Abstract
The present study was designed to define the hemoglobin [Hb] increase with altitude in Peruvian children. We suggest the normal range of [Hb] as means ±2 standard deviations (SD), with a value less than - 2 SD as a possible threshold to detect anemia. The prevalence of anemia was calculated. These values were compared to the World Health Organization (WHO) altitude correction parameter and the threshold for anemia of 11 g/dL. Likewise, polycythemia is suggested as [Hb] greater than 2 SD. 2,028,701 children aged 6–59 months were analyzed. The quadratic regression analysis shows that [Hb] is constant between sea level and 999 m. Thereafter, [Hb] increases from 11.32 g/dL (1000 m) up to ∼14.54 g/dL at 4000 m. Applying the threshold for anemia defined by WHO (11 g/dL) results in a prevalence of ∼35% for children living at altitudes <1000 m, and prevalence decreases to ∼4.5% at >4000 m. After [Hb] altitude correction, the prevalence was ∼36% (1000 m) and increases to ∼66% above 4000 m. With our proposed threshold for anemia, the prevalence was ∼15% below 1000 m and ∼5% above 4000 m. For polycythemia ([Hb] >14.5 g/dL), increases were from 1.2% at <1000 m to 39.4% at 4000 m. After [Hb] correction for altitude, the prevalence of polycythemia decreases with altitude. Excessive erythrocytosis defined as [Hb] >19 g/dL shows the highest values at 4000 m, while polycythemia defined as [Hb] greater than 2 SD was reduced at high altitude (HA). In conclusion, using WHO thresholds for anemia and [Hb] correction by altitude most likely overestimates the prevalence of anemia and may underestimate polycythemia in Peruvian children living at HA. Therefore, new threshold values for anemia and polycythemia as mean [Hb] less than 2 SD and greater than 2 SD for populations living at a specific altitude are suggested.
Keywords: altitude-corrected hemoglobin, anemia in childhood, erythrocytosis, hemoglobin, high altitude, polycythemia
Introduction
Life at high altitude (HA) in some species may require an increase in the hemoglobin concentration [Hb] as altitude increases to compensate for decreased oxygen availability (León-Velarde et al., 2000). The magnitude of increase is variable since populations with higher numbers of generations of residence at HA such as Tibetans, Ethiopians, and southern Andeans have lower [Hb] than those with fewer generations of life at the same altitudes such as northern Andeans (Wu et al., 2005; Gonzales et al., 2016; Cheong et al., 2017).
Higher [Hb] at HA than at low altitude is observed already at birth because of fetal exposure in their hypoxic mothers (Gonzales, 1998; Shah et al., 2015). Hemoglobin during the first 6 months after birth falls to lower values due to disappearance of erythrocytes containing fetal hemoglobin that are replaced with those containing adult hemoglobin, when iron reserves are normal (Lönnerdal et al., 2015). Later in childhood, levels of hemoglobin vary in relationship with age: they seem stable up to an age of ∼5 years, but increase thereafter reaching higher values in adulthood in males than in females (National Center for Health Statistics et al., 1982).
As a low [Hb] is an indicator of anemia and possibly of low iron stores, the World Health Organization (WHO) has recommended a correction of the threshold defined for sea level populations to determine anemia at HAs. The WHO has based its altitude correction of [Hb] on data, which were obtained in children living at altitudes up to 3000 m and then derived a nonlinear function from these data to generate correction factors for altitudes up to 5500 m (Centers for Disease Control and Prevention, 1989; WHO, 2001).
Because of the limitation by the rather low altitude data of the WHO these correction factors for altitudes >3000 m are an estimate and need to be considered cautiously. These problems indicate clearly the need to better redefine these correction factors, which can then be used to select the thresholds in classifying children as being anemic or polycythemic (excessive erythrocytosis).
Therefore, the present study was designed to redefine the normal magnitude of increase in hemoglobin [Hb] of children with increasing altitude in Peru. From the altitude-related mean values, the variation in normal values of [Hb] will be defined. In addition, a comparison of these findings with the WHO recommendations for correction of [Hb] for altitude will be presented, and altitude-related means [Hb] ±2 SD will be suggested as a threshold for polycythemia and anemia, respectively.
Materials and Methods
This is an analysis of a database provided by the National Center of Feeding and Nutrition (CENAN) of the National Institute of Health in Peru. The Information System of Nutritional Status (SIEN in Spanish) collected data in each of the 24 departments (provinces) of Peru.
