Abstract
The safety and efficacy of pharmacological and cellular transplantation strategies are currently being evaluated in people with spinal cord injury (SCI). In studies of people with chronic SCIs, it is thought that functional recovery will be best achieved when drug or cell therapies are combined with rehabilitation protocols. However, any functional recovery attributed to the therapy may be confounded by the conditioned state of the body and by training-induced effects on neuroplasticity. For this reason, we sought to investigate the effects of a multi-modal training program on several body systems. The training program included body-weight–supported treadmill training for locomotion, circuit resistance training for upper body conditioning, functional electrical stimulation for activation of sublesional muscles, and wheelchair skills training for overall mobility. Eight participants with chronic, thoracic-level, motor-complete SCI completed the 12-week training program. After 12 weeks, upper extremity muscular strength improved significantly for all participants, and some participants experienced improvements in function, which may be explained by increased strength. Neurological function did not change. Changes in pain and spasticity were highly variable between participants. This is the first demonstration of the effect of this combination of four training modalities. However, balancing participant and study-site burden with capturing meaningful outcome measures is also an important consideration.
Keywords: : clinical trials, conditioning, exercise, rehabilitation, spinal cord injury
Introduction
Several clinical trials evaluating the safety of pharmacological and cellular transplantation strategies in people with spinal cord injury (SCI) are ongoing (NCT02354625, NCT02302157, NCT02524379, and NCT02096913), and efficacy trials are on the horizon. In individuals with chronic SCI, however, there is agreement that functional recovery achieved through the use of drugs or cells will best be accomplished in concert with rehabilitation protocols.1 In fact, a recent systematic review of clinical trials in SCI indicated that the strongest evidence for efficacy was associated with trials including an exercise component.2 However, the measurement of functional recovery attributed to repair strategies may be confounded by the conditioned state of the body and by training-induced effects on neuroplasticity. Therefore, it is important to estimate the effects of exercise conditioning and potential neuroplasticity-inducing therapies before administration of any biological or pharmacological therapeutic.3 The present study focuses on a combination of activities, including body-weight–supported treadmill training (BWSTT) for locomotion, circuit resistance training (CRT) for upper body conditioning, functional electrical stimulation (FES) for activation of sublesional muscles, and wheelchair skills training (WST) for overall mobility.
A large body of research supports that locomotor training can influence motor recovery, particularly in individuals with incomplete SCI.4–6 (For review, see Harkema and colleagues.7) Although locomotor training by itself may have less of an influence on change in neuromotor control in persons with chronic motor complete SCI compared to those with motor incomplete SCI, small changes have been documented. Robotic assist BWSTT has been demonstrated to modulate electromyographic (EMG) muscle activity in a velocity-dependent manner in individuals with motor complete SCI ranging between cervical level 5 (C5) and lumbar level 1 (L1).8 In a single case, there was a small effect reported on neurological lower extremity motor scores in an individual 2 years after a thoracic level 7 (T7) complete SCI.9 Manual-assist BWSTT has also been reported to induce lower extremity muscle activation in an individual with a chronic C6 motor complete SCI.10 Even when BWSTT has not yielded functional motor improvements in individuals with motor complete SCI, salutary changes have been measured in bone and fat mass,10 quality of life, depression, metabolism,11 and cardiovascular health. As such, BWSTT is a viable rehabilitation technique for individuals with motor complete paraplegia.
Considerable evidence published by our investigative group, and others, has documented a rapid decline in cardiorespiratory fitness level post-SCI.12–17 This decline reduces functional capacity,18,19 and imposes early risks for all-cause cardiovascular20 and cardioendocrine21 disease. Exercise studies using upper limb cardiorespiratory activities (e.g., arm ergometry) have long been known to improve fitness post-SCI22; more substantial fitness gains have been reported when incorporating resistance exercises with cardiorespiratory reconditioning.23 A CRT model using this design is more effective for improving upper limb and trunk endurance than endurance exercise alone and also improves strength and anaerobic power.24 In addition, the circuit resistance conditioning has improved atherogenic lipid profiles25 and reduced pre-existing shoulder pain in young and middle-aged persons with SCI.26 Therefore, CRT is a viable technique for upper body conditioning.
While CRT improves fitness in persons with SCI, its benefits primarily impact the upper limb and overall conditioning, not conditioning of the sublesional musculature and circulation no longer under central autonomic control. To address conditioning of such sublesional muscles, rhythmic contractions of lower extremity muscles can be induced by sequential application of lower extremity electrical current to selected skin surface sites (FES, cycling).27 Previous work has used FES conditioning of the lower limbs to improve cardiorespiratory fitness and augment fitness acquired through upper extremity work,27–32 increase muscle strength,33 and modulate spasticity.34 As such, FES is a viable technique for lower body conditioning.
Mobility skill capacity post-SCI is a significant predictor of quality of life and participation,35,36 and mobility skill training has been shown to improve participation.37 Mobility includes such activities as the ability to transfer between the wheelchair and a bed/shower/toilet/car, maneuver a chair in tight spaces, and navigate features of the built environment, such as curbs and ramps. Under the International Classification of Functioning, Disability and Health, a biopsychosocial model of disability, mobility falls under the “activities and participation” domain, representing function at the level of the whole person.38 As a measure of “whole person” function, mobility represents the net integration of all positive and negative changes to body structures (spinal cord) and functions (motor control and sensory feedback) that are the targets of therapeutic pharmacologic and cellular transplantation strategies and the concomitant post-treatment multi-modal rehabilitation programs. Therefore, mobility assessment and training should be considered in any therapeutic trial targeting functional recovery in persons with SCI, including nonambulatory individuals with motor complete impairments.
Clinical trials examining persons with SCI have traditionally focused on outcomes based on motor and sensory function as the primary criterion of intervention success, most specifically utilizing the International Standards of Neurologic Classification of SCI (ISNCSCI), developed by the American Spinal Injury Association (ASIA).39 Neurological Level of Injury (NLI) and ASIA Impairment Scale (AIS) grade, as defined by the ISNCSCI, are often used as primary outcome measures. However, the ISNCSCI was never designed to be an all-encompassing outcome measure. Individuals living with SCI rank regaining sensory and autonomic function and reducing neuropathic pain as high priorities, in addition to improving motor control relevant to their injury level.40 Similarly, people with SCI rank decreased ability to walk or move, decreased control of bowel, decreased control of bladder, decreased sexual function, and pain to be the consequences of injury most difficult to deal with.41 In a Veterans Affairs database, spasticity was reported as the most common problematic complication post-SCI (53%) followed by pain (44%),42 or as the second-most reported complication (40%) after urinary tract infections UTIs.43 These studies highlight the importance of not only evaluating changes in neuromotor function, but also of examining the effects on autonomic and sensory function, as well as chronic pain, spasticity, and mobility, in clinical trials that test biological or pharmacological interventions to repair damaged tissue.
The individual effects of BWSTT, CRT, FES, and WST on individuals living with chronic SCI have been described in the literature. Our objective was to comprehensively evaluate the effects of a multi-modal training program on several body systems. The training program incorporated both upper limb (voluntary) and lower limb (electrically stimulated) conditioning in combination with BWSTT and WST. Determining the extent of training effects on different body systems will improve our understanding of the specific contributions of neural repair strategies.
Methods
Study design
This was a prospective, pilot trial in which 8 participants serving as their own controls received multi-modal exercise conditioning and rehabilitation interventions. After screening, two baseline assessments (B1 and B2, separated by 2 weeks) were performed to examine day-to-day measurement variability. No interventions occurred between B1 and B2. Participants then underwent three, 4-week-long multi-modal training programs, each separated by a 1-week period of multiple body systems assessments (E1, E2, E3; for details, see Supplementary Table 1) (see online supplementary material at http://www.liebertpub.com). This design was utilized because the effect of 12-week training periods were previously published for each training modality; however, little information was available regarding the timing of changes within a 12-week training period. Each participant was in the trial for 19 contiguous weeks; it took 9 months to complete all 8 participants (grouped into two cohorts).
Participants
Inclusion criteria were a trauma-induced SCI between T2 and T12; AIS grade A or B; a minimum of 1 year post-injury; and age 18–60 years. Criteria for exclusion included progressive SCI; absent or intolerable painful response to electrical stimulation; history of decubiti within the preceding 3 months; diagnosis of diabetes; history of medication for diabetes, high blood pressure, or dyslipidemia within the preceding 6 months; current fracture of the lower extremities, or stabilizing instruments in the legs that prevented full range of motion; hip, knee, and ankle joints unable to support weight bearing or limited in range of motion; and history of dizziness when upright. Written informed consent was obtained from all study participants according to the protocol approved by the Human Subjects Research Office at the University of Miami Miller School of Medicine (Miami, FL).
Interventions
BWSTT using a treadmill-based robotic gait orthosis (Lokomat; Hocoma Inc, Zurich, Switzerland) was performed two times per week on days opposing the upper extremity CRT and the lower extremity FES cycle training. The robotic orthosis was used in conjunction with a system that supports a portion of a participant's body weight, thereby reducing the load endured by the participant's legs. Training began with participants walking at a treadmill speed of 1.2 km per hour. Body weight support was adjusted to allow as much lower extremity loading as possible while maintaining normal walking kinematics (i.e., avoiding toe drag during swing or excessive knee flexion during stance). The treadmill speed was increased by 2.5% each week (provided the increased speed did not elicit greater spasticity), and the most tolerable percentage of body weight support was provided.
Upper extremity CRT sessions were performed three times per week on nonconsecutive days for approximately 30–45 min per session. The CRT protocol was modeled after a previously published training paradigm,24 except participants completed two, not three, cycles of the circuit during each training session. Participants performed two sets of 10 repetitions of each weightlifting maneuver, followed by 2 min of arm cranking on a stationary machine. Weightlifting maneuvers included overhead press, horizontal row, vertical butterfly, triceps dips, latissimus pulldowns, and bicep curls.
