Abstract
This prospective controlled observational cohort study assessed the performance of a novel panel of serum microRNA (miRNA) biomarkers on indicators of concussion, subconcussive impacts, and neurocognitive function in collegiate football players over the playing season. Male collegiate student football athletes participating in a Division I Football Bowl Subdivision of the National Collegiate Athletic Association (NCAA) were enrolled. There were a total of 53 participants included in the study, 30 non-athlete control subjects and 23 male collegiate student football athletes. Neurocognitive assessments and blood samples were taken within the week before the athletic season began and within the week after the last game of the season and measured for a panel of pre-selected miRNA biomarkers. All the athletes had elevated levels of circulating miRNAs at the beginning of the season compared with control subjects (p < 0.001). Athletes with the lowest standard assessment of concussion (SAC) scores at the beginning of the season had the highest levels of miRNAs. The area under the curve (AUC) for predicting pre-season SAC scores were miR-195 (0.90), miR-20a (0.89), miR-151-5p (0.86), miR-505* (0.85), miR-9-3p (0.77), and miR-362-3p (0.76). In athletes with declining neurocognitive function over the season, concentrations of miRNAs increased over same period. There were significant negative correlations with miR-505* (p = 0.011), miR-30d (p = 0.007), miR-92 (p = 0.033), and (p = 0.008). The miRNAs correlating with balance problems were miR-505* (p = 0.007), miR-30d (p = 0.028), and miR-151-5p (p = 0.023). Those correlating with poor reaction times were miR-20a (0.043), miR-505* (p = 0.049), miR-30d (p = 0.031), miR-92 (p = 0.015), and miR-151-5p (p = 0.044). Select miRNAs were associated with baseline concussion assessments at the beginning of the season and with neurocognitive changes from pre to post-season in collegiate football players. Should these findings be replicated in a larger cohort of athletes, these markers could potentially serve as measures of neurocognitive status in athletes at risk for concussion and subconcussive injuries.
Keywords: athletes; biomarkers; cognition, diagnosis; collegiate; concussion; football; mild traumatic brain injury; miRNA; prognosis sports; subconcussive impacts
Introduction
Concussion, also known as mild traumatic brain injury (TBI), is an unfortunately common occurrence in athletes. In the United States, the nearly 8,000,000 students who currently participate in high school athletics and the >480,000 who compete as National Collegiate Athletic Association (NCAA) athletes1 are at risk for concussion and subconcussive injuries.2 Numerous studies have documented that both clinically diagnosed concussion and subconcussive traumas induce similar changes in brain structure and functions.3,4 These changes include alterations in white matter and cerebrovascular integrity, blood flow, brain activation during working memory tasks, resting-state functional connectivity, and brain chemistry as measured by various forms of magnetic resonance imaging (MRI).3,5,6 The effect of thousands of subconcussive impacts has the potential for long-term deleterious effects on brain function and neurodegeneration in select individuals.7,8
Currently, concussion is largely a clinical diagnosis based on injury history, neurological examination, neuropsychological testing, and, on occasion, neuroimaging. Brain-specific biomarkers measured through a simple blood test could complement the clinical evaluation of concussion in athletes and potentially guide management decisions.9–13 Studies assessing biomarkers in concussion have looked at a number of potential markers that could lend not only diagnostic and prognostic, but also monitoring information.13 A novel set of biomarkers, called microRNAs (miRNA), are now being studied as the next generation of biomarkers for many diseases and disorders such as cancer and cardiovascular and neurodegenerative diseases.14 MiRNAs are small (19–28 nucleotides) endogenous RNA molecules that regulate protein synthesis at the post- transcriptional level. MiRNAs can be detected in serum and can be an indicator of disease pathology in neuronal cells. MiRNAs are relatively abundant in biofluids such as cerebrospinal fluid, serum, and urine and are relatively stable at variable pH conditions, and are resistant to repeated freeze thaw and enzymatic degradation. Because of these properties, miRNAs have advantages over protein-based markers.
The utility of miRNAs as diagnostic markers of mild TBI or concussion has recently been explored by our group and others.15–19 In 2016, Bhomia and coworkers identified specific and sensitive miRNA-based biomarkers for mild and moderate TBI using real time polymerase chain reaction (PCR) methodology.16 Samples from human subjects with mild to severe TBI were compared with samples from trauma and normal control patients and 10 miRNA signatures were identified. Eight of these miRNAs showed significant increased expression in those subjects with lesions on computed tomography (CT). Moreover, Johnson and coworkers detected a group of miRNAs in saliva that were associated with prolonged post-concussive symptoms at 4 weeks post-injury.18
Based on the important function of miRNA in neurons in the central nervous system and their association with measures of brain injury in adults and children, this prospective study assessed the performance of a panel of nine miRNA biomarkers (miR-20a, miR-505*, miR-362-3p, miR-30d, miR-92a, miR-486, miR-195, miR-9-3p, and miR-151-5p), previously reported by our group and shown to correlate with severity of injury after mild to severe TBI.16 MiRNA concentrations were compared with measures of impairment from concussion in collegiate football players during a single competitive season in a Division I NCAA Football Bowl Subdivision. This study examined the relationship between serum miRNA biomarker levels (1) with baseline standardized assessments of concussion, (2) in athletes versus non-athlete control subjects, and (3) with changes in neurocognitive function over the playing season as measured by a virtual reality (VR) graphics system evaluating balance, spatial memory, and reaction time.
Methods
Study population
This prospective controlled cohort study enrolled a sample of male collegiate student football athletes from Pennsylvania State University participating in a single competitive season in a Division I NCAA Football Bowl Subdivision. Eligibility for the study was determined by the coaches and research team. Participants were excluded if they refused the blood draws or were unable to participate in the football season. This study was approved by the Penn State Institutional Review Board. Informed consent was obtained from all participants prior to enrollment. Normal controls were obtained from a bank of serum from young healthy individuals acquired from Bioreclamation Inc.
