection. However, the magnitude of inflammation needs to be balanced as excessive levels can be detrimental (Nathan and Ding 2010). The role of the inflammasome differs according to the invading pathogen. In the early 2000s a first report emerged, indicating a detrimental role for the inflammasome/IL-1β axis in A. baumannii pneumonia. Polymorphisms in the IL-1 receptor antagonist gene were analyzed in pneumonia patients. The presence of polymorphic regions in cytokine genes can alter transcription and thus affect inflammation and in this report, the authors showed a correlation between polymorphisms in IL-1ra and A. baumannii-induced pneumonia, with certain polymorphisms resulting in severe infection (Hsu and others 2012). Immediately after, another study also showed an association between IL-1β levels and A. baumannii numbers in BALF from several patients. It was observed that increased bacterial burden was accompanied by higher IL-1β levels (Wu and others 2003a). Studies since have examined the various components of the inflammasome using genetic studies with knockout cells and mice.
It has been shown that NLRP3, caspase-1/11, and ASC are all required for IL-1β in response to A. baumannii using BMDM (Kang and others 2017). IL-1β production can still be detected in Nlrp3−/− and Casp1/11−/− neutrophils in a dose-dependent manner, that is, higher MOIs lead to IL-1β production, demonstrating an NLRP3-independent mechanism of IL-1β activation in response to A. baumannii (Kang and others 2017). The requirement for NLRP3 and caspases 1/11 was then confirmed in vivo in a model of A. baumannii pneumonia. Deletion of the inflammasome components did not impact bacterial clearance in lieu of a significant reduction of IL-1β. Despite the lack of difference in bacterial burden, Nlrp3−/−and Casp-1/11−/− mice have improved lung pathology when compared with WT mice, indicating a role for the inflammasome in immunopathology, as has been seen elsewhere (Kebaier and others 2012; Mishra and others 2013).
Infection of IL-1R knockout mice also led to improved lung pathology, without a change in bacterial burden (Kang and others 2017). BMDM from caspase 11 knockout mice can still activate NLRP3 and induce active caspase-1 and IL-1β. In vivo levels of IL-1β were higher in the absence of caspase 11 at 24 h and 72 h of infection as well as higher bacterial numbers in the airway throughout infection. Moreover, Casp11−/− infected mice had lower neutrophil numbers when compared with WT infected mice at 6 and 24 h of infection (Wang and others 2017), further indicating a deleterious role for IL-1β in A. baumannii pneumonia as well as the crucial role for early neutrophil recruitment.
Further studies have examined the role of the inflammasome with different strains of A. baumannii. Comparison of isolates with varying virulence identified a role for the inflammasome in bacterial clearance when mice were infected with a highly virulent isolate. In this case, Nlrp3−/− mice had decreased neutrophil numbers as soon as 4 h and also at 24 h after infection (Dikshit and others 2018). The authors suggested that since IL-1β is a neutrophil chemoattractant itself and that its production was abolished in Nlrp3−/− mice, this could be the mechanism behind the reduction in bacterial clearance with the highly virulent strain. The highly virulent strain also induced a very strong inflammatory response, consistent with the correlation between IL-1β levels and increased bacterial burden (Wu and others 2003a; Dikshit and others 2018). Excess IL-1β seems to be detrimental in the context of A. baumannii pneumonia; however, the role of NLRP3 seems to be dependent on the virulence of the strain making it more challenging to develop broad inflammasome-related therapies to combat A. baumannii.
Epithelial Cells and Antimicrobial Peptides
The airway epithelium is the front line of defense against respiratory pathogens and thus a significant player in airway immunity. In 2006, Lee and others (2006) using several clinical isolates demonstrated that A. baumannii was able to adhere to human bronchial cells in vitro, with different adherence capacities depending on the strain. This interaction occurs mainly through A. baumannii OmpA and TonB (Choi and others 2008; Kim and others 2008; Smani and others 2012; Zimbler and others 2013). Interaction with epithelial cells leads to production of antimicrobial peptides that display antibacterial activity against A. baumannii (March and others 2010; Bist and others 2014; Feng and others 2014). Other studies have reported the inability of different A. baumannii clinical isolates to adhere to the human lung epithelial cell line A549, opposite to what has been previously described (Lee and others 2006). These studies indicate the wide range in differences between strains of A. baumannii, highlighting that caution should be taken when making conclusions using a single strain.
Antimicrobial peptides produced by epithelial cells are one of the first mechanisms of the innate immune response against bacteria (Bals 2000; McCormick and Weinberg 2010). A. baumannii is able to survive on abiotic surfaces in nosocomial environments and form biofilms that impair antibiotic efficacy (Dijkshoorn and others 2007; Peleg and others 2008). The human antimicrobial peptide, LL-37, shows bactericidal activity against A. baumannii and is able to interfere with A. baumannii adherence to abiotic surfaces and formation of biofilms (Feng and others 2013). LL-37 can also be produced by neutrophils and has been found in neutrophil extracellular traps (NETs). Interestingly, it was shown that clarithromycin-induced NETs contained LL-37, which conferred antimicrobial activity in the NETs facilitating their ability to kill A. baumannii and also prevented biofilm formation (Konstantinidis and others 2016).
