Abstract
Endothelial cell injury in vascular arterial walls is a hallmark of atherosclerosis. Pterostilbene (Pts) has been shown to have an anti-oxidative and anti-apoptotic effect in numerous diseases via regulation of intracellular metabolism. The purpose of this study was to investigate the protective effect and possible mechanism of Pts against endothelial cell apoptosis in an atherosclerotic rat model. An atherosclerotic rat model was established using a high-fat, high glucose and high cholesterol diet. The effects of Pts on apoptosis and oxidative stress injury were measured using atherosclerotic lesion analysis, western blot analysis, hematoxylin and eosin straining, TUNEL assay and immunohistochemistry. In vivo results in an atherosclerosis rat model showed that Pts administration decreased the inflammatory response. Pts administration attenuated atherogenesis, reduced aortic plaque size, reduced macrophage infiltration, and suppressed oxidative stress and apoptosis of vascular arterial walls. In vitro assays using cultured human endothelial cells showed that Pts administration decreased hydrogen peroxide-induced cytotoxicity, oxidative stress injury and apoptosis via nuclear factor erythroid 2-related factor 2 (Nrf2) activation in endothelial cells. Additionally, Pts administration increased the expression level of Nrf2 and 5′ adenosine monophosphate-activated protein kinase (AMPK), and the phosphorylation level of AMPK and decreased signal transducer and activator of transcription 3 (STAT3) expression in these cells. Furthermore, knockdown of Nrf2 prevented Pts-decrease oxidative stress injury and apoptosis. In conclusion, these data suggest that Pts can protect endothelial cells in the vascular arterial walls against atherosclerosis-induced injury through regulation of the Nrf2-mediated AMPK/STAT3 pathway.
Keywords: pterostilbene, atherosclerosis, apoptosis, oxidative stress injury, nuclear factor erythroid 2-related factor 2, 5′ adenosine monophosphate activated protein kinase, signal transducer and activator of transcription 3
Introduction
Atherosclerosis is a common and complex chronic inflammatory disease of the vascular wall associated with lipid deposition and plaque fibrosis (1–3). Clinically, atherosclerosis is characterized by a marked dysfunction in lipid homeostasis, slow metabolism and retardation of signaling pathways that regulate the inflammatory response (4). Recently, morbidity of patients with atherosclerotic is increased significantly (5–7). A number of factors can trigger and sustain atherosclerosis, including smoke, obesity, arterial hypertension, dyslipidemia and diabetes mellitus (8). Ultimately, inflammation plays an important role in the pathogenesis of atherosclerosis, which has been recognized and confirmed at the molecular level in numerous animal models (9). In addition, endothelial cell injury in vascular arterial walls caused by inflammation frequently leads to the increased risk of atherosclerosis due to pathological changes and deposition of cholesterol (10). It is therefore crucial to investigate the association between inflammation and endothelial cell injury in atherosclerosis.
Pterostilbene (trans−3,5-dimethoxy-4-hydroxystilbene; Pts), a dimethylated analog of resveratrol, has been recognized to possess protective properties against inflammation and various diseases, such as heart reperfusion injury and atherosclerosis (11–13). Pts presents antioxidative and anti-apoptotic efficacy in numerous types of diseases via regulation of intracellular metabolism (14). Evidence suggests that Pts plays a role in suppressing the inflammatory response via NF-κB inactivation in lipopolysaccharide (LPS) or tumor necrosis factor-α (TNF-α)-induced vascular smooth muscle cells by downregulation of Toll like receptor 5 expression (13). Data indicate that Pts inhibits smooth muscle cell migration via the mitogen-activated protein kinase / matrix metallopeptidase-2 pathway and plays a novel role in the treatment of atherosclerosis (15). Pts has also been reported to have anti-inflammatory activity through the suppression of Akt kinase (16). An additional study suggested that the pro-atherogenic effect of nuclear factor erythroid 2-related factor 2 (Nrf-2) signaling was primarily mediated by its permissive role in interleukin (IL)-1 production in the chronic vascular inflammation that drives atherosclerosis (17). Nrf-2 is known to be a pro-atherogenic protein in mice, which may be mediated via positive regulation of CD36 and is a potential targeted therapy for cardiovascular diseases (18). However, the association between Pts and Nrf-2 in endothelial cells in vascular arterial walls has not been clarified.
The purpose of the present study was to explore the anti-inflammatory activity, and antioxidative and anti-apoptotic efficacy of Pts in endothelial cells in vascular arterial walls in an atherosclerosis rat model. The Pts-Nrf2-5′ adenosine monophosphate activated protein kinase (AMPK) / signal transducer and activator of transcription 3 (STAT3) signaling pathway was analyzed in endothelial cells in vascular arterial walls. The results explained the protective effects of Pts against apoptosis of endothelial cells in vascular arterial walls, and provided insight into its potential mechanism and use as an anti-atherosclerosis treatment.
