Abstract
Objective: School refusal is an important pediatric problem with significant negative short- and long-term outcomes. Specific psychosocial treatments appear effective in reducing school refusal, but many children do not respond to these treatments. Although systematic reviews have examined the efficacy of psychological interventions for school refusal, no systematic reviews on pharmacological interventions exist.
Methods: We conducted a comprehensive literature search of MEDLINE, PsycINFO, Scopus, and Embase for randomized controlled trials (RCTs) or quasi-experimental pharmacologic trials in children and adolescents with school refusal reported in English or Spanish until July 1, 2017. Two authors screened study titles and abstracts for eligibility. Data regarding the population, intervention, comparison, and outcomes for each trial were extracted and reported. Effect sizes for school attendance are presented.
Results: The search identified 6 articles, including 7 trials (6 RCTs and 1 open label) and 306 youths. Pharmacologic treatments investigated for school refusal included antidepressants (imipramine, clomipramine, and fluoxetine) and benzodiazepines (alprazolam). All pharmacotherapies studied had pretreatment to posttreatment improvements on school refusal, depression, and anxiety symptoms. However, included trials were severely underpowered and did not demonstrate significant improvement compared to placebo.
Conclusions: Data regarding pharmacological treatments for school refusal are sparse. Most trials in this area were conducted before development of newer antidepressants, were underpowered, and have significant methodological limitations that are characteristic of the time in which they were conducted. This systematic review highlights the need for more trials with newer pharmacologic agents, larger sample sizes, and improved systematic assessments of school refusal and comorbidities. School refusal represents an important functional outcome for many children, especially those with anxiety and depression. Future pharmacologic studies of anxiety and depression in children may benefit from incorporating specific school refusal measures as secondary outcomes.
Keywords: : drug therapy, school refusal behavior, systematic review, children
Introduction
School refusal is a complex problem and constitutes one of the few emergencies in child psychiatric clinics, given its significant consequences on the child, family, and school as it becomes more entrenched over time (Gittelman-Klein and Klein 1973). School refusal affects 1%–2% of children and adolescents (referred to as “children” or “youth” henceforth) in the general population and 5%–15% of children in clinic samples (Egger et al. 2003; Heyne and King 2004).
Long-term consequences of school refusal include increased risk of substance abuse, suicide attempts, risky sexual behavior, school dropout, and social adjustment problems (Kaufman et al. 2004; Maria da Conceição et al. 2006; Christle et al. 2007; Henry and Huizinga 2007; Gottfried 2009). Furthermore, school refusal causes significant economic burden associated with poor academic performance, school attendance, future underemployment, and increased risk of criminal prosecution (Egger et al. 2003; Heyne and King 2004; Kaufman et al. 2004). Longitudinal studies following children with school refusal attest to the risk for ongoing mental health problems in late adolescence and adulthood (Berg and Jackson 1985; Buitelaar et al. 1994; Flakierska-Praquin et al. 1997; McCune and Hynes 2005). Despite its prevalence and consequences, there are few effective treatments for school refusal.
School refusal has been operationalized in the literature as a child's reluctance or refusal to go to school, usually because the thought of going to school induces unhappiness or emotional distress. This in turn leads to prolonged school absences. Parents are often aware that the child is staying at home, and may make efforts to have the child attend school. This behavior is in the absence of antisocial behavior. School refusal differs from truancy in that it is primarily motivated by avoidance of emotional distress, while truancy is conceptualized as motivated by the desire for rewards other than avoidance of fear-induced stimuli (Maynard et al. 2015). Truancy, unlike school refusal, is associated with oppositional defiant disorder and conduct disorder (Egger et al. 2003). Some experts in the area have debated whether to include truancy and malingering as part of the definition of school refusal since prevalence studies have demonstrated that pure school refusal and truancy are sometimes mixed, resulting in a third category of mixed school refusal (Egger et al. 2003).
Although the population of children and adolescents with prolonged school refusal is heterogeneous, up to 50% of these youth have comorbid anxiety (Bools et al. 1990; Mcshane et al. 2001; Heyne et al. 2002; Prabhuswamy et al. 2007; Walter et al. 2010), depression (Bernstein and Garfinkel 1986; Bernstein 1991; Berg 1992), or both (Bernstein and Garfinkel 1986). Furthermore, children with school refusal and comorbid anxiety and depression tend to refuse school primarily to avoid school-related stimuli that provoke a sense of general negative affectivity and/or to escape from aversive social or evaluative situations at school (Egger et al. 2003; Kearney 2006).
Phenomenological studies of school refusal demonstrate that school refusal is complex and has variable presentations; however, there seem to be three main types of anxious school-refusing children: those with simple or specific phobia, those with separation anxiety, and those who are anxious or depressed (King and Bernstein 2001). In addition, there are school-refusing children with other comorbidities, including social anxiety, attention-deficit hyperactivity disorder, and panic disorder (Egger et al. 2003). Evaluating for these and other comorbid disorders, and for other potential contributing factors such as bullying, learning disorders, and psychosocial adversity is important in fully evaluating children with school refusal behaviors.
Most of the school refusal intervention literature has focused on school refusal with comorbid anxiety and depression. Interventions include psychosocial and pharmacologic interventions. Currently, there are systematic reviews and meta-analyses of psychosocial interventions, but none of pharmacologic treatments of school refusal. A recent meta-analysis of psychosocial interventions for school refusal identified eight small-randomized controlled and quasi-experimental trials involving 435 participants (Maynard et al. 2015). Psychosocial interventions in the Maynard et al. 2015 meta-analysis included six studies treating school refusal with psychosocial only and two with psychosocial intervention with and without medication.
Most of the psychosocial interventions included variants of Cognitive Behavioral Therapy (CBT), systematic desensitization, behavioral treatment approached with contingency plans, and a variable degree of parent and teacher involvement for 4–12 weeks. Behavioral approaches utilize concepts from social learning theory, operant and classical conditioning, and interventions that include exposure and systematic desensitization, relaxation, and social skills training, as well as contingency management procedures for the parents and school personnel. Cognitive approaches utilize cognitive restructuring to challenge distorted beliefs for the children and the parents. There are currently five CBT manuals for treating youth with school refusal (Maynard et al. 2015). They involve individual treatment with the child, and usually some level of involvement with the parent and consultation with the school. Furthermore, school refusal CBT manuals also incorporate psychoeducation and gradual return-to-school plans.
Results from previous meta-analyses demonstrated that psychosocial interventions significantly increased attendance, but did not significantly decrease anxiety in school refusal during the period of assessment (Maynard et al. 2015). However, despite improvements with psychosocial interventions, many children in the studies of school refusal did not respond to the psychosocial interventions. Effect sizes (Hedges' g) for psychosocial treatments on attendance ranged from 0.10 to 2.73, with a meta-analytic Grand Mean effect of 0.54 (Maynard et al. 2015). This may have been partly due to the differences in psychotherapy, as well as the populations being assessed and treated. Nonetheless, there is a significant need to review the current evidence for pharmacological studies in school refusal, particularly as psychosocial treatments may not be sufficient to treat the underlying comorbidities of school refusal. This systematic review aims to review the following question: in children with school refusal behavior, do pharmacologic agents (compared to other treatments or placebo) improve school refusal symptoms?
