Abstract
Objective: Hybrid closed-loop (HCL) artificial pancreas (AP) systems are now moving from research settings to widespread clinical use. In this study, the inControl algorithm developed by TypeZero Technologies was embedded to a commercial Tandem t:slim X2 insulin pump, now called Control-IQ, paired with a Dexcom G6 continuous glucose monitor and tested for superiority against sensor augmented pump (SAP) therapy. Both groups were physician-monitored throughout the clinical trial.
Research Design and Methods: In a randomized controlled trial, 24 school-aged children (6–12 years) with type 1 diabetes (T1D) participated in a 3-day home-use trial at two sites: Stanford University and the Barbara Davis Center (50% girls, 9.6 ± 1.9 years of age, 4.5 ± 1.9 years of T1D, baseline hemoglobin A1c 7.35% ± 0.68%). Study subjects were randomized 1:1 at each site to either HCL AP therapy with the Control-IQ system or SAP therapy with remote monitoring.
Results: The primary outcome, time in target range 70–180 mg/dL, using Control-IQ significantly improved (71.0% ± 6.6% vs. 52.8% ± 13.5%; P = 0.001) and mean sensor glucose (153.6 ± 13.5 vs. 180.2 ± 23.1 mg/dL; P = 0.003) without increasing hypoglycemia time <70 mg/dL (1.7% [1.3%–2.1%] vs. 0.9% [0.3%–2.7%]; not significant). The HCL system was active for 94.4% of the study period. Subjects reported that use of the system was associated with less time thinking about diabetes, decreased worry about blood sugars, and decreased burden in managing diabetes.
Conclusions: The use of the Tandem t:slim X2 with Control-IQ HCL AP system significantly improved time in range and mean glycemic control without increasing hypoglycemia in school-aged children with T1D during remote monitored home use.
Keywords: Type 1 diabetes, Artificial pancreas, Randomized controlled trial, Pediatrics, Hybrid closed loop
Artificial pancreas (AP) technology combines an insulin pump, continuous glucose monitor (CGM), and automated control algorithm to adjust basal insulin delivery in real time for patients with diabetes. AP systems have consistently demonstrated superiority to conventional therapy to improve time in range (TIR) and reduce hypoglycemia.1–3 Implementation of this technology has progressed from university-based, publicly or foundation-funded algorithm development research to industry-funded commercialization trials. AP development has thus shown a synergy between academic, foundation, public, and industry to improve therapy for patients.
One of the most rigorously tested designs in this area is the University of Virginia (UVA) closed-loop control (CLC) algorithm.4–6 This system has been tested since 2011 as the first portable, wearable AP platform on the Diabetes Assistant (DiAs) using an Android smartphone to run the CLC algorithm.7 It has been tested in inpatient trials, at diabetes camps, and in outpatient environments.7–15 The multinational home use trial of this system in 30 adults demonstrated improvement from run-in sensor augmented pump (SAP) control for TIR, hypoglycemia, and glucose variability.14 A randomized controlled trial of 32 adolescents participating in a ski camp also demonstrated significant improvement for TIR and hypoglycemia exposure of the AP over SAP therapy.15
In this study the commercial CLC algorithm developed by TypeZero Technologies was on-boarded to a commercial Tandem t:slim X2 insulin pump (called the Control-IQ system) paired with a Dexcom G6 CGM. After a successful pilot trial in adults,16 this AP system was tested for superiority against SAP therapy in children 6–12 years old during 3 days of home use as part of a randomized controlled clinical trial (RCT). We hypothesized that the AP system would be superior to SAP therapy for TIR under these real-world conditions.
Research Design and Methods
Study design
We tested the t:slim X2 with Control-IQ Technology AP system in a multisite, randomized controlled clinical trial (clinicaltrials.gov registration NCT03369067) designed to assess the efficacy of the AP to improve TIR over SAP therapy in children 6–12 years old during 3 days of home use. The research protocol was approved by the Food and Drug Administration (IDE#G170267) and UVA, University of Colorado, and Stanford University institutional review boards. Major inclusion criteria included clinical diagnosis of type 1 diabetes (T1D), daily insulin therapy for ≥6 months with pump therapy for ≥3 months, willingness to use only lispro or aspart during the trial, avoidance of acetaminophen, willingness to wear a CGM and activity monitor, and a parent/caregiver committed to receive training on the system. Major exclusion criteria included diabetic ketoacidosis (DKA) in the past 6 months; hypoglycemic seizure or loss of consciousness in the past 6 months, history of seizure disorder, history of altitude sickness, chronic pulmonary conditions, use of oral glucocorticoids, history of renal disease, requirement of intermediate or long-acting insulin or other antidiabetic medications, presence of febrile illness, and other medical and psychiatric conditions that could interfere with completion of the study (e.g., adrenal insufficiency or inpatient psychiatric treatment in the past 6 months).
Subjects and guardians provided informed assent and consent. After enrollment at each site, subjects were paired by hemoglobin A1c (HbA1c), age, and sex, and each member was randomly assigned to either remotely monitored SAP (control arm) or t:slim X2 with Control-IQ Technology AP (treatment arm). Subjects participated in a 48-h ski camp whose results are described in a separate article by Ekhlaspour et al.17 At the completion of the camp, the subjects at the Barbara Davis Center and Stanford University sites participated in 72 h of home use of either the AP system or continued SAP therapy. Parents and subjects were provided in-person teaching on how to use the t:slim X2 with Control-IQ system (AP group only). Both groups were also fitted with a Dexcom G5 Share to be able to be remotely monitored.