The database includes information on 2,105,036 children between 6 and 59 months of age. Some records had to be deleted because of missing hemoglobin values (n = 72,468), when [Hb] was unreasonably low (<3 g/dL; n = 326) or high (>30 g/dL; n = 1549), and when information on the altitude of residency was missing (n = 1992). The final sample included 2,028,701 children. Data were obtained during the period of 2012 to the first semester of 2017. Hemoglobin was measured with the HemoCue H6201 DM analyzer (HemoCue® [HB] 201+; Alglholm, Sweden) (CENAN, 2015). Blood sample was obtained, whereas child is sitting on the parent's lap.
Defining normal values of [Hb] for different residential altitudes
Ranges when [Hb] should be classified as normal were defined as mean values ±2 standard deviations (means ±2 SD) calculated from the entire population grouped by ranges of the children's residential altitude, which were <1000, 1000–1999, 2000–2999, 3000–3999, and >4000 m.
Detection of anemia
Anemia in children was diagnosed by three methods as follows: (1) when [Hb] was <11 g/dL, which is the threshold recommended by the WHO for low altitude not corrected for HA as in previous studies (Gonzales et al., 2018), (2) when [Hb] was below the value achieved after correcting the 11 g/dL for altitude [Hb] as suggested by the WHO (Centers for Disease Control and Prevention, 1989; WHO, 2001) applying the WHO recommended correction for altitude using the formula: [Hb] adjustment (g/dL) = −0.032 × (altitude in m × 0.0033) + 0.022 × (altitude in m × 0.0033)2 (Centers for Disease Control and Prevention, 1989), and (3) by applying the newly suggested threshold based on the current data, which is a [Hb] below the range of normal values, that is, the altitude-related means [Hb] minus 2 SD.
Detection of polycythemia
Abnormally high [Hb] was defined by four methods as follows: (1) according to a threshold value of 14.5 g/dL independent of altitude (Gonzales et al., 2018), (2) using a threshold of 14.5 g/dL but with correction for altitude according to the WHO, (3) when [Hb] was >19 g/dL as defined by León-Velarde et al. (2005), again independent of altitude, and (4) when [Hb] was higher than the altitude-related population means plus 2 SD.
Data were assessed using the statistical software package STATA (STATA version 14.0) for a personal computer (Stata Corporation, College Station, TX). The mean values of hemoglobin were compared for altitude and age effects using two-way analysis of variance and post hoc testing using Bonferroni correction. Differences in prevalence of anemia and in polycythemia were calculated by chi square test. A statistically significant difference was considered with a p-value <0.05.
Results
Table 1 describes the mean and normal ranges for [Hb] grouped by age and altitude of residence. At each range of altitude [Hb] increases slightly with age. The difference between the <1 year and 3- to 5-year-old children is ∼0.8 g/dL and seems to be independent of altitude. Similarly, within each age group [Hb] increases as altitude increases. For comparison, Table 2 shows the different percentiles of [Hb] for children when all age groups (6–59 months) are combined to indicate the magnitude of deviation from the altitude-related mean values. Analysis of [Hb] concentration with altitude shows a quadratic regression behavior (all age groups combined) indicating that [Hb] is quite constant at low altitudes but increases thereafter from 11.32 g/dL at 1000 m to 14.54 g/dL at ≥4000 m. The regression line describing the relationship between altitude (meters) and [Hb] (g/dL) is:
 |
Table 1.
