Abstract
Women account for 25% of all people living with HIV and 19% of new diagnoses in the United States. African American (AA) women are disproportionately affected. Yet, differences in the care continuum entry are not well understood between patient populations and healthcare sites. We aim to examine gender differences in diagnosis and linkage to care (LTC) in the Expanded HIV Testing and Linkage to Care (X-TLC) program within healthcare settings. Data were collected from 14 sites on the South and West sides of Chicago. Multivariate logistic regression analysis was used to determine the differences in HIV diagnoses and LTC by gender and HIV status. From 2011 to 2016, X-TLC performed 281,017 HIV tests; 63.7% of those tested were women. Overall HIV seroprevalence was 0.57%, and nearly one third (29.4%) of HIV-positive patients identified were cisgender women. Of newly diagnosed HIV-positive women, 89% were AA. 58.5% of new diagnoses in women were made at acute care hospitals, with the remainder at community health centers. Women who were newly diagnosed had a higher baseline CD4 count at diagnosis compared with men. Overall, women had lower odds of LTC compared with men (adjusted odds ratio = 0.58, 95% confidence interval 0.44–0.78) when controlling for patient demographics and newly versus previously diagnosed HIV status. Thus, interventions that focus on optimizing entry into the care continuum for AA women need to be explored.
Keywords: : HIV, routine testing, care continuum, linkage to care, HIV diagnosis
Introduction
In the United States, many HIV prevention efforts target men who have sex with men (MSM) and transgender women. Yet heterosexual men and cis-women remain highly impacted by HIV, particularly within the black/African American (AA) community. Women account for 19% of new HIV infections and 25% of people living with HIV; the vast majority of these infections are acquired through heterosexual contact.1,2 AA women are disproportionately affected, accounting for 61% of all new HIV infections in women.3 HIV remains a leading cause of death in AA women,4,5 reflecting disparities in timely diagnosis and care.6,7 AA women are a key target population for HIV interventions, yet face many barriers to diagnosis and engagement in the care continuum.8,9
Individual, network, and population factors contribute to HIV vulnerability in AA women. Poverty, low healthcare access, prior incarceration or incarceration of a partner, high rates of sexually transmitted infections, assortative mixing within high-prevalence communities, and concurrent sexual relationships have all been implicated.2,9–12 Heterosexual contact is the primary mode of transmission for women, yet the majority of women do not know their sex partner's risk (i.e., sex with men or injection drug use).13–15 Heterosexual women in high prevalence areas may not perceive increased individual risk,16–18 even if they recognize that their communities have a higher prevalence of HIV than other communities.19 Thus, women, including AA women, frequently self-identify as low risk and are not involved in HIV prevention and testing efforts.20
Given the under-recognized risk, routine testing in medical settings may be particularly important for the diagnosis of HIV in women in high prevalence areas. Routine opt-out testing in healthcare settings has been recommended by the Centers for Disease Control and Prevention (CDC) for all persons aged 13–64 years since 2006, regardless of risk.21 These guidelines recommend routine testing in healthcare settings with prevalence of ≥0.1% and at least annual testing for persons at high risk of infection.21 Routine testing is one of several strategies for HIV testing endorsed by the CDC; others include recruitment through social networks, partner notification, and targeted outreach in community settings.22 Despite clear guidelines, testing among high-risk heterosexuals remains suboptimal.20,23 Heterosexual persons who had a visit with a healthcare provider in the past 12 months were more likely to have been recently tested, indicating that routine testing is an important method of screening in this group.20 This suggests that routine screening in healthcare settings may be an effective strategy for identifying cisgender women, including minority women, with HIV infection.8
HIV testing, diagnosis, and notification of results are critical first steps for entry into the care continuum through linkage to care (LTC). Data on LTC show that individuals tested in healthcare settings have better rates of linkage24 but delayed entry to care for non-white populations.25,26 In assessing gender with respect to LTC, studies have been conflicting. They have shown no gender differences,27 lower rates of linkage in women,28 and an increased percentage of women linked compared with men29,30; varying populations, healthcare systems, and geographic locations may account for the discordant results. Despite well-described disparities in acquisition of HIV infection for AA women compared with their white counterparts, differences in the care continuum entry have not been extensively reported for this key population.
Understanding these differences is a crucial step in identifying interventions that encourage engagement in HIV care and prevention. We report important gender differences observed in our Expanded HIV Testing and Linkage to Care (X-TLC) program, conducted in high-prevalence areas on the South and West sides of Chicago and serving a primarily AA population.
Methods
Description of the X-TLC program
Our expanded HIV X-TLC program has been described previously.31,32 X-TLC is funded by the Chicago Department Public Health (CDPH) through CDC. The University of Chicago Medicine (UCM) is the lead organization of the X-TLC program, providing administrative support, training and technical assistance, as well as assuming responsibility for data reporting to the City of Chicago. Initiated in 2011, this program has expanded from 3 sites to 14 collaborating healthcare institutions that include acute care hospitals (ACHs, n = 5), community health centers and/or federally qualified health centers (CHC/FQHCs, n = 8), and dedicated family planning clinics (n = 1). Some participating healthcare organizations have more than one clinical site with locations across the city, but the vast majority of actively participating clinical sites are on the South and West sides of Chicago.
The location of X-TLC clinical sites originated exclusively on the South side of Chicago from 2011 to 2013 and expanded to the West side in 2014. All sites are located within areas of high HIV prevalence in Chicago, ranging from 47.3 to 110.0 cases per 100,000 population.33 Among these sites, heterogeneity in HIV linkage support and care varies across the multiple clinical locations. Some sites have an extensive HIV linkage team, others provide case management specific to HIV, whereas some smaller sites have no linkage coordinators on-site. All testing sites have access to linkage support from a licensed clinical social worker at the UCM to assist with insurance navigation and appointment scheduling in the event that a patient and/or site requires additional support. Similarly, most ACHs provide specialists for HIV care on-site, while most CHC/FQHCs and family planning clinics refer patients to an off-site HIV provider.