Devices/system
Subjects in both the SAP and AP groups wore a Dexcom G5 CGM (Dexcom, San Diego, CA) calibrated at least twice a day (before breakfast and dinner), using a study-provided blood glucose meter (ContourNext Link; Ascencia Diabetes Care, Parsippany, NJ). Subjects in the SAP group wore their own insulin pump. Subjects in the AP group wore the t:slim X2 with Control-IQ AP system (Tandem Diabetes Care, San Diego, CA) controlled by a separate Dexcom G6 CGM. As remote monitoring for Dexcom G6 was not yet available at the time of conducting this clinical trial, this group wore two CGMs, one Dexcom G5 and one Dexcom G6. The Control-IQ AP system is the commercial form of the UVA/TypeZero algorithm previously described that ran directly on the t:slim X2 pump.4–6 All subjects' insulin pump parameters were reviewed by a study physician before starting the home-use phase. Meal boluses were computed using carbohydrate counts determined by the patient and/or guardian as was developmentally necessary. Participants in the SAP group were permitted to use temporary basal rates and manual pump suspensions as desired. Insulin pump infusion set preferences for type and location were determined by subjects and parents.
Remote monitoring and safety protocols
During the home-use trial, subjects in both groups were remotely monitored through alerts by a study physician 24 h/day using the Dexcom G5 Follow App. In addition, subjects were remotely monitored by their parents/guardians using the same app. CGM alarms were set on the parents' Dexcom Follow App for 70 mg/dL (low alert) and 250 mg/dL (high alert). Glycemic treatment guidelines were provided to families for management of hypo- and hyperglycemia (Table 1). Families were permitted to take decisions on participants for both, hypo- and hyperglycemia through the CGM values. The monitoring physician was alerted for CGM values ≤60 mg/dL for 15 min or ≥300 mg/dL for 60 min.
Table 1.
Glycemic Treatment Guidelines
| Measurement | Conditions | Actions | Follow-up |
|---|---|---|---|
| A. Any SMBGa | Seizure Loss of consciousness Unable to eat or drink |
• Stop the system • Notify MD as soon as possible • Disconnect the study insulin pump • Give 1 mg of glucagonb following instructions in the glucagon emergency kit o Glucagon may be repeated in 20 min if needed o Avoid orange juice and milk ingestion after glucagon due to possible nausea and vomiting • Call 9-1-1 • Test BG and when able to eat or drink, give 16–32 g of fast acting carbs (preferably glucose tabs or gel/liquid) with NO insulin. Repeat until BG >80 mg/dL • When BG >80 mg/dL resume insulin pump therapy with HOME insulin pump (NOT STUDY PUMP) • The study will be stopped |
Treat firstc Repeat SMBG when able and every 15 min until BG >100 mg/dL |
| B. Two consecutive SMBGs <50a | Conscious Able to eat and drink |
• Notify MD as soon as possible • Consecutive is >30 and <60 min apart • Treat with 8 glucose tablets (or ∼32 g of fast-acting carbs) and check BG ∼every 15 min • Treatment screen • If there are 2 consecutive (>30 and <60 min) SMBG <50, the subject will stop participation in the study o Disconnect the study insulin pump o Notify MD as soon as possible o When BG ≥100 mg/dL resume insulin pump therapy with HOME insulin pump (NOT STUDY PUMP) |
Check SMBG every 15 min until BG ≥100 mg/dL |
| C. SMBG 50–69d | Conscious Able to eat and drink |
• Notify MD as soon as possible • Treat with 4 glucose tablets (or ∼16 g of fast-acting carbs) and check BG ∼every 15 min • Repeat as needed |
Check SMBG every 15 min until BG ≥100 mg/dL |
| D. Pre-exercise SMBG >100e | Asymptomatic and well | • Able to exercise | Follow CGM-based guidelines |
| E. Pre-exercise SMBG ≤100e,f | Before exercise | • Treat with fast acting carbs until BG >100 mg/dL before exercising | Follow CGM-based guidelines after SMBG >100 mg/dL |
| F. SMBG >300d | Feeling well | • Check infusion set and check that pump is working properly • Consider correction bolus via pen or syringe • Continue with study |
Check BG every 1 h until BG <250 mg/dL |
| G. CGM <60d | Conscious Able to eat and drink |
• Performed SMBG, if lower than 69 proceed with B or C | Check SMBG every 15 min until BG ≥100 mg/dL |
| H. CGM 60–299g | Feeling well | • Follow devices user guide and alerts (e.g., take CHO for hypoglycemia threshold or predictive alerts) | Consider checking SMBG if arrow is pointing straight down or straight up |
| I. CGM >300 for more than 1 h | Feeling well | • Performed SMBG, If SMBG >300 mg/dL follow F | Check BG every 1 h until BG <250 mg/dL |
| J. CGM >300 for more than 2 h | Feeling well | • Check ketones, if ketones >0.6 follow K or L • Performed SMBG, If SMBG >300 mg/dL follow F |
Check BG every 1 h until BG <250 mg/dL |
| Ketone measurements: test for ketones any time indicated above. Refer to table below if ketones ≥0.6 | |||
| K. Ketones 0.6–3.0a | Any | • Notify MD as soon as possible • Consider taking correction dose of insulin by syringe or pen • Change insulin in reservoir and tubing and change pump site • Drink sugar-free beverages • Once BG is 100–250 mg/dL, restart pump treatment in appropriate mode. Record the amount of insulin that was given when prompted |
Check SMBG and ketones every hour until BG <250 mg/dL and ketones ≤0.6 |
| L. Ketones >3.0a | Any | • The participant will stop the study • Notify MD as soon as possible • Disconnect the STUDY insulin pump and start HOME insulin pump once instructed by MD • Take correction dose of insulin by syringe or pen • Drink sugar-free beverages |
Check SMBG and ketones every hour until BG <250 mg/dL and ketones ≤0.6 or until transferred to an appropriate treatment environment |
| Symptoms requiring action regardless of blood sugar or ketone levels | |||
| Anya | Abdominal pain Vomiting illness Unable to eat or drink Fever ≥101.5°F Clinical need for Tylenol/acetaminophen Significant illness Use of epinephrine or glucocorticoid, for example to treat a severe allergic reaction |
• Notify MD as soon as possible • Participant STOP study • Disconnect the STUDY insulin pump and start HOME insulin pump • Subcutaneous insulin correction and insulin infusion set changeh |
Check SMBG and ketones every hour until symptoms resolve or until transferred to an appropriate treatment environment |
Subjects will be trained and instructed to respond to CGM and/or artificial pancreas alarms and treat hypoglycemia/hyperglycemia readings accordingly.