Study procedures
Assessments were made by the research team together with the team physicians and trainers. All participants completed a comprehensive pre-season interview, which included demographic information, medical and concussion history, and history of learning disabilities. The Standardized Assessment of Concussion (SAC)20 was administered at the pre-season visit to provide an estimate of baseline neurocognitive function. The SAC is most sensitive to change as a result of concussion and includes abbreviated forms of established tests best suited to measure those functions in brain injury patients.
Blood samples were taken within the week before the athletic season began (prior to any pre-season or in-season contact practices or competitions) and within the week after the last game of the season (post-season). None of the athletes were recovering from, or were diagnosed with, a concussion in the 9 months prior to the pre-season evaluation. A blood sample of 5 mL was placed in a serum separator tube and allowed to clot at room temperature before being centrifuged. The serum was placed in bar-coded aliquot containers and stored at −70°C until transport to a central laboratory where samples were analyzed in batches. Laboratory personnel conducting the miRNA analysis were blinded to the clinical data. All athletic trainers, physicians, and research personnel were blinded to the serum biomarker results.
Within the same time frame as the blood sampling, participating athletes also underwent previously validated baseline VR neurocognitive testing consisting of three components: a spatial navigation/memory module, a balance module, and a whole body reaction time module. Scores were summarized as an overall comprehensive score.21,22
A three-dimensional (3D) TV system (HeadRehab.com) with a head mounted accelerometer was used. In the balance task, subjects were instructed to hold a Tandem Romberg position for all trials. In the first trial, the virtual room was completely still (for a baseline measure) and in the subsequent six trials, the virtual room moved in various directions. In the reaction time module, subjects were instructed to stand with feet shoulder width apart, hands on their hips. The virtual room moved and they were instructed to move their body in the same direction as that of the virtual room. In the spatial memory module, subjects were shown a randomized virtual pathway with multiple turns to a door and then the return trip. Afterwards, they were instructed to repeat the exact pathway using a joystick. Detailed methods of the modules along with their sensitivity and specificity have been described previously.21,22
Outcome measurements
The primary outcome measure was the association between the pre-season SAC score and levels of the selected miRNA biomarkers. The SAC is a reliable and valid measure for evaluating the early neurocognitive effects of sports-related brain injury.20 The pre-season SAC score was dichotomized into two groups as a baseline to compare athletes with pre-season SAC results of 28–30 with those with SAC scores of <28. Previous work has shown that a three point difference between scores represents a clinically significant change in performance.20
The secondary outcome measures included the performance of the biomarkers in enrolled athletes compared with non-athlete controls, as well as changes in levels pre- and post-season. Additionally, changes in concentrations of the miRNA biomarkers were compared pre- and post-season in those with and without concussion. Diagnosis of concussion was determined clinically by certified athletic trainers and team physicians within 24 h of injury. Study investigators then screened and triaged all injuries to confirm that they met study definition and requirements. Concussion was defined as a type of TBI caused by a bump, blow, or jolt to the head or by a hit to the body that caused the head and brain to move rapidly back and forth, resulting in a change in mental status, behavior, thinking, or neurological functioning. This definition is consistent with that of the Center for Disease Control and Prevention HEADS UP initiative.23
A tertiary outcome was a change in neurocognitive function over the playing season as measured by a VR graphics system that was used to evaluate balance, spatial memory, and reaction time. In additional to individual component scores, an overall “comprehensive score” was calculated by combining the three test scores mathematically into a 10 point scale (0 = worst and 10 = best).21 These VR assessments were performed both pre- and post-season and were assessed at the same time as pre- and post-season miRNA serum levels.
Statistical analysis
Descriptive statistics with means and proportions were used to describe the data. For statistical analysis, biomarker levels were treated as continuous data, measured in copies/μL. Data were assessed for equality of variance and distribution. Paired samples were analyzed using paired sample t tests or related-samples Wilcoxon signed rank tests. Unpaired samples were assessed using independent sample t tests with variance consideration and the Mann–Whitney U test. Linear regression and correlational analysis examined changes in cognitive function over the season by changes in miRNA levels over the season.
Receiver operating characteristics (ROC) curves were created to evaluate the ability of pre-season miRNA concentrations to predict pre-season SAC scores. Estimates of the area under these curves (AUC) were obtained with 95% confidence intervals (AUC = 0.5 indicates no discrimination and an AUC = 1.0 indicates a perfect diagnostic test). All analyses were performed using the statistical software package SPSS 22.0 (IBM Corporation®, Somers, NY).
Sample size calculations
Post-hoc sample size power calculations were conducted based on differences in miRNA levels relative to pre-season SAC scores. For miRNA-505* a sample size of 21 achieved a 79% power to detect a difference of −0.70 copies/uL between the null hypothesis and the alternative hypothesis with known group standard deviations of 0.40 and 0.80 and with a significance level (α) of 0.05. The power for miR-20a was 92%, the power for miR-362-3p was 100%, the power for miR-30d was 45%, the power for miR-92a was 66%, the power for miR-486 was 26%, the power for miR-195 was 86%, the power for miR-9-3p was 70%, and the power for miR-151-5p was 90%.
MiRNA analysis
RNA isolation
Serum samples from athletes (pre- and post-season) along with healthy controls were used to isolate total RNA. RNA was isolated using 100 μL of serum using a serum/plasma miRNA isolation kit (Qiagen Inc.) as per manufacturer's recommended protocol. The RNA was eluted in 20 μL of DNAse RNAse free water and stored at −80°C for further use. For a quality check of the RNA, a bioanalyzer assay using a small RNA assay was performed to confirm the quality of the RNA.
