Abstract
Background: Nearly 20% of colorectal cancer (CRC) patients present with potentially incurable (Stage IV) disease, yet their physicians do not integrate cancer treatment with palliative care. Compared with patients treated by primary providers, surgical patients with terminal diseases are significantly less likely to receive palliative or end-of-life care.
Objective: To describe surgeon perspectives on palliative and end-of-life care for patients with Stage IV CRCs.
Design: This is a convergent mixed methods study using a validated survey instrument from the Critical Care Peer Workgroup of the Robert Wood Johnson Foundation's Promoting Excellence in End-of-Life Care Project with additional qualitative questions.
Settings: Participants were all current, nonretired members of the American Society of Colon and Rectal Surgeons.
Main Outcome Measures: Surgeon-perceived barriers to palliative and end-of-life care for patients with Stage IV CRCs were identified.
Results: Among 131 Internet survey respondents (response rate 16.5%), 76.1% reported no formal education in palliative care, and specifically noted inadequate training in techniques to forgo life-sustaining measures (37.9%) and communication (42.7%). Over half (61.8%) of surgeons cited unrealistic expectations among patients and families as a barrier to care, which also limited discussion of palliation. At the system level, absence of documentation, appropriate processes, and culture hindered the initiation of palliative care. Thematic analysis of open-ended questions confirmed and extended these findings through the following major barriers to palliative and end-of-life care: (1) surgeon knowledge and training; (2) communication challenges; (3) difficulty with prognostication; (4) patient and family factors encompassing unrealistic expectations and discordant preferences; and (5) systemic issues including culture and lack of documentation and appropriate resources.
Limitations: Generalizability is limited by the small sample size inherent to Internet surveys, which may contribute to selection bias.
Conclusions: Surgeons valued palliative and end-of-life care but reported multilevel barriers to its provision. These data will inform strategies to reduce these perceived barriers.
Keywords: : barriers, colorectal cancer, end-of-life, palliative care, surgeon, surgery
Introduction
Among 135,000 Americans diagnosed with colorectal cancer (CRC) every year, 20% present with potentially incurable disease and an additional 50% of those with early stage disease ultimately develop distant metastases.1,2 Emerging literature supports the integration of palliative care into standard care for individuals with serious illness such as metastatic CRC.3,4 However, palliative care is not yet a common component of treatment for the seriously ill; surgical patients, in particular, are less likely to receive palliative care than medical patients.5–7
In addition to patient factors that drive surgical care, surgeon factors influence the care provided to patients even at the end of life.8,9 Surgeon-specific data support the concept of an agreement between the surgeon and the patient, “surgical buy-in,” whereby the surgeon agrees to perform a procedure while the patient implicitly commits to all postoperative care. Owing to this contractual relationship, the surgeon may delay or refuse life-withdrawing measures and the initiation of palliative care referrals.10 Furthermore, limited data indicate that when surgeons are inclined to recommend palliation over curative surgery, they describe a multitude of restrictive factors outside of their control such as delayed consultation, urgency in decision making, and consulting physicians' expectations.11
With the exception of this work, exceedingly little empirical evidence exists to explain how surgeons who care for patients with CRC specifically approach end-of-life care and engage palliative care specialists. To help understand the pervasive palliative care gap among surgical patients, we sought to identify and characterize the most important surgeon-reported barriers to palliative care in patients with Stage IV CRC.
Methods
Using a mixed methods convergent research design, we assessed barriers to optimal end-of-life care.14,15 We specifically sought to identify factors contributing to the discrepancy in receipt of palliative care among CRC patients and to develop an in-depth understanding of surgeon experiences caring for patients at the end of life. We augmented a previously validated survey (quantitative) with open-ended questions (qualitative) to explore surgeons' experiences caring for seriously ill and dying patients.
Study sample
Surgeons were invited to participate through e-mail in an online survey created by SurveyMonkey (Palo Alto, CA). Eligible participants included all members of the American Society of Colon and Rectal Surgeons (ASCRS) who were not retired and ≤70 years old. This study was approved and distributed by the ASCRS Survey Task Force, and approved for exemption by the University of Michigan institutional review board.
Survey format
The survey was modified from an instrument previously validated by the Critical Care Peer Workgroup of the Robert Wood Johnson Foundation's Promoting Excellence in End-of-Life Care Project.14 The original survey contains three domains that characterize barriers to optimal end-of-life care in the ICU: clinician factors, patient/family factors, and institutional factors. A five-point Likert scale was used to assess the relative importance of each barrier. Responses ranged from one (not a barrier) to five (huge barrier). We augmented the survey to include five open-ended questions that captured surgeons' perspectives and experiences (Supplementary Data are available online at www.liebertpub.com/jpm). The questions were based on a literature review of surgeon attitudes toward end-of-life care,37 and revised based on feedback from surgeons and palliative care specialists.