FES cycle training on a cycle ergometer (Restorative Therapies RT300; RTI, Inc., Baltimore, MD) was used for submaximal lower extremity conditioning. Participants remained in their wheelchair and had their feet secured in a fixed‐angle pedal orthosis. Distance from the ergometer was set to achieve a knee angle at 15 degrees when in fullest extension. Participants cycled for 15 min three times weekly on nonconsecutive days using computer-sequenced electrical current having a frequency of 35 Hz, a current amplitude of 100–140 miliamperes (mA), and a pulse width of 350 μsec. Resistance (torque) was initially set at 0.5 Newton‐meters (Nm), but was increased for succeeding sessions to 2.39 and 3.6 Nm when the pedal cadence of 35 rpm could be independently maintained by the participant without fatigue or assistance from a motor that was interfaced with the pedal gear.32 Fatigue was defined by the inability to maintain a pedal rate greater than 35 rpm during peak current stimulation at 140 mA.32 Assistive pedaling was used for participants unable to sustain 15 min of continuous cycling.
Participants were provided with the opportunity to participate in spotter-assisted, self-driven wheelchair skills training sessions two times a week. Each session included 20–40 min of self-guided practice. Participants could opt out of WST as they desired. Spotting was provided by nonclinically trained study staff who had been trained on spotting techniques. Study staff were provided Dalhousie University's Wheelchair Skills Training Program (WSTP)© manual44 for guidance on how to provide WST.
Assessments
Physical examination and medical history
At screening, the ISNCSCI exam was performed and the NLI and AIS grade were determined.39 A physical examination verified that participants had full range of motion in the lower extremities, lacked unhealed decubiti, and exhibited no evidence of bone or joint pathology that would negatively influence participation in the interventions. Radiographical images of the lower extremities were obtained to confirm the absence of fractures, joint abnormalities, and instrumentation. Brief medical history and demographic data were collected (Table 1).
Table 1.
Participant Characteristicsa
| Participant | BC01 | BC02 | BC03 | BC04 | BC05 | BC06 | BC07 | BC08 |
|---|---|---|---|---|---|---|---|---|
| NLI, AIS grade | T10 B | T8 B | T8 A | T6 A | T5 A | T2 B | T5 B | T2 A |
| Sex | F | M | M | F | M | M | M | M |
| Age | 23 | 52 | 26 | 47 | 20 | 28 | 36 | 19 |
| Etiology of injury | MVA | MVA | Fall | MVA | MVA | MVA | Violence | MVA |
| YPI | 2 | 25 | 1 | 26 | 5 | 1.5 | 21 | 2 |
| Race | Caucasian | Hispanic | Hispanic/AA | Caucasian | Hispanic/AA | AA | Caucasian | Hispanic |
| Marital | Married | Single | Single | Married | Single | Single | Married | Single |
| Employment | Unemployed | Full-time | Unemployed | Full-time | Full-time | Unemployed | Full-time | Unemployed |
| Change from baseline to E3 | ||||||||
| PGIC | ↑↑↑ | ↑↑↑ | ↑↑ | ↑ | ↑↑ | ↑↑ | ↑ | ↑↑↑ |
| SCIM III | ↑ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ |
| Pain | N/A | ↔ | ↔ | N/A | N/A | N/A | ↔ | N/A |
| W/C skills capacity Δ in score Δ in category |
↔ (+6.3%) ↑ (to above 75%) |
↔ (−3.1%) ↔ (above 75%) |
↓ (−7.8%) ↓ (to below 75%) |
↔ (−3.1%) ↔ (below 75%) |
↔ (0.1%) ↔ (above 75%) |
↓ (−7.8%) ↔ (below 75%) |
↔ (−3.1%) ↔ (above 75%) |
↓ (−10.9%) ↓ (to below 75%) |
| UE strength | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ | ↑ |
| Fitness category | ↔ (Excellent) | ↑ (Fair to average) | ↔ (Average) | ↑ (Fair to average) | ↑ (Average to good) | ↔ (Poor) | ↔ (Good) | ↑ (Poor to average) |
| MAS | ↔ (+1) | ↑ (+2.5) | ↔ | ↔ | ↑ | ↔ (+1) | ↔ (+1) | ↔ |
| SCATS | ↑ | ↔ | ↔ | ↑ | ↑ | ↔ | ↔ | ↔ |
| FSE pendulum (degrees) | ↔ (+7) | ↔ (+2) | ↔ (−8) | ↔ (−4) | ↑ (+33) | ↑ (+20) | ↔ (−8) | ↔ (−5) |
| SL H/M ratio | ↔ | ↔ | ↔ | ↔ | ↔ | ↓ | ↔ | ↔ |
| SL H-depression | ↓ | ↓ | ↓ | ↔ | ↔ | ↓ | ↑ | ↔ |
| SL F/M ratio | ↓ | ↔ | ↓ | ↔ | ↔ | ↔ | ↓ | ↔ |
| TA F/M ratio | ↓ | ↔ | ↓ | ↓ | ↔ | ↔ | ↓ | ↓ |
Including neurological level of injury (NLI) and American Spinal Injury Association (ASIA) impairment scale (AIS) grade, sex, age, etiology of injury (motor vehicle accident = MVA), years post-injury (YPI), race (African American = AA), marital status, and employment status. Changes from baseline (average of baseline 1 [B1] and baseline 2 [B2]) to evaluation 3 (E3), including overall patient global impression of change (PGIC), spinal cord independence measure (SCIM) III total scores, wheelchair (W/C) skills capacity including change (Δ) in score and category, upper extremity (UE) strength, fitness category, modified Ashworth scale (MAS), spinal cord assessment tool for spastic reflexes (SCATS), pendulum first swing excursion (FSE) of more spastic leg, soleus (SL) H/M ratio, SL H-depression, SL F/M ratio, and tibialis anterior (TA) F/M ratio. For PGIC, ↑ = minimally improved, ↑↑ = much improved, and ↑↑↑ = very much improved. For electrophysiological measures of spasticity, decreases and increases in spasticity correspond to ↓ and ↑, respectively. For all other categories, ↑ or ↓ if change is determined to be clinically significant, otherwise ↔.
N/A, not applicable.
Peak oxygen consumption
A multi-stage, intensity-graded arm cycle ergometry test evaluated peak oxygen consumption (VO2peak) by open circuit spirometry (VMax 2130 System, Sensor Medics Model 922 Spirometer; Sensor Medics Corporation, Yorba Linda, CA). Heart rate (HR) was measured by 12-lead electrocardiography using standard limb and precordial leads. Participants performed an arm-cycling exercise test, which mirrored previously published graded exercise tests (GXTs)24,45 with the exception of the workload increasing by 30 watts for each subsequent 3-min stage.24 Criteria for terminating the test corresponded with American College of Sports Medicine guidelines (10th ed.) and included volitional fatigue or inability to maintain cadence at or above 55 revolutions per minute.46
Upper extremity muscle strength
Maximum upper extremity dynamic strength was assessed using an Equalizer 7000 Multi-Station Exercise System (Helm; Bozeman, MT) following a previously published protocol for testing isoinertial strength.24 Six different weightlifting maneuvers (overhead press, horizontal row, vertical butterfly, triceps dips, latissimus pulldowns, and bicep curls) were tested once per assessment period. One repetition-maximum (1-RM) for each maneuver was calculated using the Mayhew regression equation.47
Wheelchair skills
The WST© for Manual Wheelchair users, Version 4.2,48 was utilized to assess personal mobility skills.49,50 The 32 skills evaluated in the WST include indoor mobility skills (e.g., forward propulsion, backward propulsion, and turning) community mobility skills (taking wheelchair apart, level transfers, and gentle slope ramps), and advanced skills (e.g., wheelies, steep ramps, high curbs, and floor-to-chair transfers). According to published guidelines, each skill was scored as 0 (Fail), 1 (Pass with difficulty), or 2 (Pass) and a WST total score was calculated.50 For reporting purposes, participants were classed as low (WST total score ≤75%) or high skilled (WST total score >75%)35 and a change in WST total score was considered meaningful at ≥7%.37 Changes <7% were classed as ‘no change” (↔).
Cardiovascular function
A progressive head-up tilt (HUT) test assessed variability in blood pressure and HR in response to changes in body position. Participants were transferred to a motorized table, placed in the supine position, and secured with Velcro straps. A manual blood pressure cuff was fitted on one arm, and a heart monitor (Polar CS600X; Polar Electro Inc., Lake Success, NY) was secured to the chest. The testing protocol used two, 10-min periods of supine rest, a 5-min period of 20-degree HUT, a 5-min period of 40-degree HUT, and a 10-min period of 60-degree HUT. HR data were monitored continuously, and blood pressure data were collected during the last 2 min of each tilt period.
Blood tests
Antecubital venipuncture was performed to acquire fasting blood samples, followed by an oral glucose tolerance test. Serum collection and analysis techniques mirrored a previously published protocol.51 lood sampling included an inflammatory panel testing C-reactive protein (CRP) and interleukin-6, in addition to lipid and glycemic panels.51 Low-density lipoprotein was computed using the equation of Friedewald.52
Spasticity measures
Biomechanical/clinical
Clonus, flexor, and extensor spastic reflex activity was measured clinically using the Spinal Cord Assessment Tool for Spastic reflexes (SCATS).53 Bilateral scores were summed (range, 0–18) and reported as a composite score.54 Stretch reflex responses of the hamstring and quadriceps muscles was measured using the Modified Ashworth Scale (MAS).55,56 Scores were determined bilaterally, summed (range, 0–20) and reported as a composite score.54
Spastic activity in the quadriceps muscles in response to rapid, gravity-induced stretch was assessed using the pendulum test.57 Kinematic data related to the pendulum test were captured according to previously published protocols58–60 using a 60-Hz, eight-camera, three-dimensional motion capture system (Peak Motus® Software; Peak Performance, Centennial, CO). The examination table was positioned in the center of the capture area so that the length of the table was in the x-direction, and the participant was positioned in the supine position with the popliteal fossa 3 in beyond the end of the table. Reflective markers were placed on the greater trochanter, lateral knee, lateral malleolus, lateral calcaneus, and the metatarsal-phalangeal joint of the fifth toe. The knee of the tested limb was extended passively, and upon initiation of motion capture, the tested limb was released. The knee angle related to the first swing excursion (FSE) was identified using a custom script (MATLAB®; The MathWorks, Inc., Natick, MA). FSE for the more spastic leg was averaged over three trials.