Droplet digital PCR (ddPCR)
For absolute quantitation of miRNAs, we used a ddPCR platform (Bio Rad Inc.). For ddPCR reaction, 10 ng of total RNA was reverse transcribed using the specific miRNA TaqMan assays (Thermofisher Scientific Inc.) as per recommended protocol in a 15 μL total reaction volume; 5 μL of reverse transcribed product was used to set up the real-time PCR reaction using miRNA TaqMan assays; and 20 μL of the final real-time PCR reaction was mixed with 70 μL of droplet oil in a droplet generator (Bio Rad Inc.). Following the droplet formation, the PCR reaction was performed as per recommended thermal cycling conditions. The final PCR product within the droplets was analyzed in a droplet reader (Bio-Rad Inc). The total positive and negative droplets were measured and the concentration of the specific miRNA/μL of the PCR reaction was determined. All the reactions were performed in duplicates.
Results
There were a total of 53 participants included in the study, 30 non-athlete control subjects and 23 male collegiate student football athletes. Twenty-two athletes completed pre-season blood draws and 20 completed post-season blood draws: the first (pre-season) was within 1 week before the athletic season began (prior to any pre-season or in-season contact practices or competitions) and the second was within 1 week after the last game of the season (post-season). The missing post-season samples from two subjects resulted in a pool of 20 pairs with complete pre- and post-season blood sample data. Mean age of the enrolled athletes was 21 years (with a range from 19 to 24) with a mean height and weight of 75 inches (191 centimeters) and 270 pounds (122 kilograms) respectively. The average number of years of football experience was 11. Nine athletes (41%) had had either one or two previously diagnosed concussions (Table 1). Control subjects were healthy adults with no chronic diseases and were a mean age of 31 years (with a range from 30 to 35) and 50% were female. There were no statistically significant differences in miRNAs between male and female control subjects except for miRNA-195, which showed higher levels in female controls (p = 0.043).
Table 1.
Characteristics of Athletes Included in the Study
| SAC score 28–30 n = 7 (95%CI) | SAC Score <28 n = 15 (95%CI) | Total n = 22 (95%CI) | p value | |
|---|---|---|---|---|
| Age | 22 (21–23) | 21 (20–22) | 21 (21–22) | 0.398 |
| Range | 21–23 | 19–24 | 19–24 | |
| Height (inches) | 76 (75–77) | 75 (73–76) | 75 (74–76) | 0.416 |
| Weight (lbs) | 291 (267–315) | 261 (240–282) | 270 (254–287) | 0.076 |
| Years playing football | 13 (11–16) | 10 (8–12) | 11 (9–13) | 0.017 |
| Previous concussions | ||||
| 0 | 3 (43%) | 10 (67%) | 13 (59%) | 0.290 |
| 1 | 3 (43%) | 4 (27%) | 7 (32%) | |
| 2 | 1 (14%) | 1 (7%) | 2 (9%) | |
| History of ADHD | 1 (14%) | 2 (13%) | 3 (14%) | 0.705 |
| Player positions | 0.490 | |||
| Offense | 2 (26%) | 6 (40%) | 8 (36%) | |
| Defense | 5 (71%) | 9 (60%) | 14 (64%) |
SAC, standard assessment of concussion; CI, confidence interval; ADHD, attention-deficit/hyperactivity disorder.
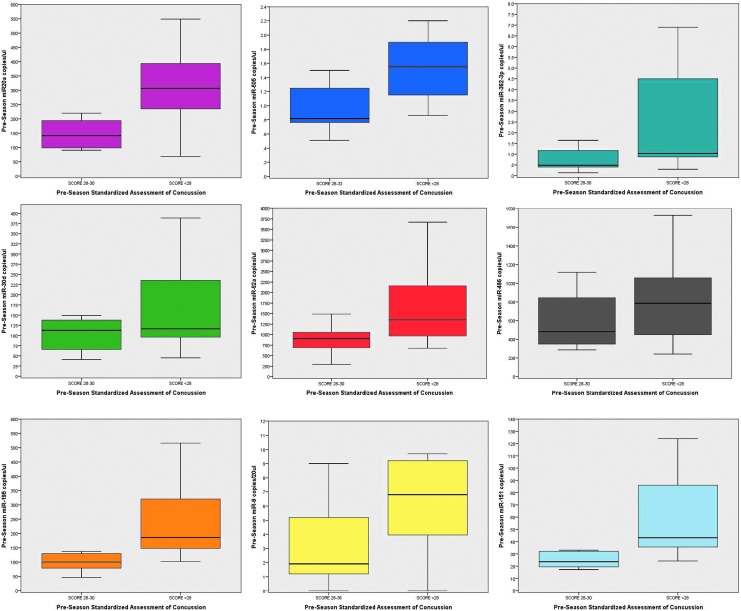
Interestingly, all the athletes had elevated levels of circulating miRNAs at the beginning of the season prior to any contact practices. To evaluate the relationship between baseline neurocognitive functioning and elevated baseline miRNA concentrations, pre-season SAC scores were compared with pre-season miRNA levels. The worse the neurocognitive score (lower scores) the higher the levels of the miRNAs. There were statistically significant negative correlations with miR-20a (correlation coefficient −0.53; p = 0.017), miR-505* (−0.54; p = 0.011), miR-195 (−0.55; p = 0.008), and miR-151-5p (−0.63; p = 0.002). Accordingly, athletes were divided into two groups based on their baseline SAC score and the distribution of clinical characteristics in each group is presented in Table 1. There were no statistically significant differences in age, height, weight, previous concussions, or player position between the groups except for years playing football.