Antimicrobial peptides also play an important role as immunomodulators, which includes functioning as neutrophil chemoattractants (De and others 2000). A potential role for the synthetic N-terminal peptide of human lactoferrin hLF (1-11) in affecting A. baumannii induced pyroptosis of alveolar macrophages has also been suggested (Dai and others 2018). hLF (1-11) corresponds to the first 11 N-terminal amino acids of human lactoferrin and displays potent antibacterial activity against a wide panel of multidrug-resistant bacteria (Nibbering and others 2001), including A. baumannii (Dijkshoorn and others 2004). Alveolar macrophages isolated from A. baumannii-infected mice have increased levels of active caspase-1 and IL-1β and alveolar macrophage pyroptosis contributes to A. baumannii-induced lung injury (Dai and others 2018). hLF (1-11) treatment led to a significant decrease of active caspase-1 and consequently a decrease in the number of pyroptotic macrophages after infection. This also led to reduced mortality and bacterial burden in the BALF and lung of A. baumannii-infected mice (Dai and others 2018).
Excess inflammation, to which pyroptosis can contribute too, has been shown to be detrimental to the host (Nathan and Ding 2010). These data suggest that control of alveolar macrophage pyroptosis can impact the outcome to A. baumannii infection and that epithelial cells can impact A. baumannii clearance through the antibacterial and immunomodulatory properties of antimicrobial peptides.
Macrophages
In naïve mice, alveolar macrophages are the predominant immune cell population; thus, these immune cells are part of the first line of defense against invading pathogens. In vitro, alveolar macrophages are able to phagocytose A. baumannii as soon as 10 min after exposure to bacterial cells (Qiu and others 2012); however, the killing capacity of alveolar macrophages is not as high as that displayed by neutrophils (Qiu and others 2012). After intranasal infection with A. baumannii, alveolar macrophages are the major immune cell type in the airway at 2 and 4 h postinfection, whereas at 24 h postinfection neutrophils become the predominant cell (Qiu and others 2012).
Initial studies examining the role of alveolar macrophages in vivo were pursued using an anti-M-CSFR antibody, which led to an 83% survival rate upon infection when compared with control mice at 100% survival, very different from the 0% survival described in the same study with neutrophil depletion (Tsuchiya and others 2012). This mild phenotype was an indication of a somewhat important but not crucial role for macrophages in response to A. baumannii. In a separate study, depletion of alveolar macrophages using clodronate liposomes resulted in an increase of susceptibility to intranasal A. baumannii infection, which was reflected in a significant increase of bacterial burden at 24 h postinfection in the BALF (Qiu and others 2012). It is interesting to note that alveolar macrophage-depleted mice also displayed lower levels of MIP-2 (Qiu and others 2012), an important neutrophil chemoattractant whose role in A. baumannii pathogenesis has been previously described (van Faassen and others 2007; Qiu and others 2009a). This suggests that alveolar macrophages can also contribute indirectly to clearance of A. baumannii infection through recruitment of neutrophils.
Infection of mice with strains of A. baumannii that vary in virulence can lead to a range of outcomes and magnitudes of immune response (Bruhn and others 2015). When alveolar macrophages are depleted and infected with a less virulent strain, a significant increase in bacterial burden (10-fold) is observed but was not reflected in lethality (Qiu and others 2012; Bruhn and others 2015). However, when mice are infected with a hypervirulent strain, a more dramatic phenotype was observed with a 100-fold increase in bacterial burden (Bruhn and others 2015). These results strongly suggest that the first hours of infection are crucial in determining the host fate and that alveolar macrophages play an important part in the initial host defense to A. baumannii, particularly with highly virulent isolates.
Neutrophils
Historically neutrophils have been known as one of the first and main line of defense against bacterial infections, which they achieve through highly effective killing mechanisms. Phagocytosis of bacteria triggers the oxidative burst that generates highly toxic molecules known as ROS (Segal 2005; Nguyen and others 2017). In more recent years, it has also been shown that neutrophils can also kill pathogens extracellularly through the extrusion of NETs, which have the ability to entrap and kill bacteria (Brinkmann and others 2004; Papayannopoulos 2018).
The role of neutrophils in the pulmonary response to A. baumannii infection has been the focus of several groups. Isolation of A. baumannii from neutropenic febrile patients was the first indication of an important role of neutrophils in A. baumannii infection (Karim and others 1991; Yadegarynia and others 2013). Several studies have established a role for neutrophils in the defense against A. baumannii infection. By using a GR-1 antibody (RB6-8C5), authors demonstrated that neutrophil depletion highly increased susceptibility of mice to A. baumannii infection, which was reflected in a 100-fold increase in bacterial burden 24 h postinfection (van Faassen and others 2007). Along with increased lethality, a decrease in production of cytokines and chemokines important for neutrophil recruitment, such as MIP-2 and KC, was observed (van Faassen and others 2007). Even though the GR-1 antibody has been extensively used to deplete neutrophils in mice, it can deplete other cells since it targets Ly6G and Ly6C, with Ly6C also expressed on some monocytes and T cell subsets, and plasmacytoid dendritic cells (Geissmann and others 2003; Pelayo and others 2005; Marshall and others 2011).