Self-monitoring blood glucose measurements schedule:
• Before exercise
• CGM hypoglycemia <60 mg/dL
• CGM hyperglycemia >300 mg/dL
• Before CGM calibration (prebreakfast and predinner could be used to calibration)
The subject may stop participation in the study.
Glucagon 1 mg can be given subcutaneously or intramuscularly. Repeat meter glucose level in 15 min.
Oral glucose treatment consists of ∼16 g of simple carbohydrate, for example, glucotabs or juice followed by a repeat meter glucose measurement after 15 min.
Treatment may be needed.
Correspond to pre-exercise.
SMBG will be performed within 30 min of exercise; if <100 mg/dL subject will be treated until >100 mg/dL before beginning exercise.
Continue the study.
Additional correction boluses may be administered no more frequently than every 4 h as needed to achieve meter glucose between 80 and 250 mg/dL and β-ketone measurement ≤0.6 mmol/L.
CGM, continuous glucose monitor; MD, medical doctor; SMBG, self-management blood glucose.
Per glycemic guidelines, parents were instructed to provide carbohydrates for a CGM value <70 mg/dL and to repeat every 15 min until the CGM value reads >80 mg/dL with optional self-management blood glucose (SMBG) confirmation. For CGM >300 mg/dL parents were instructed to test SMBG every 60 min. Individual subject stopping criteria were (1) malfunction of the system or controller that imposes upon safety, (2) hypoglycemic seizure or coma, (3) abdominal pain, vomiting, or decreased consciousness, (4) pregnancy, (5) loss of sensor for >6 h, (6) two consecutive SMBG values <50 mg/dL 30–60 min apart. Study stopping criteria included two episodes of DKA or two episodes of severe hypoglycemia resulting in stopping the study for individual subjects.
Outcomes and statistical analysis
All glucose outcomes were calculated based on the Dexcom G5 sensor glucose (SG) information. Primary outcome was the percent time in target range 70–180 mg/dL. Secondary outcomes included mean glucose, glycemic variability based on coefficient of variation (CV), percent time <70, <60, and <54, <50 mg/dL, >180, >250, and >300 mg/dL, percent time 70–140 mg/dL overnight (11 PM−7 AM), percent time 70–180 mg/dL during the day (7 AM−11 PM), total daily insulin dose, and total amount of carbohydrates required for hypoglycemia treatment. System usability was assessed by determining participant percent time with the hybrid closed-loop (HCL) algorithm being active during the study period. Participant experience was assessed by a 38-item Technology Acceptance Questionnaire where responses were quantified on a 5-point Likert Scale where 1 was strongly disagree and 5 was strongly agree (Table 2).
Table 2.
Technology Acceptance Questionnaire Responses
| Question | Mean ± SD | Percent of responses in “Best 2” categories | |
|---|---|---|---|
| Q1 | It caused too many hassles in my daily life | 1.50 ± 1.17 | 91.7 |
| Q2 | Carrying around all of the equipment was a burden | 2.25 ± 1.48 | 66.7 |
| Q3 | I worried about looking different because I had all of this stuff to carry around | 1.25 ± 0.87 | 91.7 |
| Q4 | I was annoyed by the weight of the devices I had to carry around | 1.75 ± 1.22 | 83.3 |
| Q5 | It was a big bother having to change sensors and pump sites | 1.58 ± 1.16 | 91.7 |
| Q6 | I had greater peace of mind while wearing the device | 3.83 ± 1.59 | 75.0 |
| Q7 | I felt much freer with my food choices | 3.75 ± 1.22 | 58.3 |
| Q8 | I felt freer to do the things I wanted to do | 3.25 ± 1.06 | 41.7 |
| Q9 | I spent much less time thinking about my diabetes | 4.25 ± 1.54 | 83.3 |
| Q10 | I was less worried about my blood sugars | 3.75 ± 1.48 | 66.7 |
| Q11 | I was less worried about how my insulin was working | 3.67 ± 1.61 | 58.3 |
| Q12 | I felt like I had more freedom to live my life | 3.67 ± 1.44 | 66.7 |
| Q13 | The study device was more intrusive than my typical method of diabetes care | 2.33 ± 1.67 | 75.0 |
| Q14 | It was uncomfortable to wear all of the necessary equipment | 1.91 ± 1.30 | 72.7 |
| Q15 | I was bothered by how long it took the device to respond to low blood sugars | 1.82 ± 1.33 | 72.7 |
| Q16 | I was bothered by how long it took the device to respond to high blood sugars | 2.18 ± 1.83 | 72.7 |
| Q17 | I was never entirely comfortable with allowing the device to take over my diabetes care | 1.73 ± 1.42 | 81.8 |
| Q18 | I found it hard to trust that the study device could control my blood sugars | 2.09 ± 1.58 | 72.7 |
| Q19 | I felt less burdened in managing diabetes while I was using it than I do when using my typical method of diabetes care | 3.45 ± 1.97 | 63.6 |
| Q20 | I had trouble sleeping well while wearing it | 1.73 ± 1.27 | 81.8 |
| Q21 | The study device allowed me to have more time to devote to other areas of my life | 3.09 ± 1.51 | 45.5 |
| Q22 | Wearing it made me think about diabetes too much | 2.18 ± 1.47 | 81.8 |
| Q23 | It taught me things about my diabetes that I didn't know before | 2.55 ± 1.44 | 27.3 |
| Q24 | It helped to prevent low blood sugars from happening | 3.91 ± 1.45 | 63.6 |
| Q25 | It had too many “glitches” and “bugs” | 1.27 ± 0.90 | 90.9 |
| Q26 | It helped me to relax, knowing that unwanted changes in blood sugar would be addressed automatically | 3.92 ± 1.51 | 75.0 |
| Q27 | I found the device uncomfortable to wear | 1.75 ± 1.06 | 75.0 |
| Q28 | Using the device was more trouble than it was worth | 1.42 ± 1.00 | 83.3 |
| Q29 | It helped to prevent blood sugar problems from happening | 4.08 ± 1.16 | 75.0 |
| Q30 | It helped me worry less about low blood sugars | 4.08 ± 1.24 | 66.7 |
| Q31 | It helped me to worry less about high blood sugars | 3.75 ± 1.71 | 66.7 |
| Q32 | When this study is over, I would very much like to keep using the device | 3.75 ± 1.66 | 58.3 |
| Q33 | I often challenged the study device with food and exercise to see how it would react | 3.33 ± 1.50 | 41.7 |
| Q34 | I felt confident that the device would respond well to a high or low | 3.75 ± 1.54 | 75.0 |
| Q35 | By the end of the study, I trusted the device to manage my blood glucose | 3.67 ± 1.56 | 66.7 |
| Q36 | I never stopped worrying about having a low while sleeping | 2.00 ± 1.35 | 58.3 |
| Q37 | It will be hard to give up the device once the study is over | 3.83 ± 1.64 | 66.7 |
| Q38a | How easy to use was the device? | 4.20 ± 1.48 | 80.0 |
| Q38b | How useful in managing your diabetes was the device? | 4.33 ± 1.12 | 77.8 |
| Q38c | How much did you trust the device? | 4.11 ± 1.05 | 77.8 |
Likert Scale: 1, strongly disagree; 2, disagree; 3, neither agree nor disagree; 4, agree; 5, strongly agree.