In Figure 1, baseline (pre-season) levels of the selected miRNA biomarkers were compared in athletes with pre-season SAC results of 28–30 versus those with SAC scores of <28. There were significantly higher baseline (pre-season) levels of miR-20a (p = 0.008), miR-505* (p = 0.010), miR-195 (p = 0.002), miR-151-5p (p = 0.007), miR-9-3p (p = 0.047), and miR-151-5p (p = 0.007).
FIG. 1.
Box plot comparison of pre-season microRNAs (miRNAs) in those with standard assessment of concussion (SAC) scores of <28 versus those with scores of 28–30. There were significantly higher baseline (pre-season) levels of miR-20a (p = 0.008), miR-505* (p = 0.010), miR-195 (p = 0.002), miR-151-5p (p = 0.007), miR-9-3p (p = 0.047), and miR-151-5p (p = 0.007). Box plots represent medians with interquartile ranges.
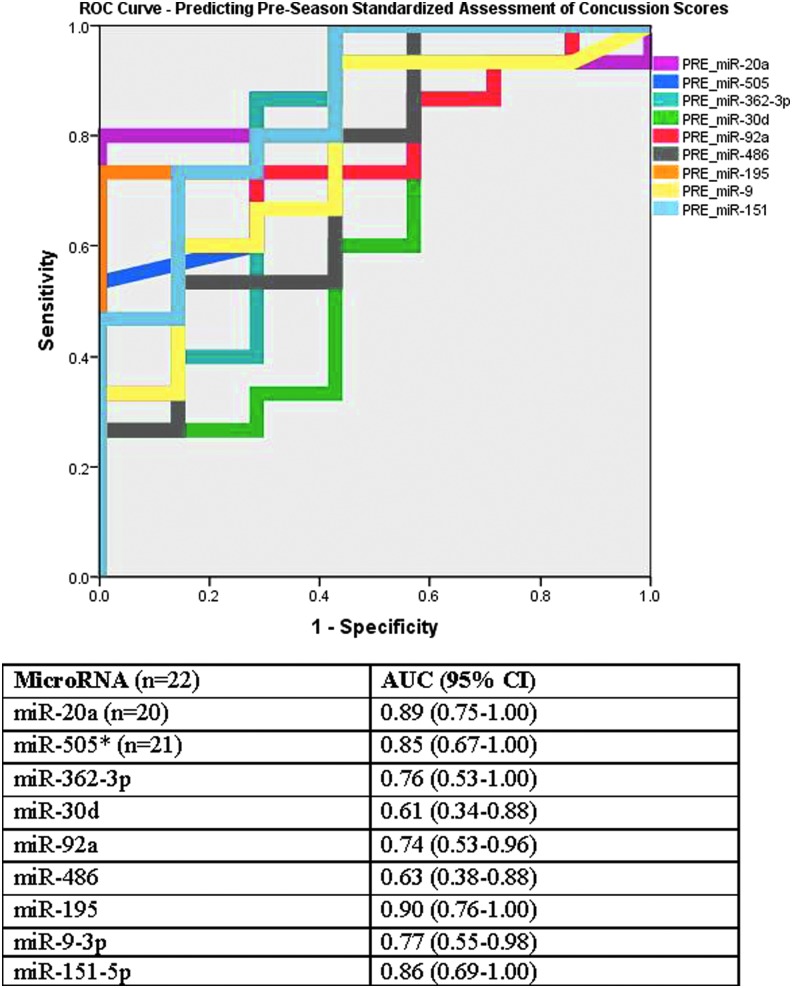
Areas under the ROC curves of the miRNAs collected pre-season for predicting pre-season SAC scores are shown in Figure 2. The miRNAs with the most significant association to SAC scores were miR-195 with an AUC of 0.90 (0.76–1.00), miR-20a with an AUC of 0.89 (0.75–1.00), miR-151-5p with an AUC of 0.86 (0.69–1.00), miR-505* with an AUC 0.85 (0.67–1.00), miR-9-3p with an AUC of 0.77 (0.55–0.98), and miR-362-3p with and AUC of 0.76 (0.53–1.00).
FIG 2.
Area under the receiver operating characteristics (ROC) curve showing the association between pre-season microRNA (miRNA) levels and pre-season standard assessment of concussion (SAC) scores. The miRNAs with the most significant association to SAC scores were miR-195 with an AUC of 0.90 (0.76–1.00), miR-20a with an AUC of 0.89 (0.75–1.00), miR-151-5p with an AUC of 0.86 (0.69–1.00), miR-505* with an AUC 0.85 (0.67–1.00), miR-9-3p with an AUC of 0.77 (0.55–0.98), and miR-362-3p with an AUC of 0.76 (0.53–1.00).