Analytic plan
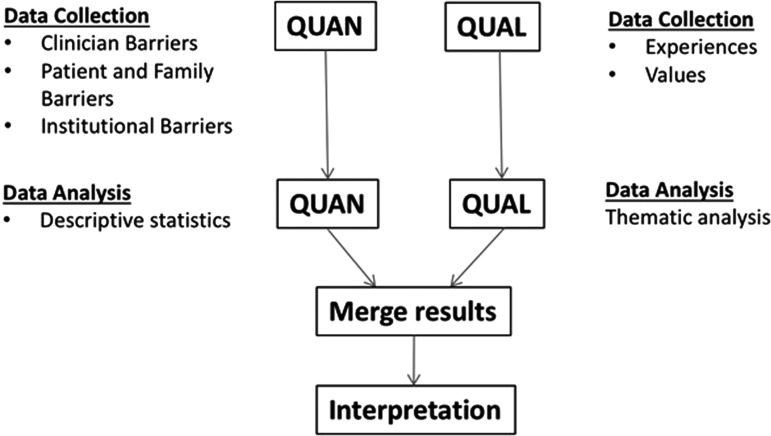
Quantitative survey responses were analyzed using SAS Version 9.4 (Cary, NC). Response rate was determined by the proportion of respondents who completed the survey after opening the e-mail invitation.15,16 Survey results were recorded in two ways: as a proportion of respondents who reported individual factors as important barriers and as a weighted average (or “average” in the Results), which indicates relative importance of each barrier and specifies which answer choice was most preferred.14 To determine proportions, the responses to the five-point Likert scale questions were dichotomized by groupings as “none/small/medium” or “large/huge.” Dichotomized responses were then reported as a proportion. Qualitative responses were analyzed by hand using thematic analysis as previously described.17 Quantitative and qualitative data sets were integrated during data collection and analysis to allow organization and structure of themes by relative importance to respondents (Fig. 1).
FIG. 1.
Convergent mixed methods design.
Results
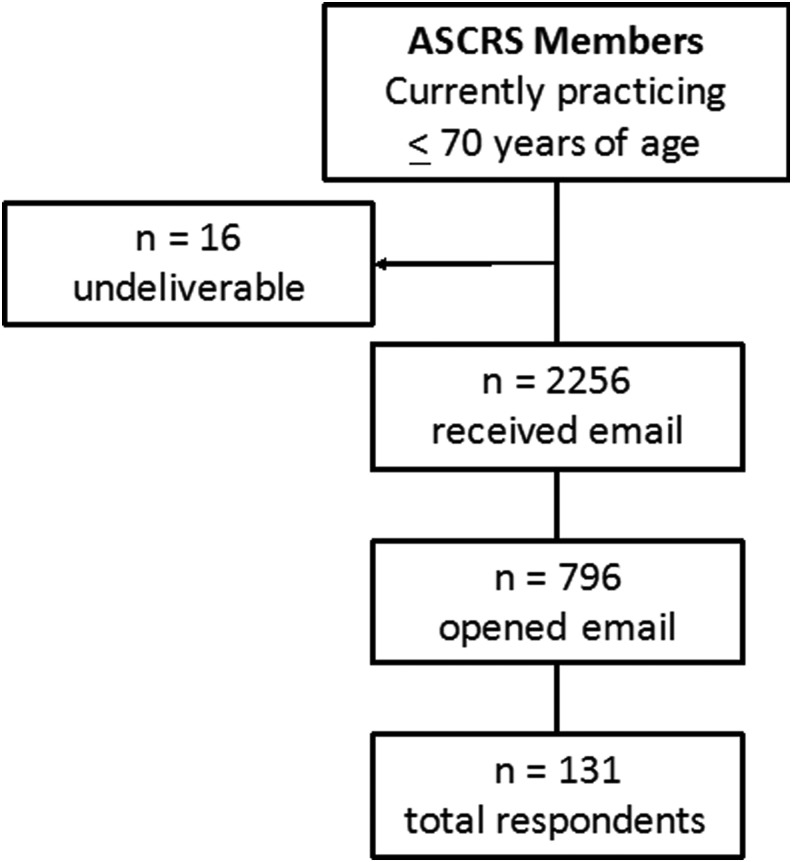
A total of 131 surgeons responded to our survey (16.5% response rate) and all were included in this study (Fig. 2). Five themes emerged from the qualitative data with regard to surgeon perceptions of major barriers to palliative care: (1) Surgeon Knowledge and Training, (2) Communication Challenges, (3) Difficulty with Prognostication, (4) Patient and Family Factors, and (5) Systemic Issues. Emergent themes were integrated with closed-ended (quantitative) survey item responses and summarized with illustrative quotes in a joint display (Table 2).
FIG. 2.
Response rate. ASCRS, American Society of Colon and Rectal Surgeons.
Table 2.