Sample size was calculated with the assumption of a medium effect size of 0.3 (based on previous algorithm trials) using a 3 × 2 within-between repeated-measures analysis of variance, with correlation of 0.5 between measures. Using G-power 3.1.9.2 (University of Dusseldorf), with a sample size of 24 subjects, there was a 91.7% power to detect a true difference of the effect size of 0.3 for the primary outcome of TIR. Significance level was set at P < 0.05. Data are reported as mean ± SD when normally distributed or as median (75% confidence interval) when skewed. The statistical analysis was performed in SPSS 25 (IBM), and data formatting and preparation were executed in Matlab 2018a (MathWorks).
Results
Subject characteristics
In total, 24 subjects were enrolled at two clinical sites, and all 24 completed the home-use portion of the study (12 at Barbara Davis Center and 12 at Stanford). By design, sexes were balanced with six male and six female participants at each site with a 50/50 split of SAP and AP users. For the entire cohort, age was 9.6 ± 1.9 years. Enrollment HbA1c was 7.35% ± 0.68%. Duration of T1D was 4.5 ± 1.9 years with 3.8 ± 1.8 years of pump use. BMI was 18.5 ± 3.2 kg/m2 and total daily insulin dose was 0.74 ± 0.2 U/(kg·day). Demographic characteristics by treatment group are broken down in Table 3. There were no differences between groups for any of the baseline/enrollment characteristics.
Table 3.
Demographics
| Control-IQ | SAP | P | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | ||
| n | 12 | 12 | - |
| Sex | 50% male, 50% female | 50% male, 50% female | ns |
| Age (years) | 10.0 ± 2.1 | 9.2 ± 1.5 | ns |
| BMI (kg/m2) | 19.2 ± 2.7 | 17.8 ± 3.5 | ns |
| Diabetes duration (years) | 4.7 ± 2.3 | 4.4 ± 1.4 | ns |
| Pump use (years) | 4.1 ± 1.9 | 3.5 ± 1.7 | ns |
| Total insulin dose (U/day) | 29.5 ± 11.2 | 23.7 ± 7.0 | ns |
| Total insulin dose [U/(kg·day)] | 0.76 ± 0.21 | 0.71 ± 0.18 | ns |
| HbA1c (%) | 7.35 ± 0.74 | 7.36 ± 0.65 | ns |
Overall glycemic control
Time in target range of 70–180 mg/dL was significantly improved in the Control-IQ group compared with the SAP group (Control-IQ: 71.0% ± 6.6% vs. SAP: 52.8% ± 13.5%; P = 0.001). This difference of 18.2% is 262 min or 4.4 h/day of increased time in target range. Mean SG was also significantly improved in the Control-IQ group compared with the SAP group (Control-IQ: 153.6 ± 13.5 vs. SAP: 180.2 ± 23.1 mg/dL; P = 0.003). Glycemic variability as assessed by CV was not significantly different between groups (Control-IQ: 36.6% ± 4.9% vs. SAP: 36.5% ± 5.4%; not significant [ns]). Hyperglycemia exposure was improved for time >180 mg/dL and >250 mg/dL in the Control-IQ group compared with the SAP group (Table 4).
Table 4.