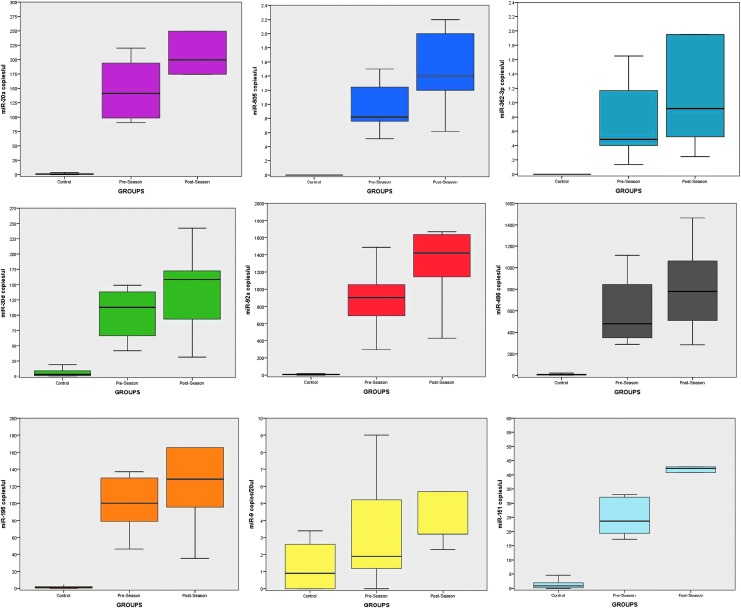
The concentrations of the miRNA biomarkers were compared among non-athlete control subjects, athletes at baseline (pre-season), and athletes post-season (Fig. 3). There were significant differences in miRNA concentrations among all three groups (p < 0.001). Specifically, there were significantly elevated levels on miRNAs in athletes pre-season compared with non-athlete control subjects (p < 0.001) for all the miRNAs. Similarly, the elevations were significantly higher in players at the end of the season compared with control subjects (p < 0.001). When pre- and post-season concentrations of miRNAs were compared, the differences were significant in players starting the season with SAC score of 28–30 but not in those with SAC score <28. The miRNAs that had significantly increases from pre- to post-season included, miR-505* (p = 0.016), miR-486 (p = 0.027), miR-30d (p = 0.030), miR-92a (p = 0.044), miR-362-3p (p = 0.049), and miR-195 (p = 0.056).
FIG. 3.
Box plots comparing microRNAs (miRNAs) in control subjects versus athletes pre- and post-season with pre-season standard assessment of concussion (SAC) scores of 28–30. There were significant differences in miRNA concentrations among all three groups (p < 0.001). Box plots represent medians with interquartile ranges.
Although there were only two players (10%) diagnosed with a concussion during the playing season, concentrations of the mi-RNA all increased from pre to post-season in these concussed players. MiRNA-92a was significant (p = 0.035) (Fig. S1) (see online supplementary material at http://www.liebertpub.com). The highest AUCs of the miRNAs for comparing concussed versus non-concussed athletes over the season in descending order were miR-195 = 0.92 (0.79–1.00), miR-92a = 0.92 (0.76–0.96), miR-30d = 0.86 (0.63–0.88), miR-505* = 0.83 (0.57–1.00), miR-151-5p = 0.81 (0.50–1.00), and miR-362-3p = 0.75 (0.49–1.00) (Fig. S2) (see online supplementary material at http://www.liebertpub.com).
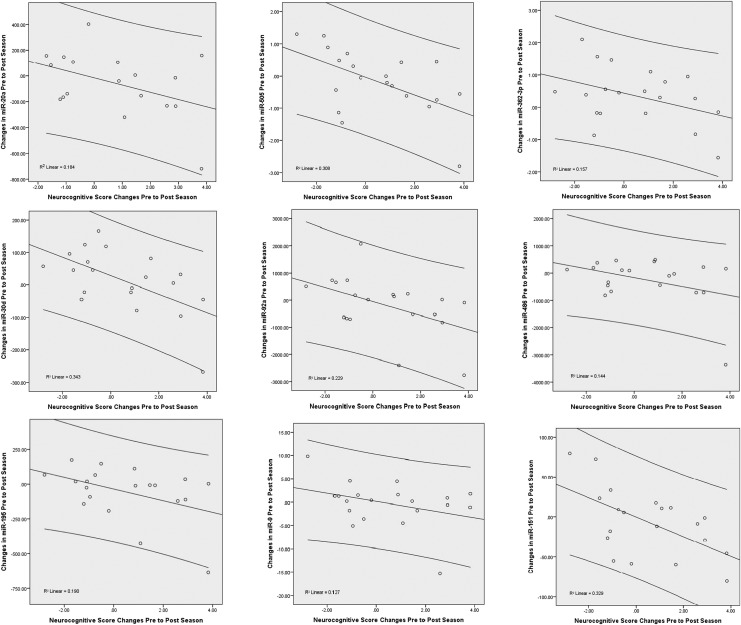
Changes in neurocognitive functioning from a VR graphics system (balance, spatial memory, reaction time, and overall score) were compared with changes in miRNA levels over the season (Table 2). In athletes with declining neurocognitive function over the season, concentrations of miRNAs increased from pre- to post-season (negative correlation). Overall comprehensive scores had significant negative correlations with miR-505* (p = 0.011), miR-30d (p = 0.007), miR-92 (p = 0.033), and miR-151-5p (p = 0.008) (Table 2). R2 values from the regression analysis for the neurocognitive function comprehensive score are shown in Figure 4.
Table 2.
Correlation between Changes in Neurocognitive Scores over the Playing Season and Changes in Concentrations of miRNAs
| MiRNA | Overall comprehensive score (correlation coefficient) | Spatial memory (correlation coefficient) | Balance (correlation coefficient) | Reaction time (correlation coefficient) |
|---|---|---|---|---|
| miR-20a | −0.429 | 0.004 | −0.283 | −0.482 |
| (p = 0.075) | (p = 0.987) | (p = 0.255) | (p = 0.043)* | |
| miR-505* | −0.555 | −0.034 | −0.581 | −0.445 |
| (p = 0.011)* | (p = 0.886) | (p = 0.007)** | (p = 0.049)* | |
| miR-362-3p | −0.397 | −0.063 | −0.422 | −0.285 |
| (p = 0.083) | (p = 0.793) | (p = 0.064) | (p = 0.223) | |
| miR-30d | −0.586 | −0.143 | −0.492 | −0.483 |
| (p = 0.007)* | (p = 0.547) | (p = 0.028)* | (p = 0.031)* | |
| miR-92a | −0.479 | −0.007 | −0.324 | −0.537 |
| (p = 0.033)* | (p = 0.977) | (p = 0.164) | (p = 0.015)** | |
| miR-486 | −0.380 | 0.054 | −0.398 | −0.363 |
| (p = 0.098) | (p = 0.821) | (p = 0.082) | (p = 0.115) | |
| miR-195 | −0.436 | −0.115 | −0.304 | −0.400 |
| (p = 0.055) | (p = 0.629) | (p = 0.193) | (p = 0.081) | |
| miR-9-3p | −0.356 | −0.045 | −0.216 | −0.390 |
| (p = 0.123) | (p = 0.850) | (p = 0.361) | (p = 0.090) | |
| miR-151-5p | −0.573 | −0.140 | −0.506 | −0.454 |
| (p = 0.008)* | (p = 0.556) | (p = 0.023)* | (p = 0.044)* |
Overall comprehensive scores had significant negative correlations with miR-505* (p = 0.011), miR-30d (p = 0.007), miR-92 (p = 0.033), and miR-151-5p (p = 0.008).