Electrophysiological
EMG activity was recorded from soleus (SL), tibialis anterior (TA), rectus femoris (RF), and the biceps femoris (BF) muscle in the leg that participants reported was most prone to muscle spasms using electrodes placed 5 cm apart (for further details, see Mayo and colleagues61). The peak-to-peak amplitude of the maximal compound action potential (M-wave) was measured for SL, TA, and RF in response to supramaximal stimulation (intensity ∼20% beyond that which evoked a maximal response; 50- or 200-μs pulse width) of the tibial, common peroneal, and femoral nerves, respectively, as an indirect measure of muscle strength.62 Values were normalized to uninjured data to show the amount of muscle atrophy.61 Three physiological assessments of spasticity were made: 1) the SL maximal H-reflex to maximal M-wave ratio (H/M ratio), a measure of reflex excitability of the motoneurons; 2) SL H-reflex depression in response to 1 Hz compared to 0.125-Hz stimulation of the tibial nerve, an indication of post-activation depression of the 1a-motoneuron synapse and one measure of spinal inhibition63; and 3) SL and TA F-wave to maximal M-wave area ratios (F/M area), a measure of motoneuron excitability.64 Each participant was also asked to contract the SL, TA, RF, and BF muscles voluntarily for 3–5 sec, in response to an auditory cue from a computer. Three attempts were made, 1 min apart. No voluntary EMG was generated in any of the muscles, confirmation that all of these leg muscles were paralyzed completely by the SCI.
Day-to-day variability was 8%, so differences that exceeded a +10% or −10% change from the baseline (M-waves) or a given scale (H/M ratio, H-reflex depression) were designated an increase or decrease in response to exercise, respectively. For example, a baseline H/M ratio of 0.5 had to reach ≥0.6 or ≤0.4 to indicate an increase or decrease attributed to exercise, respectively. Given that F/M area ratios rarely exceeded 0.10 but can reach 1.0, a 1% increase or decrease was considered a change attributed to exercise. Spearman rho correlations were analyzed for baseline physiological parameters and the differences in physiological parameters, clinical measures of spasticity (Pendulum, SCATS scores, and MAS measures for the side we used), and the global impression of change for spasticity at E3.
Pain and pain-related sensory function
Participants underwent a structured pain history interview based on the International SCI Pain Basic Dataset,65 which contains core questions for up to three separate pain problems (worst, second worst, and third worst) specifically regarding the time frame “past week.” Additionally, the severity of neuropathic pain symptoms were also assessed in face-to-face interviews using the Neuropathic Pain Symptom Inventory.66 Participants were also asked: “Overall, how hard is it for you to deal with your pain?” on a scale from 0 to 10 (0 = not hard at all; 10 = extremely hard). This question was intended to give the participant the opportunity to rate his or her pain in a “global” sense, that is, to incorporate practical and emotional aspects of dealing with pain.41
The quantitative sensory testing assessment was designed to assess the integrity of dorsal column-medial lemniscus (mechanical stimuli) and anterolateral spinothalamic (noxious and thermal stimuli)-mediated function/dysfunction. Consistent with our previous protocols,67 sensory thresholds were measured in painful and nonpainful areas in dermatomes both at- and below-level of injury. Dynamic mechanical allodynia, thermal allodynia, directional cutaneous kinesthesia, graphesthesia, and mechanical wind-up pain were measured, if present.
Self-reported function
The Spinal Cord Independence Measure (SCIM) Version III was used to measure functional mobility and independence.68,69 Total scores at B1 and B2 were averaged and reported as single baseline scores, which were compared to scores obtained at E3. An improvement of 4 points in overall score represented a “small significant improvement,” whereas an improvement of 10 points was required to obtain a “substantial improvement.”70 The International Spinal Cord Injury (ISCI) Lower Urinary Tract Function Basic Data Set was used to monitor bladder function,71 and the ISCI Bowel Function Basic Data Set was used to monitor bowel function and daily bowel habits.72 A 7-point Patient Global Impression of Change (PGIC) scale was utilized at the completion of the study to assess participants' subjective perceptions of overall change regarding strength, energy and endurance, spasticity, pain, sensation, wheelchair mobility, mood, bowel function, bladder function, and sleep.73
Statistical analyses
All data were analyzed initially. Interim data at E1 and E2 were either unchanged and remained unchanged at E3 or were changing at E1, E2, and E3. As a result, a decision was made to present the data as the average of the two baselines (B1 and B2) compared to E3. Because of a small number of participants and a great variety of assessments, much of the data were only analyzed descriptively as described in the individual sections above.
The combined baselines versus E3 for VO2peak and the blood biomarkers were analyzed using a paired t-test. For the 1-RM strength measures, we converted the resistance to a percent score (relative to the combined baseline values), which was aggregated across all exercises before running the t-test. In this way, we negated the issue of overweighting exercises for larger muscle groups. For the blood pressure values during the HUT procedure, we ran a 2 (combined baselines vs. Evaluation 3) by 6 (tilt segments) analysis of variance with repeated measures. The post-hoc individual contrasts are without adjustment. The Mann-Whitney U test was used to compare differences between participants with chronic pain and those with no chronic pain because the values were not normally distributed. Associations between baseline physiological measures of spasticity and the differences in these physiological parameters, clinical measures of spasticity (pendulum, SCATS scores, and MAS measures for the side we used), and the global impression of change for spasticity at E3 were analyzed using Spearman rho correlations.
Results
Participants were 6 males and 2 females 19–52 years of age with motor complete SCIs (AIS A, B) between the levels of T2 and T10 for a minimum of 1 year post-injury. Detailed participant characteristics and significant changes in outcomes are presented in Table 1.
Neurological motor and sensory impairment
All participants exhibited full motor strength in the upper extremities and no voluntary motor control over the muscles of the lower extremities. Four were classified as AIS A and 4 as AIS B. No changes in motor scores were observed in any participant throughout the duration of the study. Some minor variations in sensory scores were observed in 4 participants around the zone of injury. Overall, there were no clinically significant neurological changes in any participants.
Upper extremity muscle strength and peak oxygen consumption
Muscular strength increased for all resistance maneuvers. Comparison of averaged B1–B2 to E3 was statistically significant for aggregated muscular strength scores expressed as a percentage of baseline values (Table 2A). Peak fitness at averaged baseline was within the “average” range for all participants when referenced to sex and functional classification.74 Comparison of averaged baseline to E3 was not statistically significant for VO2peak (Table 2B).
Table 2.
| Baseline | E3 | p value | |
|---|---|---|---|
| A % Average 1-RM (relative to baseline) |
100.0 ± 27.6 | 119.2 ± 30.0 | 0.001 |
| B VO2peak (mL/kg/min) |
15.60 ± 4.11 | 15.80 ± 4.01 | 0.795 |
Quantified by average improvement in one repetition maximum (1-RM) for all weightlifting maneuvers at evaluation 3 (E3) relative to baseline (average of baseline 1 and baseline 2).
VO2peak in response to graded exercise testing (GXT).
Wheelchair skills
At averaged baseline, 3 participants had WST scores <75%, the threshold set as indicative of low wheelchair skill capacity.35 Of the remaining 5 participants, 2 had baseline WST scores ≥82%, and 3 had baseline scores ≥90%, an indication of average and high wheelchair skill capacity, respectively. At E3, after 12 weeks of spotter-assisted, self-driven practice, only 1 participant (BC01) of the 3 low wheelchair skill capacity participants had increased their capacity above 75% (BC01).
Blood pressure
All participants were normotensive according to 2014 Evidence-Based Guideline For The Management Of High Blood Pressure In Adults.75 No significant changes in systolic blood pressure were observed between assessment visits (omnibus analysis, p = 0.195).
Cholesterol, lipids, and biomarkers of glycemic control and inflammation
Baseline lipids and biomarkers of glycemic control were within normal range at averaged baseline and were unchanged following training. CRP was elevated at averaged baseline (4.93 ± 7.07 mg/L) and lowered after training (2.34 ± 2.24 mg/L), although this effect was not statistically significant (p = 0.194).
Clinical and electrophysiological spasticity measures
The composite MAS increased by a mean of 17% from averaged baseline to E3 for the group, whereas SCATS composite scores increased by a mean of 37%. FSE of pendulum testing for the most spastic leg increased by 8% (Table 3).
Table 3.
Clinical Measures of Spasticitya
| MAS | SCATS | Pendulum FSE | ||||
|---|---|---|---|---|---|---|
| Participant | Baseline | E3 | Baseline | E3 | Baseline (degrees) | E3 (degrees) |
| BC01 | 6.5 | 7 | 3 | 7 | 54 | 61 |
| BC02 | 5.5 | 8 | 7.5 | 6 | 19 | 21 |
| BC03 | 4 | 4 | 2 | 2 | 81 | 73 |
| BC04 | 4 | 4 | 4 | 9 | 68 | 64 |
| BC05 | 4.5 | 7 | 0 | 8 | 61 | 94 |
| BC06 | 6 | 7 | 4.5 | 6 | 42 | 62 |
| BC07 | 8 | 9 | 10 | 9 | 50 | 42 |
| BC08 | 5 | 5 | 5.5 | 3 | 84 | 79 |
Including modified Ashworth scale (MAS), spinal cord assessment tool for spastic reflexes (SCATS), and pendulum first swing excursion (FSE) for all participants at baseline (average of baseline 1 and baseline 2) and evaluation 3 (E3).