Glycemic Outcomes Measured by Continuous Glucose Monitor
| Full day (24 h) | Daytime (7 AM–11 PM) | Overnight (11 PM–7 AM) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control-IQ | SAP | P | Control-IQ | SAP | P | Control-IQ | SAP | P | |
| Percent 70–180 mg/dL | 71.2 ± 6.3 | 52.8 ± 13.5 | <0.001 | 69.3 ± 9.7 | 54.4 ± 14.2 | 0.007 | 74.9 ± 9.7 | 49.6 ± 18.8 | <0.001 |
| Time <50 mg/dL (%) | 0 (0–0.2) | 0 (0–0.4) | ns | 0 (0–0) | 0 (0–0.6) | ns | 0 (0–0) | 0 (0–0) | ns |
| Time <54 mg/dL (%) | 0.3 (0–0.5) | 0.2 (0–0.6) | ns | 0.2 (0–0.6) | 0.3 (0–0.9) | ns | 0 (0–0) | 0 (0–0) | ns |
| Time <60 mg/dL (%) | 0.7 (0.2–1.2) | 0.5 (0–0.9) | ns | 0.6 (0–1.4) | 0.7 (0–1.3) | ns | 0 (0–0.3) | 0 (0–0) | ns |
| Time <70 mg/dL (%) | 2.1 ± 1.5 | 2.1 ± 2.9 | ns | 1.7 (0.7–2.9) | 1.4 (0.5–3.4) | ns | 0.9 (0–2.8) | 0 (0–0) | 0.112 |
| Time 70–140 mg/dL (%) | 48.5 ± 9.5 | 28.7 ± 11.7 | <0.001 | 45.3 ± 12.4 | 30 ± 10.5 | 0.004 | 54.9 ± 13.3 | 26.1 ± 18.4 | <0.001 |
| Time >180 mg/dL (%) | 26.2 ± 7.1 | 44.7 ± 13.8 | <0.001 | 27.5 ± 10.8 | 42 ± 14.4 | 0.010 | 23.6 ± 9.5 | 49.9 ± 19.3 | <0.001 |
| Time >250 mg/dL (%) | 6.8 ± 4.5 | 16.1 ± 10.3 | 0.009 | 7.9 ± 6.2 | 14.8 ± 11 | 0.069 | 4.8 ± 7.8 | 18.7 ± 12.9 | 0.004 |
| Time >300 mg/dL (%) | 2.7 ± 2.7 | 5.3 ± 3.9 | 0.065 | 3.2 ± 3.9 | 4.4 ± 4.5 | ns | 1.7 ± 3.8 | 7.1 ± 6.5 | 0.021 |
| Mean glucose (mg/dL) | 152.2 ± 13.8 | 180.2 ± 23.1 | 0.002 | 155.2 ± 20.2 | 175.7 ± 24.7 | 0.038 | 146.3 ± 15.9 | 188.8 ± 30.2 | <0.001 |
| Coefficient of variation (%) | 32.6 ± 4.1 | 33.3 ± 5.4 | ns | 31.7 ± 5 | 33 ± 6.5 | 0.198 | 25.7 ± 6.6 | 20.7 ± 5.2 | ns |
| Insulin (U/kg) | 33.1 ± 14.8 | 27.8 ± 12.3 | ns | 39.7 ± 18.4 | 33.5 ± 14.4 | ns | 20.1 ± 8.1 | 16.4 ± 9 | ns |
| CHO treatment (g) | 17.5 ± 17.6 | 35.5 ± 55.5 | ns | 25.2 ± 26.3 | 51.7 ± 83.6 | ns | 2.5 ± 5.8 | 3.5 ± 7.9 | ns |
| No. of CHO treatment | 0.8 (0.3–1.4) | 0.3 (0.3–0.8) | ns | 1 (0.4–2) | 0.5 (0.4–1.3) | ns | 0 (0–0) | 0 (0–0.2) | ns |
Values are given as mean ± SD or as median (75% confidence interval).
CHO, carbohydrate; ns, not significant; SAP, sensor augmented pump.
Hypoglycemia exposure <70 mg/dL was not different between the two groups (Control-IQ: 1.7% [1.3%–2.1%] vs. SAP: 0.9% [0.3%–2.7%]; ns). The amount of carbohydrates used for hypoglycemia treatment per day was not significantly different between groups (Control-IQ: 15.5 ± 16.9 vs. SAP: 35.5 ± 55.5 g/day; ns), although this appears to have trended in the expected direction of being lower in the Control-IQ group than in the SAP group; the median number of treatments did not differ either, 0.7 (0.2–1.3) vs. 0.3 (0.3–0.8). The total daily dose of insulin was not significantly different between groups (Control-IQ: 33.2 ± 15.5 vs. SAP: 27.8 ± 12.3 U/day; ns).
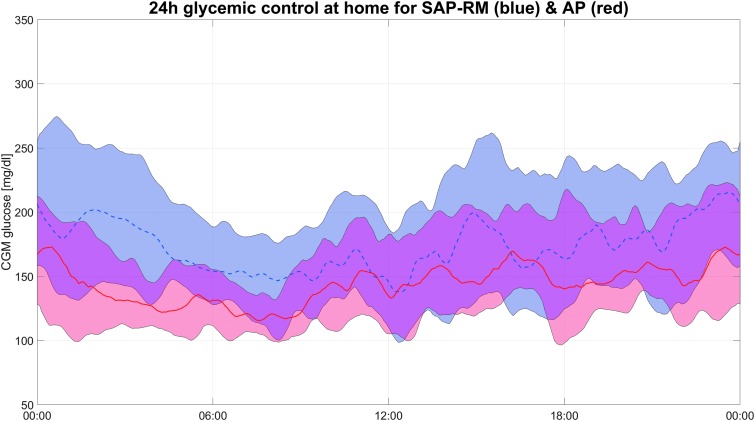
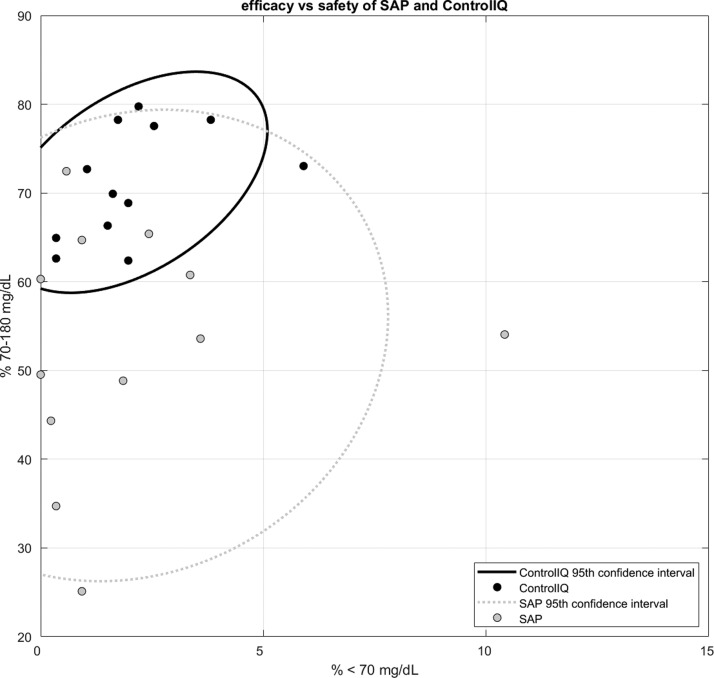
Visualization of the full-day glycemic profiles for the two groups by envelope graph (Fig. 1) demonstrates that the mean glucose values are clearly lower for virtually all periods of the day without increased exposure into the hypoglycemic regions for the Control-IQ group compared with the SAP group. The improved glycemic control is most evident during the overnight portion, particularly from midnight to 6 AM. Further visualization of the differences between glycemic control of the two groups by ellipse graph (Fig. 2) shows several notable findings. First it can be seen that for both groups improved TIR is generally associated with increased hypoglycemia (considered 54–70 mg/dL but not <54 mg/dL), exposure within the 0%–5% range. Second it can be seen that while some patients in the SAP group were able to achieve tight control with >60% TIR and <4% time <70 mg/dL, all the Control-IQ users achieved this level of control. This narrowing of the population control with automated delivery is particularly noteworthy as AP systems move toward real-world commercial use.