Methods
The authors, together with the assistance of a specialized library consultant, identified the research question, search terms and databases to be queried, and eligibility criteria before conducting the search. Published or unpublished studies that examined the effects of pharmacologic treatment of school refusal on anxiety and/or depressive symptoms and/or on attendance in children and adolescents were eligible for this review. The specialized library consultant conducted a widespread search of MEDLINE, PsycINFO, Scopus, and Embase databases (until July 1, 2017), which yielded 374 studies. The search used combinations of the following terms and keywords related to the problem: “school refus” OR “school phob” OR “school attendance AND “Anxiety” OR “Depression” OR “mental disorder,” as well as the intervention: “drug” OR “drug therapy” OR “Serotonin Uptake Inhibitor” OR “Serotonin Noradenalin Reuptake Inhibitor” OR “Anxiolytic Agent/Anti-Anxiety Agents” OR “Tricyclic Antidepressant Agent” OR “Neuroleptic Agent” OR “Mood Stabilizer” OR “Antidepressive Agents” OR “Psychotropic Drugs” OR “Psychotropic Agent.” No limiters were used for the search. The specific search protocols for each database are available upon request.
Two authors (A.L.T. and M.O.R.) independently screened the initial 374 study titles and abstracts independently for eligibility. The inclusion criteria are as follows: (1) Randomized Clinical Trial or Quasi-Experimental study design, (2) primary outcome was school refusal, (3) pharmacological trial, (4) N > 9, (5) participants included children and adolescents, and (6) studies written in English or Spanish. Documents that were not clearly ineligible or irrelevant based on title and abstract were retrieved in full text and screened independently by the two authors (A.L.T. and M.O.R). Documents with unclear eligibility were retrieved in full text for final eligibility screening. The authors compared study eligibility, and discrepancies were settled through discussion with a third reviewer (M.H.B.). Searches for additional relevant studies were conducted by contacting authors, reviewing reference lists of prior reviews and included studies, and reviewing other studies related to school refusal (King and Bernstein 2001). Eligible studies were carefully read and the following information was systematically extracted from each of the studies: population, intervention, comparison, and outcomes, including school refusal, depression, anxiety symptoms, and side effects. Effect size for attendance at school refusal outcomes in each study was calculated using comprehensive meta-analysis version 3. Standardized mean difference of the primary outcome from the trial was reported to measure school attendance. When continuous measures were not available, the effect size was estimated using dichotomous data if available. The studies were reviewed for bias and quality by evaluating for selection, ascertainment, comparison choice, and outcome choice bias. No systematic assessment of risk/bias was utilized in this review. We decided not to conduct a meta-analysis regarding pharmacological interventions for school refusal due to the small number of available clinical trials, the diversity of pharmacological treatments, and the high variability in the outcome measures of school refusal.
Results
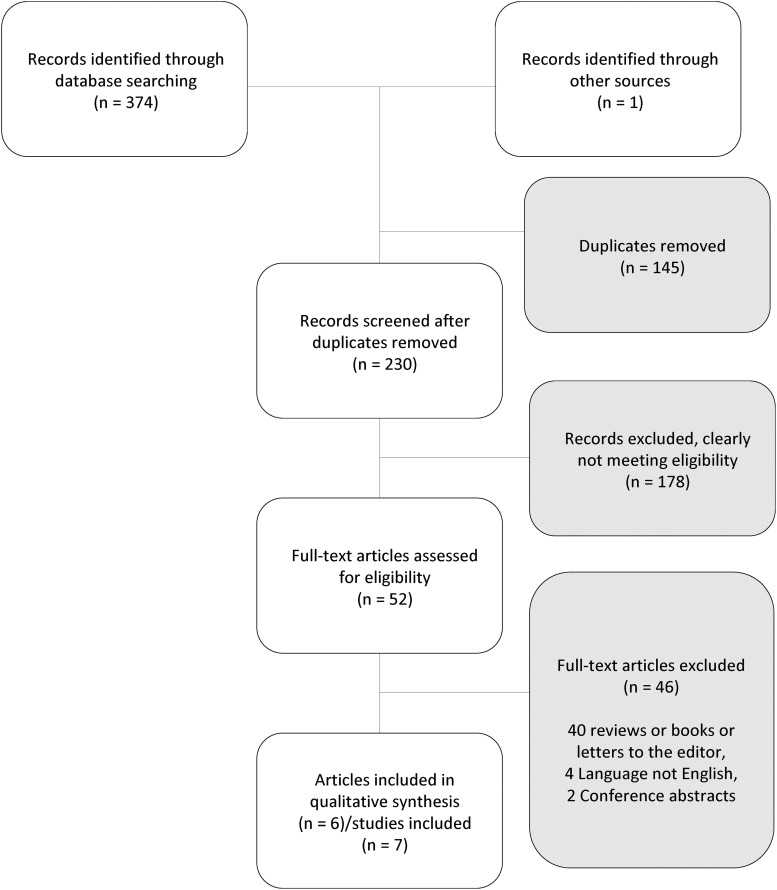
The study selection process is depicted in Figure 1. Only 6 articles/citations describing 7 studies (out of 230 unique citations) met criteria for inclusion and are reviewed here.
FIG. 1.
Study selection flowchart.
Seven studies met eligibility criteria for this review (Table 1). Specifically, two examined the effects of fluoxetine on school refusal (Wu et al. 2013; Melvin et al. 2017), four the effects of imipramine (Gittelman-Klein and Klein 1971, 1973; Bernstein et al. 1990, 2000), one the effects of clomipramine (Berney et al. 1981), and one the effects of alprazolam on school refusal (Bernstein et al. 1990) (note: some studies investigated multiple pharmacological interventions). Table 1 summarizes and provides detailed information of the characteristics across included studies. Six studies had a blinded, randomized design, and one was open label. A total of 306 school-refusing children participated in these studies; 4 countries are represented in the studies (Australia, China, England, and United States). All studies provided pharmacotherapy alongside varied psychosocial interventions, including CBT and multimodal or individual therapies, targeting school reentry and utilizing desensitization techniques.
Table 1.