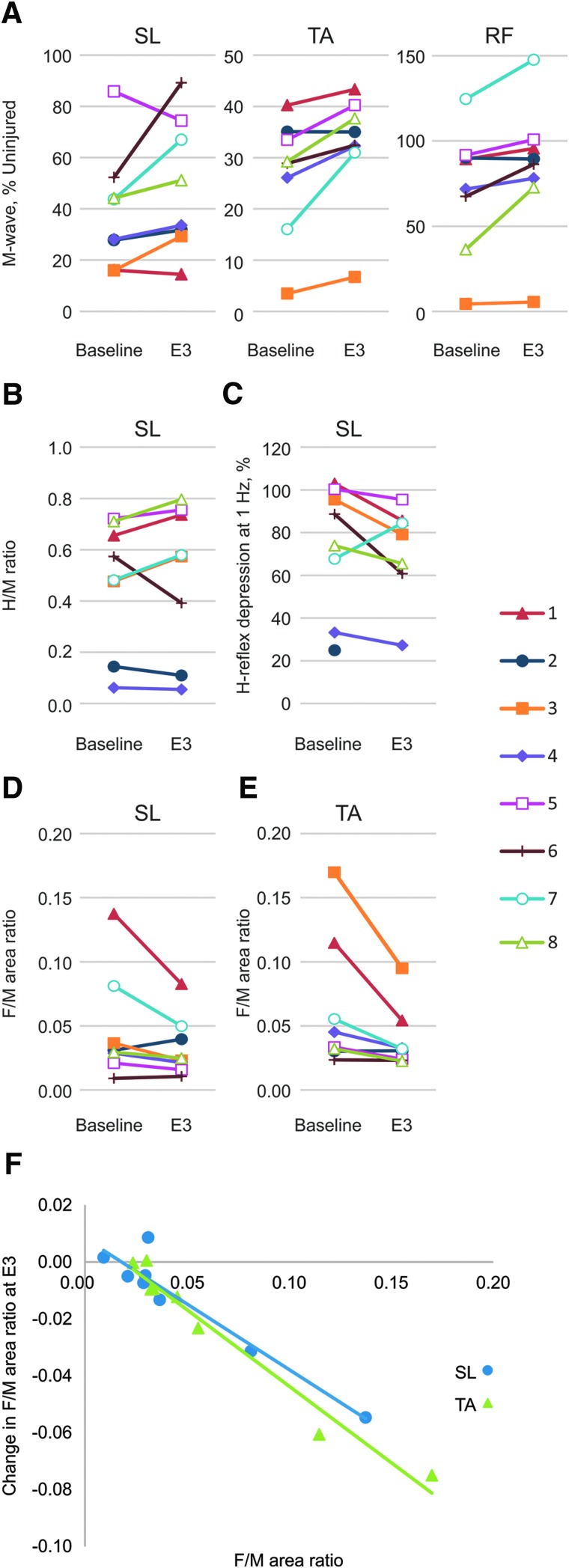
The maximal M-waves increased more than 10% beyond the baseline average for 6 SL, 7 TA, and 4 RF muscles at E3, although all but one muscle still remained atrophied relative to uninjured data (Fig. 1A). Reflex excitability, low-frequency depression of the SL reflex, and motoneuron excitability all varied widely at baseline (Fig. 1B–E, respectively). At E3, spasticity was reduced in 1 participant as measured by the SL H/M ratio (Fig. 1B), 4 participants in terms of low-frequency SL H-reflex depression (Fig. 1C), and in 3 and 5 participants for SL and TA F/M area ratios, respectively (Fig. 1D,E). Other participants either showed no change or an increase in spasticity at E3 (Table 1).
FIG. 1.
Physiological measures of spasticity. (A) Maximal M-waves from soleus (SL), tibialis anterior (TA), and rectus femoris (RF), (B) SL H-reflex/M-wave (H/M) ratios, (C) SL H-reflex depression at 1 Hz, (D) SL, and (E) TA F-wave/M-wave (F/M) area ratios by subject at baseline and evaluation 3 (E3). (F) Associations between baseline SL and TA F/M area ratios and changes at E3. Color image is available online at www.liebertpub.com/neu
Participants with high SL and TA F/M area ratios at baseline showed the greatest declines in these ratios at E3 (rho = −0.69; p = 0.047; rho = 0.952; p < 0.001, respectively; Fig. 1F). Participants with high SL F/M ratios at baseline had a decline in the SCATS extensor score at E3 (rho = −0.70; p = 0.047). Other associations between baseline physiological measures of spasticity, and differences in physiological or clinical measures of spasticity at E3, or global impression of change for spasticity, were not significant.
Pain history and pain-related sensory function
Three of the 8 participants experienced pain during the course of the study. Thus, pain related data are only reported for those participants.
Participant BC02
At the two baseline visits, the participant reported three separate and intermittent pain problems, 1 neuropathic below-level pain, and two musculoskeletal pains (Supplementary Fig. 1A) (see online supplementary material at http://www.liebertpub.com). The area of the below-level neuropathic pain and the moderate musculoskeletal shoulder pain dramatically decreased during the course of the study (Supplementary Fig. 1B) (see online supplementary material at http://www.liebertpub.com). In contrast, the intensity of the neuropathic pain, which was mild to low moderate, remained relatively stable over the testing period. Pain interference with sleep was mostly minimal, but increased toward the end of the study. With respect to sensory function, thermal allodynia, in particular, was a common sign both at- and below-level of injury. Cold allodynia was usually more severe than warm allodynia, and these sensory abnormalities lasted throughout the trial.
Participant BC03
At the two baseline visits, the participant reported two separate pain problems (Supplementary Fig. 1C) (see online supplementary material at http://www.liebertpub.com); one constant at-level neuropathic pain varying between high-moderate and severe intensity and one intermittent musculoskeletal pain with low to moderate intensity. These pains remained constant with respect to both location and intensity over the course of the study (Supplementary Fig. 1D) (see online supplementary material at http://www.liebertpub.com). Pain interference with sleep remained relatively high throughout the study. These findings covary with both spontaneous pain and mechanical allodynia. Thermal allodynia in response to a warm stimulus was consistently observed at the level of injury throughout the trial.
Participant BC07
At the two baseline visits, the participant reported two separate musculoskeletal pain problems (Supplementary Fig. 1E) (see online supplementary material at http://www.liebertpub.com). The pains varied in intensity over the course of the study (Supplementary Fig. 1F) (see online supplementary material at http://www.liebertpub.com). Although the pain frequency progressively became more constant, rather than intermittent, during the study, pain interference with sleep remained low. The sensory testing showed an absence of warm sensation but the sensation of hot pain at the level of injury at baseline. Later in the study the participant perceived both warm and cool stimuli, whereas cool sensation again disappeared at study completion.
An exploratory analysis was performed (Mann-Whitney U test) to evaluate the extent to which outcomes related to exercise performance (VO2), spasticity (clinical and electrophysiological measures), or SCIM scores differed between individuals who experienced chronic pain versus those with no pain. In this small sample size, we found no statistically significant differences.
Self-reported function
Spinal cord independence measure
Five participants had improvements, ranging from 1 to 3 points, in the self-care portion of the SCIM (Supplementary Fig. 2A) (see online supplementary material at http://www.liebertpub.com). Mobility scores improved in 3 participants, with 1 who experienced a clinically significant improvement (participant BC01, 5 points; Supplementary Fig. 2B) (see online supplementary material at http://www.liebertpub.com). Total overall SCIM scores improved in 5 participants and declined in 1 (Supplementary Fig. 2C) (see online supplementary material at http://www.liebertpub.com). A substantial improvement was achieved in 1 participant, who experienced an 11-point increase in the overall SCIM score, whereas a small clinically significant improvement was found for another participant who experienced a 5-point increase.
Lower urinary tract
Three participants experienced improvements in bladder function. Two participants who used bladder relaxant drugs at baseline no longer needed them by E3. One of these participants also reported experiencing less-frequent involuntary urine leakages.
Bowel
Two participants experienced changes in bowel function throughout the study. Both participants used enemas to perform their bowel routine at baseline. One of the participants reported having bowel accidents more than once/month and required daily use of pads/plugs. By E3, this participant was experiencing less-frequent accidents (less than once/month) and no longer required daily use of pads/plugs. The other participant reported gaining the sensation of the need to have a bowel movement, as well as normal defecation by E3; however, this participant did not gain voluntary anal contraction as measured by the ISNCSCI.
Patient Global Impression of Change
Overall, all participants reported a perceived improvement by E3, ranging from minimally (n = 2) to very much improved (n = 3). All participants reported either much improved (n = 3) or very much improved (n = 5) strength, and 7 participants reported improved energy and endurance. Impressions of change for all participants in all categories are shown in Table 4.
Table 4.
Patient Global Impression of Change at Evaluation 3 (E3)a
| Participant | Overall | Strength | Energy/ eendurance | Spasticity | Pain | Sensation | Wheelchair Mobility | Mood | Bowel | Bladder | Sleep |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC01 | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | – | ↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | – | ↑↑↑ |
| BC02 | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | ↓↓↓ | ↑↑↑ | – | ↑↑↑ | ↑↑↑ | ↑↑↑ |
| BC03 | ↑↑ | ↑↑ | ↑ | – | ↓ | – | ↑↑ | ↑ | – | – | ↓ |
| BC04 | ↑ | ↑↑ | – | – | – | – | ↑ | – | – | – | – |
| BC05 | ↑↑ | ↑↑ | ↑↑ | ↑ | – | – | – | ↑ | – | – | – |
| BC06 | ↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | – | – | ↑↑ | – | – | – | ↑↑ |
| BC07 | ↑ | ↑↑↑ | ↑↑ | ↑↑ | – | – | – | ↑↑ | – | – | – |
| BC08 | ↑↑↑ | ↑↑↑ | ↑↑↑ | ↑↑ | ↑↑↑ | U | – | ↑↑ | – | – | – |
↑ = minimally improved, ↑↑ = much improved, ↑↑↑ = very much improved, ↓ = minimally worse, ↓↓ = much worse, ↓↓↓ = very much worse; – = no change, U = undetermined by participant.