Change in the Hemoglobin Concentration (g/dL) with Altitude of Residence (Meters) in Children Aged 6 to 59 Months in Peru
| Altitude (m) | Age (months) | All ages combined | |||
|---|---|---|---|---|---|
| 6–11.9 (509,479) | 12–23.9 (605,228) | 24–35.9 (413,877) | 36–59.9 (500,117) | 6–59.9 (2,028,701) | |
| 0–999 (932,586) | 10.97a,b 8.53–13.43 (276,475) |
11.16a 8.66–13.66 (291,932) |
11.56a 9.08–14.04 (176,423) |
11.76a 9.34–14.18 (187,756) |
11.30a 8.76–13.84 (932,586) |
| 1000–1999 (156,572) | 11.47b 9.21–13.73 (30,804) |
11.61 9.37–13.85 (42,580) |
11.93 9.85–14.01 (32,859) |
12.19 10.19–14.19 (50,329) |
11.84 9.62–14.06 (156,572) |
| 2000–2999 (336,341) | 12.04b 9.58–14.5 (71,762) |
12.24 9.82–14.66 (97,352) |
12.61 10.29–14.93 (71,446) |
12.88 10.62–15.14 (95,781) |
12.46 10.0–14.92 (336,341) |
| 3000–3999 (561,008) | 12.70 9.78–15.62 (121,726) |
12.90 10.04–15.76 (161,298) |
13.26 10.58–15.94 (123,662) |
13.52 10.92–16.12 (154,322) |
13.11 10.27–15.95 (561,008) |
| ≥4000 (42,194) | 13.55 10.15–16.95 (8712) |
13.72 10.38–17.06 (12,066) |
14.03 10.95–17.11 (9487) |
14.38 11.36–17.40 (11,929) |
13.94 10.66–17.22 (42,194) |
The normal range of [Hb] was calculated as the means [Hb] ±2 SD. Numbers of individuals included are given in parentheses. Two-way ANOVA: p < 0.0001 altitude and age. Test post ANOVA Bonferroni ap < 0.001 for each group of age: [Hb] at 0–999 m versus [Hb] at 1000–1999 m, 2000–2999 m, 3000–3999 m, or >4000 m. For each altitude group: bp < 0.001 [Hb] at age 6–11 months versus at age 12–23, 24–35, or 36–59 months.
ANOVA, analysis of variance; SD, standard deviations.
Table 2.
Percentiles of Hemoglobin Concentration According to Altitude of Residence in Peruvian Children from 6 to 59 Months
| Altitude (m) | Hemoglobin (g/dL) | ||||||
|---|---|---|---|---|---|---|---|
| P5 | P10 | P25 | P50 | P75 | P90 | P95 | |
| 0–999 | 9.3 | 9.9 | 10.5 | 11.22 | 12 | 12.8 | 13.3 |
| 1000–1999 | 10 | 10.5 | 11.2 | 12 | 12.5 | 13.1 | 13.5 |
| 2000–2999 | 10.3 | 11 | 11.8 | 12.5 | 13.2 | 13.9 | 14.3 |
| 3000–3999 | 10.6 | 11.2 | 12.21 | 13.2 | 14 | 14.8 | 15.2 |
| >4000 | 11 | 11.8 | 13 | 14.1 | 15 | 15.8 | 16.3 |
| Total | 9.8 | 10.2 | 11 | 12 | 13.1 | 14.1 | 14.6 |
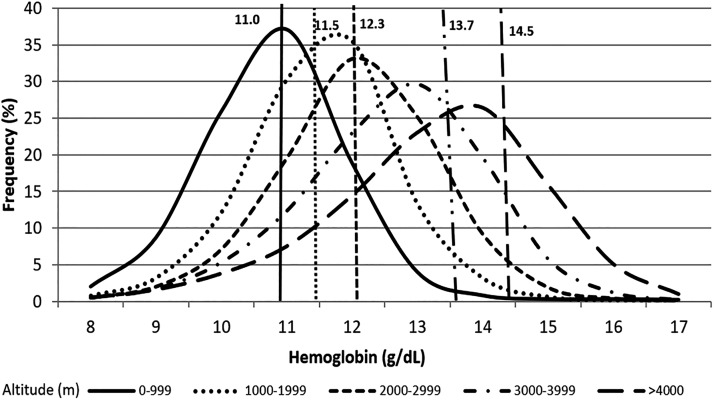
Figure 1 shows the distribution curves of [Hb] at different ranges of altitude. The vertical lines in Figure 1 indicate the WHO thresholds for detecting anemia at sea level and at the respective ranges of altitude using corrections suggested by the WHO. They show the inadequacy of those thresholds for Peruvian children, because the threshold lines of [Hb] are often higher than the population mean.
FIG. 1.
Distribution of the hemoglobin concentration in Peruvian children aged 6 to 59 months at different altitude ranges. Vertical lines indicate the cutoff values for [Hb] suggested by the WHO for detection of anemia and the change of this threshold with altitude. WHO, World Health Organization.
Table 3 indicates the prevalence of anemia in the children (all age groups combined) by comparison of the different methods used for calculation as indicated in the introduction.
Table 3.