Materials and methods
Animal study
A total of 24 male Sprague-Dawley rats (age, 8 weeks; weight, 320–350 g) were purchased from Experimental Animal Center of Shandong University. All rats were kept under 12-h light-dark cycles at (23±1)°C and (50±5)% humidity, and had free access to food and water. An atherosclerotic rat model was established through endothelial injury of the iliac arteries and feeding with a 2.5% cholesterol diet with 1% glucose (Sigma-Aldrich, Merck KGaA) for 6 weeks as described previously (19). The rats were randomly divided into two experimental groups: i) The control group that received PBS treatment orally and ii) the experimental group that orally received Pts (≥99%, purity; 10 mg/kg/day; Great Forest Biomedical, Ltd.) treatment with a regular diet for 4 weeks. Rats were sacrificed using cervical dislocation after the 4-week treatment and an anesthetic (40 mg/kg intravenous pentobarbital; Sigma-Aldrich; Merck KGaA) was used prior to euthanasia.
Evaluation of inflammatory cytokines in serum
Peripheral venous blood samples were collected from experimental rats and serum samples were obtained after centrifugation at 10,000 × g for 5 min at 4°C and analyzed for biochemical measurements. The concentrations of monocyte chemoattractant protein-1 (MCP-1; cat. no. RJE00B), Il-6 (cat. no. R6000B), IL-1β (cat. no. RLB00) and TNF-α (cat. no. RTA00) were determined using enzyme linked immunosorbent assay (ELISA) kits according to the manufacturer's instructions (R&D Systems, Inc.).
Histopathological and histomorphometric evaluation of aortic arch
Rats were sacrificed using cervical dislocation on week 5 as described above. The aortic arch samples (the remaining samples were stored at −80°C for subsequent use) obtained were fixed in 10% paraformaldehyde for 12 h at 4°C, washed with PBS, embed in paraffin, cut into 5-µm thick sections and subjected to antigen retrieval using eBioscience™ IHC Antigen Retrieval Solution (cat. no. 00-4955-58, Invitrogen, Thermo Fisher Scientific, Inc.). Thick longitudinal sections (5-µm) were stained with hematoxylin and eosin for 15 min at room temperature and images captured under a light microscope at ×40 magnification. The histopathological and histomorphometric images were evaluated by three independent pathologists.
Quantification of atherosclerotic lesion size
Quantification of lesion size was determined as described previously (20). Briefly, tissues were frozen, stored at −80°C, cut at 8-µm intervals and stained with hematoxylin and 0.5% Oil Red O (Sigma-Aldrich; Merck KGaA) for 1 h at room temperature. Atherosclerotic lesion area was quantified using Image-Pro Plus software version 5.0 (Media Cybernetics).
Cell culture
Human umbilical artery endothelial cells were purchased from Clonetics Lonza (cat. no. 199041; Lonza Group Ltd.) and cultured in endothelial growth medium (EGM-2; Lonza Group Ltd.) in 5% CO2 at 37°C. After a 24-h incubation, cells were incubated with 0, 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg/ml of Pts for 12 h at 37°C for further analysis.
Cell viability assay
Viability of endothelial cells was measured using the Cell Counting Kit-8 (CCK-8; Sigma-Aldrich, Merck KGaA). In brief, endothelial cells were seeded into 6-well plates at a density of 1×105 cells/ml and incubated with 0.2% hydrogen peroxide (H2O2) and then treated with PBS or Pts (2 mg/ml) for 24 h at 37°C. A total of 10 µl of CCK-8 solution was added to the cells and incubated for 30 min at 37°C. Absorbance at 450 nm was measured using a Microplate Reader (Bio-Rad Laboratories, Inc.).
Measurement of levels of nitric oxide (NO) and reactive oxygen species (ROS)
Endothelial cells (1×105/well) were seeded into a 6-well plate and incubated with 0.2% H2O2 to induce oxidative stress and then treated with PBS or Pts (2 mg/ml) at 37°C for 24 h. Endothelial cells were harvested and centrifuged at 2,000 × g for 10 min at 4°C. Endothelial cells were collected and lysed with RIPA buffer (Beyotime Institute of Biotechnology) for subsequent analysis of enzyme activities. The level of NO was assessed using a commercial Nitrate/Nitrite Fluorometric Assay kit (cat. no. KA1344; Abnova) following the manufacturer's protocols. Intracellular ROS production was analyzed using fluorescent probe DCFH-DA (cat. no. D6883; Sigma-Aldrich, Merck KGaA) as described previously (21).
