Abstract
Rationale: A model for stratifying progression of respiratory muscle weakness in amyotrophic lateral sclerosis (ALS) would identify disease mechanisms and phenotypes suitable for future investigations. This study sought to categorize progression of FVC after presentation to an outpatient ALS clinic.
Objectives: To identify clinical phenotypes of ALS respiratory progression based on FVC trajectories over time.
Methods: We derived a group-based trajectory model from a single-center cohort of 837 patients with ALS who presented between 2006 and 2015. We applied our model to the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database with 7,461 patients with ALS. Baseline characteristics at first visit were used as predictors of trajectory group membership. The primary outcome was trajectory of FVC over time in months.
Measurements and Main Results: We found three trajectories of FVC over time, termed “stable low,” “rapid progressor,” and “slow progressor.” Compared with the slow progressors, the rapid progressors had shorter diagnosis delay, more bulbar-onset disease, and a lower ALS Functional Rating Scale–Revised (ALSFRS-R) total score at baseline. The stable low group had a shorter diagnosis delay, lower body mass index, more bulbar-onset disease, lower ALSFRS-R total score, and were more likely to have an ALSFRS-R orthopnea score lower than 4 compared with the slow progressors. We found that projected group membership predicted respiratory insufficiency in the PRO-ACT cohort (concordance statistic = 0.78, 95% CI, 0.76–0.79).
Conclusions: We derived a group-based trajectory model for FVC progression in ALS, which validated against the outcome of respiratory insufficiency in an external cohort. Future studies may focus on patients predicted to be rapid progressors.
Keywords: amyotrophic lateral sclerosis, respiratory failure, group-based trajectory modeling, prediction modeling
At a Glance Commentary
Scientific Knowledge on the Subject
Despite heterogeneous symptom presentation and prevalence of respiratory complications in amyotrophic lateral sclerosis (ALS), an inability to identify respiratory phenotypes prevents a personalized approach to care. Progression of respiratory muscle weakness in ALS varies considerably among patients and could represent distinct disease phenotypes. While prior ALS progression models have focused on overall survival, few studies have focused on mechanisms identifying different trajectories of respiratory function, particularly using FVC.
What This Study Adds to the Field
We aimed to develop and apply a group-based trajectory model to distinguish individuals with ALS by changes in FVC over time. Using a single-center (Penn Comprehensive ALS Center) derivation cohort and an external, large, multicenter clinical trial database (Pooled Resource Open-Access ALS Clinical Trials), we demonstrated that five features at baseline (diagnosis delay, body mass index, symptom onset site, ALS Functional Rating Scale–Revised [ALSFRS-R] total score, and ALSFRS-R orthopnea score) characterize three distinct groups of decreasing FVC over time in ALS. In addition, we found that the model’s probabilities of group membership can be applied to an external dataset to predict risk of developing respiratory insufficiency. This group-based trajectory model may be an initial step toward phenotyping ALS by respiratory progression.
Amyotrophic lateral sclerosis (ALS) is a progressive neurodegenerative disease with high morbidity and universal mortality. It is the most frequent neurodegenerative disorder in middle age, with an average onset in the middle to late 50s. The incidence of ALS is approximately 1 to 2 per 100,000 people in the United States per year, with a prevalence of approximately 3 to 5 per 100,000 (1). Most cases of ALS are sporadic, although approximately 10% are familial (1). Impairment of key respiratory muscles, including the diaphragm, accessory muscles of respiration, and bulbar muscles, leads to death through a variety of mechanisms, including aspiration, diminished airway clearance due to ineffective cough, recurrent pulmonary infections, and chronic hypercapnic respiratory failure (2). The cornerstone of respiratory care in ALS involves noninvasive ventilation (NIV), which may improve quality of life and potentially survival (3).
ALS has a heterogeneous clinical presentation and symptom progression, which causes variable evolution of respiratory involvement. Despite a high risk of respiratory failure in ALS, an inability to identify mechanisms and phenotypes of disease progression prevents the delivery of personalized, effective respiratory interventions. Given the progressive nature of ALS, early discrimination of separate phenotypes of respiratory muscle decline would prove invaluable for improving precision interventions and informing clinical trial design. Baseline FVC has been shown to have prognostic importance in ALS in multiple studies (4–6).
The aim of this study was to develop and apply a group-based trajectory model (GBTM) to distinguish individuals with ALS by changes in FVC over time. We hypothesized that distinct phenotypes of individuals will be identified by differing progression of FVC decline and associated baseline characteristics.
Methods
Study Design and Study Population
We performed a retrospective cohort study. The source population used for the derivation of the GBTM was a cohort of patients evaluated at the Penn Comprehensive ALS Center (Penn) with first visit between January 1, 2006, and December 31, 2015.
The source population for the validation cohort was the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) database, which is a harmonized, de-identified, longitudinal clinical trial database from 23 phase II/III clinical trials. See online supplement for further details.
Study Samples and Data Collection
Penn cohort
The Penn cohort included 1,061 prospectively entered patients in a secure online data portal known as the Penn Integrated Neurodegenerative Disease Database. Subjects had a first visit between January 1, 2006, and December 31, 2015, and were diagnosed with ALS by an attending neurologist using the World Federation of Neurology El Escorial Criteria (7).
After each clinic visit, an attending neurologist completed clinical summary data entry. Notification of death was recorded via hospital record or caregiver notification. For this study, we excluded patients with few to no usable data or nonphysiologic values (n = 64), use of NIV before diagnosis (n = 15), and tracheostomy before diagnosis (n = 18). To ensure sufficient follow-up for the FVC trend, we excluded anyone who had fewer than two total clinic visits (n = 127). The final dataset included 837 individuals. Subjects were followed via outpatient neurology clinic visits at approximately 3-month intervals. All subjects were followed until September 1, 2016. All participating individuals provided informed consent for research data collection.
PRO-ACT cohort
The PRO-ACT cohort included de-identified data from 23 phase II/III clinical trials (see online supplement). The original PRO-ACT cohort included 10,723 subjects. We excluded those with prior tracheostomy or prior NIV (n = 153), and those with less than two clinic visits (n = 3,109). The final PRO-ACT study sample included 7,461 individuals.