Statistically significant at p < 0.05
Statistically significant at p < 0.017 (controlling for multiple comparisons)
FIG. 4.
Scatterplot showing correlation between changes in microRNAs (miRNAs) and neurocognitive function (comprehensive score) pre- to post-season. R2 values from the regression analysis for the neurocognitive function comprehensive score are shown.
The miRNAs with the most significant correlations with worsening balance were miR-505* (p = 0.007), miR-30d (p = 0.028), and miR-151-5p (p = 0.023), and the miRNAs with the most significant association with worsening reaction times were miR-20a (0.043), miR-505* (p = 0.049), miR-30d (p = 0.031), miR-92 (p = 0.015), and miR-151-5p (p = 0.044).
Discussion
This is among the first published studies to examine levels of serum miRNA in subjects who were asymptomatic but experienced multiple, high intensity subconcussive impacts over the course of a single football season. This prospective study introduces a panel of nine miRNA biomarkers (miR-20a, miR-505*, miR-362-3p, miR-30d, miR-92a, miR-486, miR-195, miR-9-3p, and miR-151-5p) previously shown to correlate with acute concussion in emergency department patients16 into the realm of sports concussion in collegiate football players. This study assessed the performance of a panel of miRNA biomarkers using not only standard methods of concussion evaluation, but also advanced VR neurocognitive assessment technology.
Interestingly, the study found significant elevations in circulating miRNA measured before the athletic season began and prior to any contact practices. All the players had significantly elevated levels compared with non-athlete controls (p < 0.001). The players had at least 6 years (average 11) of playing experience, and 41% had previously reported concussions. Moreover, these baseline levels did not reflect any recent football activity. This could suggest residual circulating miRNA biomarkers are reflecting prior concussive and subconcussive impacts.24 The significant correlations between baseline neurocognitive functioning and pre-season miRNA levels further support the explanation that elevations in miRNA signal prior injury. The higher the pre-season levels of miRNA markers, the lower the SAC scores, suggesting more neurocognitive impairment in those with higher circulating levels of miRNA. Pre-season miRNA levels predicted baseline SAC scores with very good AUCs, the highest being miR-195 (0.90), miR-20a (0.89), miR-151-5p (0.86), miR-505* (0.85), and miR-9-3p (0.77).
All the players had significantly elevated levels compared with non-athlete controls (p < 0.001) at both pre and post time points, suggesting that these football players have higher baseline levels than those who do not participate in sports. In those football players with better baseline neurocognitive function (SAC scores of 28–30), there were very significant increases in miRNAs from pre- to post-NCAA Football Bowl Subdivision competition season, including those with no reported concussions during the season. The miRNAs with the most significant increases over the course of the season were miR-505*, miR-362-3p, miR-30d, miR-92a, and miR-486. Even in the absence of a clinically diagnosed concussion, research suggests that neurocognitive changes may develop in football players as a result of frequent head impacts that occur during football games and practices.25 Our findings are consistent with this. The athletes in our study who sustained a concussion during the season did not have significant changes in VR neurocognitive performance, suggesting the role of subconcussive injuries. Cumulative effects of repetitive subconcussive impacts are a subject of great interest in sports.8 Particularly as the development of chronic traumatic encephalopathy (CTE) is being cited as a potential late effect of sport-related concussive and subconcussive head trauma.7
Research now suggests that head impacts commonly occur during contact sports in which visible signs or symptoms of neurological dysfunction may not develop despite those impacts having the potential for neurological injury.5,8 This includes studies in children 8–13 years of age who play football,6 as well as in those who play high school football.24
A novel aspect of this study was the application of VR graphics system technology and its comparison to blood-based biomarkers pre- and post-season. Advances in technology have allowed for this approach to become part of clinical concussion injury testing. VR neurocognitive assessments have the ability to detect residual abnormality in the absence of patient self-reported symptoms or traditional computer-based neuropsychological tests. Impressively, miRNAs were associated with alterations in brain function over the course of the season. Athletes who demonstrated worsening neurocognitive function from pre- to post-season on VR showed elevations in concentrations of miRNAs over the same period. Because blood sampling and neurocognitive function were conducted within the same period of time pre- and post-season, the correlations are temporally well defined. The miRNAs with consistently strong correlation to neurocognitive outcome were miR-505*, miR-30d, miR-92, and miR-151-5p. MiR-20a also showed some correlations to reaction time. When the three domains were controlled for multiple comparisons (p = 0.05/3 domains = 0.0167) the correlation was driven more specifically by domain, so that miR-505* was driven by balance and miR-92a was driven by reaction time. The findings are consistent with those of others who have found that athletes engaged in high contact sports are exposed to repetitive subconcussive head trauma and are at greater risk for lowered neuropsychological functioning.26
The properties of miRNAs such as abundance in biofluids, detection in saliva, and relative stability during variable environmental conditions make them appealing biomarkers and provide advantages over protein-based markers. Repetitive impacts and concussions in contact sports are common, but they can be elusive, because athletes often do not report their symptoms. Given the barriers to concussive symptom reporting, biomarkers could provide a more objective measure of injury and potentially identify athletes at risk for declining neurocognitive status.27
Limitations
Although these data are encouraging, the authors recognize that there are limitations to this study. Athletes were enrolled for a single season at a single site. The players, however, are representative of many players in this division of the Football Bowl Subdivision of the NCAA.