Small interfering (si-)RNA-mediated knockdown
Endothelial cells (1×105/well) were seeded into a 6-well plate. After 24 h, cells were transfected with siRNA-Nrf2 (si-Nrf2) forward, 5′-GAGACUACCAUGGUUCCAA(dTdT)-3′ and reverse, 5′-UUGGAACCAUGGUAGUCUC(dTdT)-3′ or si-RNA control (si-NC) forward, 5′-CCUACGCCACCAAUUUCGU-3′ and reverse, 5′-ACGAAAUUGGUGGCGUAGG-3′ (Invitrogen, Thermo Fisher Scientific Ltd.) using Lipofectamine® RNAiMAX (2 µl; cat. no. 13778030; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions (Thermo Fisher Scientific, Inc.). mRNA expression of Nrf2 was detected by western blot analysis 72-h after transfection. si-Nrf2-transfected cells were then treated with PBS or Pts (2 mg/ml) for 24 h at 37°C for further analysis.
Western blot analysis
A total of 1×107 endothelial cells were lysed in RIPA buffer (Bio-Rad Laboratories, Inc.). The lysates were centrifuged at 12,000 × g for 10 min at 4°C. The protein concentration was quantified using a BCA Protein Assay kit (Pierce, Thermo Fisher, Ltd.). Protein samples (40 µg) were loaded onto an SDS-PAGE (12% gel) and transferred onto polyvinylidene difluoride membranes (Sigma-Aldrich; Merck KGaA). Membranes were blocked with 5% bovine serum albumin (BSA; Sigma-Aldrich; Merck KGaA) and incubated with the following primary antibodies: Superoxide dismutase (SOD; 1:1,000; cat. no. ab13534), catalase (CAT; 1:1,000, cat. no. ab16731), heme oxygenase-1 (HO-1; 1:1,000, cat. no. ab13243), Nrf2 (1:1,000; cat. no. ab62352), AMPK (1:1,000; cat. no. ab32047), phosphorylated (p)AMPK (1:1,000; cat. no. ab92701, Abcam), STAT3 (1:1,000; cat. no. ab68153), pSTAT3 (1:1,000; cat. no. ab76315) and β-actin (1:1,000; cat. no. ab8226) for 12 h at 4°C. All antibodies were supplied by Abcam. After washing with PBS, membranes were incubated with HRP-conjugated secondary antibody (1:2,000; cat. no. ab205718; Abcam) for 2 h at room temperature. The bands were visualized using an enhanced chemiluminescence substrate kit (Beyotime Institute of Biotechnology; cat. no. P0018F). Protein expression was quantified using ImageJ software (version 4.6.2; National Institutes of Health).
Apoptosis assay
The apoptosis of cells was analyzed using a TUNEL staining kit (Roche Diagnostics). For tissue, sections were stained with TUNEL for 2 h at room temperature and analyzed using a commercial TUNEL staining kit (Roche Diagnostics) according to the manufacturer's instructions. For cells, 1×104 endothelial cells were fixed with 4% paraformaldehyde and 0.5% Triton X-100 for 30 min at room temperature, and then incubated TUNEL for 2 h at room temperature. Cells were washed with PBS three times and then incubated with 5% DAPI (Sigma-Aldrich; Merck KGaA) for 30 min at room temperature. Images were captured at ×100 magnification under Aqueous mounting medium (cat. no. ab64230; Abcam) using a ZEISS LSM 510 confocal microscope with a 488 nm laser. The apoptosis rate was measured using Developer XD 3.0 (Definiens AG) software version 1.0. Six fields of view were randomly assessed for each treatment group.
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analysis was conducted with Student's t-test or one-way ANOVA followed by Tukey's test using SPSS software (version 17.0; SPSS, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Pts improves symptoms of atherosclerosis in a rat atherosclerosis model
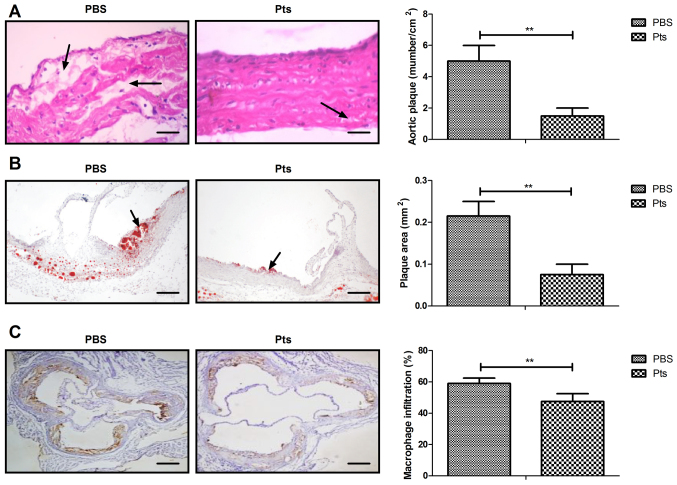
Symptoms of atherosclerosis were recorded in rats of both the Pts and PBS group. The results showed that Pts administration attenuated atherogenesis when compared with control (Fig. 1A). Administration of Pts reduced the area of aortic plaques and macrophage infiltration in the atherosclerotic rat model (Fig. 1B and C). Pts administration suppressed apoptosis of the vascular arterial wall in an atherosclerosis rat model (Fig. 1D).