For information on variables such as symptom onset site, the ALSFRS-R score, and diagnosis delay, see online supplement.
Outcomes
Our dependent variable for the trajectory analysis was FVC percent of predicted normal, recorded prospectively at each clinic visit and followed over time in months. FVC was measured using in-office spirometry by staff trained according to clinical research trial methods for reliability and reproducibility. An individual’s assigned trajectory group was determined through a multistep process (see below). The paucity of subjects with more than 36 months follow-up caused GBTM trajectories to become noisy and unreliable. We therefore truncated observations for GBTM at 36 months from the initial visit. This eliminated 386 observations for 92 people in Penn and 136 observations for 25 people in PRO-ACT, corresponding to less than 1% of total data in both cohorts.
To demonstrate the model’s performance in an external dataset, we used each individual’s group membership probabilities to measure association with development of “respiratory insufficiency” in PRO-ACT. Respiratory insufficiency was a composite endpoint that included any one of the following outcomes: initiation of NIV, tracheostomy placement, or death.
Statistical Analysis
GBTM
Data were summarized using mean ± SD or median (interquartile range) for continuous variables, and number of subjects (%) for categorical variables.
GBTM (8, 9) was used in a multistep process to identify discrete groups of FVC trajectories over time and to determine individual characteristics of each group. Baseline characteristics of each subject’s most likely trajectory group were compared using one-way ANOVA, Kruskal-Wallis, and Pearson chi-squared tests. We performed repeat pairwise analyses using Student’s t tests or Wilcoxon rank-sum tests with Bonferroni corrections, as appropriate. See the online supplement for GBTM methods, sensitivity analyses, and multiple imputation for missing data.
Validation of trajectory groups
The GBTM analysis was performed using Penn cohort data to create a group-membership probability for each of the three groups for all individuals in both cohorts. Because latent class variables cannot be externally validated, we used several analyses to assess the association of GBTM group membership with a distal outcome in a cohort distinct from the one used for the latent class analysis.
First, we used subjects’ three group membership probability variables (three independent variables) to derive a multivariable logistic regression model for respiratory insufficiency (dependent) in the Penn cohort. We validated the model in PRO-ACT using a nonparametric receiver operating characteristic curve analysis with respiratory insufficiency as the outcome producing the concordance statistic. We depicted time to respiratory insufficiency stratified by the most-likely trajectory group using Kaplan-Meier curves and compared them using the log-rank test of equality (10).
Second, using PRO-ACT we used each individual’s most-likely trajectory group (single independent variable) in a logistic regression for the outcome of respiratory insufficiency. Third, we used a Cox proportional hazards model in PRO-ACT to assess the association of the most likely group membership with the risk of respiratory insufficiency. Given violation of the proportional hazards assumption, we included a group × time interaction term. Patients were followed until they met criteria for respiratory insufficiency or were no longer followed.
Statistical significance was based on P values less than 0.05. All analyses were performed using Stata version 15.0 (StataCorp LP). A summary of our analysis is included in Figure E1 in the online supplement.
Results
Penn Cohort
The Penn cohort study sample included 837 subjects. The mean age was 63 years, 55% were self-reported male, and 83% were white individuals (Table 1). Most patients either had a normal body mass index (BMI) (n = 349) or were overweight (n = 284). The median diagnosis delay was 1.0 year. Seventy-four percent (n = 623) of the cohort had limb-onset disease, and the average FVC at baseline was 74%. The mean baseline ALSFRS-R total score was 36, and the majority of patients had no significant dyspnea (ALSFRS-R dyspnea = 4; n = 473; 57%) or orthopnea (ALSFRS-R orthopnea = 4; n = 673; 81%). Fifty-two percent classified themselves as “never” smokers. The full cohort had a median follow-up time of 1.5 years (interquartile range, 0.8–2.6 yr). The majority of the cohort (n = 717; 86%) died during follow-up. Excluded subjects with a single visit had a higher severity of disease and much shorter survival (which likely explained the lack of follow-up) compared with the study sample (Table E1).
Table 1.
Baseline Characteristics of the Penn Comprehensive ALS Center Cohort (N = 837)
| Variable | Data |
|---|---|
| Age at diagnosis, yr | 63 ± 12 |
| Sex, M, n (%) | 464 (55) |
| Race, n (%) | |
| White | 698 (83) |
| African American | 77 (9) |
| Other | 62 (8) |
| BMI class, n (%) | |
| <18.5 kg/m2 | 37 (4) |
| 18.5–24.9 kg/m2 | 349 (42) |
| 25–29.9 kg/m2 | 284 (34) |
| >30 kg/m2 | 167 (20) |
| Diagnosis delay, yr | 1.0 (0.6–1.7) |
| El Escorial criteria, n (%) | |
| Definite ALS | 175 (21) |
| Possible ALS | 217 (26) |
| Probable ALS | 252 (30) |
| Suspected ALS | 193 (23) |
| Symptom onset site, n (%) | |
| Limb | 623 (74) |
| Bulbar | 214 (26) |
| FVC seated, % predicted | 74 ± 24 |
| ALSFRS-R total score | 36 ± 7 |
| ALSFRS-R dyspnea, n (%) | |
| 4 | 473 (57) |
| 3 | 220 (26) |
| 2 | 89 (11) |
| 1 | 54 (6) |
| 0 | 1 (<1) |
| ALSFRS-R orthopnea, n (%) | |
| 4 | 673 (81) |
| 3 | 60 (7) |
| 2 | 62 (7) |
| 1 | 6 (1) |
| 0 | 36 (4) |
| Smoking history, n (%) | |
| Current | 82 (10) |
| Previous | 315 (38) |
| Never | 440 (52) |
| Coronary artery disease, n (%) | 74 (9) |
| Diabetes mellitus, n (%) | 96 (11) |
| Hypertension, n (%) | 330 (39) |
Definition of abbreviations: ALS = amyotrophic lateral sclerosis; ALSFRS-R = ALS Functional Rating Scale–Revised; BMI = body mass index.
Data are mean ± SD or median (25th–75th percentiles) unless otherwise indicated.