Studies Included in This Review (by Author's Last Name)
| Author (year) | Intervention | Inclusion criteria | Study design | Sample size | Age (mean) | Duration | Drug (mean endpoint dose) | Side effects | Outcome and measures | Effect size attendance SMD (SE) | Conclusions | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bernstein et al. (1990) | Alprazolam + school reentry program vs. imipramine + school reentry program | Not described | Open-label study, randomization not described | 17 | 9.5–17 (14.17) | 8 weeks | Alprazolam (1.43 mg/day) Imipramine (135.42 mg/day) |
Alprazolam and imipramine: sedation, occipital headache, lightheadedness, gastrointestinal discomfort, and dry mouth | Attendance: scale from 1 to 5 (1 = complete refusal, 5 = complete return) from unclear source Anxiety: none Depression: none Global: clinician global improvement scale (none, mild, moderate, and marked) |
Not applicable | Attendance = in between group Δ NS Anxiety: n/a Depression: n/a Global: in between group Δ NS |
Randomization not described Not blinded Specific SRB eligibility criteria not described Subjective measure of attendance and SRB Nonstandardized psychological intervention |
| Bernstein et al. (1990) | Alprazolam + school reentry program vs. imipramine + school reentry program vs. placebo + school reentry program | Diagnosis of an anxiety and/or depressive disorder School refusal (not defined) |
Double blind and placebo controlled | 24 | 7–18 (14.12) | 8 weeks | Alprazolam (1.82 mg/day) Imipramine (164.29 mg/day) |
Alprazolam and imipramine: abdominal pain, headaches, drowsiness, blurred vision, constipation, dry mouth, and dizziness, dizziness with standing | Attendance: scale from 1 to 5 (1 = complete refusal, 5 = complete return) from unclear source Anxiety: ARC, RMCAS Depression: CDI, CDRS Global: none |
1.14 (0.93)* | Attendance: in between group Δ NS Anxiety: significant decrease in anxiety on ARC in alprazolam > imipramine > placebo (p = 0.03) RMCAS: in between group Δ NS Depression: in between group Δ NS Global: none |
Specific SRB eligibility criteria not described Subjective measure of attendance and SRB Nonstandardized psychological intervention Short duration of intervention |
| Bernstein et al. (2000) | Imipramine + CBT vs. placebo + CBT | ≥20% absence in 4 weeks Diagnosis of depression or anxiety disorder |
RCT (double blind) | 63 started 47 completed |
12–18 (13.9) | 8 weeks | Imipramine (187 mg/day) | Not described | Attendance: Parent report of percentage of hours attended/week Successful return to school: ≥75% attendance) Anxiety: ARC-C (Remission <5) and RCMAS Depression: BDI and CDRS-R (remission <35) |
1.27 (0.28) | Attendance: imipramine group improved at a faster rate and more at the end of the intervention (p < 0.001) Successful return to school: greater for imipramine (54.2% vs. 16.7%, p < .01) Anxiety: between group Δ NS Depression: Improved in between groups on the CDRS-R favoring imipramine (p < 0.01) |
Relatively short duration of treatment |
| Berney et al. (1981) | Clomipramine + individual therapy/casework with parents vs. placebo + individual therapy/casework with parents | SRB defined as “the association of a neurotic disorder with a marked reluctance to go to school, which had persisted for at least four weeks” No truancy |
RCT double blind, randomized stratified by gender | 52 randomized 46 completed |
9–14 (not given) | 12 weeks | Clomipramine (mean dose not given) 9–10 y/o: 40 mg/day 11–12 y/o: 50 mg/day 13–14 y/o: 75 mg/day |
Not described | Attendance: clinician-rated 14-item questionnaire, 4-point scale Anxiety: clinician-rated 14-item questionnaire, 4-point scale Depression: clinician-rated 14-item questionnaire, 4-point scale Global: clinician-rated 14-item questionnaire, 4-point scale |
Unable to calculate | Attendance: in between group Δ NS Anxiety: in between group Δ NS Depression: in between group Δ NS Overall: in between group Δ NS Conclusion: This trial fails to show that Clomipramine is better than placebo for improving ability to attend school, decrease severity of anxiety, depression, and overall severity. |
Specific SRB and comorbid eligibility criteria poorly described Unvalidated measure of attendance and SRB, depression, anxiety, and global improvement Nonstandardized psychological intervention Short duration of intervention Arbitrary and low clomipramine dose selection |
| Gittelman-Klein et al. (1971) | Imipramine + multidisciplinary therapy vs. placebo + multidisciplinary therapy | Absence or difficulty attending school for 2 weeks and anxiety symptoms | RCT (double blind and placebo controlled) | 35 completed | 6–14 (10.8) | 6 weeks | Imipramine (152 mg/day) | Imipramine: including most commonly drowsiness, dry mouth, constipation, dizziness, and nausea | Attendance: Mother's report on a 7-point Likert scale Successful return to school: Mother's report on Likert scale dichotomized into “Not back to school” and “Back to school” Anxiety: Lorr IMPS, PIRF, and mother's rating of child's behavior questions Depression: Lorr IMPS, PIRF, CBQ, and mother's rating of child's behavior questions Global: Clinician, parent, and child report on a 7-point Likert scale from “Very Much Worse” to “Completely Well” |
0.87 (0.43) | Attendance: higher for imipramine (81% vs. 47%, p < 0.05). Anxiety: lower in imipramine vs. placebo in IMPS anxiety item (p < 0.01) Depression: lower in imipramine group vs. placebo in mother ratings of child's depressive symptoms (p < 0.05) and IMPS (p < 0.01) Overall: in between group significant global impression changes on clinician, parent, and child rating favoring imipramine (p < 0.005). Conclusion: Imipramine was better than placebo at improving attendance, and depression and anxiety symptoms |
Specific SRB eligibility criteria not described Subjective measure of attendance and SRB Nonstandardized and unvalidated measurements of anxiety and depression Nonstandardized psychological intervention Short duration of intervention Significant dropout in the imipramine arm |
| Melvin et al. (2017) | CBT vs. CBT + placebo vs. CBT + fluoxetine | Absence from school for >2 weeks in 4 weeks (>50% of time) Anxiety disorder No antisocial behaviors |
RCT (double blind for medication groups) | 62 | 11–16.5 (13.6) | 12 CBT sessions variable in duration | Fluoxetine (22.5 mg/day) | Fluoxetine: difficulty falling asleep, difficulty arousing in the morning, anger outbursts, headache, lethargy, and apathy CBT+ placebo: 1 suicide attempt |
Attendance: school records Successful return to school: ≥80% of school time in school over 4 weeks. Anxiety: ADIS-C, RCMAS Depression: CDI Overall: GAF, CGI Others: SEQSS, CBCL, SRP-CSQ |
0.34 (0.31)** | Attendance: in between group Δ NS Anxiety: in between group Δ NS Depression: in between group Δ NS Overall: in between group Δ NS Satisfaction: higher with CBT + fluoxetine (p < 0.05). Conclusion: this trail failed to support hypothesis that augmentation of CBT + fluoxetine improves attendance, anxiety, and depression. |
Sample size small for three comparison groups |
| Wu et al. (2013) | CBT + fluoxetine vs. CBT | Absence from school for >2 weeks in 4 weeks (>50% of time) School refusal due to mood disorder No antisocial behaviors |
Randomized, nonplacebo controlled, and nonblinded | 82 randomized 75 completed |
6–18 (13.44) | 12 weeks | Fluoxetine 20–60 mg/day (mean dose not provided) |
Fluoxetine: dry mouth, dizziness, attention problems, nausea, sleep disturbances, sleepiness, headache, increased appetite, and bellyache | Attendance: percentage of school attendance (source not specified). Successful return to school: ≥80% of school time in school in 4 weeks. Anxiety: SAS Depression: SDS Overall: CGI-S |
0.24 (0.24) | Attendance: in between group (82.1% vs. 72.22%, NS) Anxiety: in between group Δ NS Depression: in between group Δ NS Overall: in between group Δ NS Conclusion: This trial fails to show that fluoxetine can further show efficacy of CBT for school refusal |
No placebo controls. Not blinded No intent to treat analysis |
CBCL, Child Behavior Checklist; CBQ, Rutter's Children's Behavior Questionnaire; CDI, Children's Depression Inventory; CDRS, Children's Depression Rating Scale; CDRS-R, Children's Depression Rating Scale-Revised; CGI, Clinical Global Impression; CGI-S, Clinical Global Impression Severity of Illness; Lorr IMPS, Lorr Inpatient Multidimensional Psychiatric Scale; NS, nonsignificant; PIRF, Psychiatric Interview Rating Form; RCMAS, Revised Children's Manifest Anxiety Scale; SAS, Self-rating Anxiety Scale; SDS, Self-rating Depression Scale; SEQSS, Self-Efficacy Questionnaire for School Situations; SRP-CSQ, School Refusal Program Consumer Satisfaction Questionnaire; SRB, School Refusal Behavior. *Effect size for both the imipramine + school reentry program versus placebo + school reentry program and the alprazolam + school reentry program versus placebo + school reentry. **Effect size for CBT + fluoxetine compared to CBT + placebo; RCT, randomized controlled trials; CBT, Cognitive Behavioral Therapy; SE, standard error; SMD, standard mean difference.