Discussion
This study provides insight into measures of multiple domains of health and neurological function after 12 weeks of a multi-modal training program. Eight participants with thoracic-level AIS A or B chronic SCI followed a training program that included BWSTT, CRT, FES, and WST that was designed to impact the upper and lower extremities, activate spinal cord circuitry, and improve general mobility. This is the first demonstration of the effect of this combination of four training modalities. The most pronounced outcome was that upper extremity muscular strength improved significantly for all participants, and this corresponded with all participants' reporting improvements in global impression of change in strength.
This change in upper extremity strength, however, did not correspond to a significant change in cardiorespiratory fitness based on group data, though individual participants did improve (Table 1). Noteworthy was the “average” baseline fitness level when referenced by cohort ages, sex, and level of injury; baseline relative VO2peak was in the “average” category for the group and individuals distributed across the five fitness categories (2 in poor, 2 in fair, 2 in average, 1 in good, and 1 in excellent).74 These individuals appear to be a representative sample of the chronic SCI community with respect to fitness. Previous work by the investigators has reported that use of uninterrupted aerobic and resistance maneuvers performed three times weekly improves fitness by nearly 30% in young24 and middle-aged individuals26 with paraplegia. However, when used twice-weekly, a similar circuit resistance program maintained, but did not further improve, exercise benefits.77 Improvements in muscle strength subsequent twice-weekly training were both statistically and clinically significant, but much less than the 14–41% gains in strength for young and 39–60% gains reported for middle-aged persons with paraplegia undergoing three-times-weekly conditioning for 3–4 months.24,26 Activity-based therapy interventions focused on the upper extremities have also been shown to improve quality of life through increased independence and function.78 Improvements in SCIM and WST scores for some participants may be explained by improvements in upper extremity strength, given that some participants were not able to perform some transfers at baseline that could be performed at E3. Therefore, it is reasonable to conclude that upper extremity training by CRT 2–3 times per week can be expected to have a detectable impact on strength and corresponding functions that depend on upper extremity strength.
Little impact on autonomic and metabolic activity
Blood pressure responses to experimental HUT were predictably minimal and not altered by conditioning. Orthostatic intolerance is typically found at the extremes of the fitness continuum.79 In the current case series, all participants had a chronic SCI below the level of sympathetic outflow at T1 and would thus be expected to have typical pressor responses accompanying postural adjustment. At baseline, all participants were normotensive according to authoritative guidelines,75 recreationally active, and absent any complaints of lightheadedness during daily activities.
Before conditioning, all of the lipid and lipoprotein cholesterol levels were in the low-risk range category and thus unlikely to be improved by conditioning exercise. Further, exercise training volumes were below guidelines recommended to reduce cardiovascular and cardioendocrine disease risk (reviewed in previous works80,81). Fasting glucose levels were well below the cut-scores for impaired fasting glucose, although insulin levels were slightly elevated. More complex testing would be needed to identify a state of impaired fasting glucose or a benefit of exercise conditioning on this putative dysglycemia. CRP levels at baseline were elevated above the low-risk criterion of the American Heart Association, as has been reported in persons with SCI.82,83 These levels were nonsignificantly lowered after conditioning, although responses of CRP to the myriad of proinflammatory stimuli post-SCI83,84 would require more systematic testing before an effect of exercise on proatherogenic inflammatory stress could be established.
Lower extremity neurological activity does not change
Neurological function, as assessed by the ISNCSCI exam, did not change. Although some variability in sensory level within the thoracic region was measured in a few participants, this variability was not significant and can most likely be explained by normal day-to-day variability and inter-rater reliability.85 In the thoracic spinal levels, each segment has been estimated to be analogous to approximately 0.4 ISNCSCI motor points,86 and functional outcome does not differ per level in the mid-thoracic region.
The lack of change in lower extremity function is consistent with past literature indicating that, despite spinal contributions to walking function,87 lower extremity function is highly dependent on the integrity of descending pathways from supraspinal centers.88 There is a rich early history of locomotor training studies that have included individuals with chronic motor complete SCI, some of which have indicated that training improves the ability to walk in the treadmill.4,89,90 However, with the exception of three case studies, there is no evidence of restoration of lower extremity function or overground walking ability after locomotor training in individuals with chronic motor complete SCI. The case studies that make up the exceptions all relate to individuals with low paraplegia who likely retained some volitional control of proximal lower extremity muscles and who had involuntary muscle activity (spasticity) that contributed to weight bearing.9,91,92
There were increases in the strength of the paralyzed quadriceps, tibialis anterior, and soleus muscles at E3 in most participants (50%, 88%, and 75%, respectively), assessed from >10% increases in the amplitude of the respective maximal muscle compound action potentials over the average baseline (Fig. 1A). Higher EMG amplitudes would be expected from the increases in muscle size that typically occur in response to repeated functional electrical stimulation.33 Nevertheless, all but one of the 24 paralyzed leg muscles evaluated (96%) remained atrophied relative to uninjured data (Fig. 1A),61 consistent with reductions in muscle use subsequent to paralysis attributed to SCI, followed by slow recovery with FES.93
Wheelchair skills testing as a measure of whole body integration of neurological and physiological changes
Mobility skills, as assessed by the WST, were generally unchanged from baseline after 12 weeks of a multi-modal activity-based intervention that included twice-weekly, self-directed mobility skills training with spotter assistance. The lack of change is attributed to the lack of formalized training directed by a skilled trainer (i.e., clinician or other with previous experience training mobility skills in persons with SCI). A recent randomized, controlled trial (RCT) comparing five 30- to 45-min sessions of either education control or individualized mobility skills training demonstrated significant immediate and long-term (1 year post-intervention) gains in both total WST (+7%) and the advanced WST domain (+30%) in a group of highly skilled (average WST = 83%) manual wheelchair users with chronic SCI (16 years average post-injury).37 This RCT clearly demonstrates that mobility skills can be improved if a skilled trainer applies a focused training program, even among high-skilled persons.
Mobility is a measure of whole person function, representing the net integration of all positive and negative changes to body structures (spinal cord) and functions (motor control and sensory feedback) targeted by therapeutic modalities and the concomitant post-treatment multi-modal rehabilitation programs. Mobility skill measurement should include both general assessment of functional independence (e.g., SCIMmot, the sum of the SCIM self-care and mobility subscales)69 and an assessment that captures the individual's perception of mobility skill difficulty (e.g., a patient reported outcome, or PRO), such as the Spinal Cord Injury Functional Index (basic mobility, self-care, and wheelchair mobility domains).94 General assessments typically require large changes in function to meaningfully change scores, but are typically the accepted standard for judging change. PROs can provide indication of more subtle changes in function the participant perceives that may not result in a large change in functional independence. As such, it would be important for clinical trials involving rehabilitation to consider including targeted mobility skills training and measurement as a part of any comprehensive approach to maximizing and assessing the efficacy of pharmacological or biological therapeutics.
Variable impact on lower extremity spasticity
Clinical measures of spasticity were variable, both within and between participants and across time. For example, in participant BC01, MAS and pendulum test values were relatively stable, but the SCATS assessment indicated increased spasticity. In participant BC06, the MAS and SCATS scores were relatively stable, but the pendulum test indicated a decrease in quadriceps spasticity. These three clinical measures of spasticity assess somewhat different components of spasticity, and this may account for the lack of agreement among tests. Nevertheless, no clear trends in clinical measures of spasticity were identified in any of the measures.
This is at odds with past studies of locomotor training in persons with incomplete SCI, which have indicated that locomotor training is associated with reduction in spinal reflex excitability.95–97 A recent study of persons with motor complete SCI assessed common physical therapeutic approaches for reducing spasticity. Exposure to a single 30-min session of cyclic movement associated with passive walking in the Lokomat resulted in a significant reduction in spasticity (as measured by the pendulum test), which persisted for at least 45 min after the intervention.98
Functional electrical stimulation can also reduce spasticity, assessed by Ashworth scores.34 Here, SL reflex excitability decreased in only 1 participant at E3, low-frequency depression of the SL H-reflex decreased in 4 participants, and SL and TA F/M area ratios decreased in 3 and 5 participants, respectively, all indicative of reductions in spasticity (Fig. 1). Notably, individuals with high SL and TA F/M area ratios at baseline had the greatest declines in these ratios in response to 12 weeks of exercise (Fig. 1E), suggestive of reductions in motoneuron excitability,64 which would make it more difficult to excite paralyzed leg muscles by reflex inputs.
Descending supraspinal pathways also influence spinal reflex excitability, however, and there is known to be subclinical sparing of these pathways even in persons with SCI classified as AIS A and B.99 It is possible that the activity associated with BWSTT has differential influences on below-lesion spinal circuitry that are attributable to differences in subclinical levels of signal transmission in descending pathways.
Effects on pain
Severe and persistent pain of various origins are common post-SCI, with around 70% prevalence.100,101 Persistent pain negatively affects independent living post-SCI by interfering with sleep, mood, and daily function and activities, including social activities and work.102,103 Pain relief is rated by individuals with SCI as one of the top priorities, which becomes even more important with increasing time post-injury.40 Because of the high prevalence of persistent pain post-SCI, most participants in regenerative clinical trials including chronic injuries can be expected to experience chronic pain. It is not known how interventions aimed to restore or optimize lost neuronal connections will influence, in particular, chronic neuropathic pain. Although the present study showed some minor variations in pain, the symptoms and sensory signs associated with neuropathic pain remained relatively unchanged. Musculoskeletal pain types, however, may be more variable, likely dependent on both exercise regimen and pain location. Thus, this preliminary study does not support either beneficial or adverse effects of this exercise program, but it is likely that effects may vary in a larger sample of individuals with SCI. Therefore, a careful multi-modal evaluation of pain, impact of pain, and associated sensory signs is important for determining both beneficial and adverse outcomes in SCI interventional clinical trials.