In the context of other pathogens, different results have been observed between GR-1 and Ly6G depletion (Wojtasiak and others 2010; Robinson and others 2014). Although it does not seem be the case in A. baumannii infection as neutrophil depletion with Ly6G provided similar results in a model of systemic infection where several A. baumannii clinical isolates were used (van Faassen and others 2007; Breslow and others 2011), this may not be necessarily the case for pulmonary infection. To further establish the importance of early neutrophil recruitment to combat A. baumannii infection, mice have been treated with purified MIP-2 before intranasal infection to increase neutrophil chemotaxis, which also results in decreased bacterial burden (van Faassen and others 2007).
AJ mice have higher mortality and increased sensitivity toward A. baumannii respiratory infection when compared with C57BL/6J mice, which is related to a delay in neutrophil recruitment (Qiu and others 2009a). Besides a 40-fold decrease in neutrophil numbers at 4 h postinfection, AJ mice also displayed lower levels of proinflammatory cytokines and chemokines such as IL-1β and MIP-2, when compared with C57BL/6 mice. Treatment of AJ mice with MIP-2 before infection led to a significant reduction in bacterial burden and increased neutrophil numbers when compared with control mice, bringing bacterial numbers down to levels similar to those observed in infected C57BL/6 mice (Qiu and others 2009a).
A separate study that used GR-1 and NK1 monoclonal antibodies to deplete neutrophils and natural killer (NK) cells, respectively, confirmed that 100% neutropenic mice succumbed to pulmonary infection but NK cell-depleted mice also suffered significant mortality (50%) (Tsuchiya and others 2012). NK cell-depleted mice had significant reductions in recruited neutrophils compared with control mice for the first 3 days that followed infection. Furthermore, the NK-depleted mice also had lower levels of the neutrophil chemoattractant CXCL1/KC (Tsuchiya and others 2012), suggesting that NK cells can also influence neutrophil recruitment through production of KC. However, in a previous report of a systemic A. baumannii model of infection (intraperitoneal), depletion of KC did not significantly alter bacterial counts recovered from blood and several organs, including the lung (Breslow and others 2011). The lack of a phenotype in this model could have been due to a compensatory effect by other chemokines, which was not assessed in the study.
A major antimicrobial mechanism of neutrophils is their production of ROS. The main ROS generator in phagocytic cells is NADPH oxidase (Segal 2008). Deletion of gp91phox, a catalytic subunit of NADPH oxidase, impaired the ability of neutrophils to kill A. baumannii. gp91phox−/− mice infected intranasally with A. baumannii had significantly increased bacterial burden when compared with WT mice despite similar neutrophil numbers (Qiu and others 2009b), highlighting the crucial role of NADPH oxidase and ROS in controlling A. baumannii infection. In this same study the authors also used Nos2−/− mice, which cannot generate nitric oxide, another reactive free radical used by phagocytic cells to kill pathogens. But unlike gp91phox−/− mice, deletion of NOS2 did not significantly affect the ability of mice to cope with pulmonary A. baumannii infection (Qiu and others 2009b). This study shows that it is the ROS-producing capacity of neutrophils that affords neutrophils their capacity to kill A. baumannii.
NETs are another unique mechanism by which neutrophils are also able to entrap and kill bacteria (Brinkmann and others 2004). There is conflicting data concerning the capacity of neutrophils to generate NETs in response to A. baumannii (Kamoshida and others 2015; Konstantinidis and others 2016; Lazaro-Diez and others 2017). In the case of the study where NETs were generated (Lazaro-Diez and others 2017), the levels of NET formation in response to A. baumannii were always lower than neutrophils treated with PMA, a known NET stimulator (Brinkmann and others 2004; Fuchs and others 2007).
A. baumannii can escape phagocytosis by adhering to neutrophils and using them as a vehicle to disseminate (Kamoshida and others 2016). A. baumannii also has the capacity to suppress neutrophil adhesion by impairing surface expression of CD11a and thus blocking generation of NETs (Kamoshida and others 2018). In all these studies, different multiplicities of infection and incubation times with neutrophils were used, which can help explain to a certain extent the differing results.
Curiously, work showing a role for A. baumannii bacterial factors in neutrophil recruitment is beginning to emerge. In a zebrafish model, neutrophils were shown to be recruited in response to A. baumannii, similarly to what has been described in mouse models of infection (van Faassen and others 2007). Infection in the yolk, which lacks immune cells, highly increased susceptibility to infection, highlighting a pivotal role for innate immunity to combat A. baumannii. Moreover, infection using an A. baumannii strain that lacked the global virulence regulator GacS resulted in increased neutrophil influx (Bhuiyan and others 2016). One of the operons under the control of GacS is the paa operon, which is involved in the degradation of aromatic compounds, including phenylacetate. Deletion of gacS led to an accumulation of phenylacetate, which the authors demonstrated to be a neutrophil chemoattractant (Bhuiyan and others 2016).