Surgeon-Reported Clinician Level Barrier
| Substantial barrier | Minimal to no barrier | ||||
|---|---|---|---|---|---|
| Clinician barrier | n | % | n | % | Weighted average (SD) |
| Communication | |||||
| Inadequate communication between care teams and patients and/or families about appropriate goals of care | 64 | 51.6 | 60 | 48.4 | 3.35 (0.76) |
| Inadequate communication between care teams about appropriate goals of care | 59 | 47.6 | 65 | 52.4 | 3.22 (0.81) |
| Education and training | |||||
| Insufficient clinician training in communication about end-of-life care issues | 53 | 42.7 | 71 | 57.3 | 3.16 (0.74) |
| Insufficient clinician training in techniques for forgoing life-sustaining treatment without patient suffering | 47 | 37.9 | 77 | 62.1 | 3.11 (0.69) |
| Insufficient clinician training in the management of symptoms that are distressing to seriously ill patients | 50 | 40.3 | 74 | 59.7 | 3.05 (0.83) |
| Prognostication | |||||
| Unrealistic expectations by clinicians about patient prognosis or effectiveness of treatment | 56 | 45.2 | 68 | 54.8 | 3.18 (0.82) |
| Personal conflict and lack of awareness | |||||
| Psychological and/or emotional stresses of providing care to dying patients | 38 | 30.6 | 86 | 69.4 | 2.88 (0.77) |
| Insufficient attention to diverse cultural norms and customs with respect to dying, death, and grief | 27 | 21.8 | 97 | 78.2 | 2.76 (0.73) |
| Fear of legal liability for forgoing life-sustaining treatments | 31 | 25.0 | 93 | 75.0 | 2.56 (0.75) |
| Clinicians' reluctance to use opioids or sedatives because of concern about side effects | 26 | 21.0 | 98 | 79.0 | 2.36 (0.82) |
Clinician barriers: surgeon knowledge and training, communication challenges, and difficulty with prognostication
Surgeon knowledge and training
The absence of knowledge regarding opportunities for and delivery of palliative or end-of-life care, along with minimal to no training, was cited as a critical barrier. Seventy-six percent of respondents (n = 89) reported no formal training in palliative care. Forty-three percent (n = 53) reported insufficient training in communication about end-of-life issues (weighted average = 3.16, SD = 0.74), 40.3% (n = 50) in the management of symptoms that are distressing to seriously ill patients (average = 3.05, SD = 0.83), and 37.9% (n = 47) in techniques to forgo life-sustaining treatment without patient suffering (average = 3.11, SD = 0.69) (Table 1). Surgeons explicitly stated in open-ended responses that formalized training in palliative care was urgently needed (Table 2).
Table 1.
Surgeon-Reported Barriers to Palliative Care Using Mixed Methods Integration of Survey Scores and Qualitative Reports
| Perceived barriers | Survey results | Surgeons' reflections |
|---|---|---|
| Surgeon knowledge and training | 42.7% (n = 53) report insufficient training in communication about end-of-life issues | “Very difficult, little experience prior to deal with this.” “Took me a while to be comfortable with talking about death early on.” “I was frustrated and saddened by the mass exodus for the doors when it came time for the palliative care lecture at ASCRS in LA last week. We need more training in these important communication skills.” |
| 40.3% (n = 50) report insufficient training in the management of symptoms that are distressing to seriously ill patients | ||
| 76.1% (n = 89) report no formal training in palliative care | ||
| Communication challenges | 51.6% (n = 64) report inadequate communication between care teams and patients/families about appropriate goals of care | “Holding pressure on a bleeding ileostomy for hours, while the fellow and attending came in. The patient did get to see his family before surgery. The attending could have had better communication with the patient about goals and realistic outcomes.” “Could have had better communication with team and family.” |
| 47.6% (n = 59) report inadequate communication between care teams about appropriate goals of care | ||
| Difficulty with prognostication | 45.2% (n = 56) report unrealistic expectations about patient prognosis or effectiveness of treatment | “…female with small bowel obstruction of unclear etiology along with acute and worsening renal failure. It was unclear whether surgical intervention would be beneficial, and the patient also did not want to undergo surgery. She went home with hospice. What went well was that she was happy with her decision and not afraid. What could have gone better would have been me being more certain that I definitely would not help her if I offered her an operation.” “…I once operated on a young man with carcinomatosis, implants everywhere in the abdominal cavity. Went to another major center where they reoperated on patient. I still have doubts about whether or not (a) I didn't do enough for the patient or (b) the other center did too much.” “Last week I had a patient in the ICU on 4 pressors with a pH of 6.9 and a lactate of 15 for >24 hrs with flourid sepsis and multisystem organ failure. The entire care team (surgical, ICU, nursing, etc.) began to wonder how long we should continue to press on with a patient who clearly could not survive. 8 days later he is awake and alert, off pressors, on trach collar, and fully communicative. Stage IV cancer is a different story and I am a strong advocate of hospice and palliative care, but sometimes even experience clinicians cannot predict when a patient will die or recover.” |
| Patient and family factors | 61.8% (n = 81) report unrealistic patient and/or family expectations about prognosis or effectiveness of treatment | “Patient with poorly responding stage 4 colon cancer in msof (multi-system organ failure) getting same chemo that already failed. Oncologists offered treatment—family wanted everything done…” “…female dying of likely ischemic bowel and having the daughter become upset with me because she could not understand why I did not want to operate.” |
| 48.9% (n = 64) report disagreements within families about care goals | ||
| 43.5% (n = 57) report disagreements between patients/families and other care teams about care goals | ||
| Systemic issues | 43.1% (n = 56) report lack of advance directives | “…middle aged man with end stage CHF, COPD, and CKD treated laparoscopically for incarcerated ventral hernia. Prolonged postop course with recurrent arrhythmias. Bad: no advanced directive; family and patient disagreed on goals/treatment; last-minute flip flops on resuscitation/DNR; poor specialist communication of prognosis…” “I don't think that we do a good job educating families (or members of society) about end of life. Everyone just continues to think ‘you've got to do something.’” “…In my opinion, the biggest gap is that our country views death as a taboo subject and as a failure, instead of treating it like another part of life that has its own value and meaning.” “Would appreciate easier access to providers with a focus on end-of-life to collaborate in care for the patient and family.” “… women with metastatic rectal cancer, coord(ination) of home health/hospice could have been better” “We need a better palliative care service. As surgeons, we just can't fill that role adequately, though I feel we should stay involved.” |
| 39.7% (n = 52) report absence of surrogate decision maker for patients lacking decisional capacity | ||
| 49.6% (n = 66) report a culture of adding or continuing all life-sustaining therapies | ||
| 38.3% (n = 49) report insufficient recognition among staff or institutional leadership of the importance of optimal end-of-life care | ||
| 34.9% (n = 45) report inadequate support services | ||
| 32.8% (n = 42) report lack of consultants with special expertise in management of symptoms that are distressing to patients | ||
| 39.5% (n = 51) report insufficient continuity of care during transitions into higher level of care | ||
| 53.2% (n = 66) report competing demands for time |
ASCRS, American Society of Colon and Rectal Surgeons; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DNR, do-not-resuscitate order.