Recommended by the CDC since 2006, opt-out testing was not feasible in X-TLC until the Illinois AIDS Confidentiality statute changed in January 2016 to allow opt-out testing.34 The transition from opt-in testing to an opt-out model has varied across sites in adoption and implementation. While some larger clinical sites have implemented institution-wide opt-out testing with automated electronic orders, other institutions began with one or two departments internally before expanding opt-out testing site wide. Although most ACHs involved in the X-TLC program provided testing data collected in multiple departments within the hospital, a few hospitals were only able to collect testing data performed in the emergency department (ED).
Data collection
Patients screened for HIV at X-TLC sites between 2011 and 2016 were included in our cohort. We collected patient sex, race, and age at the time of screening on a monthly basis from our partnering sites. This information was generated directly from the electronic medical records (EMRs), billing records, laboratory data, or manually extrapolated by staff. For any positive HIV test, we collected additional patient information including risk factors for HIV transmission, patient notification of HIV result, and LTC.
If the HIV test was reactive, HIV diagnosis status was defined as either new or previously diagnosed. A patient was determined newly diagnosed for HIV if there was no previous reactive HIV test in the EMR and/or by patient self-report of HIV history. An existing diagnosis was determined if an individual had a previous reactive test in the EMR, was on antiretroviral treatment, or self-reported HIV positive status. For patients who could not be determined as a new or existing diagnosis, HIV status was confirmed with CDPH via surveillance data. All clients with a new diagnosis of HIV were eligible for LTC. Individuals with existing diagnoses who reported an HIV provider and/or were prescribed antiretrovirals were determined to be in care and were not eligible for linkage. All other individuals with existing diagnoses were eligible for LTC. If the patient was subsequently linked to care, we collected the initial viral load and CD4 T cell count, as well as risk factors for HIV if not previously recorded in the EMR.
X-TLC outcomes
The primary process outcomes for X-TLC include (1) number of HIV tests performed, (2) number of seropositive patients identified, (3) number of new diagnoses, (4) number of previous diagnoses, and (5) LTC rates. LTC is defined as attending an outpatient visit with an HIV care provider for both new and previously diagnosed, out of care patients. A secondary outcome includes CD4 T cell count at time of diagnosis or LTC for both new and existing, out of care diagnoses.
Our main goal in this report is to examine gender differences for HIV screening and LTC between women and men, who were then further stratified by MSM, heterosexual men, and men whose HIV risk factor was unknown. We also assess other factors affecting LTC such as diagnosis site and status (i.e., new vs. existing). We compare baseline CD4 count for new HIV diagnoses between women and men. Additionally, using data received from CDPH surveillance on HIV testing and diagnoses by gender from 2012 to 2016, we compare our X-TLC screening outcomes for women with the overall Chicago surveillance outcomes over 5 years.
Statistical analyses
We report descriptive statistics including gender and diagnosis status. For the purposes of this analysis, we excluded patients who moved out of the Chicago metropolitan area or were deceased before result notification. Patients who were identified as transgender were also excluded from our data analysis due to the very small numbers of individuals identified in this program (n = 10, 0.68%). We compared LTC outcomes using basic frequencies, chi-square tests, Kruksal–Wallis test, and generalized linear regression. Covariates were included as potential predictors of linkage such as sex, HIV risk factor, testing site type, HIV status (i.e., new vs. existing diagnosis), and CD4 count. Logistic regression was conducted to explore predictors for LTC, which were considered significant at p < 0.05. Analyses were adjusted for patient demographics (e.g., age, race), HIV status (i.e., new vs. existing diagnoses), and HIV testing site (ACH vs. CHC/FQHC). All data analyses were performed in R version 3.0.1.
Results
Gender differences in HIV testing and diagnosis
From 2011 to 2016, X-TLC conducted 281,017 HIV screening tests; 63.7% of those tested were women. Overall HIV seroprevalence was 0.57%, and almost one third (29.4%) of HIV patients identified were cisgender women (seroprevalence 0.18%; Tables 1 and 2). Similar to men, ∼44.6% (207/464) of all HIV-positive women were newly diagnosed. Women accounted for 31.3% (207/662) of all new diagnoses, the second largest demographic of newly diagnosed infections after MSM (32.2%, 213/662). For existing diagnoses, the largest group was in men with unknown risk at 39.8% (319/801), followed by women at 32.1% (257/801). Additionally, the majority of newly diagnosed patients were AA (77.3%). This disparity was greater in women (86.0%) compared with men (78.8% for heterosexual men, 73.2% for MSM, and 70.7% for men with unknown sexual partners). Heterosexual men were the smallest demographic for both new (12.8%) and existing diagnoses (7.0%).
Table 1.