All these studies make a clear point on how crucial early recruitment of fully functional neutrophils is to achieve a positive outcome to infection. Further work will have to be pursued, including in vivo NETs assessment, but it seems so far that the main mechanism for neutrophil killing of A. baumannii is their production of ROS.
Future Directions and Conclusion
The emergence of A. baumannii as a considerable threat to public health is a recent development. Thus, what is known at this point in time is reflected in that recent emergence. We have only begun to scratch the surface of the roles different aspects of the innate immune system play in response to A. baumannii. Initial studies have been able to show that both neutrophils and macrophages are important elements in the host response. What the role of the myriad of macrophage and dendritic cell subsets play, in addition to monocytes and T cell subsets remains to be determined. Studies focused on innate immune receptors have shown the variation that can occur between studies, with variable data collected for the roles of TLR2 in infection.
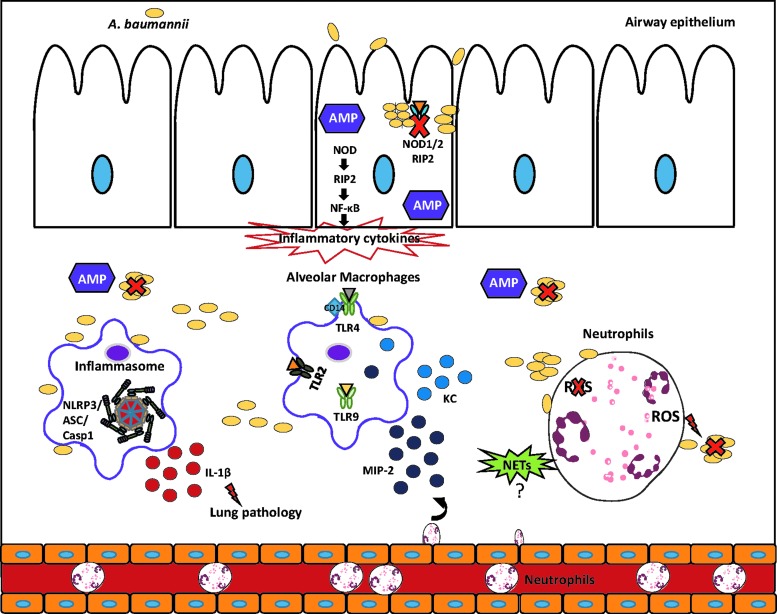
One key element that stands out from the existing studies is how much strain-to-strain variation influences the interaction with host cells and observed phenotypes in vivo. A deeper understanding of the virulence factors of A. baumannii that are important for in vivo pathogenesis is needed. This will allow us to understand the significant differences in outcome that are observed with various strains in vivo. Also, given the longtime consideration of A. baumannii as an extracellular pathogen the role of intracellular receptors is in its infancy, examination of various NLR and cytosolic receptors is to be conducted. Likewise, the innate immune pathways that are activated in response to A. baumannii are largely uncharacterized. The main findings regarding innate immuity to A. baumannii in the airway are summarized in Figure 1. With the advent of next-generation sequencing and high-throughput omics approaches, these will begin to be unraveled. Understanding the innate immune response is an important undertaking given the tremendous ability of this pathogen to develop resistance to antibiotics; thus, immune-derived therapy may provide a novel treatment in the future.
FIG. 1.
Innate immune responses to Acinetobacter baumannii in the airway. Epithelial cells are one of the first defenses encountered by A. baumannii. As a result of this interaction AMP that can effectively kill A. baumannii are produced. The NOD/RIP2 axis in epithelial cells contributes to the production of inflammatory cytokines as well as in the control of cell invasion and replication by A. baumannii. TLR receptor 4 and 9 have been shown to be important in bacterial clearance, whereas TLR2 inactivation has exhibited mixed results as far as bacterial clearance. Macrophage production of chemoattractants, namely MIP-2 and CXCL1/KC, contributes to neutrophil recruitment upon infection. Early recruitment of functional neutrophils is an essential step to kill and control A. baumannii proliferation. NET production and their role in response to A. baumannii are still inconclusive as there are conflicting results, but ROS production is essential for the ability of neutrophils to kill A. baumannii. The inflammasome is activated upon infection and although not important for clearance, excess levels of IL-1β contribute to immunopathology. AMP, antimicrobial peptide; NETS, neutrophil extracellular traps; ROS, reactive oxygen species; TLR, toll-like receptor.
Author Disclosure Statement
No competing financial interests exist.