At Penn, 742 (89%) of 837 patients developed respiratory insufficiency. In terms of the first component of the composite endpoint reached (for the 742), 454 (61%) initiated NIV and 288 (39%) died. Only 19 of these patients received a tracheostomy, and all occurred after initiating NIV.
Trajectory Groups
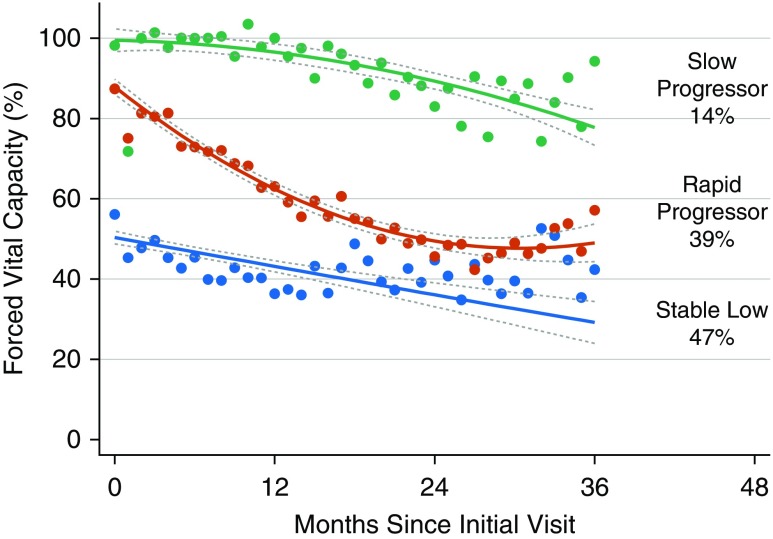
After performing several permutations (Table E2), a model with three distinct trajectory groups maximized the Bayesian information criterion and remained clinically applicable (Figure 1). The first group included 47% of the cohort, who tended to have a low FVC at diagnosis without much decrease over time (labeled “stable low”). The second group included 39% of the cohort, and suggested a higher FVC at diagnosis but a more rapid decrease over 36 months with plateauing (termed “rapid progressors”). Finally, 14% of the cohort comprised the third group (termed “slow progressors”), and suggested a normal FVC at diagnosis with very little change over time.
Figure 1.
The Penn Comprehensive ALS Center cohort trajectories of FVC percent predicted with 95% confidence intervals. Three groups are indicated by slow progressors (green), rapid progressors (red), and stable low (blue). Percentages represent proportion of the cohort with the corresponding group number as the highest predicted posterior probability. ALS = amyotrophic lateral sclerosis.
Five variables significantly predicted FVC trajectory group membership: diagnosis delay, BMI, bulbar-onset disease, ALSFRS-R orthopnea score, and ALSFRS-R total score (Table 2). When using the slow progressor group as a reference, we found that factors significantly associated with membership in the rapid progressor group included decrease in diagnosis delay (per year less; odds ratio [OR], 1.10; 95% confidence interval [CI], 1.01–1.21; P = 0.03), bulbar-onset disease (OR, 2.89; 95% CI, 1.40–5.96; P < 0.001), and decrease in ALSFRS-R total score (per 6 point decrease; OR, 2.20; 95% CI, 1.57–3.07; P < 0.001). Characteristics significantly associated with membership in the stable low group included decrease in diagnosis delay (per year less; OR, 1.15; 95% CI, 1.04–1.29; P = 0.01), decrease in BMI (per 5 units; OR, 1.52; 95% CI, 1.18–1.94; P < 0.001), bulbar-onset disease (OR, 6.13; 95% CI, 2.94–12.76; P < 0.001), decrease in ALSFRS-R total score (per 6 point decrease; OR, 4.18; 95% CI, 2.93–5.97; P < 0.001), and ALSFRS-R orthopnea score less than 4 (OR, 7.30; 95% CI, 1.73–30.78; P = 0.01).
Table 2.
Penn Comprehensive ALS Center Cohort Derivation of Baseline Covariate Association with Group Membership
| Group | Variable | OR | 95% CI | P Value |
|---|---|---|---|---|
| SP | Reference | — | — | — |
| RP | Diagnosis delay (per year less) | 1.10 | 1.01–1.21 | 0.03 |
| BMI (decrease by 5 kg/m2) | 1.17 | 0.93–1.47 | 0.18 | |
| Bulbar onset | 2.89 | 1.40–5.96 | <0.001 | |
| ALSFRS-R total (decrease by 6) | 2.20 | 1.57–3.07 | <0.001 | |
| ALSFRS-R orthopnea <4 | 1.53 | 0.33–7.07 | 0.59 | |
| SL | Diagnosis delay (per year less) | 1.15 | 1.04–1.29 | 0.01 |
| BMI (decrease by 5 kg/m2) | 1.52 | 1.18–1.94 | <0.001 | |
| Bulbar onset | 6.13 | 2.94–12.76 | <0.001 | |
| ALSFRS-R total (decrease by 6) | 4.18 | 2.93–5.97 | <0.001 | |
| ALSFRS-R orthopnea <4 | 7.30 | 1.73–30.78 | 0.01 |
Definition of abbreviations: ALS = amyotrophic lateral sclerosis; ALSFRS-R = ALS Functional Rating Scale–Revised; BMI = body mass index; CI = confidence interval; OR = odds ratio; RP = rapid progressor; SL = stable low; SP = slow progressor.
We then compared baseline characteristics as determined by the most likely trajectory group (Table 3). The three groups had significant differences across all characteristics. The stable low group had an older age of diagnosis, more bulbar-onset disease (compared with slow progressors), lower FVC, lower ALSFRS-R scores, and shorter survival. The slow progressor group had significantly longer time from symptom onset to first visit, higher proportion of limb-onset disease (compared with stable low), higher FVC, higher ALSFRS-R score, and longer survival.
Table 3.