Prevalence of Anemia in Peruvian Children (6–59 Months) Living at Different Altitudes
| Altitude (m) | Threshold 11 g/dL, without correction prevalence (%) n | Threshold with WHO correction for altitude prevalence (%) n | Threshold means minus 2 SDaprevalence (%) n |
|---|---|---|---|
| 1: 0–999 | 35.24b,c 328,675 |
35.99c 335,648 |
15.57c 145,233 |
| 2: 1000–1999 | 17.01b 26,632 |
28.93 45,297 |
9.60 5038 |
| 3: 2000–2999 | 9.65b 32,469 |
40.94 137,704 |
6.66 22,412 |
| 4: 3000–3999 | 6.98b 39,164 |
55.78 312,894 |
6.42 36,014 |
| 5: ≥4000 | 4.49b 1894 |
65.83 27,775 |
5.22 2201 |
| Overall | 21.14 428,834 |
42.36 859,318 |
10.89 220,898 |
A comparison of different evaluation methods. Prevalence was estimated with the threshold value of [Hb] = 11 g/dL suggested by the WHO for low altitude without (left) and with (middle) correction for altitude and by use of the newly suggested thresholds shown in Table 1 (right column). Chi-square test: p < 0.0001.
Altitude related threshold points obtained from data in Table 1.
p < 0.0001 between anemia with [Hb] without correction versus [Hb] with correction and versus [Hb] means-minus-2 SD.
p < 0.0001 between anemia at altitude 0–999 m with respect to anemia at altitude 1000–1999 m, altitude 2000–2999 m, altitude 3000–3999 m, and altitude >4000 m.
WHO, World Health Organization.
Applying the threshold for anemia defined by WHO for sea level (11 g/dL) results in a prevalence of ∼35% for children living at altitudes <1000 m, and the prevalence decreases to ∼4.5% at ≥4000 m. After correcting the WHO threshold for altitude, the prevalence was ∼36% below 1000 m and increases to ∼66% above 4000 m. Basing the threshold for anemia on [Hb] minus 2 SD measured in our large population, the prevalence was ∼15% below 1000 m and ∼5% above 4000 m.
Table 4 summarizes data on polycythemia, where prevalence based on thresholds of [Hb] of 14.5 g/dL without (Gonzales et al., 2018) and with [Hb] correction by altitude according to WHO suggestion, a threshold of 19 g/dL to diagnose excessive erythrocytosis as for adults (León-Velarde et al., 2005), and the newly suggested threshold of [Hb] minus 2 SD calculated from our database were compared.
Table 4.
Prevalence of Polycythemia in Peruvian Children (6–59 Months) Living at Different Altitudes (Meters) Calculated Using (A) a Threshold of [Hb] = 14.5 g/dL Without Correction, (B) After WHO Suggested [Hb] Correction, (C) a Threshold of 19 g/dL, and (D) a Threshold Corresponding to [Hb] > Means Plus 2 SD of the Data Evaluation (Table 1)
| Altitude (meters) | Threshold 14.5 g/dL, without correction prevalence (%) n | Threshold 14.5 g/dL with WHO correction for altitude prevalence (%) n | Threshold 19 g/dL (León-Velarde et al., 2005) prevalence (%) n | Thresholds of means plus 2 SD+ prevalence (%) n |
|---|---|---|---|---|
| 1: 0–999 | 1.20*,b (11,202) | 1.19* (11,110) | 0.01a (102) | 3.31* (30,845) |
| 2: 1000–1999 | 0.82c (1286) | 0.42 (662) | 0.01 (19) | 2.62 (4098) |
| 3: 2000–2999 | 3.58c (12,048) | 0.31 (1.031) | 0.02 (58) | 2.45 (8248) |
| 4: 3000–3999 | 13.36c (74,926) | 0.21 (1168) | 0.03 (175) | 1.76 (9868) |
| 5: ≥4000 | 39.36c (16,609) | 0.29 (122) | 0.16 (69) | 1.41 (595) |
| Total | 5.72 (116,071) | 0.71 (14,093) | 0.03 (643) | 2.64 (53.654) |
Chi-square test: *p < 0.001 for the prevalence of polycythemia at altitude 0–999 m versus at altitude 1000–1999 m, versus at altitude 2000–2999 m, versus at altitude 3000–3999 m, versus at altitude >4000 m. ap < 0.001 for the prevalence of polycythemia at altitude 0–999 m versus altitude 3000–3999 m, altitude and versus altitude >4000 m. Comparing polycythemia within each altitude group: bp < 0.001 polycythemia defined as [Hb] >14.5 g/dL without correction by altitude versus polycythemia (excessive erythrocytosis) [Hb] >19 g/dL, and versus polycythemia [Hb] means plus 2 SD. cp < 0.001 polycythemia defined as [Hb] without correction by altitude >14.5 g/dL versus polycythemia defined as [Hb] >14.5 g/dL after correction by altitude, polycythemia [Hb] >19 g/dL, polycythemia defined as [Hb] means plus 2 SD.