Figure 1.
Pts reduces symptoms of atherosclerosis in an atherosclerosis rat model. (A) The number of aortic plaques/cm2. (B) Plaque area. (C) Macrophage infiltration. (D) Apoptosis of vascular arterial walls in atherosclerosis rat model. Scale bar, 50 μm. **P<0.01 vs. PBS. Pts, pterostilbene.
Pts suppresses the inflammatory response in an atherosclerosis rat model
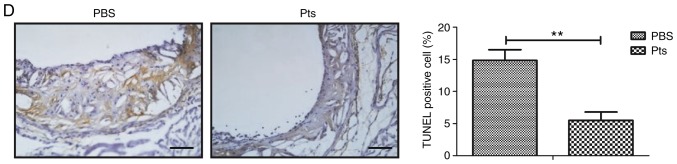
Inflammatory cytokines play a crucial role in regulating the inflammatory response in atherosclerosis. Serum levels of inflammatory cytokines including MCP-1, IL-6, IL-1β and TNF-α were examined to determine whether Pts could regulate their expression. In vivo results showed that Pts administration corresponded with decreased serum levels of MCP-1, IL-6, IL-1β and TNF-α (Fig. 2A-D).
Figure 2.
Pts decreases the inflammatory response in an atherosclerosis rat model. Inflammatory cytokines (A) MCP-1, (B) IL-6, (C) IL-1β and (D) TNF-α in serum samples of Pts and PBS groups of rat models of atherosclerosis. **P<0.01 vs. PBS. IL, interleukin; MCP-1, monocyte chemoattractant protein-1; Pts, pterostilbene; TNF-α, tumor necrosis factor-α.
Pts decreases H2O2-induced cytotoxicity in cultured endothelial cells
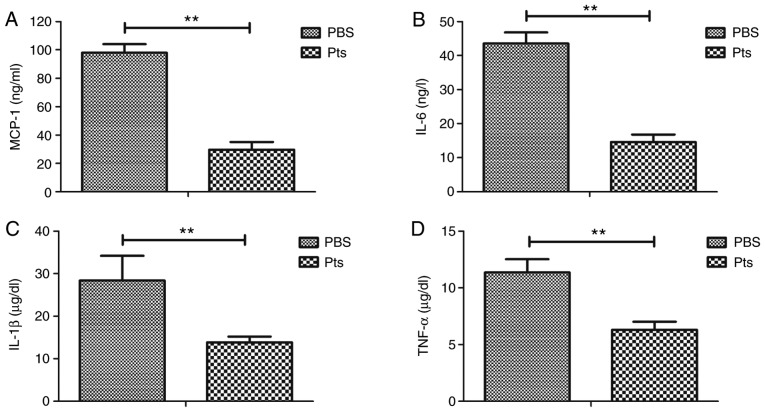
The protective effects of Pts on endothelial cells were investigated in vitro. The dose of 2.0 mg/ml Pts showed the optimal protective effect on H2O2-induced cytotoxicity in endothelial cells (Fig. S1). The results showed that Pts administration decreased H2O2-induced cytotoxicity compared with the PBS group (Fig. 3A). Oxidative stress injury-associated ROS production and NO generation was reduced by Pts, and the expression levels of antioxidant proteins SOD, CAT and HO-1 were upregulated by Pts in these endothelial cells (Fig. 3B and C). Apoptosis of endothelial cells was reduced after Pts treatment when compared with control (Fig. 3D).
Figure 3.
Pts decreases H2O2-induced cytotoxicity in endothelial cells. (A) Effect of Pts on cell viability in H2O2-induced endothelial cell cytotoxicity. (B) Oxidative stress injury induces ROS production and NO generation in endothelial cells treated with Pts and PBS. (C) Expression levels of antioxidant proteins SOD, CAT and HO-1 in endothelial cells. (D) Apoptosis of endothelial cells in Pts and control groups. *P<0.05 and **P<0.01 vs. control. CAT, catalase; H2O2, hydrogen peroxide; HO-1, heme oxygenase-1; NO, nitric oxide; Pts, pterostilbene; ROS, reactive oxygen species; SOD, superoxide dismutase.