Baseline Penn Comprehensive ALS Center Cohort Characteristics by Most Likely Trajectory Group
| Variable | Stable Low (n = 397) | Rapid Progressor (n = 329) | Slow Progressor (n = 111) | P Value |
|---|---|---|---|---|
| Age at diagnosis, yr | 65 ± 12*† | 62 ± 12* | 60 ± 13† | <0.001 |
| Sex, M, n (%) | 199 (50)* | 199 (61)* | 67 (60) | 0.01 |
| Race, n (%) | <0.001 | |||
| White | 312 (79)*† | 283 (86)* | 105 (94)† | |
| African American | 54 (13) | 19 (6) | 3 (3) | |
| Other | 31 (8) | 27 (8) | 3 (3) | |
| BMI class, n (%) | <0.001 | |||
| <18.5 kg/m2 | 30 (7)*† | 7 (2)* | 0 (0)† | |
| 18.5–24.9 kg/m2 | 173 (44) | 131 (40) | 44 (40) | |
| 25–29.9 kg/m2 | 123 (31) | 122 (37) | 40 (36) | |
| >30 kg/m2 | 71 (18) | 69 (21) | 27 (24) | |
| Diagnosis delay, yr | 1.0 (0.6–1.4) | 1.0 (0.5–1.5)‡ | 1.4 (0.6–2.7)‡ | <0.001 |
| Symptom onset to first visit, yr | 1.1 (0.7–2.0)† | 1.2 (0.8–2.0)‡ | 2.0 (1.0–3.3)†‡ | <0.001 |
| El Escorial criteria, n (%) | <0.001 | |||
| Definite ALS | 117 (29)*† | 54 (16)*‡ | 5 (5)†‡ | |
| Possible ALS | 103 (26) | 84 (26) | 29 (26) | |
| Probable ALS | 106 (27) | 117 (36) | 30 (27) | |
| Suspected ALS | 71 (18) | 74 (22) | 47 (42) | |
| Symptom onset site, n (%) | <0.001 | |||
| Limb | 271 (68)*† | 255 (78)* | 98 (88)† | |
| Bulbar | 126 (32) | 74 (22) | 13 (12) | |
| FVC seated, % predicted | 56 ± 18*† | 88 ± 14*‡ | 99 ± 13†‡ | <0.001 |
| ALSFRS-R total score | 33 ± 7*† | 38 ± 5*‡ | 41 ± 4†‡ | <0.001 |
| ALSFRS-R dyspnea, n (%) | <0.001 | |||
| 4 | 177 (45)*† | 218 (66)* | 78 (70)† | |
| 3 | 103 (26) | 90 (27) | 27 (24) | |
| 2 | 68 (17) | 15 (5) | 6 (6) | |
| 1 | 48 (12) | 6 (2) | 0 (0) | |
| 0 | 1 (<1) | 0 (0) | 0 (0) | |
| ALSFRS-R orthopnea, n (%) | <0.001 | |||
| 4 | 252 (64)*† | 312 (95)* | 109 (98)† | |
| 3 | 49 (12) | 9 (3) | 1 (1) | |
| 2 | 55 (14) | 7 (2) | 1 (1) | |
| 1 | 6 (1) | 0 (0) | 0 (0) | |
| 0 | 35 (9) | 1 (<1) | 0 (0) | |
| Smoking history, n (%) | 0.02 | |||
| Never | 191 (48)† | 175 (53) | 73 (66)† | |
| Previous | 165 (42) | 119 (36) | 32 (29) | |
| Current | 41 (10) | 35 (11) | 6 (5) | |
| Coronary artery disease, n (%) | 46 (12)† | 25 (8) | 3 (3)† | 0.009 |
| Diabetes mellitus, n (%) | 56 (14)† | 37 (11)‡ | 3 (3)†‡ | 0.004 |
| Hypertension, n (%) | 176 (44) | 118 (36) | 37 (33) | 0.02 |
| Survival, mo | 15 (9–25)*† | 25 (17–37)*‡ | 46 (28–69)†‡ | <0.001 |
| Survival from symptom onset, mo | 28 (19–46)*† | 39 (28–57)*‡ | 66 (49–97)†‡ | <0.001 |
For definition of abbreviations, see Table 1.
Survival is indicated by time between diagnosis and last follow-up date or date of death. Data are mean ± SD or median (25th–75th percentiles) unless otherwise indicated. Groups were compared using one-way ANOVA, Kruskal-Wallis, or Pearson chi-squared test, as appropriate.
For items in the same row, pairwise significant differences with Bonferroni correction.
For items in the same row, pairwise significant differences with Bonferroni correction.
For items in the same row, pairwise significant differences with Bonferroni correction.
Sensitivity Analyses
Because significant bulbar weakness can cause FVC measurement error, we removed any observations in which significant bulbar symptoms (salivation, speech, or swallowing score ≤2) were noted (n = 1,992 observations). In a separate analysis, we removed all individuals who ever developed significant bulbar disease during follow-up (n = 561). The proportions of the three groups and appearance of the trajectories were similar to those found in the full cohort (data not shown).
PRO-ACT Cohort
The PRO-ACT cohort included 7,461 subjects. The mean age was 55 years, 62% were self-reported male, and 95% were white (Table 4). The median delay between diagnosis and symptom onset was 0.8 years, and 79% of individuals had limb-onset symptoms. The mean baseline ALSFRS-R total score was 37. The majority of patients had no significant baseline dyspnea (ALSFRS-R dyspnea = 4; n = 5,442; 73%) or orthopnea (ALSFRS-R orthopnea = 4; n = 6,564; 88%). The cohort had a median follow-up time of 0.9 years (interquartile range, 0.3–1.2 yr). Excluded patients with a single visit, shown in Table E3, were more likely to be underweight, were more likely to have bulbar onset, and had lower FVC but a similar survival.
Table 4.