FIG. 1.
Full-day glycemic control during home use. The red line and shaded area represent Control-IQ use and the blue line and shaded area represent SAP use. The shaded area represents the 25th to 75th percentile for glucose values in each group. The center line (plan and dotted) represents the mean. SAP, sensor augmented pump.
FIG. 2.
Time in target range 70–180 mg/dL and time in hypoglycemia <70 mg/dL. Subjects in Control-IQ (black) and SAP (gray) along with the 95th confidence interval for the fit around each group.
Overnight glycemic control
Additional analysis was conducted focusing on the overnight period from 11 PM to 7 AM (8 h). TIR was significantly improved overnight in the Control-IQ group compared with the SAP group (Control-IQ: 74.9% ± 10.1% vs. SAP: 49.6% ± 18.8%; P = 0.001). This difference of 25.3% is 121 min or 2 h of improved time per day in target range overnight. About half of the total daily improved time in target range occurred during the night. Time in the narrow target range of 70–140 mg/dL overnight was 54.9% versus 26.1%, P ≤ 0.001, an almost 2.5 h/day improvement. The mean SG was significantly improved overnight (Control-IQ: 146.3 ± 15.9 vs. SAP: 188.8 ± 30.2 mg/dL; P ≤ 0.001). Glycemic variability as assessed by CV was not significantly different between groups (Control-IQ: 33.4% ± 7.1% vs. SAP: 32.9% ± 6.4%; ns). Hyperglycemia exposure was improved for time >180 mg/dL, >250 mg/dL, and >300 mg/dL in the Control-IQ group compared with the SAP group overnight. Hypoglycemia exposure, treatment for hypoglycemia, and total insulin use were not significantly different between groups during the overnight period.
System usability
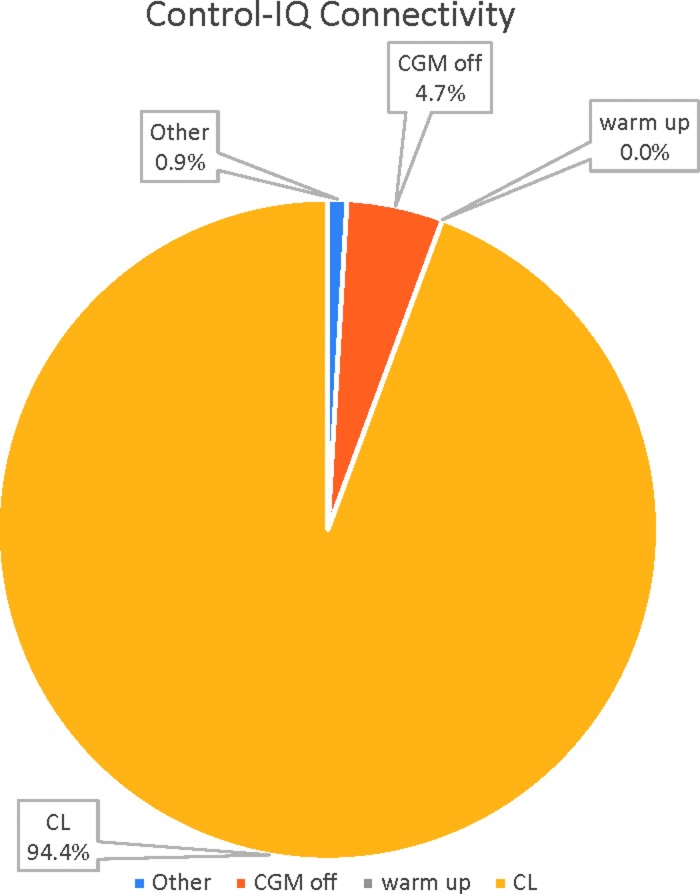
System usability was assessed by objective analysis of percent time for which the HCL control was active and subjective responses to the Technology Acceptance Questionnaire using Likert scale. During remote-monitored real-world use, the Control-IQ system was in closed loop for 94.4% of the time (Fig. 3). Subjects were out of closed loop (CL) for 4.7% of the time because of loss of CGM communication with the system. Subjects were out of CL for <1% of the time because of other system issues. It is worth noting that the 2-h CGM warm-up occurred during the ski camp study before home use and thus there was no down time because of the warm-up for a new sensor during this study period.
FIG. 3.
Connectivity of the Control-IQ system during home use. System was in closed loop 94.4% of the time, CGM was not connected 4.7% of the time, system was inactive for other system issues 0.9% of the time, and CGM was in warm up for 0% of the time. CGM, continuous glucose monitor; CL.
Overall, subjects had favorable subjective responses to the system as assessed by the Technology Acceptance Questionnaire (Table 2). Subjects disagreed or strongly disagreed with the statements that “Using the device was more trouble than it was worth” (1.42 ± 1.0; 83.3%), “It had too many glitches and bugs” (1.27 ± 0.9; 90.9%), and “It caused too many hassles in my daily life” (1.50 ± 1.17; 91.7%). Subjects agreed or strongly agreed with the statements that “I spent much less time thinking about my diabetes” (4.25 ± 1.54; 83.3%), the devices were “easy to use” (4.2 ± 1.48; 80.0%), “useful in managing your diabetes” (4.33 ± 1.12; 77.8%), and that they could “trust the device” (4.11 ± 1.05; 77.8%). Majority of subjects also agreed that “It will be hard to give up the device once the study is over” (3.83 ± 1.64; 66.7%).