Characteristics of HIV-Positive Clients: New Diagnoses by Risk Group (n = 662)
| Women (N = 207) | Men-heterosexual (N = 85) | Men-MSM (N = 213) | Men-unknown (N = 157) | Total (N = 662) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Age, mean ± SD** | 35.0 ± 13.3 | 41.3 ± 14.8 | 28.9 ± 10.2 | 34.9 ± 14.2 | 33.8 ± 13.3 | |||||
| Race | ||||||||||
| White | 11 | 5.3 | 7 | 8.2 | 32 | 15.0 | 19 | 12.1 | 69 | 10.4 |
| Black | 178 | 86.0 | 67 | 78.8 | 156 | 73.2 | 111 | 70.7 | 512 | 77.3 |
| Other | 13 | 6.3 | 10 | 11.8 | 16 | 7.5 | 23 | 14.6 | 62 | 9.4 |
| Unknown | 5 | 2.4 | 1 | 1.2 | 9 | 4.2 | 4 | 2.5 | 19 | 2.9 |
| Insurance** | ||||||||||
| Uninsured | 16 | 7.7 | 13 | 15.3 | 37 | 17.4 | 23 | 14.6 | 89 | 13.4 |
| Public | 58 | 28.0 | 16 | 18.8 | 30 | 14.1 | 29 | 18.5 | 133 | 20.1 |
| Private | 20 | 9.7 | 15 | 17.6 | 45 | 21.1 | 18 | 11.5 | 98 | 14.8 |
| Unknown | 113 | 54.6 | 41 | 48.2 | 101 | 47.4 | 87 | 55.4 | 342 | 51.7 |
| Site type* | ||||||||||
| Hospitals | 121 | 58.5 | 54 | 63.5 | 114 | 53.5 | 113 | 72.0 | 402 | 60.7 |
| ED | 45 | 21.7 | 24 | 28.2 | 66 | 31.0 | 32 | 20.4 | 167 | 25.2 |
| Outpatient | 6 | 2.9 | 2 | 2.4 | 9 | 4.2 | 11 | 7.0 | 28 | 4.2 |
| Inpatient | 16 | 7.7 | 7 | 8.2 | 4 | 1.9 | 15 | 9.6 | 42 | 6.3 |
| Unknown | 33 | 15.9 | 15 | 17.6 | 28 | 13.1 | 41 | 26.1 | 117 | 17.7 |
| CHC/FQHCs | 85 | 41.1 | 31.0 | 36.5 | 99 | 46.5 | 44 | 28.0 | 259 | 39.1 |
| Family planning clinic | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| Laboratory results | ||||||||||
| Viral load** | 200,462.3 ± 912,091.6 | 193,518.5 ± 380,637.3 | 1,144,350.5 ± 8,579,657.9 | 782,211.5 ± 2211,662.5 | 663,582 ± 5,514,296 | |||||
| CD4 count** | 417.5 ± 306.7 | 290.6 ± 250.5 | 372.8 ± 236.4 | 285.4 ± 275.7 | 361.2 ± 269.5 | |||||
| Linked to care** | 135 | 66.2a | 75 | 89.3a | 192 | 92.3a | 84 | 56.4a | 486 | 76.1 |
Denominator: eligible to be linked includes those who are not deceased or moved.
p < 0.05; **p < 0.01.
CHC/FQHCs, community health center/federally qualified health centers; ED, emergency department; MSM, men who have sex with men; SD, standard deviation.
Table 2.
Characteristics of HIV-Positive Clients: Existing Diagnoses by Risk Group (n = 801)
| Women (N = 257) | Men-heterosexual (N = 56) | Men-MSM (N = 169) | Men-unknown (N = 319) | Total (N = 801) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | |
| Age, mean ± SD** | 39.7 ± 13.9 | 42.9 ± 14.8 | 34.0 ± 12.6 | 44.6 ± 13.6 | 40.7 ± 14.2 | |||||
| Race** | ||||||||||
| White | 12 | 4.7 | 3 | 5.4 | 25 | 14.8 | 21 | 6.6 | 61 | 7.6 |
| Black | 214 | 83.3 | 47 | 83.9 | 110 | 65.1 | 253 | 79.3 | 624 | 77.9 |
| Other | 22 | 8.6 | 5 | 8.9 | 25 | 14.8 | 37 | 11.6 | 89 | 11.1 |
| Unknown | 9 | 3.5 | 1 | 1.8 | 9 | 5.3 | 8 | 2.5 | 27 | 3.4 |
| Insurance | ||||||||||
| Uninsured | 12 | 4.7 | 6 | 10.7 | 23 | 13.6 | 21 | 6.6 | 62 | 7.7 |
| Public | 59 | 23.0 | 19 | 33.9 | 37 | 21.9 | 95 | 29.8 | 210 | 26.2 |
| Private | 25 | 9.7 | 9 | 16.1 | 31 | 18.3 | 32 | 10.0 | 97 | 12.1 |
| Unknown | 161 | 62.6 | 22 | 39.3 | 78 | 46.2 | 171 | 53.6 | 432 | 53.9 |
| Site type** | ||||||||||
| Hospitals | 202 | 78.6 | 31 | 55.4 | 89 | 52.7 | 262 | 82.1 | 584 | 72.9 |
| ED | 48 | 18.7 | 10 | 17.9 | 31 | 18.3 | 54 | 16.9 | 143 | 17.9 |
| Outpatient | 10 | 3.9 | 3 | 5.4 | 6 | 3.6 | 13 | 4.1 | 32 | 4.0 |
| Inpatient | 16 | 6.2 | 1 | 1.8 | 8 | 4.7 | 25 | 7.8 | 50 | 6.2 |
| Unknown | 92 | 35.8 | 6 | 10.7 | 19 | 11.2 | 126 | 39.5 | 243 | 30.3 |
| CHC/FQHCs | 55 | 21.4 | 25 | 44.6 | 80 | 47.3 | 57 | 17.9 | 217 | 27.1 |
| Laboratory results | ||||||||||
| Viral load** | 59,022.3 ± 139,847.6 | 57,229.1 ± 104,581.5 | 144,776 ± 506,827.5 | 159,080 ± 553,517.1 | 114,917 ± 425,343.5 | |||||
| CD4 count** | 452.8 ± 337.1 | 381.1 ± 286.0 | 471.4 ± 306.3 | 340.7 ± 295.9 | 416.4 ± 311.7 | |||||
| Linked to care** | 77 | 53.8a | 19 | 76.0a | 100 | 90.1a | 55 | 41.0a | 251 | 65.2 |
Denominator: eligible to be linked includes those who are not deceased, moved, or already in care.
p < 0.05; **p < 0.01.