Baseline Characteristics of Pooled Resource Open-Access ALS Clinical Trials Cohort (N = 7,461)
| Variable | Data |
|---|---|
| Age at diagnosis, yr | 55 ± 12 |
| Sex, M, n (%) | 4,626 (62) |
| Race, n (%) | |
| White | 7,088 (95) |
| African American | 149 (2) |
| Other | 224 (3) |
| BMI class, n (%) | |
| <18.5 kg/m2 | 597 (8) |
| 18.5–24.9 kg/m2 | 3,059 (41) |
| 25–29.9 kg/m2 | 2,387 (32) |
| >30 kg/m2 | 1,418 (19) |
| Diagnosis delay, yr | 0.8 (0.3–1.4) |
| Symptom onset site, n (%) | |
| Limb | 5,894 (79) |
| Bulbar | 1,567 (21) |
| FVC seated, % predicted | 87 ± 23 |
| ALSFRS-R total score | 37 ± 6 |
| ALSFRS-R dyspnea | |
| 4 | 5,442 (73) |
| 3 | 1,114 (15) |
| 2 | 666 (9) |
| 1 | 219 (3) |
| 0 | 20 (<1) |
| ALSFRS-R orthopnea | |
| 4 | 6,564 (88) |
| 3 | 596 (8) |
| 2 | 223 (3) |
| 1 | 73 (1) |
| 0 | 5 (<1) |
For definition of abbreviations, see Table 1.
Data are mean ± SD or median (25th–75th percentiles) unless otherwise indicated.
In PRO-ACT, 2,777 (37%) of 7,461 subjects developed respiratory insufficiency. The first component of the composite endpoint reached for the 2,777 included 1,216 (44%) starting NIV, 139 (5%) receiving tracheostomy, and 1,498 (54%) who died. Seventy-six of the patients starting NIV underwent tracheostomy afterwards.
We applied our GBTM derived from the Penn cohort to the PRO-ACT cohort. Our analysis characterized the cohort as 23% stable low, 52% rapid progressors, and 25% slow progressors. Baseline characteristics by group are shown in Table 5. The groups had significant differences for all characteristics. Similar to the Penn cohort, the stable low group was older and had a lower proportion of males and a higher proportion of bulbar-onset disease; each individual had a lower FVC (compared with rapid progressors), a lower ALSFRS-R total score, and shorter survival.
Table 5.
Baseline Pooled Resource Open-Access ALS Clinical Trials Cohort Characteristics by Most Likely Trajectory Group
| Variable | Stable Low (n = 1,698) | Rapid Progressor (n = 3,867) | Slow Progressor (n = 1,896) | P Value |
|---|---|---|---|---|
| Age at diagnosis, yr | 57 ± 12*† | 55 ± 12*‡ | 54 ± 12†‡ | <0.001 |
| Sex, M, n (%) | 916 (54)*† | 2,397 (62)*‡ | 1,286 (68)†‡ | <0.001 |
| Race, n (%) | 0.01 | |||
| White | 1,591 (94)† | 3,677 (95) | 1,824 (96)† | |
| African American | 34 (2) | 68 (2) | 24 (1) | |
| Other | 73 (4) | 122 (3) | 48 (3) | |
| BMI class, n (%) | <0.001 | |||
| <18.5 kg/m2 | 218 (13)*† | 259 (7)*‡ | 74 (4)†‡ | |
| 18.5–24.9 kg/m2 | 816 (48) | 1,585 (41) | 661 (35) | |
| 25–29.9 kg/m2 | 437 (26) | 1,235 (32) | 732 (39) | |
| >30 kg/m2 | 227 (13) | 788 (20) | 429 (22) | |
| Diagnosis delay, yr | 0.7 (0.3–1.2)*† | 0.7 (0.3–1.3)*‡ | 0.9 (0.4–1.6)†‡ | <0.001 |
| Symptom onset to first visit, yr | 1.5 (0.9–2.2)*† | 1.5 (0.9–2.3)*‡ | 1.7 (1.1–2.5)†‡ | <0.001 |
| Symptom onset site | <0.001 | |||
| Limb | 1,038 (61)*† | 3,061 (79)*‡ | 1,761 (93)†‡ | |
| Bulbar | 660 (39) | 806 (21) | 135 (7) | |
| FVC seated, % predicted | 64 ± 18* | 88 ± 18*‡ | 104 ± 19‡ | <0.001 |
| ALSFRS-R total score | 33 ± 7*† | 37 ± 6*‡ | 40 ± 5†‡ | <0.001 |
| ALSFRS-R dyspnea, n (%) | <0.001 | |||
| 4 | 1,017 (60)*† | 2,875 (75)*‡ | 1,574 (83)†‡ | |
| 3 | 352 (21) | 580 (15) | 183 (9) | |
| 2 | 252 (15) | 315 (8) | 104 (6) | |
| 1 | 64 (4) | 89 (2) | 31 (2) | |
| 0 | 13 (<1) | 8 (<1) | 4 (<1) | |
| ALSFRS-R orthopnea, n (%) | <0.001 | |||
| 4 | 1,301 (77)*† | 3,480 (90)*‡ | 1,781 (94)†‡ | |
| 3 | 259 (15) | 254 (6) | 81 (4) | |
| 2 | 110 (7) | 109 (3) | 30 (2) | |
| 1 | 20 (1) | 19 (1) | 2 (<1) | |
| 0 | 8 (<1) | 5 (<1) | 2 (<1) | |
| Time to respiratory insufficiency, mo | 11.4 (7.5–16.4)*† | 19.2 (13.1–27.4)*‡ | 34.0 (24.3–40.2)†‡ | <0.001 |
| Survival, mo | 18.8 (13.0–26.6)*† | 21.7 (14.8–29.0)*‡ | 25.6 (17.1–33.4)†‡ | <0.001 |
| Survival from symptom onset, mo | 29.0 (21.1–38.1)*† | 31.5 (24.0–41.8)*‡ | 35.1 (27.2–45.3)†‡ | <0.001 |
For definition of abbreviations, see Table 1.
Survival is indicated by time between diagnosis and last follow-up date or date of death. Data are mean ± SD or median (25th–75th percentiles). Groups were compared using one-way ANOVA, Kruskal-Wallis, or Pearson chi-squared test, as appropriate.
For items in the same row, pairwise significant differences with Bonferroni correction.
For items in the same row, pairwise significant differences with Bonferroni correction.
For items in the same row, pairwise significant differences with Bonferroni correction.