Conclusion
There is growing evidence that exercise and rehabilitation in animal models of SCI influence neuroprotection, regeneration, plasticity, spinal and cortical organization, and neuronal properties.104,105 The literature on SCI clinical trials indicates that interventions that incorporate rehabilitation have the strongest evidence for efficacy.2 As such, it is important to capture exercise and rehabilitation activities, given that they may modify or confound outcomes of pharmacological or cellular interventions being tested in clinical trials. Accordingly, there is a need to accurately document the content and dosing of these exercise and rehabilitation activities, in addition to measuring relevant outcome measures.
Various other considerations must also be addressed when designing clinical trials that incorporate exercise or rehabilitation. In particular, it is important to balance participant as well as study-site burden with capturing meaningful changes. In this study, each individual participated for 19 continuous weeks and came to the research center 5 days/week every week. Overburdening of participants creates a high risk of noncompliance and dropouts, which could lead to missing data. Further, only highly motivated individuals may be study participants, and thus the study sample may not be representative of the larger group of people with SCI. Fewer assessments and evaluation time points make compliance easier for research participants. The field should also consider adapting some exercise training activities so they can be performed at home to increase compliance. In addition, multi-center, large-scale clinical trials may not be able to produce consistent high-quality data if protocols are complicated and lengthly. As a single-center study, we were able to perform a wide array of specialized testing in a consistent manner; however, we were at maximum capacity and could not manage more than 5 participants simutaneously. For trials larger than phase I, selecting simple, yet meaningful, clinical or self-report tests that are relevant to the intervention(s) being tested and can reliable be administered are preferred over more complex, invasive, and time-consuming measures. Comprehensive testing at baseline and end of study can be valuable, but extensive testing between those time points may not be necessary. The selection of outcome measures should take into account the intervention and subpopulation being tested and the reliability with which the data can be collected.
Supplementary Material
Acknowledgments
The authors acknowledge Luisa Betancourt, Patricia Burns-Drecq, Deena Cilien, Kelly Ginnity Hearne, Meagan Mayo, and Jacqueline Tibbet for assistance with data collection. Importantly, the authors thank all of their participants for deciding to participate in this study and contribute to the advancement of our field. Funded by the Robert J. Kleberg, Jr., and Helen C. Kleberg Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Thuret S., Moon L.D., and Gage F.H. (2006). Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 7, 628–643 [DOI] [PubMed] [Google Scholar]
- 2.Gomes-Osman J., Cortes M., Guest J., and Pascual-Leone A. (2016). A systematic review of experimental strategies aimed at improving motor function after acute and chronic spinal cord injury. J. Neurotrauma 33, 425–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steeves J.D., Kramer J.L., and Zariffa J. (2012). Traversing the translational trail for trials. Top. Spinal Cord Inj. Rehabil. 18, 79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dobkin B.H., Harkema S., Requejo P., and Edgerton V.R. (1995). Modulation of locomotor-like EMG activity in subjects with complete and incomplete spinal cord injury. J. Neurol. Rehabil. 9, 183–190 [PubMed] [Google Scholar]
- 5.Wirz M., Colombo G., and Dietz V. (2001). Long term effects of locomotor training in spinal humans. J. Neurol. Neurosurg. Psychiatry 71, 93–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field-Fote E.C., and Roach K.E. (2011). Influence of a locomotor training approach on walking speed and distance in people with chronic spinal cord injury: a randomized clinical trial. Phys. Ther. 91, 48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harkema S.J., Hillyer J., Schmidt-Read M., Ardolino E., Sisto S.A., and Behrman A.L. (2012). Locomotor training: as a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch. Phys. Med. Rehabil. 93, 1588–1597 [DOI] [PubMed] [Google Scholar]
- 8.Lunenburger L., Bolliger M., Czell D., Muller R., and Dietz V. (2006). Modulation of locomotor activity in complete spinal cord injury. Exp. Brain Res. 174, 638–646 [DOI] [PubMed] [Google Scholar]
- 9.Manella K.J., Torres J., and Field-Fote E.C. (2010). Restoration of walking function in an individual with chronic complete (AIS A) spinal cord injury. J. Rehabil. Med. 42, 795–798 [DOI] [PubMed] [Google Scholar]
- 10.Forrest G.F., Sisto S.A., Barbeau H., Kirshblum S.C., Wilen J., Bond Q., Bentson S., Asselin P., Cirnigliaro C.M., and Harkema S. (2008). Neuromotor and musculoskeletal responses to locomotor training for an individual with chronic motor complete AIS-B spinal cord injury. J. Spinal Cord Med. 31, 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hicks A.L., and Ginis K.A. (2008). Treadmill training after spinal cord injury: it's not just about the walking. J. Rehabil. Res. Dev. 45, 241–248 [DOI] [PubMed] [Google Scholar]
- 12.Cowan R.E., and Nash M.S. (2010). Cardiovascular disease, SCI and exercise: unique risks and focused countermeasures. Disabil. Rehabil. 32, 2228–2236 [DOI] [PubMed] [Google Scholar]
- 13.Davis G.M. (1993). Exercise capacity of individuals with paraplegia. Med. Sci. Sports Exerc. 25, 423–432 [PubMed] [Google Scholar]
- 14.Hoffman M.D. (1986). Cardiorespiratory fitness and training in quadriplegics and paraplegics. Sports Med. (Auckland, N.Z.) 3, 312–330 [DOI] [PubMed] [Google Scholar]
- 15.Figoni S.F. (1990). Perspectives on cardiovascular fitness and SCI. J. Am. Paraplegia Soc. 13, 63–71 [DOI] [PubMed] [Google Scholar]
- 16.Lavis T.D., Scelza W.M., and Bockenek W.L. (2007). Cardiovascular health and fitness in persons with spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 18, 317–331, vii. [DOI] [PubMed] [Google Scholar]
- 17.Nash M.S., Jacobs P.L., Woods J.M., Clark J.E., Pray T.A., and Pumarejo A.E. (2002). A comparison of 2 circuit exercise training techniques for eliciting matched metabolic responses in persons with paraplegia. Arch. Phys. Med. Rehabil. 83, 201–209 [DOI] [PubMed] [Google Scholar]
- 18.Dallmeijer A.J., van der Woude L.H., Hollander A.P., and van As H.H. (1999). Physical performance during rehabilitation in persons with spinal cord injuries. Med. Sci. Sports Exerc. 31, 1330–1335 [DOI] [PubMed] [Google Scholar]
- 19.Hjeltnes N., and Vokac Z. (1979). Circulatory strain in everyday life of paraplegics. Scand. J. Rehabil. Med. 11, 67–73 [PubMed] [Google Scholar]
- 20.Bauman W.A., and Spungen A.M. (2008). Coronary heart disease in individuals with spinal cord injury: assessment of risk factors. Spinal Cord 46, 466–476 [DOI] [PubMed] [Google Scholar]
- 21.Bauman W.A., and Spungen A.M. (2001). Carbohydrate and lipid metabolism in chronic spinal cord injury. J. Spinal Cord Med. 24, 266–277 [DOI] [PubMed] [Google Scholar]
- 22.Valent L., Dallmeijer A., Houdijk H., Talsma E., and van der Woude L. (2007). The effects of upper body exercise on the physical capacity of people with a spinal cord injury: a systematic review. Clin. Rehabil. 21, 315–330 [DOI] [PubMed] [Google Scholar]
- 23.Jacobs P.L., Mahoney E.T., Nash M.S., and Green B.A. (2002). Circuit resistance training in persons with complete paraplegia. J. Rehabil. Res. Dev. 39, 21–28 [PubMed] [Google Scholar]
- 24.Jacobs P.L., Nash M.S., and Rusinowski J.W. (2001). Circuit training provides cardiorespiratory and strength benefits in persons with paraplegia. Med. Sci. Sports Exerc. 33, 711–717 [DOI] [PubMed] [Google Scholar]
- 25.Nash M.S., Jacobs P.L., Mendez A.J., and Goldberg R.B. (2001). Circuit resistance training improves the atherogenic lipid profiles of persons with chronic paraplegia. J. Spinal Cord Med. 24, 2–9 [DOI] [PubMed] [Google Scholar]
- 26.Nash M.S., van de Ven I., van Elk N., and Johnson B.M. (2007). Effects of circuit resistance training on fitness attributes and upper-extremity pain in middle-aged men with paraplegia. Arch. Phys. Med. Rehabil. 88, 70–75 [DOI] [PubMed] [Google Scholar]
- 27.Jacobs P.L., and Nash M.S. (2001). Modes, benefits, and risks of voluntary an delectrically induced exercise in persons with spinal cord injury. J. Spinal Cord Med. 24, 10–18 [DOI] [PubMed] [Google Scholar]
- 28.Ragnarsson K.T. (1988). Physiologic effects of functional electrical stimulation-induced exercises in spinal cord-injured individuals. Clin. Orthop. Relat. Res. (2003), 53–63 [PubMed] [Google Scholar]
- 29.Griffin L., Decker M.J., Hwang J.Y., Wang B., Kitchen K., Ding Z., and Ivy J.L. (2009). Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J. Electromyogr. Kinesiol. 19, 614–622 [DOI] [PubMed] [Google Scholar]