Communication challenges
Inadequate communication across care teams (average = 3.22, SD = 1.20) and between care teams and patients and/or families about goals of care (average = 3.35, SD = 1.15) was reported as “large or huge” barriers for 47.6% and 51.6% of surgeons, respectively (Table 1). Surgeons reported specific experiences when communication would have improved end-of-life care (Table 2). Conversely, surgeons also reported specific examples of how good communication led to improved decision making. For example, one surgeon stated, “I was asked to discuss futility of treatment…Going over his difficult course and highlighting his personal strength through it somehow freed him to finally say it was time to stop all interventions…”
Difficulty with prognostication
Nearly half (45.2%) of respondents reported that clinicians had unrealistic expectations about patient prognosis or the effectiveness of treatment (average = 3.18, SD = 0.82), and recalled uncertainty in their own decision making (Table 1). One surgeon reported, “… I had a patient in the ICU with florid sepsis and multisystem organ failure. The entire care team began to wonder how long we should continue to press on with a patient who clearly could not survive…days later he is awake and alert, off pressors, on trach collar, and fully communicative…sometimes even experienced clinicians cannot predict when a patient will die or recover” (Table 2).
Personal conflict and lack of awareness
Additional clinician-level barriers included psychological and/or emotional stress (30.6%, average = 2.88, SD = 0.77), insufficient attention to diverse cultural norms and customs with respect to dying, death, and grief (21.8%, average = 2.76, SD = 0.73), fear of legal liability for forgoing life-sustaining treatments (25.0%, average = 2.56, SD = 0.75), and fear of prescribing opioids and sedatives because of concern about side effects (21%, average = 2.36, SD = 0.82) (Table 1).
Patient and family barriers: unrealistic expectations and discordance
Unrealistic expectations
The most commonly reported external barriers to providing optimal end-of-life care were unrealistic patient and/or family expectations about prognosis or effectiveness of treatment (average = 3.61, SD = 1.15), reported by 61.8% of respondents (Table 3). Surgeons recalled experiences with families desiring aggressive interventions despite poor prognoses. One surgeon noted, “Patient with poorly responding stage 4 colon cancer in multisystem organ failure getting same chemo that already failed…family wanted everything done…” (Table 2).
Table 3.
Surgeon-Reported Patient or Family Level Barrier
| Substantial barrier | Minimal to no barrier | ||||
|---|---|---|---|---|---|
| Patient or family barrier | n | % | n | % | Weighted average (SD) |
| Unrealistic expectations | |||||
| Unrealistic patient and/or family expectations about prognosis or effectiveness of treatment | 81 | 61.8 | 50 | 38.2 | 3.61 (0.77) |
| Discordance | |||||
| Disagreements within families about care goals | 64 | 48.9 | 67 | 51.1 | 3.27 (0.85) |
| Disagreements between patients and/or families and other care teams about care goals | 57 | 43.5 | 74 | 56.5 | 3.21 (0.78) |
| Patient factors | |||||
| Inability of many patients to participate in treatment discussions | 35 | 26.7 | 96 | 73.3 | 2.73 (0.80) |
| Cultural beliefs about death and dying | 29 | 22.1 | 102 | 77.9 | 2.71 (0.72) |
| Refusals by patients and/or families to forgo life-sustaining treatments for religious reasons | 29 | 22.1 | 102 | 77.9 | 2.41 (0.79) |
| Communication with patients and/or families due to language | 17 | 13.0 | 114 | 87.0 | 2.24 (0.72) |
In contrast, surgeons reported occasions when the patient and family were accepting of death, they were able to provide better end-of-life care and families expressed appreciation. For example, “85 y/o with carcinomatosis due to appendiceal cancer, presenting with SBO, refused surgery despite her brother disagreement. Ultimately sent home with iv hydration, venting PEG and palliative care. She survived 4 months. Her daughter stated that she and her mom spent the best time together over those 4 months… Her daughter thanked me for helping them understand her mom's prognosis so they could enjoy the life she had, one day at a time.”