Prediction of Respiratory Insufficiency or Death
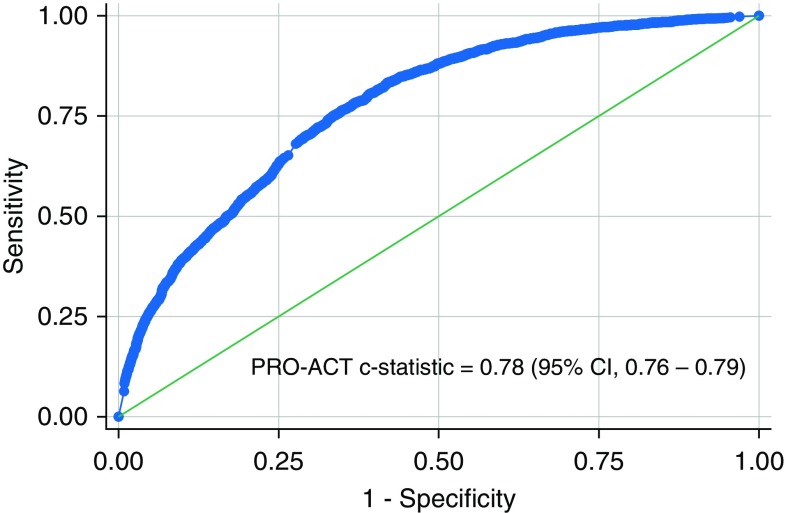
We created a multivariable logistic regression model for the association of the three group membership probabilities and the outcome of respiratory insufficiency in the Penn cohort. We then used the model in PRO-ACT in a nonparametric receiver operating characteristic analysis yielding a concordance statistic of 0.78 (95% CI, 0.76–0.79) (Figure 2).
Figure 2.
Receiver operating characteristic curve for external validation of the Penn Comprehensive ALS Center cohort logistic regression predicted odds of respiratory insufficiency in the Pooled Resource Open-Access ALS Clinical Trials (PRO-ACT) cohort. ALS = amyotrophic lateral sclerosis; CI = confidence interval.
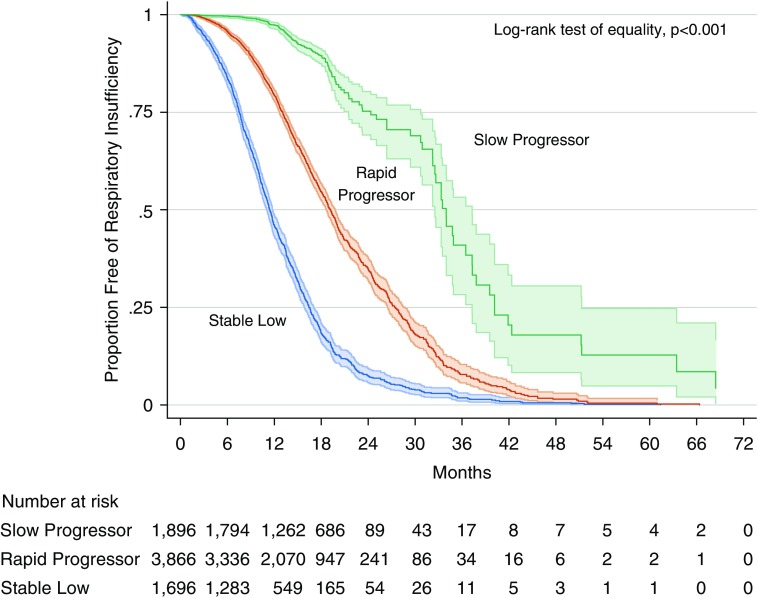
The three groups had significantly different risks for respiratory insufficiency (P < 0.001; Figure 3). Median time to respiratory insufficiency was 11.4 months (interquartile range [IQR], 7.5–16.4 mo) for the stable low group, 19.2 months (IQR, 13.1–27.4 mo) for rapid progressors, and 34.0 months (IQR, 24.3–40.2 mo) for slow progressors.
Figure 3.
Kaplan-Meier curves with 95% confidence intervals for the respiratory insufficiency outcome in the Pooled Resource Open-Access ALS Clinical Trials cohort, stratified by most likely trajectory group. ALS = amyotrophic lateral sclerosis.
We also validated our GBTM model by using each PRO-ACT individual’s most likely trajectory group in a logistic regression model to assess the risk of respiratory insufficiency (Table 6). Using the slow progressors as a reference, both the rapid progressor and the stable low group had significantly increased associations with respiratory insufficiency, OR 6.0 (95% CI, 5.08–7.12; P < 0.001) and OR 21.3 (95% CI, 17.70–25.68; P < 0.001), respectively.
Table 6.
Risk of Respiratory Insufficiency, by Most Likely Trajectory Group (N = 7,461)
| Group | OR | 95% CI | P Value |
|---|---|---|---|
| Slow progressor (ref) | — | — | — |
| Rapid progressor | 6.0 | 5.1–7.1 | <0.001 |
| Stable low | 21.3 | 17.7–25.7 | <0.001 |
Definition of abbreviations: CI = confidence interval; OR = odds ratio; ref = reference.
Group membership was also associated with the time to respiratory insufficiency using a Cox proportional hazards model and Nelson-Aalen cumulative hazard function (Table E4 and Figure E2).
Discussion
We created a GBTM that identified three distinct trajectory groups of FVC in ALS, termed stable low, rapid progressor, and slow progressor. Five variables were found to have significant association with the trajectory groups: diagnosis delay, BMI, bulbar-onset disease, ALSFRS-R orthopnea score, and ALSFRS-R total score. We found that, compared with the slow progressor group, the rapid progressors tended to have shorter diagnosis delay, lower FVC, and lower ALSFRS-R total scores. This three-group model remained significant even after removing observations with significant bulbar dysfunction and separately removing all individuals who ever developed bulbar dysfunction during follow-up. In addition, our Penn-derived model and three FVC trajectory groups were validated by demonstrating similar groups in the large, multicenter PRO-ACT cohort with a strong association with the risk of respiratory insufficiency.