- 30.Thijssen D.H., Heesterbeek P., van Kuppevelt D.J., Duysens J., and Hopman M.T. (2005). Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med. Sci. Sports Exerc. 37, 1112–1118 [DOI] [PubMed] [Google Scholar]
- 31.Nash M.S., Bilsker M.S., Kearney H.M., Ramirez J.N., Applegate B., and Green B.A. (1995). Effects of electrically-stimulated exercise and passive motion on echocardiographically-derived wall motion and cardiodynamic function in tetraplegic persons. Paraplegia 33, 80–89 [DOI] [PubMed] [Google Scholar]
- 32.Nash M.S., Bilsker S., Marcillo A.E., Isaac S.M., Botelho L.A., Klose K.J., Green B.A., Rountree M.T., and Shea J.D. (1991). Reversal of adaptive left ventricular atrophy following electrically-stimulated exercise training in human tetraplegics. Paraplegia 29, 590–599 [DOI] [PubMed] [Google Scholar]
- 33.Bickel C.S., Yarar-Fisher C., Mahoney E.T., and McCully K.K. (2015). Neuromuscular Electrical Stimulation-Induced Resistance Training After SCI: A Review of the Dudley Protocol. Top. Spinal Cord Inj. Rehabil. 21, 294–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuhn D., Leichtfried V., and Schobersberger W. (2014). Four weeks of functional electrical stimulated cycling after spinal cord injury: a clinical cohort study. Int. J. Rehabil. Res. 37, 243–250 [DOI] [PubMed] [Google Scholar]
- 35.Hosseini S.M., Oyster M.L., Kirby R.L., Harrington A.L., and Boninger M.L. (2012). Manual wheelchair skills capacity predicts quality of life and community integration in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 93, 2237–2243 [DOI] [PubMed] [Google Scholar]
- 36.Lemay V., Routhier F., Noreau L., Phang S.H., and Ginis K.A. (2012). Relationships between wheelchair skills, wheelchair mobility and level of injury in individuals with spinal cord injury. Spinal Cord 50, 37–41 [DOI] [PubMed] [Google Scholar]
- 37.Kirby R.L., Mitchell D., Sabharwal S., McCranie M., and Nelson A.L. (2016). Manual wheelchair skills training for community-dwelling veterans with spinal cord injury: a randomized controlled trial. PLoS One 11, e0168330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization (2002). Towards a Common Language for Functioning, Disability and Health: ICF The International Classification of Functioning, Disability and Health. Available at: http://www.who.int/classifications/icf/icfbeginnersguide.pdf (last accessed March17, 2017)
- 39.Kirshblum S.C., Waring W., Biering-Sorensen F., Burns S.P., Johansen M., Schmidt-Read M., Donovan W., Graves D., Jha A., Jones L., Mulcahey M.J., and Krassioukov A. (2011). Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J. Spinal Cord Med. 34, 547–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anderson K.D. (2004). Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma 21, 1371–1383 [DOI] [PubMed] [Google Scholar]
- 41.Widerstrom-Noga E.G., Felipe-Cuervo E., Broton J.G., Duncan R.C., and Yezierski R.P. (1999). Perceived difficulty in dealing with consequences of spinal cord injury. Arch. Phys. Med. Rehabil. 80, 580–586 [DOI] [PubMed] [Google Scholar]
- 42.Walter J.S., Sacks J., Othman R., Rankin A.Z., Nemchausky B., Chintam R., and Wheeler J.S. (2002). A database of self-reported secondary medical problems among VA spinal cord injury patients: its role in clinical care and management. J. Rehabil. Res. Dev. 39, 53–61 [PubMed] [Google Scholar]
- 43.Noreau L., Proulx P., Gagnon L., Drolet M., and Laramee M.T. (2000). Secondary impairments after spinal cord injury: a population-based study. Am. J. Phys. Med. Rehabil. 79, 526–535 [DOI] [PubMed] [Google Scholar]
- 44.Kirby RL, Smith C., Parker K, McAllister M, Boyce J, Rushton PW, Routhier F, Best KL, Diane MacKenzie, Mortenson B, and Brandt A. (2017). The Wheelchair Skills Program Manual. Published electronically at Dalhousie University, Halifax, Nova Scotia, Canada: Available at: http://www.wheelchairskillsprogram.ca/eng/manual.php (last accessed March17, 2017). [Google Scholar]
- 45.Jacobs P.L., Nash M.S., and Mintz C.D. (1999). Assessment of fractional expired gases and air flow by an ambulatory metabolic analyzer. J. Exerc. Physiol. 2, 1–10 [Google Scholar]
- 46.Armstrong L. (2006). ACSM's guidelines for exercise testing and prescription/American College of Sports Medicine. Lippincott Williams & Wilkins: Philadelphia, PA [Google Scholar]
- 47.Mayhew J.L., Ball T.E., Arnold M.D., and Bowen J.C. (1992). Relative muscular endurance performance as a predictor of bench press strength in college men and women. J. Appl. Sport Sci. Res. 6, 200–206 [Google Scholar]
- 48.Dalhousie University. (2013). Wheelchair Skills Test (WST). Available at: http://www.wheelchairskillsprogram.ca/eng/testers.php (last accessed March17, 2017)
- 49.Kirby R.L., Dupuis D.J., Macphee A.H., Coolen A.L., Smith C., Best K.L., Newton A.M., Mountain A.D., Macleod D.A., and Bonaparte J.P. (2004). The wheelchair skills test (version 2.4): measurement properties. Arch. Phys. Med. Rehabil. 85, 794–804 [DOI] [PubMed] [Google Scholar]
- 50.Lindquist N.J., Loudon P.E., Magis T.F., Rispin J.E., Kirby R.L., and Manns P.J. (2010). Reliability of the performance and safety scores of the wheelchair skills test version 4.1 for manual wheelchair users. Arch. Phys. Med. Rehabil. 91, 1752–1757 [DOI] [PubMed] [Google Scholar]
- 51.Groah S.L., Nash M.S., Ward E.A., Libin A., Mendez A.J., Burns P., Elrod M., and Hamm L.F. (2011). Cardiometabolic risk in community-dwelling persons with chronic spinal cord injury. J. Cardiopulm. Rehabil. Prev. 31, 73–80 [DOI] [PubMed] [Google Scholar]
- 52.Friedewald W.T., Levy R.I., and Fredrickson D.S. (1972). Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 18, 499–502 [PubMed] [Google Scholar]
- 53.Benz E.N., Hornby T.G., Bode R.K., Scheidt R.A., and Schmit B.D. (2005). A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch. Phys. Med. Rehabil. 86, 52–59 [DOI] [PubMed] [Google Scholar]
- 54.Thompson C.K., and Hornby T.G. (2013). Divergent modulation of clinical measures of volitional and reflexive motor behaviors following serotonergic medications in human incomplete spinal cord injury. J. Neurotrauma 30, 498–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bohannon R.W., and Smith M.B. (1987). Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 67, 206–207 [DOI] [PubMed] [Google Scholar]
- 56.Ashworth B. (1964). Preliminary trial of carisoprodol in multiple sclerosis. Practitioner 192, 540–542 [PubMed] [Google Scholar]
- 57.Wartenberg R. (1951). Pendulousness of the legs as a diagnostic test. Neurology 1, 18–24 [DOI] [PubMed] [Google Scholar]
- 58.Ness L.L., and Field-Fote E.C. (2009). Effect of whole-body vibration on quadriceps spasticity in individuals with spastic hypertonia due to spinal cord injury. Restor. Neurol. Neurosci. 27, 621–631 [DOI] [PubMed] [Google Scholar]
- 59.Fowler E.G., Nwigwe A.I., and Ho T.W. (2000). Sensitivity of the pendulum test for assessing spasticity in persons with cerebral palsy. Dev. Med. Child Neurol. 42, 182–189 [DOI] [PubMed] [Google Scholar]
- 60.Stillman B., and McMeeken J. (1995). A video-based version of the pendulum test: technique and normal response. Arch. Phys. Med. Rehabil. 76, 166–176 [DOI] [PubMed] [Google Scholar]
- 61.Mayo M., DeForest B.A., Castellanos M., and Thomas C.K. (2017). Characterization of involuntary contractions after spinal cord injury reveals associations between physiological and self-reported measures of spasticity. Front. Integr. Neurosci. 11, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomas C.K. (1997). Contractile properties of human thenar muscles paralyzed by spinal cord injury. Muscle Nerve 20, 788–799 [DOI] [PubMed] [Google Scholar]
- 63.Schindler-Ivens S., and Shields R.K. (2000). Low frequency depression of H-reflexes in humans with acute and chronic spinal-cord injury. Exp. Brain Res. 133, 233–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Butler J.E., and Thomas C.K. (2003). Effects of sustained stimulation on the excitability of motoneurons innervating paralyzed and control muscles. J. Appl. Physiol. (1985) 94, 567–575 [DOI] [PubMed] [Google Scholar]
- 65.Widerstrom-Noga E., Biering-Sorensen F., Bryce T., Cardenas D.D., Finnerup N.B., Jensen M.P., Richards J.S., and Siddall P.J. (2008). The international spinal cord injury pain basic data set. Spinal Cord 46, 818–823 [DOI] [PubMed] [Google Scholar]
- 66.Bouhassira D., Attal N., Fermanian J., Alchaar H., Gautron M., Masquelier E., Rostaing S., Lanteri-Minet M., Collin E., Grisart J., and Boureau F. (2004). Development and validation of the Neuropathic Pain Symptom Inventory. Pain 108, 248–257 [DOI] [PubMed] [Google Scholar]
- 67.Widerstrom-Noga E., Felix E.R., Adcock J.P., Escalona M., and Tibbett J. (2016). Multidimensional neuropathic pain phenotypes after spinal cord injury. J. Neurotrauma 33, 482–492 [DOI] [PubMed] [Google Scholar]
- 68.Itzkovich M., Gelernter I., Biering-Sorensen F., Weeks C., Laramee M.T., Craven B.C., Tonack M., Hitzig S.L., Glaser E., Zeilig G., Aito S., Scivoletto G., Mecci M., Chadwick R.J., El Masry W.S., Osman A., Glass C.A., Silva P., Soni B.M., Gardner B.P., Savic G., Bergstrom E.M., Bluvshtein V., Ronen J., and Catz A. (2007). The Spinal Cord Independence Measure (SCIM) version III: reliability and validity in a multi-center international study. Disabil. Rehabil. 29, 1926–1933 [DOI] [PubMed] [Google Scholar]