References
- Bals R. 2000. Epithelial antimicrobial peptides in host defense against infection. Respir Res 1(3):141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer S, Kirschning CJ, Hacker H, Redecke V, Hausmann S, Akira S, Wagner H, Lipford GB. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc Natl Acad Sci U S A 98(16):9237–9242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuiyan MS, Ellett F, Murray GL, Kostoulias X, Cerqueira GM, Schulze KE, Mahamad Maifiah MH, Li J, Creek DJ, Lieschke GJ, Peleg AY. 2016. Acinetobacter baumannii phenylacetic acid metabolism influences infection outcome through a direct effect on neutrophil chemotaxis. Proc Natl Acad Sci U S A 113(34):9599–9604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bist P, Dikshit N, Koh TH, Mortellaro A, Tan TT, Sukumaran B. 2014. The Nod1, Nod2, and Rip2 axis contributes to host immune defense against intracellular Acinetobacter baumannii infection. Infect Immun 82(3):1112–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasius AL, Beutler B. 2010. Intracellular toll-like receptors. Immunity 32(3):305–315 [DOI] [PubMed] [Google Scholar]
- Breslow JM, Meissler JJ, Jr., Hartzell RR, Spence PB, Truant A, Gaughan J, Eisenstein TK. 2011. Innate immune responses to systemic Acinetobacter baumannii infection in mice: neutrophils, but not interleukin-17, mediate host resistance. Infect Immun 79(8):3317–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. 2004. Neutrophil extracellular traps kill bacteria. Science 303(5663):1532–1535 [DOI] [PubMed] [Google Scholar]
- Bruhn KW, Pantapalangkoor P, Nielsen T, Tan B, Junus J, Hujer KM, Wright MS, Bonomo RA, Adams MD, Chen W, Spellberg B. 2015. Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J Infect Dis 211(8):1296–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caruso R, Warner N, Inohara N, Nunez G. 2014. NOD1 and NOD2: signaling, host defense, and inflammatory disease. Immunity 41(6):898–908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapartegui-Gonzalez I, Lazaro-Diez M, Bravo Z, Navas J, Icardo JM, Ramos-Vivas J. 2018. Acinetobacter baumannii maintains its virulence after long-time starvation. PLoS One 13(8):e0201961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi CH, Lee JS, Lee YC, Park TI, Lee JC. 2008. Acinetobacter baumannii invades epithelial cells and outer membrane protein A mediates interactions with epithelial cells. BMC Microbiol 8:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai M, Pan P, Li H, Liu S, Zhang L, Song C, Li Y, Li Q, Mao Z, Long Y, Su X, Hu C. 2018. The antimicrobial cathelicidin peptide hlF(1-11) attenuates alveolar macrophage pyroptosis induced by Acinetobacter baumannii in vivo. Exp Cell Res 364(1):95–103 [DOI] [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, Chertov O. 2000. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med 192(7):1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L, Brouwer CP, Bogaards SJ, Nemec A, van den Broek PJ, Nibbering PH. 2004. The synthetic N-terminal peptide of human lactoferrin, hLF(1–11), is highly effective against experimental infection caused by multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 48(12):4919–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkshoorn L, Nemec A, Seifert H. 2007. An increasing threat in hospitals: multidrug-resistant Acinetobacter baumannii. Nat Rev Microbiol 5(12):939–951 [DOI] [PubMed] [Google Scholar]
- Dikshit N, Kale SD, Khameneh HJ, Balamuralidhar V, Tang CY, Kumar P, Lim TP, Tan TT, Kwa AL, Mortellaro A, Sukumaran B. 2018. NLRP3 inflammasome pathway has a critical role in the host immunity against clinically relevant Acinetobacter baumannii pulmonary infection. Mucosal Immunol 11(1):257–272 [DOI] [PubMed] [Google Scholar]
- Feng X, Sambanthamoorthy K, Palys T, Paranavitana C. 2013. The human antimicrobial peptide LL-37 and its fragments possess both antimicrobial and antibiofilm activities against multidrug-resistant Acinetobacter baumannii. Peptides 49:131–137 [DOI] [PubMed] [Google Scholar]
- Feng Z, Jia X, Adams MD, Ghosh SK, Bonomo RA, Weinberg A. 2014. Epithelial innate immune response to Acinetobacter baumannii challenge. Infect Immun 82(11):4458–4465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. 2007. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 176(2):231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaddy JA, Tomaras AP, Actis LA. 2009. The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infect Immun 77(8):3150–3160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher LA, Ramage E, Weiss EJ, Radey M, Hayden HS, Held KG, Huse HK, Zurawski DV, Brittnacher MJ, Manoil C. 2015. Resources for genetic and genomic analysis of emerging pathogen Acinetobacter baumannii. J Bacteriol 197(12):2027–2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Patino MG, Garcia-Contreras R, Licona-Limon P. 2017. The immune response against Acinetobacter baumannii, an emerging pathogen in nosocomial infections. Front Immunol 8:441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes R, Edwards JR, National Nosocomial Infections Surveillance S. 2005. Overview of nosocomial infections caused by gram-negative bacilli. Clin Infect Dis 41(6):848–854 [DOI] [PubMed] [Google Scholar]