CHC/FQHCs, community health center/federally qualified health centers; ED, emergency department; MSM, men who have sex with men; SD, standard deviation.
We compared screening among women and men by HIV testing site type and found that a higher proportion of men whose risk is unknown (75.6%) were identified in ACHs compared with their peers, whereas a smaller proportion of MSM were identified in ACHs (52.6%). Yet, MSM had the largest proportion diagnosed in CHC/FQHCs, regardless of new or previously diagnosed status (p < 0.01, data not shown); this relationship between risk groups and testing site types remained significant when stratified by HIV diagnosis status (p < 0.01).
Of all women, 41.1% of those newly diagnosed were identified in CHC/FQHCs, 21.7% in the ED, and 7.7% as inpatient units; whereas previously diagnosed women were mostly identified in ACHs (78.6%), followed by CHC/FQHCs (21.4%). By comparison, heterosexual men and MSM show similar results by site type: CHC/FQHCs (40.1% and 47.4%, respectively), ED (26.1% and 27.8%, respectively), and inpatient units (5.6% and 3.1%, respectively). At the UCM alone, 43.5% of women were diagnosed in the ED and 20.0% were diagnosed in the inpatient setting.
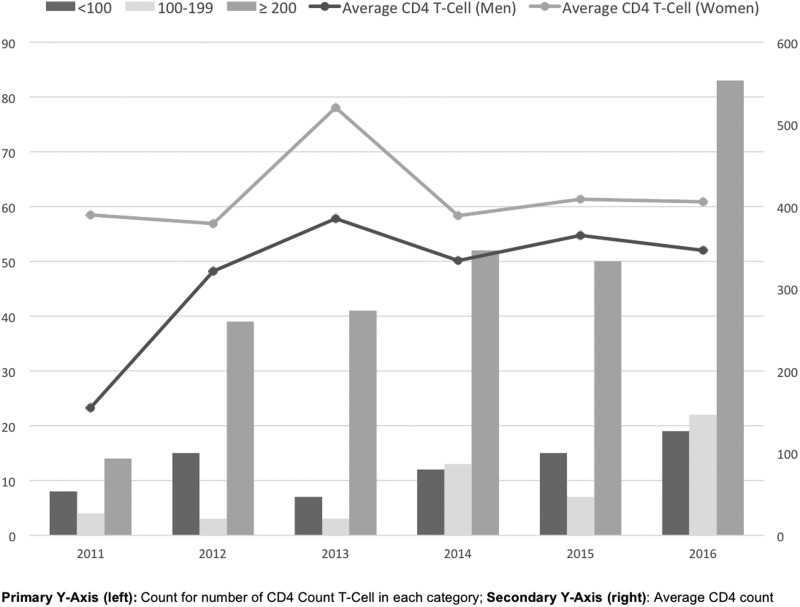
The mean age for newly diagnosed women was 35.0 ± 13.3 years compared with 28.9 ± 10.2 years for MSM, 41.3 ± 14.8 years for heterosexual men, and 34.9 ± 14.2 years for men with unknown risk. Women identified through X-TLC as newly diagnosed were younger (35.0 ± 13.3 years) compared with previously diagnosed women (39.7 ± 13.9 years). We found a significant difference between the baseline absolute CD4 T cell counts of women who were newly diagnosed with HIV (417.5 ± 306.7) compared with heterosexual men (290.6 ± 250.5), MSM (372.8 ± 236.4), and men whose unknown risk (285.4 ± 275.7; p < 0.01). In Table 3, we identified that persons who had a CD4 count ≥200 were 2.6 times more likely to be linked to care than those who had a CD4 count <100 [adjusted odds ratio (aOR) = 2.64, 95% confidence interval (CI) 1.31–5.20]. Figure 1 displays CD4 T cell count categorized by <100, 100–199, and >200 or greater, in addition to average CD4 T cell count over time, stratified by men and women. While men demonstrated an increase in CD4 count over time from 2011 to 2013, women showed a stable CD4 count over the 5 years.
Table 3.
Logistic Regression: Predictors of X-TLC Outcomes
| Estimated odds ratio linked vs. not linked | ||
|---|---|---|
| uOR (95% CI) | aOR (95% CI) | |
| Gender | ||
| Men | Ref. | Ref. |
| Women* | 0.61 (0.46–0.80) | 0.59 (0.44–0.78) |
| Risk | ||
| Women | Ref. | Ref. |
| Men-heterosexual* | 4.58 (2.55–8.86) | 4.11 (2.21–8.26) |
| Men-MSM* | 6.33 (4.14–9.97) | 7.16 (4.41–12.10) |
| Men-unknown** | 0.55 (0.40–0.75) | 0.61 (0.43–0.85) |
| Testing site type | ||
| Hospitals | Ref. | Ref. |
| CHC/FQHC* | 1.79 (1.37–2.35) | 2.23 (1.62–3.10) |
| HIV status | ||
| New diagnoses | Ref. | Ref. |
| Existing diagnoses* | 0.47 (0.37–0.61) | 0.56 (0.42–0.74) |
| CD4 T cell count | ||
| <100 | Ref. | Ref. |
| 100–199 | 1.05 (0.47–2.41) | 0.97 (0.42–2.29) |
| ≥200** | 2.43 (1.27–4.51) | 2.64 (1.31–5.20) |
Controlling for patient demographics, HIV status, and testing site.
p < 0.001, **p < 0.01.
aOR, adjusted odds ratio; CHC/FQHC, community health center/federally qualified health center; CI, confidence interval; MSM, men who have sex with men; OR, odds ratio; uOR, unadjusted odds ratio; X-TLC, Expanded HIV Testing and Linkage to Care.