Altitude related threshold points obtained from the data in Table 1.
Using the sea level threshold of [Hb] >14.5 g/dL, the prevalence of polycythemia increased from 1.2% at <1000 m to 39.4% at >4000 m. After adjusting the 14.5 g/dL for altitude (WHO) prevalence of polycythemia was decreased as altitude increased. Excessive erythrocytosis defined as [Hb] >19 g/dL resulted in a very low prevalence at low altitude and an increase with greater altitudes, with the highest values above 4000 m. When the threshold is based on means plus 2 SD the prevalence of polycythemia was ∼3% at low altitude and tended to decrease with altitude.
Using a database containing serum ferritin values and hemoglobin concentration in 133 children from Puno (3800 m) reported in a recent publication (Gonzales et al., 2018), it was calculated that the serum levels of ferritin in those with anemia diagnosed according to the means minus 2 SD definition were 8.3 ± 1.3 ng/mL, whereas ferritin was 13.32 ± 0.12 ng/mL (p = 0.0016) in those children whose [Hb] was within the new normal range. According to WHO, iron deficiency is diagnosed when serum ferritin levels were below 12 ng/mL in children aged 6–59 months (WHO, 2011).
Discussion
Our results shown in Table 1 confirm previously reported results of increased [Hb] in children with greater altitude of residency (Dirren et al., 1994; Garruto et al., 2003) but shows that the increase is less pronounced than what has been proposed by the WHO correction guidelines (WHO, 2001). This puts into question the values provided by the WHO as thresholds to diagnose anemia. We therefore suggest new values, which are based on the data obtained on a large population (>2,000,000) of Peruvian children between 5 and 59 months of age whose altitude of residence ranges from sea level to ∼5000 m.
Distribution curves for [Hb] for the different altitude ranges (Fig. 1) also indicate a shift to the right with increasing ranges of altitude (<1000, 1000–1999, 2000–2999, 3000–3999, and ≥4000 m). The figure also shows that most values, which might be considered normal, are below the thresholds defined by the WHO for anemia ([Hb] <11 g/dL plus correction for altitude). This would indicate an extremely high prevalence of anemia in children at low (1000–1999 m) and even more so at the higher altitudes, where prevalence reaches values well above 50% (middle column, Table 2). There is no proof whether this is true, since we lack important other hematological indices, such as ferritin, vitamin B12, folate, and mean corpuscular volume, but it may point to considerable overestimation.
Indirect evidence for the validity of the newly proposed criteria comes from ferritin values measured in children from Puno (3800 m), where using the new criteria proposed in this study resulted in lower ferritin in those children considered anemic than in those with [Hb] within the normal range. We have previously reported that also with the WHO threshold for anemia at low altitude children with anemia in Puno (3800 m) had below-normal serum ferritin levels. However, after [Hb] correction for altitude according to the WHO, serum ferritin was higher in those defined as anemic by this standard likely because now more children with normal [Hb] and thus likely normal ferritin levels were included. This supports the notion that the adjustment for altitude as suggested by the WHO may not be applicable for the children studied here (Gonzales et al., 2018). Additional laboratory parameters, as indicated above, may be needed to verify this.
It has already been considered by the WHO (Pasricha et al., 2018) to adapt threshold values specific for regions to account for differences among ethnicities in different regions of the world and even within a country with mixed populations. Lack of applicable threshold values is also the reason why many laboratories in different countries use different threshold values, most of which are lower than the corrections suggested by the WHO. Again, this indicates insufficient guidelines and population-specific thresholds to determine whether a specific [Hb] is abnormal (Colman et al., 2018). In fact, it has previously been suggested that the threshold for anemia should be reevaluated in children at low and at HA (Domellöf et al., 2002; Gonzales et al., 2018).