Selective serotonin reuptake inhibitors
Fluoxetine
Two studies examined the effects of fluoxetine with CBT for school refusal behavior (Wu et al. 2013; Melvin et al. 2017). Both studies showed significant in-group pretreatment to posttreatment improvements, but failed to show superiority of CBT + fluoxetine over CBT in attendance and other secondary outcomes, including depression and anxiety. Below is a more detailed description of the trials.
Wu et al. (2013). Eighty-two school-refusing children recruited from a hospital setting in China were randomized to CBT only (no placebo pill) or CBT + fluoxetine for 12 weeks. School refusal was defined as refusal to go to school due to mood disorder in the absence of antisocial behaviors for 2 weeks in the 4 weeks before assessment. Fluoxetine doses started at 10 mg/day for the first week and then increased gradually based on response and side effects. CBT protocol included relaxation, problem solving, emotion regulation, social communication training, and cognitive restructuring and systematic desensitization for the children, and problem solving and strategies to help children attend school for parents. The main outcome was return to school defined as child attending 80% of school time in a 4-week period. The secondary outcomes were anxiety, depression, and global impression.
Results demonstrated that the CBT + fluoxetine group had an 82.1% back-to-school rate compared to 72.2% in the CBT-only group, and the effect size for improvement in school attendance was 0.24 (standard error [SE] = 0.24) for fluoxetine compared to placebo when combined with CBT. However, this difference was not statistically significant (chi square = 1.032, p = 0.310). The anxiety, depression, and global impression scores significantly improved in preanalysis to postanalysis in all groups (p < 0.001). However, the difference between CBT and CBT + fluoxetine treatment was not significant for anxiety (F = 0.23, p = 0.631), depression (F = 1.78, p = 0.186), or global improvement (F = 0.08, p = 0.773). Seven patients dropped out from the study. The authors did not provide information whether the difference between dropouts in each group was statistically different. Significant limitations in this trial include that it was not placebo controlled, not blinded, and there was no intent-to-treat analysis.
Melvin et al. (2017). Sixty-two school-refusing children with <50% attendance in the past 4 weeks (based on school records), and meeting criteria for an anxiety disorder through the Anxiety Disorders Interview Schedule for DSM-IV Child Version, were randomized to CBT + fluoxetine or CBT + placebo, or CBT only. Children completed 12 CBT sessions. Children were also followed naturalistically for 6 and 12 months posttreatment. Two clinicians were assigned to each case: one worked with the parents and one with the child. Clinicians utilized an existing evidence-based CBT manual for school refusal, slightly modified to emphasize social skills training and depressive symptoms. Fluoxetine was titrated using a flexible-dose design for clinical response and tolerability. Primary outcome was school attendance for the prior 4 weeks using official school records. The primary outcome was acceptable attendance, which was defined by greater than 80% school attendance.
Similar, to the Wu et al. findings, this trial found pretreatment to posttreatment effects, but no differences between groups posttreatment. All treatments had improvement in school attendance with medium effect size from preassessments to postassessments (d = 0.59) and with successful return to school ranging from 44% to 56%. All treatments had improvements in anxiety and depressive symptoms over time with small to medium effect sizes (d = 0.35, range 0.23–0.58). However, there were no significant differences between treatment groups on primary or secondary outcome measures. When comparing CBT + fluoxetine to CBT + placebo, the effect size for improvement of attendance was 0.34 (SE = 0.31). The only statistically significant difference between groups was treatment satisfaction, which was greater in the CBT + fluoxetine group compared to the CBT-only group (p < 0.05). Moreover, naturalistic follow-up results suggested that school attendance improvements were maintained in all three groups. Anxiety and depressive symptoms showed a trend of improvement at 6 and 12 months posttreatment in all groups. All treatments were well tolerated, and the CBT + fluoxetine group had the lowest rates of suicidal ideation [F(2623) = 3.64 p < 0.05]. Otherwise, there were no significant side effect differences between groups.
Tricyclic antidepressants
Three randomized controlled trials (RCTs) and one open-label study examined the use of imipramine for school refusal (Gittelman-Klein and Klein 1971, 1973; Bernstein et al. 1990, 2000). In addition, one RCT examined the use of clomipramine (Berney et al. 1981) for school refusal. For imipramine, two studies reported significant improvement in school refusal and depression/anxiety symptoms and two reported no statistically significant differences between medication and control. The clomipramine study did not show statistically significant differences between clomipramine and placebo in school refusal or depression/anxiety symptoms. All RCTs had variations in controls and psychological interventions.
Imipramine
Bernstein et al. (2000). Sixty-three children with school refusal were randomized to CBT + imipramine versus CBT + placebo for 8 weeks. Inclusion criteria defined school refusal as a minimum of 20% days absent in the 4 weeks before assessment, and a diagnosis of an anxiety or depressive disorder. Imipramine and placebo were increased in a fixed schedule until week 2 and titrated based on drug levels and side effects. CBT sessions were conducted using a manualized school refusal protocol. The sessions were primarily conducted with the adolescent, and the parents and adolescent at the end of each session. Sessions included psychoeducation, school reentry program, and cognitive and behavioral techniques for school refusal. The main outcome was attendance and successful return to school (defined as ≥75% of school hours attended) as per parent report. Secondary outcomes included anxiety and depression.
This double-blinded RCT concluded that CBT + imipramine was superior to CBT + placebo in improving school attendance and depression, but not anxiety. For return-to-school, the imipramine group improved more and at a faster rate compared to the placebo group [70.1% vs. 27.6%, F (1,39) = 13.3, p < .001 and z = 2.39, p = 0.017, d = 0.287]. The effect size for improvement of attendance was 1.27 (SE = 0.28) of imipramine compared to placebo when combined with CBT. Anxiety symptoms improved significantly within groups, but not between imipramine and control. Only the Children's Depression Rating Scale-Revised (CDRS-R) showed in-between group differences, showing improvements on depressive symptoms for the imipramine group more than the control group (z = 2.08, p = 0.037, d = 0.33). However, there was no difference between imipramine versus control group in a priori definitions of remission for depression and anxiety. Sixteen participants dropped out of the trial, but there were no statistical differences between the two groups. Limitations to this study include relatively small sample size and a large number of comparisons without appropriate statistical correction.