FIG. 1.
CD4 T cell count for newly diagnosed HIV-positive patients, over 5 years. Primary Y-axis (left): number of patients in each CD4 T cell count category; secondary Y-axis (right): average CD4 count.
Gender differences in LTC outcomes
Men were more likely to be linked than women for both new (79.3% vs. 66.2%, p < 0.01) and previously diagnosed (69.0% vs. 53.8%, p < 0.01). Women had lower odds of LTC compared with men (aOR = 0.59, 95% CI 0.44–0.78) when controlling for patient demographics and HIV status (Table 3). When we observe by risk group, compared with women, MSM had seven times the odds (aOR = 7.16, 95% CI 4.41–12.10) and heterosexual men had almost four times the odds (aOR = 4.11, 95% CI 2.21–8.26) to be linked to HIV care. Men whose risk is unknown had lesser odds of being linked compared with women (aOR = 0.61, 95% CI 0.43–0.85). For both men and women, existing diagnoses were less likely to be linked compared with newly diagnosed individuals (aOR = 0.56, 95% CI 0.42–0.74).
Comparison between X-TLC and citywide Chicago data
Women accounted for 31.3% (207/662) of all new diagnoses in X-TLC, which is a greater proportion than the 15.9% for Chicago overall according to the CDPH data. Table 4 displays the results of X-TLC testing data, citywide CDPH-sponsored testing programs, and HIV surveillance data for Chicago by year from 2012 to 2016. X-TLC accounted for the majority of women diagnosed with HIV infection through CDPH-sponsored testing programs, 60.9% of all women testing positive, and 74.4% of the women with a new diagnosis. X-TLC reaches primarily AA women, who accounting for 90.5% of the AA women diagnosed. Additionally, women diagnosed through X-TLC accounted for 21.9% (160/731) of all the women newly diagnosed in Chicago during this time period.
Table 4.
Five-Year Surveillance of Newly Diagnosed HIV Infections in Chicago, by Gender
| 2012 | 2013 | 2014 | 2015 | 2016 | Total | |
|---|---|---|---|---|---|---|
| Total HIV infections | 1036 | 966 | 886 | 896 | 824 | 4608 |
| New HIV infection, male, n (%) | 858 (82.2) | 809 (83.7) | 763 (86.1) | 764 (85.3) | 683 (82.9) | 3877 (84.1) |
| New HIV infection, female, n (%) | 178 (17.2) | 157 (16.3) | 123 (13.9) | 132 (14.7) | 141 (17.1) | 731 (15.9) |
| New HIV infections, females only, n (%) | ||||||
| CDPH testinga (AA) | 40 (87.5) | 66 (97.0) | 37 (83.8) | 20 (65.0) | 52 (78.8) | 215 (85.6) |
| X-TLCb (AA) | 23 (95.7) | 27 (88.9) | 32 (87.5) | 22 (86.4) | 56 (87.5) | 160 (88.8) |
| UCMc (AA) | 7 (100.0) | 7 (100.0) | 8 (100.0) | 9 (100.0) | 8 (100.0) | 39 (100.0) |
Data source: surveillance data from the Chicago Department of Public Health. HIV/STI Surveillance Report 2017. Chicago, IL: City of Chicago, December 2017. https://www.cityofchicago.org/content/dam/city/depts/cdph/HIV_STI/HIV_STISurveillanceReport2016_12012017.pdf
Data source: X-TLC program data.
Data source: University of Chicago Medicine, hospital-specific data.
AA, African American; CDPH, Chicago Department Public Health; STI, sexually transmitted infection; UCM, University of Chicago Medicine; X-TLC, Expanded HIV Testing and Linkage to Care.
Discussion
In this study, we examined trends in HIV diagnosis and LTC through a routine healthcare-based testing program on the South and West sides of Chicago. We found that routine healthcare-based testing is very effective at identifying women with HIV infection, particularly AA women within this geographic area in Chicago. On average, women were older and found to have a higher CD4 count at diagnosis compared with men. However, women newly diagnosed with HIV were less likely to be linked to care than men. Finally, testing through X-TLC identified 21.9% of all new diagnoses in women in Chicago during this time period.
Routine opt-out HIV testing in healthcare settings has been recommended by the CDC since 2006.21 We found, through this routine healthcare-based testing program across multiple sites, that more women were tested for HIV than men. Yet, men were more likely to be diagnosed with HIV than women, reflecting the demographics of the epidemic.33 Despite this, women accounted for nearly one third of new diagnoses through X-TLC, which is a higher proportion than reported at national-wide and citywide levels.1,33 In addition, most of the women diagnosed through X-TLC were cisgender AA women, a group disproportionately affected by HIV. During this time period, the University of Chicago also conducted social network testing, partner notification, and community-based testing, none of which identified new diagnoses of HIV in cisgender women. Clearly, there are still women with undiagnosed HIV infection who are tested in healthcare settings.
Our results underscore the importance of routine screening, particularly for heterosexual women who may not have identifiable risk factors for HIV other than residing within a high prevalence area. Routine HIV screening is important in identifying patients with undiagnosed HIV infection, particularly those who may not know of their presumed risk, which is the case with many high-risk heterosexual individuals.7 A significant portion of heterosexually exposed women and men lack complete information of the HIV status and/or risk of their sex partners.15 Thus, women may not take part in programs that perform targeted outreach for HIV prevention and are less likely to be diagnosed through other screening methods. At the University of Chicago, partner notification and social network strategies did not identify ciswomen with HIV; however, these strategies can be effective in identifying women and other high-risk heterosexuals in some settings.35 The finding that women were more likely to be screened for HIV than men through routine testing has been previously seen36 and may reflect higher utilization of healthcare services by women, often related to reproductive health including pregnancy.37,38 Accordingly, if women access healthcare at higher rates than men, they may be more likely to encounter opportunities for routine HIV screening. Furthermore, routine testing can be leveraged to identify women who would benefit from more frequent HIV screening and biomedical HIV prevention such as pre-exposure prophylaxis (PrEP).