Our results suggest that using a very large database can be helpful to set [Hb] thresholds to define anemia for children living in Peru. Comparing the prevalence of anemia measured by the different methods shows pronounced differences. This has important implications for clinical medicine and public health. Using the wrong thresholds could be one important reason why the prevalence of anemia is minimally reduced in different countries despite intervention programs. It is now well recognized that supplementation with iron in a child with normal [Hb] will not change [Hb] because of changes in iron absorption regulated by hepcidin (Brannon and Taylor, 2017).
It is interesting to note that the prevalence of anemia was lower at HA than at low altitude when using both the uncorrected low-altitude threshold [Hb] of 11 g/dL (not corrected by altitude) and using our new threshold based on means minus 2 SD. While the former is to be expected because mean [Hb] increases with altitude (Table 1) and the distribution curves shift to the right (Fig. 1) thus moving away from the threshold and can thus not be applied, the reason for the latter is less clear. One possibility is a difference in the populations, for example, the proportion between Quechuas and Aymaras, differing in [Hb] (Moulin, 1971; Quilici and Vergnes, 1977; Arnaud et al., 1981), which might be indicated by different skewness of the distribution curves. Our data cannot determine which sample is Aymara or Quechua. However, both results contradict the published data from the Ministry of Health and National Institute of Statistics and Informatics (INEI) in Peru indicating that the prevalence of anemia is higher in children from HA than in those from low altitude (INEI, 2018). However, this latter statement is owing to the fact that the threshold of [Hb] to define anemia is corrected by altitude according to the WHO recommendations (INEI, 2018).
Besides the diagnosis of anemia, it is also important to diagnose polycythemia or erythrocytosis because high [Hb] is associated with chronic mountain sickness (León-Velarde et al., 2005). Thus, it is of importance to define the appropriate threshold. Threshold values are not as well defined as those for anemia. Previous studies in pregnant women suggested a threshold of 14.5 g/dL (Scanlon et al., 2000) for sea level. We have also used this value in children living in Peru (Gonzales et al., 2018) and compared it with data obtained with and without [Hb] correction for altitude using the WHO corrections. A [Hb] >19 g/dL for adult women and >21 g/dL in adult men has been suggested to indicate chronic mountain sickness (León-Velarde et al., 2005) without any further correction for altitude.
As discussed above, without applying altitude corrections of predefined thresholds (14.5 g/dL), the percentage of children with polycythemia increases with greater altitude because the distribution curves move away from the threshold value. Thus, this procedure cannot be applied, which is strong support for the use of threshold values that account for increasing [Hb] with altitude. Furthermore, only 0.03% of the children had values of [Hb] >19 g/dL indicative of excessive erythrocytosis or even chronic mountain sickness. This is consistent with the epidemiology of chronic mountain sickness (CMS), which is a disease of adults that increases with age. When [Hb] is corrected by altitude as suggested by the WHO recommendations, the prevalence of polycythemia is reduced to 0.02%. Thus, factors such as oxygen saturation and family history have to be evaluated to better interpret these findings.
Excessive erythrocytosis is a cardinal sign of chronic mountain sickness (León-Velarde et al., 2005). The prevalence of chronic mountain sickness in HA dwellers ranges from 1.2% in native Tibetans to 5.6% in Chinese Han; 6% to 8% in male residents of La Paz, Bolivia; and 15.6% in the Peruvian Andes (Pasha and Newman, 2010). The fact that prevalence of excessive erythrocytosis increases with higher altitude may explain why CMS is more frequent as altitude increases (Penaloza and Arias-Stella, 2007).
In summary, we have attempted to define normal ranges of [Hb] for Peruvian children living at different altitudes by use of a database provided by the CENAN in 2017. These tables can serve as reference tables for clinicians in evaluating measured [Hb] values. More importantly, these tables can also be used to detect anemia and polycythemia, where we define the threshold values as being below the means minus 2 SD and the means plus 2 SD, respectively. They also provide additional ranges (percentiles) for more detailed evaluation (Table 2), because the 2 SD-ranges, which contain ∼95% of all samples, may not be conservative enough and thresholds at lower percentiles may be more stringent, which need further evaluation and correlation with confounding factors.