Bernstein et al. (1990). This article describes two studies, an open-label trial and an RCT. The open-label study included 17 children with school refusal referred from clinics. Inclusion criteria are not specifically described for this study; however, children completed a clinical structured interview and most had either an anxiety or depression diagnosis, or had symptoms of depression and anxiety not meeting diagnostic thresholds. Participants received alprazolam + school reentry therapy versus imipramine + school reentry therapy for 8 weeks; randomization was not described. School reentry therapy consisted of school reentry program and individual psychotherapy.
Results demonstrated that 55% of children taking alprazolam versus 50% of children taking imipramine returned to school (there is no description of how this was measured, and there was insufficient information regarding primary outcome for effect size calculation). Clinician ratings indicated that 67% of children receiving alprazolam and 67% receiving imipramine had moderate to marked improvements in anxiety and depression (measured by a clinician rating of global improvement). There were no significant adverse drug effects in patients taking either of the medications. This was an open-label study and ratings were not blinded, which may have significantly compromised the results of this trial.
The double-blinded controlled part of this report included 24 children with school refusal and comorbid chart diagnosis of anxiety and/or depression randomized to alprazolam + school reentry therapy versus imipramine + school reentry therapy versus placebo + school reentry therapy for 8 weeks. School reentry therapy was individualized to participant's needs, but mainly consisted of gradually exposing children to more hours at school, providing a support person at school, “attending possibly 1 to several hours per day in a classroom for emotionally and behaviorally disturbed students,” and unspecified individual psychotherapy (Bernstein et al. 1990).
The primary outcome was return to school quantified by a 1–5 scale (1 = complete refusal to 5 = complete return to school). The effect size for return to school (as a dichotomous outcome) was 1.14 (SE = 0.93) when comparing the medication arms (for both imipramine and alprazolam) + school reentry program versus placebo + school reentry program. Secondary outcomes included anxiety and depression. The study did not find significant differences in school attendance. In both medication groups, all participants returned to school, and 5/6 children in the placebo group returned to school. After adjustment for baseline scores, there were no significant differences between groups in anxiety or depression scales.
In terms of side effects, most subjects identified mild side effects, including abdominal pain and headaches with both medications. One subject dropped out from the alprazolam arm, three from the imipramine arm, and one from the placebo arm. There were no significant differences between the subjects who dropped out and the cohort who completed the study. Limitations to this study include imprecise definitions of school refusal and nonstandardized measures of school refusal and return to school.
Gittelman-Klein and Klein (1971, 1973). Thirty-five clinic-referred, school-refusing children were randomized to imipramine + multidisciplinary therapy versus placebo + multidisciplinary therapy for 6 weeks. School refusal was defined by marked distress in school or inability to attend school for a 2-week period. Multidisciplinary therapy included working with parents and school, and persuasive and desensitization techniques with a school reentry program and consultation to school and parents. The primary outcome was return to school rated by the mother on a 7-point Likert scale from “complete school refusal” to “attends classes regularly—goes by himself and feels comfortable” and dichotomized to “back to school” and “not back to school.” The effect size of imipramine compared to placebo when combined with multidisciplinary therapy for school attendance was 0.87 (SE = 0.43).
Secondary outcomes included psychiatric symptoms of anxiety and depression, mother's ratings of children's behavior questions, and global improvement measured by clinician, parent, and child. Results of the study showed that 81% of participants in the imipramine arm versus 47% in the placebo arm returned to school (p < 0.05). Psychiatric interviews also showed improvement in the imipramine group compared to placebo on depressive symptoms (p < 0.01) and phobic behavior (p < 0.01), and mother's rating of child's depressive symptoms (p < 0.05). Clinician, parent, and child report of global improvement were higher in the imipramine group versus placebo (p < 0.005).
Children on imipramine reported more side effects (81% versus 47%, p < 0.025). Most common side effects for imipramine included drowsiness and dry mouth, and constipation, with only dry mouth being significantly different between imipramine and placebo, and most improving with time. There was one case of orthostatic hypotension that required dosage adjustment in the imipramine group. The trial had seven dropouts; five were from the imipramine arm. This study has several significant limitations, including not specifying reporter and specific criteria for school refusal, using parent-report measures of attendance without school-record validation and dichotomizing them, using nonstandardized measures of anxiety and depression, and providing nonstandardized or manualized psychological interventions.
Clomipramine
There is only one double-blind RCT study on clomipramine for school refusal. Results from this trial failed to show superior improvement of clomipramine compared to placebo on ability to attend school, depression severity, and separation anxiety severity.
Berney (1981). Forty-six school-refusing children with “marked reluctance” to attend school for at least 4 weeks + neurotic disorder (not clearly defined) were included in the study. Participants were randomized to clomipramine + individual therapy versus placebo + individual therapy for 12 weeks. Nonstandardized individual therapy tailored to each patient was provided together with casework with parents. Primary outcome was ability to attend school, and secondary outcome included severity of anxiety, severity of depression, and global functioning, measured by a psychiatrist-rated 14 behavior questionnaire.
The study found that for ability to go to school, anxiety, depression, and global functioning, both imipramine and placebo had significant pretreatment to posttreatment improvements. For all of these measures, in-between group analysis differences were significant, favoring imipramine. Information about side effects was not provided in the article. This study has significant limitations, including poorly defined school refusal, poorly defined inclusion criteria and psychiatric comorbidities, use of unvalidated scales for all outcomes, low clomipramine dose selection, and nonstandardized and variable therapy provided.
Benzodiazepines
As described in the section on tricyclic antidepressants (TCAs), only one trial systematically studied the use of benzodiazepines for school refusal behavior compared to imipramine (Bernstein et al. 1990). In the Bernstein trial, alprazolam was not significantly different from imipramine or placebo in improving school attendance. Alprazolam resulted in greater reductions in anxiety on some measures, but not all. However, after adjusting for baseline symptoms, there were no significant differences between alprazolam and imipramine.
Discussion
Although a large number of children exhibit school refusal behavior and its consequences are significant, this review highlights the lack of current pharmacologic studies available to guide clinical decision-making. Few studies have rigorously studied the use of pharmacotherapy in school refusal. Even fewer studies examined the efficacy of commonly used antidepressant agents (e.g., selective serotonin reuptake inhibitors [SSRIs] or serotonin-norepinephrine reuptake inhibitor(s) [SNRIs]) for school refusal. The trials that do exist are severely underpowered to detect the small-to-medium sized expected treatment benefits of antidepressants.