Discordance within patients and families
Disagreements within families (average = 3.27, SD = 1.03) and between patients/families and care teams about care goals (average = 3.21, SD = 1.11) were reported by 48.9% and 43.5% of surgeons, respectively (Table 3). Surgeons noted multiple occasions when disagreements negatively impacted end-of-life care (Table 2), including “… the patient's advanced disease meant he had weeks to months at the most… I attempted to convince him to do hospice… he wanted testing and options for cure… Unfortunately, by the time he was discharged he was much weaker and no longer able to do any activities he might have been able to do had he decided quickly to accept the inevitable.” In contrast, a number of surgeons also reported how agreement between family members and care teams led to their perception of a better death including, “…The patient and his family accepted the inevitability of death and had a chance to spend their remaining days together at home…”
Systemic barriers: lack of documentation, lack of appropriate resources, and culture
Systemic barriers represented deficiencies in documentation or resources or reflected cultural attributes of the hospital and health system. Forty-three percent of surgeons reported the absence of advance directives (average = 3.24, SD = 1.15), and 39.7% reported the absence of a surrogate decision maker for patients who lacked decisional capacity (average = 3.10, SD = 1.12) as large or huge barriers. Multiple respondents reported challenges in the lack of appropriate documentation and specialists available to assist in end-of-life care (Table 2).
Competing demands for clinicians' time were reported as a large or huge barrier by 53.2% of surgeons (average = 3.45, SD = 1.22). Fewer surgeons reported that inadequate support services or a lack of consultants with special expertise in management of symptoms that are distressing to patients was large or huge barriers (34.9% and 32.8% respectively). Finally, a lack of palliative care services for dying patients was reported as large or huge barriers by 25.6% of surgeons (average = 2.29, SD = 0.88) (Table 4).
Table 4.
Surgeon-Reported Systemic Barrier
| Substantial barrier | Minimal to no barrier | ||||
|---|---|---|---|---|---|
| Systemic barrier | n | % | n | % | Weighted average (SD) |
| Lack of documentation | |||||
| Lack of advance directives | 56 | 43.1 | 74 | 56.9 | 3.24 (0.76) |
| Absence of surrogate decision maker for patients lacking decisional capacity | 52 | 39.7 | 79 | 60.3 | 3.11 (0.81) |
| Failure to locate existing advance directives | 31 | 24.0 | 98 | 76.0 | 2.59 (0.80) |
| Lack of appropriate resources | |||||
| Competing demands for clinicians' time | 66 | 53.2 | 58 | 46.8 | 3.45 (0.76) |
| Insufficient continuity of care during transitions into higher level of care | 51 | 39.5 | 78 | 60.5 | 2.95 (0.82) |
| Inadequate support services | 45 | 34.9 | 84 | 65.1 | 2.83 (0.84) |
| Lack of consultants with special expertise in management of symptoms that are distressing to patients | 42 | 32.8 | 86 | 67.2 | 2.69 (0.83) |
| Lack of palliative care services for dying patients | 33 | 25.6 | 96 | 74.4 | 2.29 (0.89) |
| Suboptimal space for meeting with patients and/or families | 24 | 18.6 | 105 | 81.4 | 2.18 (0.80) |
| Culture | |||||
| There is a culture of adding or continuing all life-sustaining therapies | 66 | 51.2 | 63 | 48.8 | 3.30 (0.80) |
| There is insufficient recognition among staff or institutional leadership of the importance of optimal end-of-life care | 49 | 38.3 | 79 | 61.7 | 2.91 (0.82) |
When palliative care specialists were available, however, surgeons were appreciative and valued the role experts played in providing end-of-life care, noting, “I am lucky to have a good palliative care doc,” and when asked what went well in their experiences caring for dying patients, they said, “collaboration with palliative care team” and “smooth transition from acute care to palliative care.”
One respondent expressed concern regarding cultural or experiential differences between surgeons and other specialists, whereas multiple respondents expressed satisfaction when cultural differences were bridged with multidisciplin ary care, noting, “22yo with metastatic colon cancer had sudden respiratory failure at home…MICU attending ruled out all reversible causes of respiratory failure then recommended withdrawal of support. MICU attending took the time to make sure family, primary oncology physicians and patients adolescent friends were all on same page…Communication between ICU attending and surgeon and other oncology care providers for patient made a huge positive impact.”
Half (51.2%) of surgeons reported that a culture of adding or continuing all life-sustaining therapies was a large or huge barrier (average = 3.30, SD = 1.31) (Table 4). Surgeons reported a lack of public understanding of the limitations of medical care, with one respondent reporting, “…the biggest gap is that our country views death as a taboo subject and as a failure, instead of treating it like another part of life that has its own value and meaning” (Table 2). Whereas 38.3% of surgeons reported insufficient recognition among staff or institutional leadership of the importance of optimal end-of-life care (average = 2.91, SD = 1.33) (Table 4). One surgeon reported, “85 year old filled with liver tumor found on exploration…Family relieved with me admin(istering) meds directly for comfort. Contact from hosp(ital) that what I did was inappropriate after patient died. Hosp(ital) needed to educate their pall care staff/nursing staff.”