Baseline FVC has been shown to have prognostic importance in ALS in multiple studies (4–6, 11, 12). In addition, four of the five variables (excluding orthopnea score) used in our model have demonstrated association with overall ALS disease progression (4, 6, 11, 13–16). Crowdsourcing initiatives have found several predictors (ie, time from symptom onset, site of symptom onset, FVC, and ALSFRS-R total score) using advanced machine-learning algorithms to identify predictors for relatively “fast” versus “slow” disease progressors (17). A prior study identified differing slopes of FVC in ALS (12); however, they did not explore the characteristics associated with each group as was performed in the current study.
We have previously derived and externally validated a clinical prediction rule for onset of respiratory insufficiency within 6 months of presentation to an ALS clinic that could be used at the bedside for an individual patient (18). Respiratory insufficiency was defined as a composite outcome of initiation of NIV, FVC less than 50% predicted, tracheostomy placement, or death. This prior study focused on short-term outcomes and did not incorporate changes or trajectories of FVC over time. In the current paper, GBTM incorporated complex data to identify distinct subphenotypes that could provide mechanistic insights, further understanding of disease progression, or assist with planning of clinical trials, for example.
In the current study, the PRO-ACT cohort differed from the Penn cohort by having a higher proportion of slow progressors (25% vs. 14%, respectively) and a lower proportion of the stable low group (23% vs. 39%, respectively). There are several possible reasons for this. First, the Penn cohort included most patients evaluated at one center over a 10-year period, whereas the PRO-ACT cohort is composed of selected patients from clinical trials with multiple inclusion and exclusion criteria, which may exclude patients with advanced disease. Therefore, the relative proportions for trajectory group membership in the two cohorts may differ. For example, the lower proportion of stable low and higher proportion of slow progressor individuals in PRO-ACT is likely attributable to clinical trial cohorts selecting healthier subjects with fewer comorbidities and thus lower rates of respiratory outcomes during follow-up. Finally, there were significantly more missing data in the PRO-ACT cohort compared with the Penn cohort. Despite the differences in the two cohorts, our model’s strong generalizability is evident by its ability to separate individuals into groups that differ in their FVC trajectories, with similar group characteristics in both the Penn and PRO-ACT cohorts, and the association with the risk of respiratory insufficiency. A rapid progressor took longer to reach respiratory insufficiency than a stable low individual; the names describe the FVC progression, not the risk of respiratory insufficiency.
Our study has several strengths. Our model’s ability to distinguish trajectory groups with different outcomes in a large multicenter cohort attests to its generalizability. Respiratory phenotypes in ALS have yet to be defined and, to our knowledge, this is one of the largest studies investigating this topic. We accounted for the relationship between bulbar symptoms and FVC measurement by demonstrating the robustness of our model in multiple sensitivity analyses.
Although prior studies have developed approaches to mapping ALS disease progression, those alternate approaches require many variables not available in typical clinical practice, necessitate repeat assessments over time, or focus on overall disease progression rather than respiratory events (15, 17, 19). However, the variables used in our model (diagnosis delay, BMI, symptom onset site, ALSFRS-R total, and ALSFRS-R orthopnea) are readily available at initial evaluation. Within the first visit, such a model could provide rapid assessment of patients’ future trajectories of respiratory progression.
The relationship that these five different variables have with the rapidity of respiratory muscle decline suggests mechanisms of disease progression. For example, the presence of orthopnea may indicate significant diaphragm muscle weakness. The increased association between orthopnea and respiratory muscle decline may be explained by individuals who are predisposed to hypoventilation, earlier hypercapnia, progressive dyspnea, and fatigue, thus creating a cycle that contributes to perpetual respiratory muscle decline.
Our study has several limitations. Measurement error could have affected FVC. However, the Penn cohort had trained personnel to ensure spirometry reliability and reproducibility. In addition, PRO-ACT is comprised of randomized controlled trial data, using rigorous clinical research-grade methodologies. Measurement error would likely bias to the null, unless differential in regard to the exposures and outcomes.
Bulbar symptoms could have lowered FVC rather than respiratory muscle weakness. We performed two sensitivity analyses, which eliminated 1) observations with significant bulbar symptoms or 2) individuals who ever developed significant bulbar disease during follow-up, and found similar results, making confounding by bulbar symptoms less likely.
Supine FVC may detect diaphragm weakness in ALS (20). However, most individuals in the study cohorts lacked these data. Alternative invasive measurements of respiratory strength have been highly predictive of ventilator-free survival in ALS (21). However, these measurements are uncomfortable for patients, require skilled personnel, and are not routinely measured in ALS centers or trials.
As with any GBTM analysis, there are necessary inherent assumptions. Most important, we assumed that groups with differing slopes of FVC over time actually exist. However, it has been well-described in the ALS literature and anecdotally by neurologists that there are subsets of patients with ALS with dramatically variable disease progression. GBTM-building allows for multiple explanations of the same data, and our results reflect our interpretation of the most clinically feasible model with the highest Bayesian information criterion.
Exclusion of single-visit, higher-severity subjects likely introduces some selection bias; however, this was a relatively small proportion of patients and exclusion of more severe patients would more likely bias to the null (spectrum bias).
The slope differences between the groups could be interpreted as separate groups of patients presenting at different stages of disease rather than representing a progression from baseline. However, we accounted for this by 1) using initial clinic presentation as time zero (as opposed to diagnosis date), 2) controlling for diagnosis delay, and 3) indicating time from symptom onset to first visit and survival since symptom onset. Time from symptom onset to presentation was longest for the slow progressor group, opposite to expectations if this group represented presentation at the early stage of disease. The model’s strong ability to discriminate trajectories in PRO-ACT despite these significant differences in “start” time of observation testify to robustness of the model. Accounting for diagnosis delay allowed us to control for time between symptom onset and diagnosis, which is often used as a surrogate for rapidity of disease progression in ALS.
Outcomes such as initiation of NIV, tracheostomy, and death could be misclassified. However, it is unlikely that this would have substantially altered results. Unmeasured confounding is possible; however, this would not affect the ability to determine trajectory groups.
Conclusions
A model for early stratification of respiratory progression in ALS may facilitate several future studies, such as examining the association of rapid progressors with respiratory outcomes in response to interventions such as NIV, airway clearance, or muscle stimulation. By further refining characteristics associated with different trajectories of respiratory function, we may tailor goals of care and respiratory interventions to provide personalized medicine for ALS.