This first systematic review of pharmacologic treatment of school refusal found six articles, involving seven total trials. Most of the studies available are on TCAs, which often have more side effects than newer antidepressants (e.g., SSRIs). Effect sizes of the studies reviewed ranged from small (standard mean difference [SMD] = 0.24) to large (SMD = 1.27). As a comparison, a 2015 meta-analysis found that psychosocial school refusal interventions had effect sizes ranging from 0.10 to 2.73, with a meta-analytic SMD of 0.54 (Maynard et al. 2015). While the reviewed studies were underpowered and did not reach statistical significance, our review suggests that children with school refusal and depression/anxiety comorbidity generally had improvements in their school refusal with pharmacotherapy and psychotherapy combined and psychotherapy alone. There is very limited trial data to determine whether combined treatment with psychotherapy and pharmacotherapy is significantly more effective than psychotherapy alone.
Notably, some of the negative findings may be due to school refusal cases unrelated to depression and anxiety, and instead due to factors such as bullying, learning disorders, adversity, and stressors in the home. Similarly, pharmacotherapy targeting anxiety and depression may be more effective when combined with interventions addressing these other factors. In addition, this literature review needs to be taken in the context of this review's limitations, the quality and limited number of studies that are currently available for school refusal, and the broader more robust literature on the pharmacologic treatment of depression and anxiety in children.
Limitations to this review include not explicitly incorporating a systematic assessment of risk of bias. However, information about potential sources of bias such as small sample sizes, lack of intention-to-treat analysis, and lack of blinded assessments was provided. In addition, publication bias was a concern, given that many of the studies were published decades ago and have small sample sizes. Other limitations to this review include that only Spanish and English reports were included. Furthermore, a formally written a priori search protocol was not included.
The studies reviewed here have significant methodological limitations. For instance, the small sample sizes resulted in underpowered studies. Underpowering may explain why the reviewed studies failed to demonstrate superiority of pharmacotherapy combined with psychotherapy (relative to psychotherapy alone) for anxiety and depression with comorbid school refusal, despite the robust evidence to the contrary in the nonschool refusal anxiety and depression literature (March et al. 2004).
Other limitations of the reviewed studies include the following: (1) high placebo and control response rate, given the use of effective psychotherapies in the control groups, (2) use of unvalidated ratings of return-to-school, rather than objective measures like school attendance based on school records, (3) utilization of dichotomous rather than continuous outcomes for school refusal, depression, and anxiety, (4) use of varied and inconsistent inclusion criteria and definition for school refusal behavior, and (5) use of nonstandardized or nonmanualized psychotherapies for some studies. Furthermore, some studies used complex behavioral interventions that may be difficult to replicate in community settings, which may limit generalizability and clinical applicability of these studies. That is, many communities lack access to intensive behavioral and psychological therapies, and pharmacotherapy may be especially useful when such interventions are unavailable.
Another limitation of the reviewed studies is that most of the studies were conducted from the 1970s to 1990s and studied a limited number of TCAs (imipramine and clomipramine), one SSRI (fluoxetine), and no SNRIs. This is in contrast to a larger literature on pediatric anxiety that has shown that SSRIs and SNRIs have a medium effect size (d = 0.62) in the treatment of pediatric anxiety disorders (Strawn et al. 2015; Locher et al. 2017). Evidence also suggests that SSRI/SNRIs are better tolerated and result in fewer treatment discontinuations relative to TCAs (Anderson 2000; Peretti et al. 2000). In addition, one study examined the benefit of alprazolam, and while benzodiazepines may have short-term anxiolytic benefits in children (Kuang et al. 2017), there is limited evidence supporting the use of benzodiazepines in childhood anxiety disorders. Antipsychotics have also been considered for school refusal; however, rigorous studies are lacking (Abe 1975a, 1975b).
When considering the evidence of augmenting psychosocial interventions with medication for school refusal, it is important to remember that substantial evidence suggests that medication combined with therapy is indeed superior to therapy alone for childhood anxiety disorders (Taylor et al. 2017b). For instance, the largest pediatric anxiety trial to date, the Child/Adolescent Anxiety Multimodal Study (CAMS), found that pharmacotherapy (sertraline) combined with psychotherapy (CBT) was better than CBT alone and sertraline alone, both of which outperformed placebo (Walkup et al. 2008). Combination treatment was particularly beneficial for youths with severe anxiety in CAMS (Taylor et al. 2017a). However, in CAMS, combination treatment was similar to sertraline alone for youths with social anxiety disorder (which is commonly comorbid with school refusal), and sertraline alone and combined treatment were better than CBT alone, which did not differentiate from placebo (Compton et al. 2014). While CAMS excluded children with school refusal, future childhood anxiety studies should include school refusal youth.
Several meta-analyses of SSRIs for depression have shown that SSRIs are effective for pediatric depression, with fluoxetine having the best evidence of efficacy (Usala et al. 2008; Cipriani et al. 2016). The largest study of pediatric depression, Treatment for Adolescents with Depression Study (TADS), examined the use of CBT + fluoxetine (combined treatment) versus fluoxetine versus CBT versus placebo in an RCT. TADS found that combined treatment was most effective, and that 71% of participants in combined versus 61% in fluoxetine versus 43% in CBT versus 35% in placebo treatment arms had significant improvement in depressive symptoms (March et al. 2004). That is, similar to anxiety outcomes in CAMS, combination treatment outperformed monotherapy for depression in TADS. Also, like CAMS, TADS excluded school-refusing children, which they defined as missing ≥25% of school in the 2 months preceding randomization. Again, given the exclusion of children with school refusal, it is possible that TADS findings may not extend to depressed youth with school refusal.
In the context of pediatric anxiety and depression literature reviewed, future studies should consider studying medications that are effective for pediatric anxiety and depression, and should address the methodological limitations of the existing school refusal literature described above. Specifically, future trials of school refusal behavior should be (1) powered adequately such that they have the ability to detect differences between medication and placebo similar in magnitude to those that have been reported in previous pediatric anxiety and depression trials, and (2) conducted on children who have not responded to previous behavioral treatments. Previous controlled trials of school refusal have suggested that response rates to behavioral treatments alone are substantial enough that adding randomized medication versus placebo assignment on top of behavioral treatments is likely to yield failed trials because of the high rate of response in the control condition (Berney et al. 1981; Bernstein et al. 1990; Wu et al. 2013; Melvin et al. 2017).
Furthermore, larger anxiety and depression (and perhaps oppositional defiant disorder) trials should consider adding school refusal as a secondary outcome. Future studies should also quantify changes in attendance based on school hours attended relative to expected hours attended, preferably with official school records and corroborated by parental report. Furthermore, studies should consider using the School Refusal Assessment Scale-Revised (Kearney and Silverman 1993) to assess the factors responsible for maintenance of school refusal and the Self-Efficacy Questionnaire for School Situations, which provides a standardized assessment of the child's perceptions to cope with anxiety-provoking stimuli in school situations (Heyne et al. 1998).
Of utmost importance, studies should use standardized inclusion criteria and a consistent definition for school refusal, such as the definition proposed by Berg in 1997: “(1) seeks the comfort and security of home, preferring to remain close to parental figures, especially during school hours; (2) displays evidence of emotional upset when faced with the prospect of having to attend school, although this may only take the form of unexplained physical symptoms; (3) manifests no severe antisocial tendencies, apart from possible aggressiveness when attempts are made to force school attendance; and (4) does not attempt to conceal the problem from parents (Berg 1997).” This has been operationalized by some of the studies reviewed here as follows: severe difficulty attending school (≥25%–50% of school hours missed in the 4 weeks before screening), severe emotional upset (a diagnosis of anxiety or depression), and exclusion of antisocial tendencies (absence of conduct disorder) (Melvin et al. 2017).