- Geisinger E, Isberg RR. 2015. Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11(2):e1004691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19(1):71–82 [DOI] [PubMed] [Google Scholar]
- Gottig S, Gruber TM, Higgins PG, Wachsmuth M, Seifert H, Kempf VA. 2014. Detection of pan drug-resistant Acinetobacter baumannii in Germany. J Antimicrob Chemother 69(9):2578–2579 [DOI] [PubMed] [Google Scholar]
- Hsu MJ, Lu YC, Hsu YC, Liu WS, Wu WT. 2012. Interleukin-1 receptor antagonist gene polymorphism in patients with multidrug-resistant Acinetobacter baumannii-associated pneumonia. Ann Thorac Med 7(2):74–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kale SD, Dikshit N, Kumar P, Balamuralidhar V, Khameneh HJ, Bin Abdul Malik N, Koh TH, Tan GGY, Tan TT, Mortellaro A, Sukumaran B. 2017. Nod2 is required for the early innate immune clearance of Acinetobacter baumannii from the lungs. Sci Rep 7(1):17429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshida G, Kikuchi-Ueda T, Nishida S, Tansho-Nagakawa S, Ubagai T, Ono Y. 2018. Pathogenic bacterium Acinetobacter baumannii inhibits the formation of neutrophil extracellular traps by suppressing neutrophil adhesion. Front Immunol 9:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoshida G, Kikuchi-Ueda T, Tansho-Nagakawa S, Nakano R, Nakano A, Kikuchi H, Ubagai T, Ono Y. 2015. Acinetobacter baumannii escape from neutrophil extracellular traps (NETs). J Infect Chemother 21(1):43–49 [DOI] [PubMed] [Google Scholar]
- Kamoshida G, Tansho-Nagakawa S, Kikuchi-Ueda T, Nakano R, Hikosaka K, Nishida S, Ubagai T, Higashi S, Ono Y. 2016. A novel bacterial transport mechanism of Acinetobacter baumannii via activated human neutrophils through interleukin-8. J Leukoc Biol 100(6):1405–1412 [DOI] [PubMed] [Google Scholar]
- Kang MJ, Jo SG, Kim DJ, Park JH. 2017. NLRP3 inflammasome mediates interleukin-1beta production in immune cells in response to Acinetobacter baumannii and contributes to pulmonary inflammation in mice. Immunology 150(4):495–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim M, Khan W, Farooqi B, Malik I. 1991. Bacterial isolates in neutropenic febrile patients. J Pak Med Assoc 41(2):35–37 [PubMed] [Google Scholar]
- Kawai T, Akira S. 2006. TLR signaling. Cell Death Differ 13(5):816–825 [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Kawai T. 2014. Toll-like receptor signaling pathways. Front Immunol 5:461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebaier C, Chamberland RR, Allen IC, Gao X, Broglie PM, Hall JD, Jania C, Doerschuk CM, Tilley SL, Duncan JA. 2012. Staphylococcus aureus alpha-hemolysin mediates virulence in a murine model of severe pneumonia through activation of the NLRP3 inflammasome. J Infect Dis 205(5):807–817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Kim DJ, Lee SJ, Jeong YJ, Kang MJ, Lee JY, Choi JA, Kwon SJ, Park JH, Park JH. 2014. Tolllike receptor 2 promotes bacterial clearance during the initial stage of pulmonary infection with Acinetobacter baumannii. Mol Med Rep 9(4):1410–1414 [DOI] [PubMed] [Google Scholar]
- Kim SA, Yoo SM, Hyun SH, Choi CH, Yang SY, Kim HJ, Jang BC, Suh SI, Lee JC. 2008. Global gene expression patterns and induction of innate immune response in human laryngeal epithelial cells in response to Acinetobacter baumannii outer membrane protein A. FEMS Immunol Med Microbiol 54(1):45–52 [DOI] [PubMed] [Google Scholar]
- Kim SW, Choi CH, Moon DC, Jin JS, Lee JH, Shin JH, Kim JM, Lee YC, Seol SY, Cho DT, Lee JC. 2009. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol Lett 301(2):224–231 [DOI] [PubMed] [Google Scholar]
- Knapp S, Wieland CW, Florquin S, Pantophlet R, Dijkshoorn L, Tshimbalanga N, Akira S, van der Poll T. 2006. Differential roles of CD14 and toll-like receptors 4 and 2 in murine Acinetobacter pneumonia. Am J Respir Crit Care Med 173(1):122–129 [DOI] [PubMed] [Google Scholar]