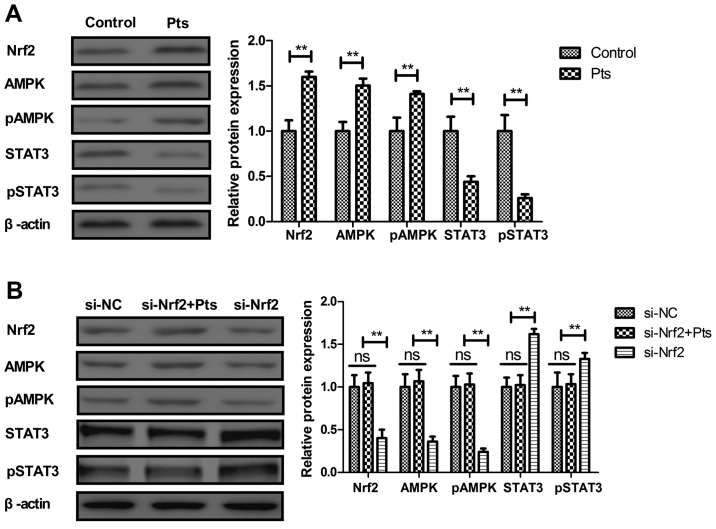
Pts inhibits the Nrf2-mediated AMPK/STAT3 pathway in cultured endothelial cells
The Nrf2-mediated AMPK/STAT3 pathway was analyzed in cultured endothelial cells. Pts administration increased Nrf2, STAT3 and AMPK expression in endothelial cells (Fig. 4A). Knockdown of Nrf2 (si-Nrf2) abolished Pts-regulated AMPK and pAMPK and increased STAT3 and pSTAT3 levels in endothelial cells (Fig. 4B). The results revealed that knockdown of Nrf2 increased Nrf2 expression and increased the ratio of p-Nrf2/t-Nrf2 in endothelial cells (Fig. S2).
Figure 4.
Pts inhibits the Nrf2-mediated AMPK/STAT3 pathway in endothelial cells. (A) Effect of Pts on Nrf2, AMPK, pAMPK, STAT3 and pSTAT3 levels in endothelial cells. (B) Effects of Nrf2 knockdown on Pts-regulated AMPK, pAMPK, STAT3 and pSTAT3 levels in endothelial cells. **P<0.01. AMPK, 5′ adenosine monophosphate-activated protein kinase; Nrf2, nuclear factor erythroid 2-related factor 2; p, phosphorylated; Pts, pterostilbene; STAT3, signal transducer and activator of transcription 3; ns, not significant; NC, negative control; si, small interfering RNA.
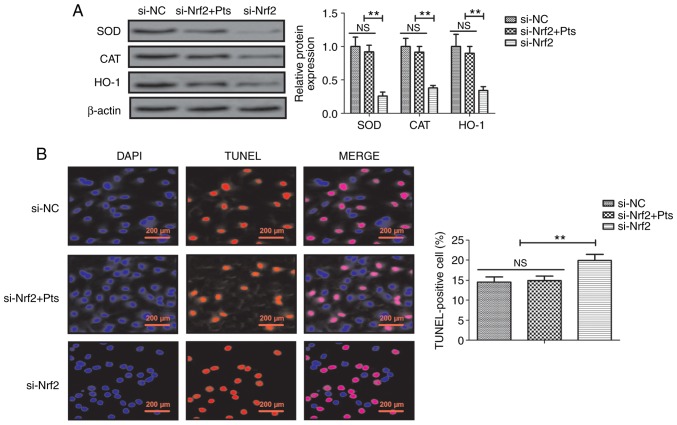
Knockdown of Nrf2 reduces Pts-regulated oxidative stress prevention of injury and apoptosis in endothelial cells
The role of Nrf2 knockdown was further investigated in Pts-regulated oxidative stress injury and apoptosis in endothelial cells. The results demonstrated that si-Nrf2 decreased SOD, CAT and HO-1 protein expression compared with si-NC and that there was no significant difference between si-NC and si-Nrf2+ Pts group in endothelial cells (Fig. 5A). Similarly, knockdown of Nrf2 increased apoptosis of endothelial cells and reduced Pts-induced prevention of apoptosis in endothelial cells when compared with the si-NC group (Fig. 5B).
Figure 5.
Effect of si-Nrf2 and Pts on oxidative stress injury and apoptosis in endothelial cells. (A) Effects of Nrf2 knockdown on Pts-regulated SOD, CAT and HO-1 protein expression in endothelial cells. (B) Effects of Nrf2 knockdown on apoptosis in endothelial cells. **P<0.01. CAT, catalase; HO-1, heme oxygenase-1; Nrf2, nuclear factor erythroid 2-related factor 2; Pts, pterostilbene; si, small interfering RNA; SOD, superoxide dismutase; NC, negative control; ns, not significant.
Discussion
Pts is a stilbene belonging to a family of polyphenols reported to have anti-inflammatory effects in fructose-fed diabetic and acute renal ischemia reperfusion injury rats (14,22). Previously, Nrf2 was reported to play a pivotal role in inflammasome activation and oxidative stress (18). An additional study reported that Pts ameliorated streptozotocin-induced diabetes through enhancement of antioxidant signaling pathways mediated by Nrf2 (23). Notably, Pts was shown to inhibit high fat-induced atherosclerosis inflammation via regulation of the NF-κB signaling pathway in experimental mice (13). The current study aimed to clarify the therapeutic mechanism of Pts action on endothelial cells in the atherosclerotic rat. The results revealed that Pts administration attenuated atherogenesis, aortic plaque formation, macrophage infiltration, oxidative stress and apoptosis of vascular arterial walls in an atherosclerosis rat model. Data suggested that Pts decreased oxidative stress and inhibited apoptosis via an Nrf2-mediated AMPK/STAT3 signaling pathway in endothelial cells, which ameliorated atherosclerosis.