Conclusions
Results from this home-use study of the commercial Tandem t:slim X2 with Control-IQ HCL AP system in school-aged children indicate that this system significantly improved glycemic control in children 6–12 year old. Although this system is currently undergoing pivotal study, we report a decrease in average SG of 27 mg/dL for the full day and 42 mg/dL overnight. The increase in TIR by 4.4 h/day also indicates that profoundly greater glycemic control can be possible with AP system use. This improvement was seen through hyperglycemia reduction, primarily overnight, without increased hypoglycemia exposure. The system was demonstrated to be extremely dependable as it operated in closed-loop mode for 94.4% of the possible time. The system also scored very high for usability by subjects as measured by responses to the Technology Acceptance Questionnaire.
This study system is a commercial evolution of the DiAs HCL AP system that underwent extensively outpatient testing in adults as reported by Anderson et al.14 The Anderson study investigated multinational home use in 30 participants for 3 weeks of SAP run-in, 2 weeks of overnight-only AP use, and 2 weeks of full-day HCL. They reported TIR of 73% (68%–76%) for the full day with HCL therapy, a mean SG of 153 ± 12 mg/dL, and percent <70 mg/dL of 1.7% (1.1%–2.7%). This study of the Control-IQ system in children produced similar HCL results with TIR of 71.0% ± 6.6%, mean SG of 153.6 ± 13.5 mg/dL, and percent <70 mg/dL of 1.7% (1.3%–2.1%). It is notable however that the baseline comparison data for the Anderson study showed much better glycemic control than the SAP group for this study.
In this trial, the SAP control arm consisted of patients using their own insulin pump with settings adjusted by a pediatric endocrinologist several times before the home-use phase. Furthermore, the patients in the SAP group had continuous remote monitoring of their CGM by both a parent and pediatric endocrinologist. This level of supervision, which was deemed necessary owing to the experimental group because it was the first use of the HCL system in an at-home setting, is substantially greater than typical monitoring for patients conducting normal SAP therapy.
The patient population studied in this trial, children 6–12 years of age, has been largely understudied in AP trials to date. The pivotal trial of the commercially available MiniMed 670G HCL AP was conducted in patients who were 14+ years old.18 A subsequent pivotal trial of the 670G has since been conducted in children 7–13 years of age.19,20 This trial reports data on 105 children 7–13 years of age using the 670G for 3 months. The subjects in this trial had an average SG of 161.7 ± 12.4 mg/dL, with 65.0% ± 7.7% TIR, and 3.0% ± 1.6% time <70 mg/dL. The MiniMed 670G contains safety features that can cause it to revert from HCL function (Auto Mode) to traditional open-loop pump function (Manual Mode).21 In the pediatric pivotal trial, the subjects spent 80.6% (70.0%–87.7%) time in Auto Mode.20 Russell et al. also report results on use of a bihormonal (insulin and glucagon) AP system in children 6–11 years of age.22 They reported average SG of 136.8 ± 10.8 mg/dL, with 80.6% ± 7.4% TIR, 1.2% ± 1.1% time <60 mg/dL, and loss of CGM communication with the pump 4.2%–6.1% of the time. Direct comparison of the brief present trial with the 3-month 670G pivotal data and the bihormonal data is highly limited because of differences in study design. Synthesis of the data from the three trials, however, demonstrates that in elementary school-aged children with T1D, HCL AP systems can achieve control of >65% TIR with ≤3% hypoglycemia <70 mg/dL.
Beyond simply improving glycemic control, a major proposed benefit of AP systems is their potential to reduce diabetes burden and improve quality of life for patients and their families. A study by Garza et al. investigated the hopes and expectations of children, adolescents, and adults with T1D and their families regarding new AP systems.23 This study identified three main themes: (1) expectation that diabetes technology will alleviate diabetes-specific worry and burden, (2) that technology may reduce day-to-day stress, and (3) that technology may improve family relationships. The third theme was not addressed in this study; however, participant responses to several of the technology acceptance questions showed support for benefit in two other themes. A majority of subjects endorsed spending less time thinking about diabetes, having greater peace of mind, improved ability to relax, decreased worry, less burden, and more freedom to live their lives while using the Control-IQ system. In addition, subjects were able to keep the HCL system active for 94.4% of the time while strongly endorsing that the system did not have too many glitches or cause hassles in their daily lives. These factors strongly support a high degree of usability for this device.
There were several limitations to this study. Because of this being the first outpatient use of this build of the system in children, a high degree of physician oversight was provided to both groups through continuous remote monitoring by a pediatric endocrinologist. This may have biased both the experimental and control groups toward better control than could be seen in the real world, thereby limiting generalizability. Subjects had enrollment HbA1c values of <7.5% on average in both groups, which indicates selection bias for study recruitment, although balanced between study groups, which may further limit generalizability. As noted in the methods, subjects participated in a 3-day ski camp session before the home-use study. High-intensity exercise in the preceding 3 days, although not generally uncommon in school-aged children, may have impacted the insulin sensitivity and glycemic profiles of subjects during this study period although not differentially between arms of the study.
In conclusion, the Tandem t:slim X2 with Control-IQ HCL AP System significantly improved TIR and mean SG without increasing hypoglycemia during remote-monitored home use in elementary school-aged children with T1D. Subjects found the system to be very easy to use and were able to maintain the system in closed loop for almost the entirety of home use. These data will help inform pivotal trials of this system with larger patient numbers and longer duration are ongoing in adults, adolescents, and children.
Acknowledgments
The authors thank all the families who participated in this study, Mary Oliveri for regulatory work, Tandem Diabetes care and Dexcom for providing the funding and equipment and the University of Virginia strategic investment in type 1 diabetes fund-PriMeD project.