We also found that women with a new diagnosis of HIV were older than men and had higher average CD4 count at diagnosis. This appears to be acquisition of HIV at an older age rather than late diagnosis. Recent CDC data on HIV testing show that the time from HIV infection to diagnosis has significantly decreased in the United States from 3 years and 7 months to 3 years overall, but the decrease is very different for the various populations at risk.23 Heterosexual women have a shorter delay compared with heterosexual men, 2.4 and 4.9 years, respectively.23 These changes in time to diagnosis are associated with increased testing in all risk groups, but testing in heterosexual men and women still remains low compared with MSM and people who inject drugs (PWID), although women were more likely than men to have been tested in the past 12 months,23 similar to our findings. Our study's results also reflect a shorter delay in diagnosis for heterosexual women given the higher CD4 count at diagnosis.
Importantly, we observed a substantial difference in LTC for women with a new diagnosis of HIV compared with men, as well as with a previous diagnosis who were out of care. Women were less likely to be linked to care compared with men even when adjusting for factors, such as demographics, HIV diagnosis status, and site of care. This occurred despite collaborative agreements and standard operating procedures for LTC both within and outside our network. This difference was surprising, given the greater number of women tested and historical higher utilization of healthcare by women. However, in additional analyses, we found that despite linkage being low, retention remained high once a woman was linked to care.39
Some previous reports have indicated that women are more likely to be linked to care than men, which is contrary to what we have observed in our cohort of patients. Using data from the National HIV Surveillance System (NHSS), it was reported that a higher proportion of heterosexual women were linked to care at 30 and 90 days compared with MSM or heterosexual men29; this has also been the conclusion from analyses of the NHSS data comparing only black women and men.30,40 Literature reporting on gender differences for other care continuum outcomes has similarly found lower retention in care and viral suppression for black males compared with females.40–42 AA individuals overall had lower odds of being linked to care, retained in care, and virally suppressed compared with white individuals.29,41–43 Other studies have identified women as being less likely to establish care following a diagnosis of HIV.28 Barriers for entry into care for women include poverty, housing insecurity, lack of transportation or child care, lack of insurance, substance use, mental illness, and stigma.44–46 While our results cannot determine the cause of the gender differences in LTC, the program sites are located within areas of high economic hardship within Chicago, which may play a role in these findings.
Routine HIV screening is reaching a significant number of AA women in Chicago with HIV infection who are unaware of their diagnosis. The proportion of seropositive women identified was higher than the national average and for the City of Chicago as well. Although the number of new HIV diagnoses both nationwide and in Chicago has been decreasing over time, including for women,3,33 the number of new diagnoses identified by X-TLC has been increasing. This may reflect the addition of new sites and an increase in the total number of tests performed over time. Still, it is clear that routine testing in healthcare settings is identifying a very high proportion of new diagnoses of HIV in women.
A major limitation to our study is the limited risk assessment data with routine screening. As a result, we were unable to determine the risk factors for HIV in a significant proportion of patients. This may be due to the difficulty in obtaining risk factor information in patients who were referred out of our network for specialty HIV care. Due to referrals out of our network, we were also unable to obtain information on outcomes at points in the care continuum after linkage. Another limitation is that we did not specify a time frame for linkage. Before 2015, the recommended time frame for LTC was within 90 days.47 The updated National HIV/AIDS Strategy (NHAS) changed this goal to within 30 days of diagnosis.48 Some sites were only able to report a binary (yes/no) linkage result rather than a date by which to accurately determine time to linkage. Finally, we are unable to determine from our data why women are less likely to be linked to care in this routine testing program.
Routine testing through the X-TLC program was successful in identifying new HIV diagnoses, particularly in AA women. Due to the importance of routine testing in identifying new HIV infections, it is crucial that support for routine test in healthcare settings continues to improve testing rates, which can be accomplished through initiatives such as incorporating opt-out testing and automatic orders for HIV tests through the EMR.49,50 Additionally, identifying high-risk HIV-negative women through routine testing can be an important method for implementing PrEP within this population. Some of our findings are consistent with national trends, such as a higher proportion of women tested for HIV through routine testing; while others, such as delayed entry into care for women, are not. This highlights the importance of monitoring local and programmatic outcomes to identify areas for improvement. Early entry and retention in the care continuum improve health outcomes and survival for persons living with HIV as well as decreases transmission events.51–53 Recommendations for improving care continuum outcomes include immediate referral to HIV care; active case management with insurance, housing, and transportation support; intensive outreach for those not engaged in care at 1 month following a diagnosis; and active re-engagement of persons lost to care.44,54 The X-TLC program performs these functions, yet future research will need to identify underlying causes for our findings and develop interventions to address lower LTC rates in women.
Acknowledgments
We acknowledge the Chicago Department of Public Health for their support of the Expanded Testing and Linkage to Care program. We also thank Monique Rucker, MPH, for her work as the X-TLC program manager and epidemiologist at the Mt. Sinai Hospital from 2012 to 2015. In addition, we would like to recognize all X-TLC participating sites for the work they do in diagnosing and linking individuals to care. M.C.M. would like to thank the Center for Prevention Implementation Methodology (P30 DA027828) for their support. We acknowledge funding from Gilead's Frontlines of Communities in the United States (FOCUS) program. FOCUS funding supports HIV, HCV, and HBV screening and linkage to the first medical appointment after diagnosis. FOCUS funding does not support any activities beyond the first medical appointment and is agnostic to how FOCUS partners handle subsequent patient care and treatment.