- Konstantinidis T, Kambas K, Mitsios A, Panopoulou M, Tsironidou V, Dellaporta E, Kouklakis G, Arampatzioglou A, Angelidou I, Mitroulis I, Skendros P, Ritis K. 2016. Immunomodulatory role of clarithromycin in Acinetobacter baumannii infection via formation of neutrophil extracellular traps. Antimicrob Agents Chemother 60(2):1040–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Diez M, Chapartegui-Gonzalez I, Redondo-Salvo S, Leigh C, Merino D, Segundo DS, Navas J, Icardo JM, Acosta F, Ocampo-Sosa A, Martinez-Martinez L, Ramos-Vivas J. 2017. Human neutrophils phagocytose and kill Acinetobacter baumannii and A. pittii. Sci Rep 7(1):4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Koerten H, van den Broek P, Beekhuizen H, Wolterbeek R, van den Barselaar M, van der Reijden T, van der Meer J, van de Gevel J, Dijkshoorn L. 2006. Adherence of Acinetobacter baumannii strains to human bronchial epithelial cells. Res Microbiol 157(4):360–366 [DOI] [PubMed] [Google Scholar]
- Li YJ, Pan CZ, Fang CQ, Zhao ZX, Chen HL, Guo PH, Zhao ZW. 2017. Pneumonia caused by extensive drug-resistant Acinetobacter baumannii among hospitalized patients: genetic relationships, risk factors and mortality. BMC Infect Dis 17(1):371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski S, Till A, Sina C, Arlt A, Grasberger H, Schreiber S, Rosenstiel P. 2009. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J Cell Sci 122(19):3522–3530 [DOI] [PubMed] [Google Scholar]
- Lu YC, Yeh WC, Ohashi PS. 2008. LPS/TLR4 signal transduction pathway. Cytokine 42(2):145–151 [DOI] [PubMed] [Google Scholar]
- March C, Regueiro V, Llobet E, Moranta D, Morey P, Garmendia J, Bengoechea JA. 2010. Dissection of host cell signal transduction during Acinetobacter baumannii-triggered inflammatory response. PLoS One 5(4):e10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall HD, Chandele A, Jung YW, Meng H, Poholek AC, Parish IA, Rutishauser R, Cui W, Kleinstein SH, Craft J, Kaech SM. 2011. Differential expression of Ly6C and T-bet distinguish effector and memory Th1 CD4(+) cell properties during viral infection. Immunity 35(4):633–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick TS, Weinberg A. 2010. Epithelial cell-derived antimicrobial peptides are multifunctional agents that bridge innate and adaptive immunity. Periodontol 2000 54(1):195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra BB, Rathinam VA, Martens GW, Martinot AJ, Kornfeld H, Fitzgerald KA, Sassetti CM. 2013. Nitric oxide controls the immunopathology of tuberculosis by inhibiting NLRP3 inflammasome-dependent processing of IL-1beta. Nat Immunol 14(1):52–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Price LS, Weinstein RA. 2008. Acinetobacter infection. N Engl J Med 358(12):1271–1281 [DOI] [PubMed] [Google Scholar]
- Nathan C, Ding A. 2010. Nonresolving inflammation. Cell 140(6):871–882 [DOI] [PubMed] [Google Scholar]
- Nguyen GT, Green ER, Mecsas J. 2017. Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front Cell Infect Microbiol 7:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbering PH, Ravensbergen E, Welling MM, van Berkel LA, van Berkel PH, Pauwels EK, Nuijens JH. 2001. Human lactoferrin and peptides derived from its N terminus are highly effective against infections with antibiotic-resistant bacteria. Infect Immun 69(3):1469–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto MJ, Boyd KL, Burns WJ, Varga MG, Peek RM, Jr., Skaar EP. 2015. Toll-like receptor 9 contributes to defense against Acinetobacter baumannii infection. Infect Immun 83(10):4134–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira-Nascimento L, Massari P, Wetzler LM. 2012. The role of TLR2 in infection and immunity. Front Immunol 3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozgur ES, Horasan ES, Karaca K, Ersoz G, Nayci Atis S, Kaya A. 2014. Ventilator-associated pneumonia due to extensive drug-resistant Acinetobacter baumannii: risk factors, clinical features, and outcomes. Am J Infect Control 42(2):206–208 [DOI] [PubMed] [Google Scholar]
- Papayannopoulos V. 2018. Neutrophil extracellular traps in immunity and disease. Nat Rev Immunol 18(2):134–147 [DOI] [PubMed] [Google Scholar]
- Pelayo R, Hirose J, Huang J, Garrett KP, Delogu A, Busslinger M, Kincade PW. 2005. Derivation of 2 categories of plasmacytoid dendritic cells in murine bone marrow. Blood 105(11):4407–4415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21(3):538–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, KuoLee R, Harris G, Chen W. 2009a. High susceptibility to respiratory Acinetobacter baumannii infection in A/J mice is associated with a delay in early pulmonary recruitment of neutrophils. Microbes Infect 11(12):946–955 [DOI] [PubMed] [Google Scholar]