Author Contributions
G.P.F. wrote the article, reviewed the protocol, and conducted the clinical study at the Barbara Davis Center. L.E. reviewed the protocol and article and conducted the clinical study at Stanford. M.B. co-wrote the investigational device exemption oversaw all phases of the study, and reviewed the article and protocol. D.M.M. reviewed the protocol and article and assisted with conducting the clinical study at Stanford. R.P.W. reviewed the protocol and article and assisted with conducting the clinical study at BDC. M.D. reviewed the protocol and article and assisted with conducting the study at UVA. L.H.M. reviewed the protocol and article and coordinated the study at BDC. M.T. reviewed the protocol and article and coordinated the study at Stanford. J.P. reviewed the protocol and manuscript and coordinated the study at UVA. G.K. reviewed the IDE and protocol and provided engineering support at all three sites. B.A.B. reviewed the protocol and article and conducted the clinical study at Stanford. D.C. wrote the clinical protocol, co-wrote the IDE, reviewed the article, and conducted the clinical study at UVA.
Author Disclosure Statement
G.P.F. conducts research sponsored by Medtronic, Dexcom, Abbott, Tandem, Insulet, Bigfoot, Beta Bionics, and TypeZero; he has served as a consultant/speaker for Medtronic, Dexcom, Abbott, and Tandem. D.C. has research support from NLM, the higher education council of Virginia (SCHEV) and his institution (UVA). He is part time CMO of TypeZero Technologies, Inc. (recently acquired by Dexcom, Inc.) and has served as speaker for Tandem and JDRF. L.H.M. has served as a consultant for Tandem Diabetes Care, Clinical Sensors, and Capillary Biomedical. D.M.M. has research support from the NIH, JDRF, NSF, and the Helmsley Charitable Trust and his institution has research support from Medtronic, Dexcom, Insulet, Bigfoot Biomedical, Tandem, and Roche. He has also consulted for Abbott, the Helmsley Charitable Trust, Sanofi, Novo Nordisk, Eli Lilly, and Insulet. M.B. has research support handled by the UVA: Dexcom, Roche, SANOFI, Tandem; Patent Royalties handled by the UVA: SANOFI; Dexcom. Consultant: SANOFI, TANDEM; Ascencia Speaker's Bureau: Roche, Ascencia.
References
- 1. Bekiari E, Kitsios K, Thabit H, et al. : Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weisman A, Bai JW, Cardinez M, et al. : Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 3. Dai X, Luo ZC, Zhai L, et al. : Artificial pancreas as an effective and safe alternative in patients with type 1 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther 2018;9:1269–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kovatchev B, Patek S, Dassau E, et al. : Control to range for diabetes: functionality and modular architecture. J Diabetes Sci Technol 2009;3:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patek SD, Magni L, Dassau E, et al. : Modular closed-loop control of diabetes. IEEE Trans Biomed Eng 2012;59:2986–2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hughes CS, Patek SD, Breton MD, Kovatchev BP: Hypoglycemia prevention via pump attenuation and red-yellow-green “traffic” lights using continuous glucose monitoring and insulin pump data. J Diabetes Sci Technol 2010;4:1146–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cobelli C, Renard E, Kovatchev BP, et al. : Pilot studies of wearable outpatient artificial pancreas in type 1 diabetes. Diabetes Care 2012;35:e65–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kovatchev BP, Renard E, Cobelli C, et al. : Safety of outpatient closed-loop control: first randomized crossover trials of a wearable artificial pancreas. Diabetes Care 2014;37:1789–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kovatchev BP, Renard E, Cobelli C, et al. : Feasibility of outpatient fully integrated closed-loop control: first studies of wearable artificial pancreas. Diabetes Care 2013;36:1851–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeSalvo DJ, Keith-Hynes P, Peyser T, et al. : Remote glucose monitoring in cAMP setting reduces the risk of prolonged nocturnal hypoglycemia. Diabetes Technol Ther 2014;16:1–7 [DOI] [PubMed] [Google Scholar]
- 11. Ly TT, Breton MD, Keith-Hynes P, et al. : Overnight glucose control with an automated, unified safety system in children and adolescents with type 1 diabetes at diabetes camp. Diabetes Care 2014;37:2310–2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown SA, Kovatchev BP, Breton MD, et al. : Multinight “bedside” closed-loop control for patients with type 1 diabetes. Diabetes Technol Ther 2015;17:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Favero S, Place J, Kropff J, et al. : Multicenter outpatient dinner/overnight reduction of hypoglycemia and increased time of glucose in target with a wearable artificial pancreas using modular model predictive control in adults with type 1 diabetes. Diabetes Obes Metab 2015;17:468–476 [DOI] [PubMed] [Google Scholar]
- 14. Anderson SM, Raghinaru D, Pinsker JE, et al. : Multinational home use of closed-loop control is safe and effective. Diabetes Care 2016;39:1143–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breton MD, Chernavvsky DR, Forlenza GP, et al. : Closed loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the Artificial Pancreas Ski Study. Diabetes Care 2017;40:1644–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brown S, Raghinaru D, Emory E, Kovatchev B: First look at control-IQ: a new-generation automated insulin delivery system. Diabetes Care 2018;41:2634–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ekhlaspour L, Wadwa RP, Chernavvsky D, et al. : Artificial Pancreas (AP) Ski Camp 2018: successful use of the tandem control-IQ AP system in adolescents and children during winter sports and at home (abstract). Pediatric Diabetes 2018;19 (suppl 26):34–35 [Google Scholar]
- 18. Garg SK, Weinzimer SA, Tamborlane WV, et al. : Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technol Ther 2017;19:155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Buckingham B, Shulman D, Forlenza G, et al. : Glycemic outcomes during minimed (TM) 670G system use in children with T1D. Diabetes Technol Ther 2018;20:A19. [DOI] [PubMed] [Google Scholar]
- 20. Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. : Safety evaluation of the MiniMed 670G system in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Messer LH, Forlenza GP, Sherr JL, et al. : Optimizing hybrid closed-loop therapy in adolescents and emerging adults using the MiniMed 670G system. Diabetes Care 2018;41:789–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Russell SJ, Hillard MA, Balliro C, et al. : Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol 2016;4:233–243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garza KP, Jedraszko A, Weil LEG, et al. : Automated insulin delivery systems: hopes and expectations of family members. Diabetes Technol Ther 2018;20:222–228 [DOI] [PMC free article] [PubMed] [Google Scholar]