Author Disclosure Statement
D.P., J.P.R., and N.G. are supported through Gilead's Frontlines of Communities in the United States (FOCUS) program. N.G. reports additional funding from Gilead.
References
- 1.Centers for Disease Control and Prevention. Diagnoses of HIV Infection in the United States and Dependent Areas, 2016. HIV Surveillance Report. 2017;28. Available at: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html (Last accessed May22, 2018)
- 2.Hodder SL, Justman J, Hughes JP, et al. HIV acquisition among women from selected areas of the United States: A cohort study. Ann Intern Med 2013;158:10–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCree DH, Sutton M, Bradley E, et al. Changes in the disparity of HIV diagnosis rates among Black women—United States, 2010–2014. MMWR Morb Mortal Wkly Rep 2017;66:104–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Leading Causes of Death in Females, 2014. 2017. Available at: https://www.cdc.gov/women/lcod/2014/black/index.htm (Last accessed May22, 2018)
- 5.Murphy SL, Xu J, Kochanek KD, et al. Deaths: Final Data for 2015. Natl Vital Stat Rep 2017;66:1–120 [PubMed] [Google Scholar]
- 6.Hirschhorn LR, McInnes K, Landon BE, et al. Gender differences in quality of HIV care in Ryan White CARE Act-funded clinics. Womens Health Issues 2006;16:104–112 [DOI] [PubMed] [Google Scholar]
- 7.Hodder SL, Justman J, Haley DF, et al. Challenges of a hidden epidemic: HIV prevention among women in the United States. J Acquir Immune Defic Syndr 2010;55(Suppl 2):S69–S73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stein R, Pierce T, Hollis N, et al. HIV testing and service delivery among black females—61 health department jurisdictions, United States, 2012–2014. MMWR Morb Mortal Wkly Rep 2016;65:83–85 [DOI] [PubMed] [Google Scholar]
- 9.Adimora AA, Schoenbach VJ, Floris-Moore MA. Ending the epidemic of heterosexual HIV transmission among African Americans. Am J Prev Med 2009;37:468–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno CL, Morrill AC, El-Bassel N. Sexual risk factors for HIV and violence among Puerto Rican women in New York City. Health Soc Work 2011;36:87–97 [DOI] [PubMed] [Google Scholar]
- 11.Adimora AA, Schoenbach VJ, Doherty IA. Concurrent sexual partnerships among men in the United States. Am J Public Health 2007;97:2230–2237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hallfors DD, Iritani BJ, Miller WC, et al. Sexual and drug behavior patterns and HIV and STD racial disparities: The need for new directions. Am J Public Health 2007;97:125–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McLellan-Lemal E, O'Daniels CM, Marks G, et al. Sexual risk behaviors among African-American and Hispanic women in five counties in the southeastern United States: 2008–2009. Womens Health Issues 2012;22:e9–e18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings L, Rompalo AM, Wang J, et al. Prevalence and correlates of knowledge of male partner HIV testing and serostatus among African-American women living in high poverty, high HIV prevalence communities (HPTN 064). AIDS Behav 2015;19:291–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenness SM, Neaigus A, Hagan H, et al. Heterosexual HIV and sexual partnerships between injection drug users and noninjection drug users. AIDS Patient Care STDS 2010;24:175–181 [DOI] [PubMed] [Google Scholar]
- 16.Kalichman SC. HIV transmission risk behaviors of men and women living with HIV/AIDS: Prevalence, predictors, and emerging clinical interventions. Clin Psychol Sci Pract 2000;7:32–47 [Google Scholar]
- 17.Younge SN, Salem D, Bybee D. Risk revisited: The perception of HIV risk in a community sample of low-income African American women. J Black Psychol 2008;36:49–74 [Google Scholar]
- 18.Ford CL, Daniel M, Miller WC. High rates of HIV testing despite low perceived HIV risk among African-American sexually transmitted disease patients. J Natl Med Assoc 2006;98:841–844 [PMC free article] [PubMed] [Google Scholar]
- 19.Blackstock OJ, Frew P, Bota D, et al. Perceptions of community HIV/STI risk among U.S. women living in areas with high poverty and HIV prevalence rates. J Health Care Poor Underserved 2015;26:811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sionean C, Le BC, Hageman K, et al. HIV risk, prevention, and testing behaviors among heterosexuals at increased risk for HIV infection-National HIV Behavioral Surveillance System, 21 US cities, 2010. MMWR Surveill Summ 2014;63:1–39 [PubMed] [Google Scholar]
- 21.Branson BM, Handsfield HH, Lampe MA, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep 2006;55:1–17; quiz CE1–4. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Implementing HIV Testing in Nonclinical Settings: A Guide for HIV Testing Providers. Atlanta, GA: CDC, 2016 [Google Scholar]
- 23.Dailey AF, Hoots BE, Hall HI, et al. Vital signs: Human immunodeficiency virus testing and diagnosis delays—United States. MMWR Morb Mortal Wkly Rep 2017;66:1300–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seth P, Wang G, Collins NT, et al. Identifying new positives and linkage to HIV medical care—23 testing site types, United States, 2013. MMWR Morb Mortal Wkly Rep 2015;64:663–667 [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS 2005;19:423–431 [DOI] [PubMed] [Google Scholar]
- 26.Torian LV, Wiewel EW. Continuity of HIV-related medical care, New York City, 2005–2009: Do patients who initiate care stay in care? AIDS Patient Care STDS 2011;25:79–88 [DOI] [PubMed] [Google Scholar]