- 69.Anderson K.D., Acuff M.E., Arp B.G., Backus D., Chun S., Fisher K., Fjerstad J.E., Graves D.E., Greenwald K., Groah S.L., Harkema S.J., Horton J.A., III, Huang M.N., Jennings M., Kelley K.S., Kessler S.M., Kirshblum S., Koltenuk S., Linke M., Ljungberg I., Nagy J., Nicolini L., Roach M.J., Salles S., Scelza W.M., Read M.S., Reeves R.K., Scott M.D., Tansey K.E., Theis J.L., Tolfo C.Z., Whitney M., Williams C.D., Winter C.M., and Zanca J.M. (2011). United States (US) multi-center study to assess the validity and reliability of the Spinal Cord Independence Measure (SCIM III). Spinal Cord 49, 880–885 [DOI] [PubMed] [Google Scholar]
- 70.Scivoletto G., Tamburella F., Laurenza L., and Molinari M. (2013). The spinal cord independence measure: how much change is clinically significant for spinal cord injury subjects. Disabil. Rehabil. 35, 1808–1813 [DOI] [PubMed] [Google Scholar]
- 71.Biering-Sorensen F., Craggs M., Kennelly M., Schick E., and Wyndaele J.J. (2008). International lower urinary tract function basic spinal cord injury data set. Spinal Cord 46, 325–330 [DOI] [PubMed] [Google Scholar]
- 72.Krogh K., Perkash I., Stiens S.A., and Biering-Sorensen F. (2009). International bowel function basic spinal cord injury data set. Spinal Cord 47, 230–234 [DOI] [PubMed] [Google Scholar]
- 73.Guy W. (1976). Clinical global impression scale, in: The ECDEU Assessment Manual for Psychopharmacology—Revised. U.S. Department of Health, Education, and Welfare Publication (ADM), Rockville, MD, pps. 218–222 [Google Scholar]
- 74.Simmons O.L., Kressler J., and Nash M.S. (2014). Reference fitness values in the untrained spinal cord injury population. Arch Phys Med Rehabil. 95, 2272–2278 [DOI] [PubMed] [Google Scholar]
- 75.James P.A., Oparil S., Carter B.L., Cushman W.C., Dennison-Himmelfarb C., Handler J., Lackland D.T., LeFevre M.L., MacKenzie T.D., and Ogedegbe O. (2014). 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 311, 507–520 [DOI] [PubMed] [Google Scholar]
- 76.Geloneze B., Vasques A.C., Stabe C.F., Pareja J.C., Rosado L.E., Queiroz E.C., and Tambascia M.A.; BRAMS Investigators. (2009). HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS). Arq. Bras. Endocrinol. Metabol. 53, 281–287 [DOI] [PubMed] [Google Scholar]
- 77.Ditor D.S., Latimer A.E., Ginis K.A., Arbour K.P., McCartney N., and Hicks A.L. (2003). Maintenance of exercise participation in individuals with spinal cord injury: effects on quality of life, stress and pain. Spinal Cord 41, 446–450 [DOI] [PubMed] [Google Scholar]
- 78.Quel de Oliveira C., Refshauge K., Middleton J., de Jong L., and Davis G.M. (2016). Effects of activity-based therapy interventions on mobility, independence, and quality of life for people with spinal cord injuries: a systematic review and meta-analysis. J. Neurotrauma 34, 1726–1743 [DOI] [PubMed] [Google Scholar]
- 79.Levine B.D. (1993). Regulation of central blood volume and cardiac filling in endurance athletes: the Frank-Starling mechanism as a determinant of orthostatic tolerance. Med. Sci. Sport Exerc. 25, 727–732 [PubMed] [Google Scholar]
- 80.Kressler J., Cowan R.E., Bigford G.E., and Nash M.S. (2014). Reducing cardiometabolic disease in spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 25, 573–604, viii. [DOI] [PubMed] [Google Scholar]
- 81.Nash M.S., Cowan R.E., and Kressler J. (2012). Evidence-based and heuristic approaches for customization of care in cardiometabolic syndrome after spinal cord injury. J. Spinal Cord Med. 35, 278–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gibson A.E., Buchholz A.C., and Martin Ginis K.A. (2008). C-Reactive protein in adults with chronic spinal cord injury: increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord 46, 616–621 [DOI] [PubMed] [Google Scholar]
- 83.Ellenbroek D., Kressler J., Cowan R.E., Burns P.A., Mendez A.J., and Nash M.S. (2014). Effects of prandial challenge on triglyceridemia, glycemia, and pro-inflammatory activity in persons with chronic paraplegia. J. Spinal Cord Med. 38, 468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nash M., Dalal K., Martinez-Barrizonte J., and Cardenas D. (2011). Suppression of proatherogenic inflammatory cytokines as a therapeutic countermeasure to CVD risks accompanying SCI. Top. Spinal Cord Inj. Rehabil. 16, 14–32 [Google Scholar]
- 85.Hales M., Biros E., and Reznik J.E. (2015). Reliability and validity of the sensory component of the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI): a systematic review. Top. Spinal Cord Inj. Rehabil. 21, 241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van Hedel H.J., and Curt A. (2006). Fighting for each segment: estimating the clinical value of cervical and thoracic segments in SCI. J. Neurotrauma 23, 1621–1631 [DOI] [PubMed] [Google Scholar]
- 87.Grillner S., and El Manira A. (2015). The intrinsic operation of the networks that make us locomote. Curr. Opin. Neurobiol. 31, 244–249 [DOI] [PubMed] [Google Scholar]
- 88.Field-Fote E.C., Yang J.F., Basso D.M., and Gorassini M.A. (2017). Supraspinal control predicts locomotor function and forecasts responsiveness to training after spinal cord injury. J. Neurotrauma 34, 1813–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dietz V., Colombo G., Jensen L., and Baumgartner L. (1995). Locomotor capacity of spinal cord in paraplegic patients. Ann. Neurol. 37, 574–582 [DOI] [PubMed] [Google Scholar]
- 90.Hicks A.L., Adams M.M., Martin Ginis K., Giangregorio L., Latimer A., Phillips S.M., and McCartney N. (2005). Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord 43, 291–298 [DOI] [PubMed] [Google Scholar]
- 91.Spiess M.R., Jaramillo J.P., Behrman A.L., Teraoka J.K., and Patten C. (2012). Unexpected recovery after robotic locomotor training at physiologic stepping speed: a single-case design. Arch. Phys. Med. Rehabil. 93, 1476–1484 [DOI] [PubMed] [Google Scholar]
- 92.Murillo N., Kumru H., Opisso E., Padulles J.M., Medina J., Vidal J., and Kofler M. (2012). Recovery of assisted overground stepping in a patient with chronic motor complete spinal cord injury: a case report. NeuroRehabilitation 31, 401–407 [DOI] [PubMed] [Google Scholar]
- 93.Peckham P.H., Mortimer J.T., and Marsolais E.B. (1976). Alteration in the force and fatigability of skeletal muscle in quadriplegic humans following exercise induced by chronic electrical stimulation. Clin. Orthop. Relat. Res. 326–333 [PubMed] [Google Scholar]
- 94.Sinha R., Slavin M.D., Kisala P.A., Ni P., Tulsky D.S., and Jette A.M. (2015). Functional ability level development and validation: providing clinical meaning for Spinal Cord Injury Functional Index scores. Arch. Phys. Med. Rehabil. 96, 1448–1457 [DOI] [PubMed] [Google Scholar]
- 95.Manella K.J., and Field-Fote E.C. (2013). Modulatory effects of locomotor training on extensor spasticity in individuals with motor-incomplete spinal cord injury. Restor. Neurol. Neurosci. 31, 633–646 [DOI] [PubMed] [Google Scholar]
- 96.Khan A.S., Patrick S.K., Roy F.D., Gorassini M.A., and Yang J.F. (2016). Training-specific neural plasticity in spinal reflexes after incomplete spinal cord injury. Neural Plast. 2016, 6718763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adams M.M., and Hicks A.L. (2011). Comparison of the effects of body-weight-supported treadmill training and tilt-table standing on spasticity in individuals with chronic spinal cord injury. J. Spinal Cord Med. 34, 488–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Estes S.P., Iddings J.A., and Field-Fote E.C. (2017). Priming neural circuits to modulate spinal reflex excitability. Front. Neurol. 8, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McKay W.B., Lim H.K., Priebe M.M., Stokic D.S., and Sherwood A.M. (2004). Clinical neurophysiological assessment of residual motor control in post-spinal cord injury paralysis. Neurorehabil. Neural Repair 18, 144–153 [DOI] [PubMed] [Google Scholar]
- 100.Siddall P.J., McClelland J.M., Rutkowski S.B., and Cousins M.J. (2003). A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257 [DOI] [PubMed] [Google Scholar]
- 101.Cruz-Almeida Y., Martinez-Arizala A., and Widerström-Noga E.G. (2005). Chronicity of pain associated with spinal cord injury: a longitudinal analysis. J. Rehabil. Res. Dev. 42, 585–594 [DOI] [PubMed] [Google Scholar]
- 102.Ravenscroft A., Ahmed Y.S., and Burnside I.G. (2000). Chronic pain after SCI. A patient survey. Spinal Cord 38, 611–614 [DOI] [PubMed] [Google Scholar]
- 103.Widerstrom-Noga E.G., Felipe-Cuervo E., and Yezierski R.P. (2001). Chronic pain after spinal injury: interference with sleep and daily activities. Arch. Phys. Med. Rehabil. 82, 1571–1577 [DOI] [PubMed] [Google Scholar]
- 104.Fouad K., and Tetzlaff W. (2012). Rehabilitative training and plasticity following spinal cord injury. Exp. Neurol. 235, 91–99 [DOI] [PubMed] [Google Scholar]
- 105.Sandrow-Feinberg H.R., and Houle J.D. (2015). Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 1619, 12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.