Discussion
To our knowledge, this is the first study aimed at characterizing perceived barriers to optimal palliative and end-of-life care among surgeons who care for patients with CRC. We found that participating surgeons reported multiple types of barriers to optimal palliative and end-of-life care, including (1) surgeon knowledge and training, (2) communication challenges, (3) difficulty with prognostication, (4) patient and family factors, and (5) systemic issues.
We found that surgeons reported the most important barriers to be their own. Specifically, most surgeons reported that they lacked formal training in the areas of communication, symptom management, and techniques to discontinue or withhold life-sustaining therapies at the end of life. Although very few surgeons explicitly stated discomfort with providing end-of-life care, a large proportion reported difficulties, and many provided specific examples of the challenges they had encountered. Consistent with our findings, a number of studies have demonstrated that despite substantial exposure to seriously ill and dying patients, surgical education lacks training in palliative and end-of-life care despite recommendations by both the Association of American Medical Colleges and the American College of Surgeons (ACS).18–20,37 As such, individual programs are tasked with providing and maintaining structured curricula and/or exposure for their trainees, which are limited across the United States and heterogeneous in their approaches.38,39 This deficiency in training is associated with recommendations for major surgery and fewer referrals to hospice for patients with poor prognosis cancers, suggesting that palliative care curricula may provide more meaningful and impactful clinical encounters.22–26 Until palliative care training becomes a mandatory part of medical and residency training, alternative methods to achieve competency at the training level include structured curricula developed and implemented by experienced faculty, identifying mentors with skill in palliative care approaches, undergoing additional training such as the ACS Palliative Surgical Care Course, and collaborating with local palliative care specialists.
Surgeons reported communication challenges in providing care for seriously ill and dying patients. We found a persistent theme of anxiety and stress caused by obscure, incomplete, and inaccurate counseling by both the respondents and other providers. Surgeons reported frustration with inadequate communication and expressed satisfaction or fulfillment when they were able to provide appropriate communication and participate in discussions that allowed the care team and the patient to come to an agreement. In contrast to the notion that surgeons are primarily technicians, these data indicate that surgeons act as guides and recognize that the patient–surgeon relationship is based on empathic communication, and not simply the procedures performed.27–30
Our findings are complementary to a few studies of palliative care among general surgeons. A focus group study of 37 seniors and 17 surgeons found that despite a strong belief that maintaining independence and quality of life are critical for patients, surgeons have difficulty conveying their professional opinion to not pursue surgery. The authors proposed a trajectory of care outside of their own control that would inevitably lead to surgery once a surgical diagnosis was made, termed “clinical momentum.”11 Factors contributing to this process include patient and family belief that a surgical consultation is indicative of surgery being the optimal therapy, and the consulting physician's expectations about the benefits of surgery. Surgeons in our own study may share similar experiences although we did not explicitly inquire about this nor did we ask respondents to describe their decision making around palliative care versus surgery. Another qualitative study demonstrated that, despite a sense of responsibility for preoperative conversations, surgeon communication was hindered by poor availability of medical records and low access to palliative care services, as well as time constraints and surgeons' own attitudes about palliative and end-of-life care.31
Supporting the need for systematic organization and structure, surgeons reported that optimal end-of-life care relied on a multidisciplinary team-based approach. There was an appreciation for palliative care when such services were available. Conversely, when palliative care was unavailable or inadequate, desperation among respondents was evident in those seeking assistance with end-of-life decision making and the dying process. For example, the lack of timely resources may lead to ineffective or poorly executed conversations, led by more junior residents or other inexperienced providers. The widespread lack of consistent access to specialty palliative care services often renders the surgical team the sole resource for palliative care. Most surgeons recognized that both surgeons and palliative care specialists are essential for patients with end-stage CRC and cannot exist without the other. Patients who are older, malnourished, or with multiple comorbid conditions or otherwise more likely to experience poor outcomes after surgery may benefit from introducing palliative care earlier in their disease trajectory.40–43
We also found that patients' and/or families' unrealistic expectations as well as discordance within families and between families and care teams were important barriers. Multiple respondents recalled experiences of family insistence on aggressive measures despite poor prognoses. Family members, surgeons reported, have a substantial impact on the care team's end-of-life recommendations and goals. As dying patients exerted less direct control over treatment decision making, agency was displaced to the family member who may or may not have had prior discussions about preferences.