Similarly, an a priori definition of return-to-school criteria is needed if the outcomes will be dichotomized (e.g., attending ≥80% of school required hours in the last week of the study). Finally, given that many youth with school refusal and comorbid anxiety and depression do not respond to SSRIs and TCAs, antidepressant and anxiolytic agents with novel mechanisms of action should be considered for future studies (Dwyer et al. 2017; Taylor et al. 2018).
Conclusions
Clinically, taken together, the literature on school refusal treatment suggests that providers should first thoroughly evaluate the school refusal behavior of the child, including underlying/comorbid conditions such as mood and anxiety disorders, social factors (e.g., bullying and access to care and support), neurocognitive factors (e.g., learning disabilities), and the level of impairment. After evaluation, clinicians may consider treating the school refusal and comorbid disorder with evidence-based psychotherapies (e.g., school refusal CBT) first, or combined with pharmacotherapy.
Combination treatment may be indicated as first-line treatment because school refusal is considered an emergency and requires urgent treatment, given that school refusal prognosis worsens the longer school refusal continues. If the child partially responds or is refractory to psychotherapy, the risks and benefits of pharmacotherapy can be considered with the caregivers and the child. In selecting medications, the reviewed studies indicate that TCAs (imipramine) and SSRIs (fluoxetine) may be beneficial for children with school refusal with comorbid anxiety and depression, with TCAs having more severe side effects compared to SSRIs. However, these studies are limited and the larger pediatric depression and anxiety literature must also be taken into account.
Clinical Significance
School refusal represents an important functional outcome for many children, especially those with anxiety and depression. This report provides the first systematic review of pharmacologic treatments for school refusal behavior. While limited data exist, this systematic review suggests that combined psychosocial and pharmacotherapy, particularly antidepressants and anxiolytics, may be warranted in school refusal if there are comorbid anxiety or depression symptoms. It is also important to note that in addition to anxiety and depressive symptoms, factors like bullying, learning disorders, and psychosocial adversity may result is school refusal. A careful assessment of these factors is important, and the treatment plan should address these factors.
Acknowledgment
We would like to thank Janis Glover, librarian, for her aid in conducting the systematic search for this article.
Disclosures
A.L.T. is supported by the NIH R25 MH 077823-09, American Psychiatric Association SAMHSA Fellowship program, American Academy of Child and Adolescent Psychiatry Award, and the Alan B. Slifka Foundation through the Riva Ariella Ritvo award. M.H.B. receives research support from Therapix Biosciences and Biohaven Pharmaceuticals. M.H.B. gratefully acknowledges additional research support from NIH, NARSAD, and the Patterson Foundation. J.H.T. is supported by the NIMH (5T32MH019112). The State of Connecticut also provided resource support through the Abraham Ribicoff Research Facilities at the Connecticut Mental Health Center. M.O.R. has no support to disclose.
References
- Abe K: Sulpiride in depressive school phobic children. Psychopharmacologia 43:101, 1975a [DOI] [PubMed] [Google Scholar]
- Abe K: Sulpiride in school phobia. Psychiatria Clinica 8:95–98, 1975b [DOI] [PubMed] [Google Scholar]
- Anderson IM: Selective serotonin reuptake inhibitors versus tricyclic antidepressants: A meta-analysis of efficacy and tolerability. J Affect Disord 58:19–36, 2000 [DOI] [PubMed] [Google Scholar]
- Berg I: Absence from school and mental health. Br J Psychiatry 161:154–166, 1992 [DOI] [PubMed] [Google Scholar]
- Berg I: School refusal and truancy. Arch Dis Child 76:90–91, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg I, Jackson A: Teenage school refusers grow up: A follow-up study of 168 subjects, ten years on average after in-patient treatment. The British Journal of Psychiatry 147:366–370, 1985 [DOI] [PubMed] [Google Scholar]
- Berney T, Kolvin I, Bhate SR, Garside RF, Jeans J, Kay B, Scarth L: School phobia: A therapeutic trial with clomipramine and short-term outcome. Br J Psychiatry 138:110–118, 1981 [DOI] [PubMed] [Google Scholar]
- Bernstein GA: Comorbidity and severity of anxiety and depressive disorders in a clinic sample. J Am Acad Child Adolesc Psychiatry 30:43–50, 1991 [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Borchardt CM, Perwien AR, Crosby RD, Kushner MG, Thuras PD, Last CG: Imipramine plus cognitive-behavioral therapy in the treatment of school refusal. J Am Acad Child Adolesc Psychiatry 39:276–283, 2000 [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Garfinkel BD: School phobia: The overlap of affective and anxiety disorders. J Am Acad Child Psychiatry 25:235–241, 1986 [DOI] [PubMed] [Google Scholar]
- Bernstein GA, Garfinkel BD, Borchardt CM: Comparative studies of pharmacotherapy for school refusal. J Am Acad Child Adolesc Psychiatr 29:773–781, 1990 [DOI] [PubMed] [Google Scholar]
- Bools C, Foster J, Brown I, Berg I: The identification of psychiatric disorders in children who fail to attend school: A cluster analysis of a nonclinical population. Psychol Med 20:171–181, 1990 [DOI] [PubMed] [Google Scholar]
- Buitelaar JK, van Andel H, Duyx JH, van Strien DC: Depressive and anxiety disorders in adolescence: A follow-up of adolescents with school refusal. Acta Paedopsychiatrica: International Journal of Child & Adolescent Psychiatry, 1994 [PubMed] [Google Scholar]
- Christle CA, Jolivette K, Nelson CM: School characteristics related to high school dropout rates. Remedial Spec Educ 28:325–339, 2007 [Google Scholar]
- Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, Coghill D, Zhang Y, Hazell P, Leucht S, Cuijpers P, Pu J, Cohen D, Ravindran AV, Liu Y, Michael KD, Yang L, Liu L, Xie P: Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: A network meta-analysis. Lancet 388:881–890, 2016 [DOI] [PubMed] [Google Scholar]
- Compton SN, Peris TS, Almirall D, Birmaher B, Sherrill J, Kendall PC, March JS, Gosch EA, Ginsburg GS, Rynn MA, Piacentini JC, McCracken JT, Keeton CP, Suveg CM, Aschenbrand SG, Sakolsky D, Iyengar S, Walkup JT, Albano AM: Predictors and moderators of treatment response in childhood anxiety disorders: Results from the CAMS trial. J Consult Clin Psychol 82:212–224, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JB, Beyer C, Wilkinson ST, Ostroff RB, Qayyum Z, Bloch MH: Ketamine as a treatment for adolescent depression: A case report. J Am Acad Child Adolesc Psychiatry 56:352–354, 2017 [DOI] [PubMed] [Google Scholar]
