Abstract
Studies of cardiac function in adolescent and young adult survivors have been performed at least a decade after anthracycline exposure, with little knowledge of short-term changes in cardiac function in this age group. To this end, we evaluated cardiac function within a 2-year period among 18 patients who received high-dose anthracyclines for osteosarcoma treatment. At 2 years, there was a significant decline in left ventricular ejection fraction (p = 0.005), with 8 of 18 patients having a >10% reduction. There was a significant change in E-wave velocity (p = 0.04). To our knowledge, this is the first study assessing short-term change in systolic and diastolic function in osteosarcoma patients receiving anthracyclines.
Keywords: cardiac function, anthracyclines, osteosarcoma, adolescent and young adult cancer survivors
Long-term survival rates among adolescent and young adults (AYAs) with osteosarcoma presenting without metastasis have improved to ∼70% due to the advent of anthracycline-based chemotherapy. Unfortunately, improvements in cancer-related outcomes have been accompanied by a competing risk of anthracycline cardiotoxicity.1 Current measures are in place to monitor cardiac function, as determined by left ventricular ejection fraction (LVEF). Although two-dimensional (2D) echocardiogram is the standard imaging technique used to assess change in LVEF, there is a lack of consensus regarding the timing of serial echocardiograms to monitor for anthracycline cardiotoxicity. This is due, in part, to prior studies of cardiac function in AYA survivors being performed at least a decade after anthracycline exposure, with little knowledge of short-term changes in cardiac function in this age group.
To this end, we evaluated systolic and diastolic function within a 2-year period for patients who received high-dose anthracyclines (>250 mg/m2) for osteosarcoma treatment at Children's Cancer Hospital and Sarcoma Center at M.D. Anderson Cancer Center.2 We identified 18 AYAs who were treated for osteosarcoma between 2000 and 2016 according to standard protocols consisting of combination chemotherapy with doxorubicin (cumulative mean dose 436 mg/m2, [range 270–540 mg/m2]), cisplatin, and high-dose methotrexate followed by surgery and adjuvant chemotherapy and had 2D echocardiograms at baseline and at 2-years post-diagnosis as part of their follow-up care. Images were acquired in standard windows and interpreted independently by two experienced cardiologists. Standard 2D and Doppler echocardiographic parameters were obtained. LVEF and diastolic measures were obtained quantitatively using the biplane method of disks technique.3 Frequencies and descriptive statistics were used to determine patient characteristics. Standard t-tests were used to examine differences in cardiac function for a 2-year period. The study was approved by the IRB at the University of Texas M.D. Anderson Cancer Center.
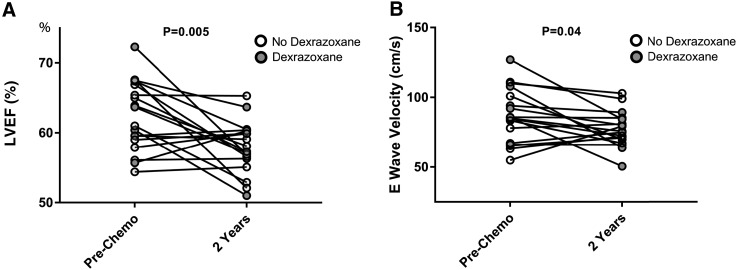
Mean age at diagnosis was 16.7 years (range 15–19). Mean body mass index was 24.2 ± 6.1 kg/m2 and blood pressure was 122 ± 17/68 ± 8 mmHg. No patients were on cardiac medications at the time of the first echocardiogram and all were asymptomatic at the time of the second echocardiogram. Six patients (33.3%) received dexrazoxane at the start of anthracycline-based therapy. At the 2-year follow-up, there was a significant decline in LVEF (Fig. 1A, p = 0.005), with 8 of the 18 patients having a >10% reduction. There was a significant decrease in E-wave velocity (Fig. 1B, p = 0.04) and change in left ventricular (LV) posterior wall thickness (PWT)/LV end diastolic dimension (EDD) (p = 0.03). E/A ratio and mitral valve deceleration time were unchanged (p > 0.10). No changes in tissue Doppler were noted (p > 0.10 for both). Of note, we were statistically underpowered to determine a change in cardiac function stratifying on dexrazoxane treatment; however, we have highlighted dexrazoxane-treated patients in the figure.
FIG. 1.
Two-year change in left ventricular ejection fraction (A) and E-wave velocity (B) in osteosarcoma patients receiving anthracyclines with dexrazoxane (gray) and without dexrazoxane (white). LVEF, left ventricular ejection fraction.
The main finding of this report is that a significant decline in LVEF is detectable within 2 years of anthracycline-based treatment among AYAs with osteosarcoma. This finding is clinically significant given prior work in adult patients has shown that LVEF decline after anthracycline-based chemotherapy is prognostic of future congestive heart failure, although potentially mitigated with early initiation of medical therapy.4 Our results are in line with the study of Cardinale et al. in adult cancer patients as well as a recent meta-analysis of cardiac function in children and AYAs5 suggesting that anthracycline cardiotoxicity is not a late-onset disease, but rather a late diagnosis of an early developed disease.4
Regarding the diastolic measures assessed in this study, the significant change in E-wave velocity is intriguing. It is well known that there is a basic dependence of the peak E wave on filling pressure and loading conditions and less of a mechanical dependence on the atrial contraction in this age group. However, without a significant change in E/A ratio, A wave and or tissue e' wave, it is unclear whether this is an early diastolic phenomena associated with impairment in LV compliance or LV suction capacity related to a patient's underlying disease or treatment, or alternatively, type 1 error or a specific age-related variation. Of note, we did observe a change in LV PWT/LV EDD, a surrogate of wall stress and LV remodeling. This measure has been linked to adverse outcomes in childhood cancer survivors.6 These results support our recent data in mice showing a significant change in diastolic PWT and LVEF after anthracycline exposure.7 Taken together, these findings deserve further study with detailed cardiac measurements and documentation of preventative therapies (e.g., dexrazoxane and exercise) in a larger study population of osteosarcoma patients undergoing anthracycline-based chemotherapy.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Mulrooney DA, Yeazel MW, Kawashima T, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: retrospective analysis of the Childhood Cancer Survivor Study cohort. BMJ. 2009;339:b4606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lipshultz SE, Alvarez JA, Scully RE. Anthracycline associated cardiotoxicity in survivors of childhood cancer. Heart. 2008;94:525–33 [DOI] [PubMed] [Google Scholar]
- 3. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 4. Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8 [DOI] [PubMed] [Google Scholar]
- 5. Tuzovic M, Wu PT, Kianmahd S, Nguyen KL. Natural history of myocardial deformation in children, adolescents, and young adults exposed to anthracyclines: systematic review and meta‐analysis. Echocardiography. 2018;35:922–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lipshultz SE, Landy DC, Lopez-Mitnik G, et al. Cardiovascular status of childhood cancer survivors exposed and unexposed to cardiotoxic therapy. J Clin Oncol. 2012;30:1050–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang F, Schadler K, Chandra J, Kleinerman ES. Abstract 3008: effect of exercise on acute and late onset doxorubicin-induced cardiotoxicity. Cancer Res. 2018;78:3008 [Google Scholar]