These population-specific normal values and thresholds may be more precise than the values suggested by the WHO, because the latter overestimate the prevalence of anemia at HA. This discrepancy points to another important aspect, which is indicated by differences in [Hb] between ethnic groups around the world. In fact, Tibetan and Han-Chinese living at the same altitude in the Himalayas have different [Hb] levels (Wu et al., 2005) likely because of genetic adaptation (Simonson, 2010), which is already established in children (Garruto et al., 2003; Wu et al., 2005). In addition, Ethiopians have a pattern of change in [Hb] with altitude that differs from those reported from South Americans (Beall, 2006).
Thus it is of great clinical significance to establish ranges of normal [Hb] based on local databases to account for differences in the response of [Hb] to life at HA between ethnic groups.
Acknowledgments
The authors thank Dr. Nelly Zavaleta from CENAN and Dr. César Cabezas from National Institute of Health (INS, Peru) for providing the national anemia database. The authors also acknowledge the significant contributions of colleagues in the field that could not always be cited due to space limitations. The authors acknowledge Dr. Heimo Mairbäurl, University of Heidelberg (Germany) for helpful discussion to strengthen the article. This study was supported by a Grant U01TW010107 (1/2 Regional GeoHealth hub centered in Peru) of the National Institute of Health of the United States of America (Fogarty Program). This study was supported by the NIH Fogarty International Center, National Institutes of Environmental Health Sciences, National Cancer Institute, centers for disease control and the NIH under award number [for Research Grant U01TW010107] [for Training Grant U2RTW010114]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author Disclosure Statement
No competing financial interests exist.
References
- Arnaud J, Quilici JC, and Rivière G. (1981). High-altitude haematology: Quechua-Aymara comparisons. Ann Hum Biol 8:573–578 [DOI] [PubMed] [Google Scholar]
- Beall CM. (2006). Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol 46:18–24 [DOI] [PubMed] [Google Scholar]
- Brannon PM, and Taylor CL. (2017). Iron supplementation during pregnancy and infancy: Uncertainties and implications for research and policy. Nutrients 9:1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CENAN. (2015). Technical Report. Nutritional status for life stages in the Peruvian population; 2013–2014. Lima:INS. 1–224 [Google Scholar]
- Centers for Disease Control and Prevention. (1989). Criteria for anemia in children and childbearing-aged women. MMWR Morb Mortal Wkly Rep 38:400–404 [PubMed] [Google Scholar]
- Cheong HI, Janocha AJ, Monocello LT, Garchar AC, Gebremedhin A, Erzurum SC, and Beall CM. (2017). Alternative hematological and vascular adaptive responses to high-altitude hypoxia in East African highlanders. Am J Physiol Lung Cell Mol Physiol 312:L172–L177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman KS, Wood EM, De La Salle B, Stanworth SJ, and Pasricha SR. (2018). Heterogeneous hemoglobin lower thresholds in clinical laboratories. Am J Hematol 93:E142–E144 [DOI] [PubMed] [Google Scholar]
- Dirren H, Logman MH, Barclay DV, and Freire WB. (1994). Altitude correction for hemoglobin. Eur J Clin Nutr 48:625–632 [PubMed] [Google Scholar]
- Domellöf M, Lonnerdal B, Dewey KG, Cohen RJ, Rivera LL, and Hernell O. (2002). Sex differences in iron status during infancy. Pediatrics 110:545–552 [DOI] [PubMed] [Google Scholar]
- Garruto RM, Chin CT, Weitz CA, Liu JC, Liu RL, and He X. (2003). Hematological differences during growth among Tibetans and Han Chinese born and raised at high altitude in Qinghai, China. Am J Phys Anthropol 122:171–183 [DOI] [PubMed] [Google Scholar]
- Gonzales GF. (1998). Contribución peruana a la hematología en poblaciones nativas de altura. Acta Andina 8:105–130 [Google Scholar]
- Gonzales GF, Alarcón DE, and Zevallos-Concha A. (2016). The biochemistry of oxidative stress. In: Advances in Biochemistry in Health and Disease. Gelpi RJ, Boveris A, Poderoso JJ, eds. Basel, Switzerland: Springer International Publishing; pp. 109–125 [Google Scholar]