- Qiu H, Kuolee R, Harris G, Chen W. 2009b. Role of NADPH phagocyte oxidase in host defense against acute respiratory Acinetobacter baumannii infection in mice. Infect Immun 77(3):1015–1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, KuoLee R, Harris G, Van Rooijen N, Patel GB, Chen W. 2012. Role of macrophages in early host resistance to respiratory Acinetobacter baumannii infection. PLoS One 7(6):e40019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson KM, McHugh KJ, Mandalapu S, Clay ME, Lee B, Scheller EV, Enelow RI, Chan YR, Kolls JK, Alcorn JF. 2014. Influenza A virus exacerbates Staphylococcus aureus pneumonia in mice by attenuating antimicrobial peptide production. J Infect Dis 209(6):865–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo TA, Luke NR, Beanan JM, Olson R, Sauberan SL, MacDonald U, Schultz LW, Umland TC, Campagnari AA. 2010. The K1 capsular polysaccharide of Acinetobacter baumannii strain 307-0294 is a major virulence factor. Infect Immun 78(9):3993–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneberger D, Caldwell S, Kanthan R, Singh B. 2013. Expression of toll-like receptor 9 in mouse and human lungs. J Anat 222(5):495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweppe DK, Harding C, Chavez JD, Wu X, Ramage E, Singh PK, Manoil C, Bruce JE. 2015. Host-microbe protein interactions during bacterial infection. Chem Biol 22(11):1521–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal AW. 2005. How neutrophils kill microbes. Annu Rev Immunol 23:197–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal AW. 2008. The function of the NADPH oxidase of phagocytes and its relationship to other NOXs in plants, invertebrates, and mammals. Int J Biochem Cell Biol 40(4):604–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smani Y, McConnell MJ, Pachon J. 2012. Role of fibronectin in the adhesion of Acinetobacter baumannii to host cells. PLoS One 7(4):e33073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacconelli E, Carrara E, Savoldi A, Harbarth S, Mendelson M, Monnet DL, Pulcini C, Kahlmeter G, Kluytmans J, Carmeli Y, Ouellette M, Outterson K, Patel J, Cavaleri M, Cox EM, Houchens CR, Grayson ML, Hansen P, Singh N, Theuretzbacher U, Magrini N, Group WHOPPLW. 2018. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 18(3):318–327 [DOI] [PubMed] [Google Scholar]
- Tipton KA, Chin CY, Farokhyfar M, Weiss DS, Rather PN. 2018. Role of capsule in resistance to disinfectants, host antimicrobials, and desiccation in Acinetobacter baumannii. Antimicrob Agents Chemother 62(12):pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchiya T, Nakao N, Yamamoto S, Hirai Y, Miyamoto K, Tsujibo H. 2012. NK1.1(+) cells regulate neutrophil migration in mice with Acinetobacter baumannii pneumonia. Microbiol Immunol 56(2):107–116 [DOI] [PubMed] [Google Scholar]
- van Faassen H, KuoLee R, Harris G, Zhao X, Conlan JW, Chen W. 2007. Neutrophils play an important role in host resistance to respiratory infection with Acinetobacter baumannii in mice. Infect Immun 75(12):5597–5608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang-Lin SX, Olson R, Beanan JM, MacDonald U, Balthasar JP, Russo TA. 2017. The capsular polysaccharide of Acinetobacter baumannii is an obstacle for therapeutic passive immunization strategies. Infect Immun 85(12):pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Shao Y, Li S, Xin N, Ma T, Zhao C, Song M. 2017. Caspase-11 plays a protective role in pulmonary Acinetobacter baumannii infection. Infect Immun 85(10):pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Zhang X, Feng X, Liu X, Deng L, Liang ZA. 2016. Expression of toll-like receptor 4 in lungs of immune-suppressed rat with Acinetobacter baumannii infection. Exp Ther Med 12(4):2599–2605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtasiak M, Pickett DL, Tate MD, Londrigan SL, Bedoui S, Brooks AG, Reading PC. 2010. Depletion of Gr-1+, but not Ly6G+, immune cells exacerbates virus replication and disease in an intranasal model of herpes simplex virus type 1 infection. J Gen Virol 91(Pt 9):2158–2166 [DOI] [PubMed] [Google Scholar]
- Wu CL, Lee YL, Chang KM, Chang GC, King SL, Chiang CD, Niederman MS. 2003a. Bronchoalveolar interleukin-1 beta: a marker of bacterial burden in mechanically ventilated patients with community-acquired pneumonia. Crit Care Med 31(3):812–817 [DOI] [PubMed] [Google Scholar]
- Wu CL, Lee YL, Chang KM, Chang GC, King SL, Chiang CD, Niederman MS. 2003b. Bronchoalveolar interleukin-1 beta: a marker of bacterial burden in mechanically ventilated patients with community-acquired pneumonia. Crit Care Med 31(3):812–817 [DOI] [PubMed] [Google Scholar]
- Wu HG, Liu WS, Zhu M, Li XX. 2018. Research and analysis of 74 bloodstream infection cases of Acinetobacter baumannii and drug resistance. Eur Rev Med Pharmacol Sci 22(6):1782–1786 [DOI] [PubMed] [Google Scholar]
- Yadegarynia D, Fatemi A, Mahdizadeh M, Kabiri Movahhed R, Alizadeh MA. 2013. Current spectrum of bacterial infections in patients with nosocomial fever and neutropenia. Caspian J Intern Med 4(3):698–701 [PMC free article] [PubMed] [Google Scholar]
- Zimbler DL, Arivett BA, Beckett AC, Menke SM, Actis LA. 2013. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect Immun 81(9):3382–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]