There were only two athletes diagnosed with a concussion during the season. Although the concentrations of the miRNAs all increased from pre- to post-season in these concussed players, only miRNA-92a (p = 0.035) met statistical significance. This may be because of the limited number of concussion cases. Another explanation is that other athletes may have experienced subconcussive injuries that also elevated the miRNA levels. Accordingly, those athletes in our study who sustained a concussion during the season did not have significant changes in VR neurocognitive performance, further supporting the role of subconcussive injuries in contributing to changes in neurocognitive functioning. Further study with a larger number of athletes will be required to confirm this finding.
Despite the small sample size, post-hoc sample size calculations determined that the study achieved more than adequate power for the majority of the markers (79%, 80%, 86%, 90%, 92%, and 100%) to detect differences in pre-season SAC scores. There was also an advantage to the samples being paired, as each athlete served as his own control pre- and post-season.
We cannot confirm that the selected microRNAs are brain specific and may be released from other organs. However, in this study, the levels of selected miRNAs in healthy controls were significantly lower than in the athletes. A previous study by our group comparing mild TBI to trauma control subjects has shown good discrimination between trauma controls and subjects with mild TBI for the selected miRNA markers.16 According to the data from a recent report based on miRNA sequencing analysis of various human organs, miR-9-3p, miR-151-5p, miR-195, and miR-486 highly enriched in the brain. Additionally, miRNAs miR-20a, miR-30d, miR-92a, miR-505*, and miR-362-3p are reported to be expressed in human brain.28,29 Further study is needed to examine this.
Further limitations include a lack of access to the concussion histories of the healthy controls and an inability to assess gender differences among the athletes, as they were all male. There were no statistically significant differences between genders in the control group except for miRNA-195 which showed higher levels in females. The range in ages between athletes and control subjects was 19–24 and 30–35, respectively, so the differences were not exceedingly large. Age and gender differences need further exploration, and studies are currently underway.
Conclusion
This study introduces a panel of microRNA biomarkers, previously shown to correlate with acute concussion in emergency department patients, into the realm of sports concussion. In collegiate football players, the candidate miRNAs were associated with neurocognitive functioning at the beginning of the season and over the course of season, even in those not diagnosed with concussion. Should these findings be replicated in a larger cohort of athletes, these markers could potentially serve as measures of neurocognitive status in athletes at risk for concussion and subconcussive trauma.
Supplementary Material
Acknowledgments
The authors thank Dr. Zhao Zhang Li, Bio-Instrumentation Center (BIC), UHUHS, for her help with digital microRNA PCR experiments. The microRNA analysis was funded by DMRDP grant D10_I_AR_J6_855.
Contributor Information
Collaborators: on behalf of the Concussion Neuroimaging Consortium (CNC)
Author Disclosure Statement
Drs. Papa and Bhomia are inventors of a United States patent application filed by Uniformed Services University of the Health Sciences (USUHS) regarding the potential utilities of selected miRNAs as diagnostic biomarkers for TBI. The other authors have nothing to disclose. The opinions expressed herein are those of authors and are not necessarily representative of those of the USUHS, Department of Defense or, the United States Army, Navy, Air Force and Defense Medical Research and Development Program (DMRDP).
References
- 1. NCAA. (2018). Estimated Probability of Competing in College Athletics. NCAA Research; http://www.ncaa.org/about/resources/research/estimated-probability-competing-college-athletics (last accessed October16, 2018) [Google Scholar]
- 2. Bailes J.E., Petraglia A.L., Omalu B.I., Nauman E., and Talavage T. (2013). Role of subconcussion in repetitive mild traumatic brain injury. J. Neurosurg. 119, 1235–1245 [DOI] [PubMed] [Google Scholar]
- 3. Slobounov S.M., Walter A., Breiter H.C., Zhu D.C., Bai X., Bream T., Seidenberg P., Mao X., Johnson B., and Talavage T.M. (2017). The effect of repetitive subconcussive collisions on brain integrity in collegiate football players over a single football season: a multi-modal neuroimaging study. Neuroimage Clin. 14, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reynolds B.B., Stanton A.N., Soldozy S., Goodkin H.P., Wintermark M., and Druzgal T.J. (2017). Investigating the effects of subconcussion on functional connectivity using mass-univariate and multivariate approaches. Brain Imaging Behav. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Johnson B., Neuberger T., Gay M., Hallett M., and Slobounov S. (2014). Effects of subconcussive head trauma on the default mode network of the brain. J. Neurotrauma 31, 1907–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bahrami N., Sharma D., Rosenthal S., Davenport E.M., Urban J.E., Wagner B., Jung Y., Vaughan C.G., Gioia G.A., Stitzel J.D., Whitlow C.T., and Maldjian J.A. (2016). Subconcussive head impact exposure and white matter tract changes over a single season of youth football. Radiology 281, 919–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gavett B.E., Stern R.A., and McKee A.C. (2011). Chronic traumatic encephalopathy: a potential late effect of sport-related concussive and subconcussive head trauma. Clin. Sports Med. 30, 179–188, xi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bailes J.E., Dashnaw M.L., Petraglia A.L., and Turner R.C. (2014). Cumulative effects of repetitive mild traumatic brain injury. Prog. Neurol. Surg. 28, 50–62 [DOI] [PubMed] [Google Scholar]