- Egger HL, Costello JE, Angold A: School refusal and psychiatric disorders: A community study. J Am Acad Child Adolesc Psychiatry 42:797–807, 2003 [DOI] [PubMed] [Google Scholar]
- Flakierska-Praquin N, Lindström M, Gillberg C: School phobia with separation anxiety disorder: A comparative 20-to 29-year follow-up study of 35 school refusers. Comprehensive Psychiatry 38:17–22, 1997 [DOI] [PubMed] [Google Scholar]
- Gittelman-Klein R, Klein DF: Controlled imipramine treatment of school phobia. Arch Gen Psychiatry 25:204–207, 1971 [PMC free article] [PubMed] [Google Scholar]
- Gittelman-Klein R, Klein DF: School phobia: Diagnostic considerations in the light of imipramine effects. J Nerv Mental Dis 156:199–215, 1973 [PubMed] [Google Scholar]
- Gottfried MA: Excused versus unexcused: How student absences in elementary school affect academic achievement. Educ Eval Policy Anal 31:392–415, 2009 [Google Scholar]
- Henry KL, Huizinga DH: School-related risk and protective factors associated with truancy among urban youth placed at risk. J Prim Prev 28:505–519, 2007 [DOI] [PubMed] [Google Scholar]
- Heyne D, King N, Tonge B, Rollings S, Pritchard M, Young D, Myerson N: The self-efficacy questionnaire for school situations: Development and psychometric evaluation. Behav Change 15:31–40, 1998 [Google Scholar]
- Heyne D, King NJ: Treatment of school refusal. In: Barrett PM, Ollendick TH. (eds). Handbook of interventions that work with children and adolescents: prevention and treatment. London: John Wiley & Sons; pp. 243–272, 2004 [Google Scholar]
- Heyne D, Rollings S, King N, Tonge B: School Refusal. BPS Blackwell, Oxford, 2002 [Google Scholar]
- Kaufman P, Alt MN, Chapman CD: Dropout Rates in the United States: 2001. Statistical Analysis Report NCES 2005-046. US Department of Education, 2004 [Google Scholar]
- Kearney CA: Confirmatory factor analysis of the School Refusal Assessment Scale-Revised: Child and parent versions. J Psychopathol Behav Assess 28:139–144, 2006 [Google Scholar]
- Kearney CA, Silverman WK: Measuring the function of school refusal behavior: The School Refusal Assessment Scale. J Clin Child Psychol 22:85–96, 1993 [Google Scholar]
- King NJ, Bernstein GA: School refusal in children and adolescents: A review of the past 10 years. J Am Acad Child Adolesc Psychiatry 40:197–205, 2001 [DOI] [PubMed] [Google Scholar]
- Kuang H, Johnson JA, Mulqueen JM, Bloch MH: The efficacy of benzodiazepines as acute anxiolytics in children: A meta-analysis. Depress Anxiety 34:888–896, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locher C, Koechlin H, Zion SR, Werner C, Pine DS, Kirsch I, Kessler RC, Kossowsky J: Efficacy and safety of selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, and placebo for common psychiatric disorders among children and adolescents: A systematic review and meta-analysis. JAMA Psychiatry 74:1011–1020, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- March J, Silva S, Petrycki S, Curry J, Wells K, Fairbank J, Burns B, Domino M, McNulty S, Vitiello B, Severe J: Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA Psychiatry 292:807–820, 2004 [DOI] [PubMed] [Google Scholar]
- Maria da Conceição CA, Aquino EM, de Barros AP: School trajectory and teenage pregnancy in three Brazilian state capitals [In Portuguese]. Cad. Saúde Pública 22:1397–1409, 2006 [DOI] [PubMed] [Google Scholar]
- Maynard BR, Heyne D, Brendel KE, Bulanda JJ, Thompson AM, Pigott TD: Treatment for school refusal among children and adolescents: A systematic review and meta-analysis. Res Soc Work Pract 2015. DOI: 10.1177/1049731515598619 [DOI] [Google Scholar]
- McCune N, Hynes J: Ten year follow-up of refusal. Ir J Psych Med 22:56–58, 2005 [Google Scholar]
- Mcshane G, Walter G, Rey JM: Characteristics of adolescents with school refusal. Aust N Z J Psychiatry 35:822–826, 2001 [DOI] [PubMed] [Google Scholar]
- Melvin GA, Dudley AL, Gordon MS, Klimkeit E, Gullone E, Taffe J, Tonge BJ: Augmenting cognitive behavior therapy for school refusal with fluoxetine: A randomized controlled trial. Child Psychiatry Hum Dev 48:485–497, 2017 [DOI] [PubMed] [Google Scholar]
- Peretti S, Judge R, Hindmarch I: Safety and tolerability considerations: Tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand Suppl 403:17–25, 2000 [DOI] [PubMed] [Google Scholar]
- Prabhuswamy M, Srinath S, Girimaji S, Seshadri S: Outcome of children with school refusal. Indian J Pediatr 74:375–379, 2007 [DOI] [PubMed] [Google Scholar]
- Strawn JR, Welge JA, Wehry AM, Keeshin B, Rynn MA: Efficacy and tolerability of antidepressants in pediatric anxiety disorders: A systematic review and meta-analysis. Depress Anxiety 32:149–157, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Landeros-Weisenberger A, Coughlin C, Mulqueen J, Johnson JA, Gabriel D, Reed MO, Jakubovski E, Bloch MH: Ketamine for social anxiety disorder: A randomized, placebo-controlled crossover trial. Neuropsychopharmacology 43:325, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Lebowitz ER, Jakubovski E, Coughlin CG, Silverman WK, Bloch MH: Monotherapy insufficient in severe anxiety? Predictors and moderators in the child/adolescent anxiety multimodal study. J Clin Child Adolesc Psychol 53:1–16, 2017a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JH, Lebowitz ER, Silverman WK: Anxiety disorders. In: Martin A, Volkmar FR, Bloch MH. (eds.) Lewis's Child and Adolescent Psychiatry: A Comprehensive Textbook (5th ed.). Philidelphia, PA, Wolters Kluwer, 2017b, pp. 509–518 [Google Scholar]
- Usala T, Clavenna A, Zuddas A, Bonati M: Randomised controlled trials of selective serotonin reuptake inhibitors in treating depression in children and adolescents: A systematic review and meta-analysis. Eur Neuropsychopharmacol 18:62–73, 2008 [DOI] [PubMed] [Google Scholar]
- Walkup JT, Albano AM, Piacentini J, Birmaher B, Compton SN, Sherrill JT, Ginsburg GS, Rynn MA, McCracken J, Waslick B, Iyengar S, March JS, Kendall PC: Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 359:2753–2766, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter D, Hautmann C, Rizk S, Petermann M, Minkus J, Sinzig J, Lehmkuhl G, Doepfner M: Short term effects of inpatient cognitive behavioral treatment of adolescents with anxious-depressed school absenteeism: An observational study. Eur Child Adolesc Psychiatry 19:835–844, 2010 [DOI] [PubMed] [Google Scholar]
- Wu X, Liu F, Cai H, Huang L, Li Y, Mo Z, Lin I: Cognitive behaviour therapy combined fluoxetine treatment superior to cognitive behaviour therapy alone for school refusal. Int J Pharmacol 9:197–203, 2013 [Google Scholar]