Inflammation plays a key role in the pathogenesis of atherosclerosis and has gained considerable attention in clinical practice (24). MCP-1 is expressed by endothelial cells and has been reported to play an important role in the pathogenesis of atherosclerosis and to influence cell growth within the atherosclerotic lesion (25). In the current study Pts reduced the serum level of MCP-1 in a rat model of atherosclerosis. Release of the pro-inflammatory cytokine IL-6 affects the histological features of plaque composition (26). In addition, studies have found that proinflammatory IL-1 family cytokine production was increased in atherosclerosis patients due to a reduction in wall shear stress (27). Further findings suggested that TNF-inhibitory intervention should be added to conventional therapy as a novel strategy for treating the elderly patients with atherosclerosis (28). In the current study Pts administration effectively decreased serum levels of IL-6, IL-1β and TNF-α, indicating that Pts may reduce the apoptosis of endothelial cells by modulating the expression of inflammatory cytokines.
Antioxidative and anti-inflammatory efficacy contributes to Pts anti-atherosclerosis activity and it has been confirmed to regulate endothelial function in a rat model (29). In addition, the excessive release of ROS leads to enhanced lipid peroxidation, aggravated atherosclerosis and oxidative stress (30). Furthermore, SOD has potential beneficial effects with respect to the development of atherosclerosis (31). A reduction in NO generation leads to an improvement in LPS-induced apoptosis in RAW 264.7 macrophages (32). Previous studies have also found that atherosclerosis is a chronic inflammatory cardiovascular disease and is characterized by an increased ROS and NO production in arterial endothelial cells (30,33). In the current study the efficacy of Pts was reported in preventing oxidative stress injury. In addition, Pts treatment decreased ROS and NO production in endothelial cells, which further improved the endothelium function and may be regarded as a therapeutic drug for the treatment of atherosclerosis. These findings may be attributed to the antioxidative efficacy of Pts, due to Nrf2-mediated signal transduction in endothelial cells.
Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis (17). Nrf2-mediated pathways are associated with antioxidant defense in atherosclerosis (34). AMPK-dependent phosphorylation of sterol regulatory element-binding protein may offer therapeutic strategies to atherosclerosis (35–37). Furthermore, inhibition of STAT3 activation may be a potential therapeutic target in the treatment of atherosclerosis (38). In the current study, in vivo assays revealed that Pts treatment attenuated atherogenesis, reduced the number and area of aortic plaques and macrophage infiltration, and suppressed oxidative stress and apoptosis of vascular arterial walls in atherosclerosis rat model. In vitro assays showed that Pts regulated oxidative stress injury and apoptosis via Nrf2-mediated AMPK/STAT3 pathway in endothelial cells.
In conclusion, the key findings of the current study are that Pts may be an efficient drug in endothelial cells in the pathology of atherosclerosis. The results indicated that the protective effects of Pts may be associated with the regulation of Nrf2-mediated AMPK/STAT3 pathway, which provides a potential novel anti-atherosclerosis agent for patients.
Supplementary Material
Acknowledgements
Not applicable.
Funding
This study was supported by the Foundation of Jiangsu Provincial Commission of Health and Family Planning (grant no. QNRC2016353), and the National Key Research and Develeopment Program of China (grant no. 2016YFE0126000).
Availability of data and materials
The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request.
Authors' contributions
TT designed and conceived the current study, and provided intellecutal content. ZD performed statistical analysis and wrote/revised the manuscript. JX collected and analyzed the data, prepared the figures and revised the manuscript. JL and SZ established the rat model and revised the manuscript. XZ and HZ performed the literature search and generated the rat model. YW collected the data and approved the final manuscript for publication.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Yangzhou University.