- 9. Papa L. (2016) Potential blood-based biomarkers for concussion. Sports Med. Arthrosc. Rev. 24, 108–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Papa L., Mittal M.K., Ramirez J., Ramia M., Kirby S., Silvestri S., Giordano P., Weber K., Braga C.F., Tan C.N., Ameli N.J., Lopez M., and Zonfrillo M. (2016). In children and youth with mild and moderate traumatic brain injury, glial fibrillary acidic protein out-performs S100beta in detecting traumatic intracranial lesions on computed tomography. J. Neurotrauma 33, 58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Papa L., Zonfrillo M.R., Ramirez J., Silvestri S., Giordano P., Braga C.F., Giordano P., Weber K., Braga C.F., Tan C.N., Ameli N.J., Lopez M., and Zonfrillo M. (2015). Performance of glial fibrillary acidic protein in detecting traumatic intracranial lesions on computed tomography in children and youth with mild head trauma. Acad Emerg Med 22, 1274–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Papa L., Ramia M.M., Kelly J.M., Burks S.S., Pawlowicz A., and Berger R.P. (2013). Systematic review of clinical research on biomarkers for pediatric traumatic brain injury. J. Neurotrauma 30, 324–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Papa L., Ramia M.M., Edwards D., Johnson B.D., nd Slobounov S.M. (2015). Systematic review of clinical studies examining biomarkers of brain injury in athletes after sports–related concussion. J. Neurotrauma 32, 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin X.F., Wu N., Wang L., and Li J. (2013). Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell Mol. Neurobiol. 33, 601–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Balakathiresan N., Bhomia M., Chandran R., Chavko M., McCarron R.M., and Maheshwari R.K. (2012). MicroRNA let-7i is a promising serum biomarker for blast-induced traumatic brain injury. J. Neurotrauma 29, 1379–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bhomia M., Balakathiresan N.S., Wang K.K., Papa L., and Maheshwari R.K. (2016). A panel of serum MiRNA biomarkers for the diagnosis of severe to mild traumatic brain injury in humans. Sci. Rep. 6, 28148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hicks S.D., Johnson J., Carney M.C., Bramley H., Olympia R.P., Loeffert A.C., and Thomas N.J. (2017). Overlapping microRNA expression in saliva and cerebrospinal fluid accurately identifies pediatric traumatic brain injury. J Neurotrauma 35, 64–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson J.J., Loeffert A.C., Stokes J., Olympia R.P., Bramley H., and Hicks S.D. (2018). Association of salivary microRNA changes with prolonged concussion symptoms. JAMA Pediatr. 172, 65–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitra B., Rau T.F., Surendran N., Brennan J.H., Thaveenthiran P., Sorich E., Fitzgerald M.C., Rosenfeld J.V., and Patel S.A. (2017). Plasma micro–RNA biomarkers for diagnosis and prognosis after traumatic brain injury: a pilot study. J. Clin. Neurosci. 38, 37–42 [DOI] [PubMed] [Google Scholar]
- 20. McCrea M. (2001). Standardized mental status assessment of sports concussion. Clin. J. Sport. Med. 11, 176–181 [DOI] [PubMed] [Google Scholar]
- 21. Teel E., Gay M., Johnson B., and Slobounov S. (2016). Determining sensitivity/specificity of virtual reality-based neuropsychological tool for detecting residual abnormalities following sport-related concussion. Neuropsychology 30, 474–483 [DOI] [PubMed] [Google Scholar]
- 22. Teel E.F., and Slobounov S.M. (2015). Validation of a virtual reality balance module for use in clinical concussion assessment and management. Clin. J. Sport Med. 25, 144–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Centers for Disease Control and Prevention (2017). HEADS UP to Health Care Providers: Tools for Providers. Centers for Disease Control and Prevention, HEADS UP Concussion Prevention Initiative: Atlanta [Google Scholar]
- 24. Abbas K., Shenk T.E., Poole V.N., Breedlove E.L., Leverenz L.J., Nauman E.A., Talavage T.M., and Robinson M.E. (2015). Alteration of default mode network in high school football athletes due to repetitive subconcussive mild traumatic brain injury: a resting-state functional magnetic resonance imaging study. Brain Connect. 5, 91–101 [DOI] [PubMed] [Google Scholar]
- 25. Campolettano E.T., Gellner R.A., and Rowson S. (2017). High-magnitude head impact exposure in youth football. J. Neurosurg. Pediatr. 20, 604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsushima W.T., Ahn H.J., Siu A.M., Yoshinaga K., Choi S.Y., and Murata N.M. (2018). Effects of repetitive subconcussive head trauma on the neuropsychological test performance of high school athletes: a comparison of high, moderate, and low contact sports. Appl. Neuropsychol. Child. 2, 1–8 [DOI] [PubMed] [Google Scholar]
- 27. Chrisman S.P., Quitiquit C., and Rivara F.P. (2013). Qualitative study of barriers to concussive symptom reporting in high school athletics. J. Adolesc. Health 52, 330–335 [DOI] [PubMed] [Google Scholar]
- 28. Papa L., Robicsek S.A., Brophy G.M., Wang K.K.W., Hannay H.J., Heaton S., Schmalfuss I., Gabrielli A., Hayes R.L., and Robertson C.S. (2018). Temporal profile of microtubule-associated protein 2: a novel indicator of diffuse brain injury severity and early mortality after brain trauma. J Neurotrauma 35, 32–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fehlmann T., Ludwig N., Backes C., Meese E., and Keller A. (2016). Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 13, 1084–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.