- Gonzales GF, Rubín de Celis V, Begazo J, del Rosario Hinojosa M, Yucra S, Zevallos-Concha A, and Tapia V. (2018). Correcting the cut-off point of hemoglobin at high altitude favors misclassification of anemia, erythrocytosis and excessive erythrocytosis. Am J Hem 93:E12–E16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Statistic and Informatics. Peru: Demographic and Family Health Survey. (2017). Available at: https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1525/index.html (last accessed September18, 2018)
- León-Velarde F, Gamboa A, Chuquiza JA, Esteba WA, Rivera-Chira M, and Monge CC. (2000). Hematological parameters in high altitude residents living at 4,355, 4,660, and 5,500 meters above sea level. High Alt Med Biol 1:97–104 [DOI] [PubMed] [Google Scholar]
- León-Velarde F, Maggiorini M, Reeves JT, Aldashev A, Asmus I, Bernardi L, Ge RL, Hackett P, Kobayashi T, Moore LG, Penaloza D, Richalet JP, Roach R, Wu T, Vargas E, Zubieta-Castillo G, and Zubieta-Calleja G. (2005). Consensus statement on chronic and subacute high-altitude diseases. High Alt Med Biol 6:147–157 [DOI] [PubMed] [Google Scholar]
- Lönnerdal B, Georgieff MK, and Hernell O. (2015). Physiology of the development of iron absorption, homeostasis and metabolism in the healthy term child. J Pediatr 167:S8–S14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin J. (1971). Hematimetrie et cytologie en milieu tropical d'l Amérique du Sud: variations raciales el écologiques. These, Université Paul Sabatier, Toulouse [Google Scholar]
- National Center for Health Statistics, Fulwood R, Johnson CL, Bryner JE, Gunter EW, and McGrath CR. (1982). Hematological and nutritional biochemistry reference data for persons 6 months-74 years of age: United States, 1976–1980. Vital and Health Statistics. Series 11—No. 232. DHHS Pub. No. (PHS) 83-l 682. Public Health Service. Washington. U.S. Government Printing Office, December:1– 183 [PubMed] [Google Scholar]
- Pasha MA, and Newman JH. (2010). High-altitude disorders: pulmonary hypertension: pulmonary vascular disease: The global perspective. Chest 137(6 Suppl):13S–19S [DOI] [PubMed] [Google Scholar]
- Pasricha SR, Colman K, Centeno-Tablante E, Garcia-Casal MN, and Peña-Rosas JP. (2018). Revisiting WHO haemoglobin thresholds to define anaemia in clinical medicine and public health. Lancet Haematol 5:e60–e62 [DOI] [PubMed] [Google Scholar]
- Penaloza D, and Arias-Stella J. (2007). The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation 115:1132–1146 [DOI] [PubMed] [Google Scholar]
- Quilici JC, and Vergnes H. (1977). The haematological characteristics of high altitude population. In: The Biology of High-Altitude Peoples. International Biological Programme 14. Baker PT, ed. Cambridge, New York: Cambridge University Press; pp. 189–218 [Google Scholar]
- Scanlon KS, Yip R, Schieve LA, and Cogswell ME. (2000). High and low hemoglobin levels during pregnancy: differential risks for preterm birth and small for gestational age. Obstet Gynecol 96(5 Pt 1):741–748 [DOI] [PubMed] [Google Scholar]
- Shah J, Shah PS, and Jefferies A. (2015). Hematological parameters immediately after birth in twins and higher order multiples. Am J Perinatol 32:653–658 [DOI] [PubMed] [Google Scholar]
- Simonson TS, Yang Y, Huff CD, Yun H, Qin G, Witherspoon DJ, Boi Z, Lorenzo FR, Xing J, Jorde LB, and Prchal JT. (2010). Genetic evidence for high-altitude adaptation in Tibet. Science 329:72–75 [DOI] [PubMed] [Google Scholar]
- WHO. (2001). Iron Deficiency Anemia. Assessment, Prevention and Control. In: A Guide for Programme Managers. Geneva: World Health Organization [Google Scholar]
- WHO. (2011). Serum Ferritin Concentrations for the Assessment of Iron Sttaus and Iron Deficiency in Populations. World Health organization, Geneva. Available at: www.who.int/vmnis/indicators/serum_ferritin.pdf (last accessed March16, 2018)
- Wu T, Wang X, Wei C, Cheng H, Wang X, Li Y, Ge-Dong, Zhao H, Young P, Li G, and Wang Z. (2005). Hemoglobin levels in Qinghai-Tibet: different effects of gender for Tibetans vs. Han. J Appl Physiol (1985) 98:598–604 [DOI] [PubMed] [Google Scholar]