Footnotes
Supported by NIH grants T32 HL-007891, K24 HL-103844, P01 AG032953-A1, and F32 HL144145.
Author Contributions: J.A., J.H.-F., B.L.J., E.P.W., R.J.S., L.E., and S.M.K. designed the study, implemented the project and manuscript, and managed the submission. J.A., B.L.J., and E.P.W. performed the statistical analysis. J.A. and L.E. collected the data. All authors revised the manuscript critically for important intellectual content and gave final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201902-0344OC on July 19, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Brown RH, Al-Chalabi A. Amyotrophic lateral sclerosis. N Engl J Med. 2017;377:162–172. doi: 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Tsai C-P, Chang B-H, Lee CT-C. Underlying cause and place of death among patients with amyotrophic lateral sclerosis in Taiwan: a population-based study, 2003-2008. J Epidemiol. 2013;23:424–428. doi: 10.2188/jea.JE20130045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: a randomised controlled trial. Lancet Neurol. 2006;5:140–147. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 4.Czaplinski A, Yen AA, Appel SH. Amyotrophic lateral sclerosis: early predictors of prolonged survival. J Neurol. 2006;253:1428–1436. doi: 10.1007/s00415-006-0226-8. [DOI] [PubMed] [Google Scholar]
- 5.Czaplinski A, Yen AA, Appel SH. Forced vital capacity (FVC) as an indicator of survival and disease progression in an ALS clinic population. J Neurol Neurosurg Psychiatry. 2006;77:390–392. doi: 10.1136/jnnp.2005.072660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westeneng H-J, Debray TPA, Visser AE, van Eijk RPA, Rooney JPK, Calvo A, et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 2018;17:423–433. doi: 10.1016/S1474-4422(18)30089-9. [DOI] [PubMed] [Google Scholar]
- 7.Brooks BR, Miller RG, Swash M, Munsat TL.the World Federation of Neurology Research Group on Motor Neuron Diseases El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis Amyotroph Lateral Scler Other Motor Neuron Disord 20001293–299. [DOI] [PubMed] [Google Scholar]
- 8.Nagin DS. Group-based trajectory modeling: an overview. Ann Nutr Metab. 2014;65:205–210. doi: 10.1159/000360229. [DOI] [PubMed] [Google Scholar]
- 9.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109–138. doi: 10.1146/annurev.clinpsy.121208.131413. [DOI] [PubMed] [Google Scholar]
- 10.Dudley WN, Wickham R, Coombs N. An introduction to survival statistics: Kaplan-Meier analysis. J Adv Pract Oncol. 2016;7:91–100. doi: 10.6004/jadpro.2016.7.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnus T, Beck M, Giess R, Puls I, Naumann M, Toyka KV. Disease progression in amyotrophic lateral sclerosis: predictors of survival. Muscle Nerve. 2002;25:709–714. doi: 10.1002/mus.10090. [DOI] [PubMed] [Google Scholar]
- 12.Schiffman PL, Belsh JM. Pulmonary function at diagnosis of amyotrophic lateral sclerosis: rate of deterioration. Chest. 1993;103:508–513. doi: 10.1378/chest.103.2.508. [DOI] [PubMed] [Google Scholar]
- 13.Calvo A, Moglia C, Lunetta C, Marinou K, Ticozzi N, Ferrante GD, et al. Factors predicting survival in ALS: a multicenter Italian study. J Neurol. 2017;264:54–63. doi: 10.1007/s00415-016-8313-y. [DOI] [PubMed] [Google Scholar]
- 14.Clavelou P, Blanquet M, Peyrol F, Ouchchane L, Gerbaud L. Rates of progression of weight and forced vital capacity as relevant measurement to adapt amyotrophic lateral sclerosis management for patient Result of a French multicentre cohort survey. J Neurol Sci. 2013;331:126–131. doi: 10.1016/j.jns.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Elamin M, Bede P, Montuschi A, Pender N, Chiò A, Hardiman O. Predicting prognosis in amyotrophic lateral sclerosis: a simple algorithm. J Neurol. 2015;262:1447–1454. doi: 10.1007/s00415-015-7731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Esquinas Rodriguez AM, Paladini L, Vianello A. Respiratory function deterioration and the effect of non-invasive mechanical ventilation in amyotrophic lateral sclerosis: the crucial importance of bulbar muscle involvement. Eur J Neurol. 2013;20:e65. doi: 10.1111/ene.12106. [DOI] [PubMed] [Google Scholar]
- 17.Küffner R, Zach N, Norel R, Hawe J, Schoenfeld D, Wang L, et al. Crowdsourced analysis of clinical trial data to predict amyotrophic lateral sclerosis progression. Nat Biotechnol. 2015;33:51–57. doi: 10.1038/nbt.3051. [DOI] [PubMed] [Google Scholar]
- 18.Ackrivo J, Hansen-Flaschen J, Wileyto EP, Schwab RJ, Elman L, Kawut SM. Development of a prognostic model of respiratory insufficiency or death in amyotrophic lateral sclerosis. Eur Respir J. 2019;53:1802237. doi: 10.1183/13993003.02237-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du W, Cheung H, Goldberg I, Thambisetty M, Becker K, Johnson CA. A longitudinal support vector regression for prediction of ALS score. IEEE Int Conf Bioinform Biomed Workshops. 2015;2015:1586–1590. doi: 10.1109/BIBM.2015.7359912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lechtzin N, Wiener CM, Shade DM, Clawson L, Diette GB. Spirometry in the supine position improves the detection of diaphragmatic weakness in patients with amyotrophic lateral sclerosis. Chest. 2002;121:436–442. doi: 10.1378/chest.121.2.436. [DOI] [PubMed] [Google Scholar]
- 21.Polkey MI, Lyall RA, Yang K, Johnson E, Leigh PN, Moxham J. Respiratory muscle strength as a predictive biomarker for survival in amyotrophic lateral sclerosis. Am J Respir Crit Care Med. 2017;195:86–95. doi: 10.1164/rccm.201604-0848OC. [DOI] [PMC free article] [PubMed] [Google Scholar]