There are several limitations to our study. First, the response rate was low and, therefore, selection bias may be a limitation to generalizability. However, our response rate was consistent with other Internet surveys of surgeons, and we note that response rate thresholds alone are no longer the primary measure of survey quality.16,32–35 Second, we did not compare surgeon-perceived barriers with barriers perceived by medical doctors or other surgical subspecialties. The goal of this study, however, was not to compare differences in perceived barriers between providers but to understand the drivers of palliative and end-of-life care among surgeons as critical members of the clinical team. Finally, our study did not inquire about how quality measures may impact end-of-life decision making after complications of surgery.36 We acknowledge this important issue and anticipate that additional studies will clarify the implications of quality measures in end-of-life care going forward. Despite these limitations, this novel study reveals commonly encountered barriers in providing care to seriously ill and dying patients as perceived by surgeons.
In conclusion, we have identified and characterized important surgeon-perceived barriers to palliative and end-of-life care, which include clinician level factors, patient and/or family level factors, and systemic factors. A better understanding of the challenges that providers encounter may provide critical insight into strategies to improve care at the end of life for patients with CRC or other end-stage diseases.
Supplementary Material
Acknowledgment
The authors would like to thank Michael D. Fetters, MD, MPH, MA, from the University of Michigan Mixed Methods Research and Scholarship Program for his feedback on survey and study design.
Author Disclosure Statement
No competing financial interests exist.
P.A.S. is currently funded by the Division of Geriatric and Palliative Medicine at the University of Michigan and the Society for Surgery of the Alimentary Tract and Research Foundation of the American Society of Colon and Rectal Surgeons Joint Research Award. L.M. is supported by RO1AG032298, K24 AG050685, RO1AG041780, and by the Claude D. Pepper Older American Independence Centers funding from the National Institute on Aging
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. : Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177–193 [DOI] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Nordlinger B, Cervantes A, Group EGW: Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol 2010;21 Suppl 5:v93–v97 [DOI] [PubMed] [Google Scholar]
- 3.Smith TJ, Temin S, Alesi ER, et al. : American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol 2012;30:880–887 [DOI] [PubMed] [Google Scholar]
- 4.Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2017;35:96–112 [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez R, Marr L, Rajput A, Fahy BN: Utilization of palliative care consultation service by surgical services. Ann Palliat Med 2015;4:194–199 [DOI] [PubMed] [Google Scholar]
- 6.Olmsted CL, Johnson AM, Kaboli P, et al. : Use of palliative care and hospice among surgical and medical specialties in the Veterans Health Administration. JAMA Surg 2014;149:1169–1175 [DOI] [PubMed] [Google Scholar]
- 7.Kross EK, Engelberg RA, Downey L, et al. : Differences in end-of-life care in the ICU across patients cared for by medicine, surgery, neurology, and neurosurgery physicians. Chest 2014;145:313–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnato AE, Herndon MB, Anthony DL, et al. : Are regional variations in end-of-life care intensity explained by patient preferences?: A study of the US Medicare Population. Med Care 2007;45:386–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lipitz-Snyderman A, Sima CS, Atoria CL, et al. : Physician-driven variation in nonrecommended services among older adults diagnosed with cancer. JAMA Intern Med 2016;176:1541–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarze ML, Bradley CT, Brasel KJ: Surgical “buy-in”: the contractual relationship between surgeons and patients that influences decisions regarding life-supporting therapy. Crit Care Med 2010;38:843–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nabozny MJ, Kruser JM, Steffens NM, et al. : Constructing high-stakes surgical decisions: It is better to die trying. Ann Surg 2016;263:64–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nelson JE, Angus DC, Weissfeld LA, et al. : End-of-life care for the critically ill: A national intensive care unit survey. Crit Care Med 2006;34:2547–2553 [DOI] [PubMed] [Google Scholar]
- 13.Creswell JW, Klassen AC, Plano Clark VL, Smith KC: Best Practices for Mixed Methods Research in Health Sciences. Commissioned by the Office of Behavioral and Social Sciences Research of the National Institutes of Health, 2011. https://obssr.od.nih.gov/wp-content/uploads/2016/02/Best_Practices_for_Mixed_Methods_Research.pdf (last accessed January4, 2016.)