Patient consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Okuyama H, Hamazaki T, Hama R, Ogushi Y, Kobayashi T, Ohara N, Uchino H. A critical review of the consensus statement from the European Atherosclerosis Society Consensus Panel 2017. Pharmacology. 2018;101:184–218. doi: 10.1159/000486374. [DOI] [PubMed] [Google Scholar]
- 2.Henrot P, Foret J, Barnetche T, Lazaro E, Duffau P, Seneschal J, Schaeverbeke T, Truchetet ME, Richez C. Assessment of subclinical atherosclerosis in systemic lupus erythematosus: A systematic review and meta-analysis. Joint Bone Spine. 2018;85:155–163. doi: 10.1016/j.jbspin.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Martins P, Castela E, Rocha G, Sena C, Seiça R. Premature atherosclerosis in HIV-infected pediatric patients: Literature review and clinical approach. Acta Med Port. 2017;30:742–749. doi: 10.20344/amp.8726. (In Portuguese) [DOI] [PubMed] [Google Scholar]
- 4.Zhao TX, Mallat Z. Targeting the immune system in atherosclerosis: JACC State-of-the-art review. J Am Coll Cardiol. 2019;73:1691–1706. doi: 10.1016/j.jacc.2018.12.083. [DOI] [PubMed] [Google Scholar]
- 5.Parolin M, Dassie F, Martini C, Mioni R, Russo L, Fallo F, Rossato M, Vettor R, Maffei P, Pagano C. Preclinical markers of atherosclerosis in acromegaly: A systematic review and meta-analysis. Pituitary. 2018;21:653–662. doi: 10.1007/s11102-018-0911-5. [DOI] [PubMed] [Google Scholar]
- 6.Song P, Xia W, Zhu Y, Wang M, Chang X, Jin S, Wang J, An L. Prevalence of carotid atherosclerosis and carotid plaque in Chinese adults: A systematic review and meta-regression analysis. Atherosclerosis. 2018;276:67–73. doi: 10.1016/j.atherosclerosis.2018.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Stachyra K, Kiepura A, Olszanecki R. Air pollution and atherosclerosis - a brief review of mechanistic links between atherogenesis and biological actions of inorganic part of particulate matter. Folia Med Cracov. 2017;57:37–46. [PubMed] [Google Scholar]
- 8.Fava C, Montagnana M. Atherosclerosis is an inflammatory disease which lacks a common anti-inflammatory therapy: How human genetics can help to this issue. A narrative review. Front Pharmacol. 2018;9:55. doi: 10.3389/fphar.2018.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartman J, Frishman WH. Inflammation and atherosclerosis: A review of the role of interleukin-6 in the development of atherosclerosis and the potential for targeted drug therapy. Cardiol Rev. 2014;22:147–151. doi: 10.1097/CRD.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 10.Patel TN, Shishehbor MH, Bhatt DL. A review of high-dose statin therapy: Targeting cholesterol and inflammation in atherosclerosis. Eur Heart J. 2007;28:664–672. doi: 10.1093/eurheartj/ehl445. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Ding XQ, Gu TT, Song L, Li JM, Xue QC, Kong LD. Pterostilbene and allopurinol reduce fructose-induced podocyte oxidative stress and inflammation via microRNA-377. Free Radic Biol Med. 2015;83:214–226. doi: 10.1016/j.freeradbiomed.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 12.Lv M, Liu K, Fu S, Li Z, Yu X. Pterostilbene attenuates the inflammatory reaction induced by ischemia/reperfusion in rat heart. Mole Med Rep. 2015;11:724–728. doi: 10.3892/mmr.2014.2719. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y. Pterostilbene, a novel natural plant conduct, inhibits high fat-induced atherosclerosis inflammation via NF-kappaB signaling pathway in Toll-like receptor 5 (TLR5) deficient mice. Biomed Pharmacother. 2016;81:345–355. doi: 10.1016/j.biopha.2016.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Gao D, Jing S, Zhang Q, Wu G. Pterostilbene protects against acute renal ischemia reperfusion injury and inhibits oxidative stress, inducible nitric oxide synthase expression and inflammation in rats via the Toll-like receptor 4/nuclear factor-κB signaling pathway. Exp Ther Med. 2018;15:1029–1035. doi: 10.3892/etm.2017.5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin HC, Hsieh MJ, Peng CH, Yang SF, Huang CN. Pterostilbene inhibits vascular smooth muscle cells migration and matrix metalloproteinase-2 through modulation of MAPK pathway. J Food Sci. 2015;80:H2331–H2335. doi: 10.1111/1750-3841.13002. [DOI] [PubMed] [Google Scholar]
- 16.Park ES, Lim Y, Hong JT, Yoo HS, Lee CK, Pyo MY, Yun YP. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascul Pharmacol. 2010;53:61–67. doi: 10.1016/j.vph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. doi: 10.1002/eji.201041316. [DOI] [PubMed] [Google Scholar]
- 18.Sussan TE, Jun J, Thimmulappa R, Bedja D, Antero M, Gabrielson KL, Polotsky VY, Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chernukha IM, Fedulova LV, Kotenkova EA, Takeda S, Sakata R. Hypolipidemic and anti-inflammatory effects of aorta and heart tissues of cattle and pigs in the atherosclerosis rat model. Anim Sci J. 2018;89:784–793. doi: 10.1111/asj.12986. [DOI] [PubMed] [Google Scholar]