- 27.Horberg MA, Hurley LB, Klein DB, et al. The HIV care cascade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDS 2015;29:582–590 [DOI] [PubMed] [Google Scholar]
- 28.Mugavero MJ, Lin HY, Allison JJ, et al. Failure to establish HIV care: Characterizing the “no show” phenomenon. Clin Infect Dis 2007;45:127–130 [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2015. HIV Surveill Suppl Rep 2017;22:1–63 [Google Scholar]
- 30.Dailey AF, Johnson AS, Wu B. HIV care outcomes among blacks with diagnosed HIV—United States, 2014. MMWR Morb Mortal Wkly Rep 2017;66:97–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bares S, Eavou R, Bertozzi-Villa C, et al. Expanded HIV testing and linkage to care: Conventional vs. point-of-care testing and assignment of patient notification and linkage to care to an HIV care program. Public Health Rep 2016;131(Suppl 1):107–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rucker MG, Eavou R, Allgood KL, et al. Implementing routine HIV screening in three Chicago hospitals: Lessons learned. Public Health Rep 2016;131(Suppl 1):121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chicago Department of Public Health. HIV/STI Surveillance Report 2017. Chicago, IL: City of Chicago, 2017 [Google Scholar]
- 34.Illinois AIDS Confidentiality Act, 410 ILCS 305, 2015 [Google Scholar]
- 35.Gwadz M, Cleland CM, Perlman DC, et al. Public health benefit of peer-referral strategies for detecting undiagnosed HIV infection among high-risk heterosexuals in New York City. J Acquir Immune Defic Syndr 2017;74:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bond L, Lauby J, Batson H. HIV testing and the role of individual- and structural-level barriers and facilitators. AIDS Care 2005;17:125–140 [DOI] [PubMed] [Google Scholar]
- 37.Green CA, Pope CR. Gender, psychosocial factors and the use of medical services: A longitudinal analysis. Soc Sci Med 1999;48:1363–1372 [DOI] [PubMed] [Google Scholar]
- 38.Lassman D, Hartman M, Washington B, et al. US health spending trends by age and gender: Selected years 2002–2010. Health Aff (Millwood) 2014;33:815–822 [DOI] [PubMed] [Google Scholar]
- 39.Almirol EA, Lancki N, Schmitt J, et al. HIV care and engagement: Demographics and risk factors associated with retention and viral suppression in Chicago, IL. In: 11th International Workshop on HIV Transmission. Reviews in Antiviral Therapy & Infectious Diseases; Chicago, IL, 2016 [Google Scholar]
- 40.Whiteside YO, Cohen SM, Bradley H, et al. Progress along the continuum of HIV care among blacks with diagnosed HIV-United States, 2010. MMWR Morb Mortal Wkly Rep 2014;63:85–89 [PMC free article] [PubMed] [Google Scholar]
- 41.Sheehan DM, Fennie KP, Mauck DE, et al. Retention in HIV care and viral suppression: Individual- and neighborhood-level predictors of racial/ethnic differences, Florida, 2015. AIDS Patient Care STDS 2017;31:167–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasgupta S, Oster AM, Li J, et al. Disparities in consistent retention in HIV care—11 states and the District of Columbia, 2011–2013. MMWR Morb Mortal Wkly Rep 2016;65:77–82 [DOI] [PubMed] [Google Scholar]
- 43.Vaughan Sarrazin MS, Ohl ME, Richardson KK, et al. Patient and facility correlates of racial differences in viral control for black and white veterans with HIV infection in the Veterans Administration. AIDS Patient Care STDS 2018;32:84–91 [DOI] [PubMed] [Google Scholar]
- 44.Aziz M, Smith KY. Challenges and successes in linking HIV-infected women to care in the United States. Clin Infect Dis 2011;52(Suppl 2):S231–S237 [DOI] [PubMed] [Google Scholar]
- 45.Rumptz MH, Tobias C, Rajabiun S, et al. Factors associated with engaging socially marginalized HIV-positive persons in primary care. AIDS Patient Care STDS 2007;21(Suppl 1):S30–S39 [DOI] [PubMed] [Google Scholar]
- 46.Pollini RA, Blanco E, Crump C, et al. A community-based study of barriers to HIV care initiation. AIDS Patient Care STDS 2011;25:601–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Office of National AIDS Policy. National HIV/AIDS Strategy Improving Outcomes: Accelerating Progress Along the HIV Care Continuum. Washington, DC: Office of National AIDS Policy, 2013 [Google Scholar]
- 48.Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. Washington, DC: Office of National AIDS Policy, 2015 [Google Scholar]
- 49.Lin J, Mauntel-Medici C, Heinert S, et al. Harnessing the power of the electronic medical record to facilitate an opt-out HIV screening program in an urban academic emergency department. J Public Health Manag Pract 2017;23:264–268 [DOI] [PubMed] [Google Scholar]
- 50.Felsen UR, Cunningham CO, Heo M, et al. Expanded HIV testing strategy leveraging the electronic medical record uncovers undiagnosed infection among hospitalized patients. J Acquir Immune Defic Syndr 2017;75:27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hall HI, Tang T, Johnson AS, et al. Timing of linkage to care after HIV diagnosis and time to viral suppression. J Acquir Immune Defic Syndr 2016;72:e57–e60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011;365:493–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lundgren JD, Babiker AG, Gordin F, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med 2015;373:795–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.International Advisory Panel on HIV Care Continuum Optimization. IAPAC guidelines for optimizing the HIV care continuum for adults and adolescents. J Int Assoc Provid AIDS Care 2015;14(Suppl 1):S3–S34 [DOI] [PubMed] [Google Scholar]