- 14.Creswell JW, CV: Designing and Conducting Mixed Methods Research, 2nd ed. Thousand Oaks, CA: SAGE, 2011 [Google Scholar]
- 15.Groves RM, Fowler FJ, Couper MP, et al. :. Survey Methodology. Hoboken, NJ: John Wiley & Sons, Inc.; 2004 [Google Scholar]
- 16.Groves RM, Peytcheva E: The impact of nonresponse rates on non response bias: A meta-analysis. Public Opin Q 2008;72:167–189 [Google Scholar]
- 17.Dixon-Woods M, Agarwal S, Jones D, et al. : Synthesising qualitative and quantitative evidence: A review of possible methods. J Health Serv Res Policy 2005;10:45–53 [DOI] [PubMed] [Google Scholar]
- 18.McCahill LE, Krouse R, Chu D, et al. : Indications and use of palliative surgery-results of Society of Surgical Oncology survey. Ann Surg Oncol 2002;9:104–112 [DOI] [PubMed] [Google Scholar]
- 19.Amini A, Miura JT, Larrieux G, et al. : Palliative care training in surgical oncology and hepatobiliary fellowships: A national survey of the fellows. Ann Surg Oncol 2015;22:1761–1767 [DOI] [PubMed] [Google Scholar]
- 20.Cooper Z, Meyers M, Keating NL, et al. : Resident education and management of end-of-life care: The resident's perspective. J Surg Educ 2010;67:79–84 [DOI] [PubMed] [Google Scholar]
- 21.Bradley CT, Webb TP, Schmitz CC, et al. : Structured teaching versus experiential learning of palliative care for surgical residents. Am J Surg 2010;200:542–547 [DOI] [PubMed] [Google Scholar]
- 22.Klaristenfeld DD, Harrington DT, Miner TJ: Teaching palliative care and end-of-life issues: A core curriculum for surgical residents. Ann Surg Oncol 2007;14:1801–1806 [DOI] [PubMed] [Google Scholar]
- 23.Lesnock JL, Arnold RM, Meyn LA, et al. : Palliative care education in gynecologic oncology: A survey of the fellows. Gynecol Oncol 2013;130:431–435 [DOI] [PubMed] [Google Scholar]
- 24.Galante JM, Bowles TL, Khatri VP, et al. : Experience and attitudes of surgeons toward palliation in cancer. Arch Surg 2005;140:873–878; discussion 878–880. [DOI] [PubMed] [Google Scholar]
- 25.Walbert T, Glantz M, Schultz L, Puduvalli VK: Impact of provider level, training and gender on the utilization of palliative care and hospice in neuro-oncology: A North-American survey. J Neurooncol 2016;126:337–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wancata LM, Hinshaw DB, Suwanabol PA: Palliative care and surgical training: Are we being trained to be unprepared? Ann Surg 2017;265:32–33 [DOI] [PubMed] [Google Scholar]
- 27.McCahill LE, Dunn GP, Mosenthal AC, et al. : Palliation as a core surgical principle: Part 1. J Am Coll Surg 2004;199:149–160 [DOI] [PubMed] [Google Scholar]
- 28.Dunn GP, Milch RA: Introduction and historical background of palliative care: Where does the surgeon fit in? J Am Coll Surg 2001;193:325–328 [DOI] [PubMed] [Google Scholar]
- 29.Milch RA: The surgeon-patient relationship in advanced illness. Surg Oncol Clin N Am 2001;10:25–30 [PubMed] [Google Scholar]
- 30.Regenbogen SE, Veenstra CM, Hawley ST, et al. : The effect of complications on the patient-surgeon relationship after colorectal cancer surgery. Surgery 2014;155:841–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cauley CE, Block SD, Koritsanszky LA, et al. : Surgeons' perspectives on avoiding nonbeneficial treatments in seriously ill older patients with surgical emergencies: A qualitative study. J Palliat Med 2016;19:529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yarger JB, James TA, Ashikaga T, et al. : Characteristics in response rates for surveys administered to surgery residents. Surgery 2013;154:38–45 [DOI] [PubMed] [Google Scholar]
- 33.Cunningham CT, Quan H, Hemmelgarn B, et al. : Exploring physician specialist response rates to web-based surveys. BMC Med Res Methodol 2015;15:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groves RM: Nonresponse rates and nonresponse bias in household surveys. Public Opin Q 2006;70:646–675 [Google Scholar]
- 35.Sur RL, Scales CD, Preminger GM, Dahm P: Evidence-based medicine: A survey of American Urological Association members. J Urol 2006;176:1127–1134 [DOI] [PubMed] [Google Scholar]
- 36.Schwarze ML, Brasel KJ, Mosenthal AC: Beyond 30-day mortality: Aligning surgical quality with outcomes that patients value. JAMA Surg 2014;149:631–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suwanabol PA, Kanters AE, Reichstein AC, et al. :. Characterizing the role of U.S. surgeons in the provision of palliative care: A systematic review and mixed-methods meta-synthesis. J Pain Symptom Manage 2017. [Epub ahead of print; DOI: 10.1016/j.jpainsymman.2017.11.031.] [DOI] [PubMed]
- 38.Horowitz R, Gramling R, Quill T: Palliative care education in US medical schools. Med Ed 2014:48:59–66 [DOI] [PubMed] [Google Scholar]
- 39.Koettewar SA, Bearelly D, Bearelly S, et al. : Residents' end-of-life training experience: A literature review of interventions. J Palliat Med 2014;17:725–732 [DOI] [PubMed] [Google Scholar]
- 40.Turrentine FE, Wang H, Simpson VB, Jones RS: Surgical risk factors, morbidity, and mortality in elderly patients. J Am Coll Surg 2006;203:865–877 [DOI] [PubMed] [Google Scholar]
- 41.Thomas DR, Ritchie CS: Preoperative assessment of older adults. J Am Geriatr Soc 1995;43:811–821 [DOI] [PubMed] [Google Scholar]
- 42.Brennan TA, Leape LL, Laird NM, et al. : Incidence of adverse events and negligence in hospitalized patients. Results of the Harvard Medical Practice Study I. N Engl J Med 1991;324:370–376 [DOI] [PubMed] [Google Scholar]
- 43.Rangel EL, Cooper Z, Olufajo OA, et al. : Mortality after emergency surgery continues to rise after discharge in the elderly: Predictors of 1-year mortality. J Trauma Acute Care Surg 2015;79:349–358 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.