- 20.Chai JT, Biasiolli L, Li L, Alkhalil M, Galassi F, Darby C, Halliday AW, Hands L, Magee T, Perkins J, et al. Quantification of lipid-rich core in carotid atherosclerosis using magnetic resonance T2 mapping: Relation to clinical presentation. JACC Cardiovasc Imaging. 2017;10:747–756. doi: 10.1016/j.jcmg.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bombaca ACS, Viana PG, Santos ACC, Silva TL, Rodrigues ABM, Guimarães ACR, Goulart MOF, da Silva Júnior EN, Menna-Barreto RFS. Mitochondrial disfunction and ROS production are essential for anti-Trypanosoma cruzi activity of beta-lapachone-derived naphthoimidazoles. Free Radical Biol Med. 2018;130:408–418. doi: 10.1016/j.freeradbiomed.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Kosuru R, Kandula V, Rai U, Prakash S, Xia Z, Singh S. Pterostilbene decreases cardiac oxidative stress and inflammation via activation of AMPK/Nrf2/HO-1 pathway in fructose-fed diabetic rats. Cardiovasc Drugs Ther. 2018;32:147–163. doi: 10.1007/s10557-018-6780-3. [DOI] [PubMed] [Google Scholar]
- 23.Elango B, Dornadula S, Paulmurugan R, Ramkumar KM. Pterostilbene ameliorates streptozotocin-induced diabetes through enhancing antioxidant signaling pathways mediated by Nrf2. Chem Res Toxicol. 2016;29:47–57. doi: 10.1021/acs.chemrestox.5b00378. [DOI] [PubMed] [Google Scholar]
- 24.Geovanini GR, Libby P. Atherosclerosis and inflammation: Overview and updates. Clin Sci (Lond) 2018;132:1243–1252. doi: 10.1042/CS20180306. [DOI] [PubMed] [Google Scholar]
- 25.Lin J, Kakkar V, Lu X. Impact of MCP-1 in atherosclerosis. Curr Pharm Des. 2014;20:4580–4588. doi: 10.2174/1381612820666140522115801. [DOI] [PubMed] [Google Scholar]
- 26.Bernberg E, Ulleryd MA, Johansson ME, Bergström GM. Social disruption stress increases IL-6 levels and accelerates atherosclerosis in ApoE−/− mice. Atherosclerosis. 2012;221:359–365. doi: 10.1016/j.atherosclerosis.2011.11.041. [DOI] [PubMed] [Google Scholar]
- 27.Grebe A, Hoss F, Latz E. NLRP3 Inflammasome and the IL-1 pathway in atherosclerosis. Circ Res. 2018;122:1722–1740. doi: 10.1161/CIRCRESAHA.118.311362. [DOI] [PubMed] [Google Scholar]
- 28.Park KY, Heo TH. Critical role of TNF inhibition in combination therapy for elderly mice with atherosclerosis. Cardiovasc Ther. 2017;35:35. doi: 10.1111/1755-5922.12280. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Han X, Li R, Zhao W, Bai B, Yan C, Dong X. Anti-atherosclerosis of oligomeric proanthocyanidins from Rhodiola rosea on rat model via hypolipemic, antioxidant, anti-inflammatory activities together with regulation of endothelial function. Phytomedicine. 2018;51:171–180. doi: 10.1016/j.phymed.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Wu T, Peng Y, Yan S, Li N, Chen Y, Lan T. Andrographolide ameliorates atherosclerosis by suppressing pro-inflammation and ROS generation-mediated foam cell formation. Inflammation. 2018;41:1681–1689. doi: 10.1007/s10753-018-0812-9. [DOI] [PubMed] [Google Scholar]
- 31.Décordé K, Ventura E, Lacan D, Ramos J, Cristol JP, Rouanet JM. An SOD rich melon extract Extramel prevents aortic lipids and liver steatosis in diet-induced model of atherosclerosis. Nutr Metab Cardiovasc Dis. 2010;20:301–307. doi: 10.1016/j.numecd.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Yao Y, Liu K, Zhao Y, Hu X, Wang M. Pterostilbene and 4′-Methoxyresveratrol inhibited lipopolysaccharide-induced inflammatory response in RAW264.7 macrophages. Molecules. 2018;23:23. doi: 10.3390/molecules23051148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitra R, O'Neil GL, Harding IC, Cheng MJ, Mensah SA, Ebong EE. Glycocalyx in atherosclerosis-relevant endothelium function and as a therapeutic target. Curr Atheroscler Rep. 2017;19:63. doi: 10.1007/s11883-017-0691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai G, Vaughn S, Zhang Y, Wang ET, Garcia-Cardena G, Gimbrone MA., Jr Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 35.Zeng Y, Li C, Guan M, Zheng Z, Li J, Xu W, Wang L, He F, Xue Y. The DPP-4 inhibitor sitagliptin attenuates the progress of atherosclerosis in apolipoprotein-E-knockout mice via AMPK- and MAPK-dependent mechanisms. Cardiovasc Diabetol. 2014;13:32. doi: 10.1186/1475-2840-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fullerton MD, Steinberg GR, Schertzer JD. Immunometabolism of AMPK in insuliresistance and atherosclerosis. Mol Cell Endocrinol. 2013;366:224–234. doi: 10.1016/j.mce.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: Potential role in atherosclerosis. Diabetes. 2015;64:2028–2041. doi: 10.2337/db14-1225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the study are available from the corresponding author on reasonable request.