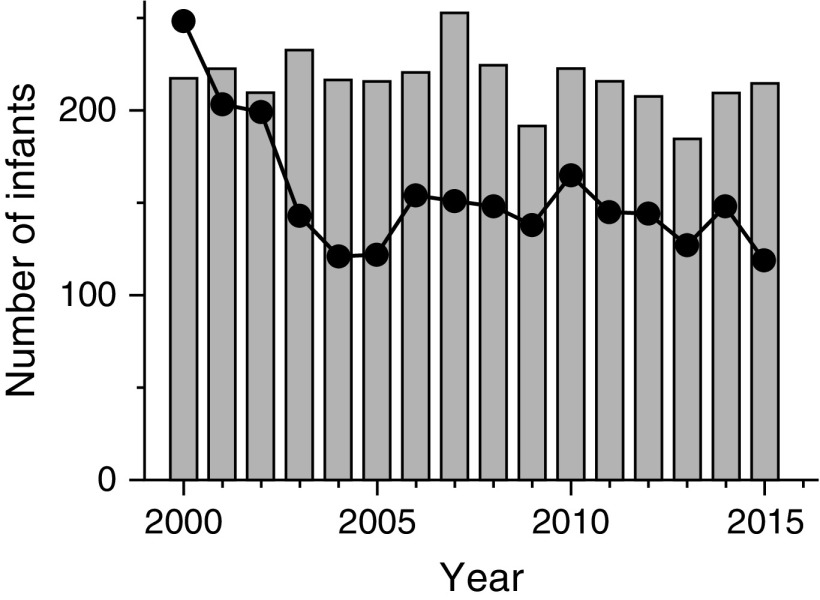
Congenital diaphragmatic hernia (CDH) represents one of the most challenging causes of respiratory failure managed in neonatal critical care. These challenges arise from the pathophysiological triad of pulmonary hypoplasia, pulmonary vascular disease, and left ventricular (LV) dysfunction associated with herniated abdominal contents, impaired fetal lung growth, and perturbations in umbilical venous return to the developing left ventricle (1). The management of CDH has evolved from a primarily surgical problem to one of integrated prenatal, surgical, respiratory, and cardiac care, principally focusing on lung-protective approaches to support the hypoplastic lungs and reducing the burden of pulmonary hypertension (2–4). Unfortunately, mortality remains persistently high (25–30%) (5), and, in contrast to what has been observed with other causes of severe neonatal respiratory failure, the rates of extracorporeal membrane oxygenation use have not decreased with incremental improvements in ventilatory care (Figure 1). LV hypoplasia has long been recognized as a fetal manifestation of CDH (6), and resultant cardiac dysfunction has been postulated as an important and underappreciated determinant of outcome (1, 7).
Figure 1.
Use of extracorporeal membrane oxygenation for congenital diaphragmatic hernia (gray bars) and meconium aspiration syndrome (solid circles) over the 15-year period after the U.S. Food and Drug Administration approved inhaled nitric oxide for persistent pulmonary hypertension in newborns (16). The marked reduction in the use of extracorporeal membrane oxygenation for meconium aspiration syndrome contrasts with little change in its use for congenital diaphragmatic hernia over this period.
Early cardiac function in CDH is poorly understood, and investigations have been limited to small observational studies (8, 9). The study presented in this issue of the Journal by Patel and colleagues (pp. 1522–1530) is thus timely and provides valuable insight into CDH pathophysiology (10). They report echocardiographic categorizations of LV and right ventricular function performed in the first 48 hours after delivery in 1,173 infants enrolled in the CDH Study Group Registry (59 centers) from 2015 to 2018, a period encompassing current lung-protective ventilatory strategies and a multimodal pharmacological approach to pulmonary hypertension. Some form of ventricular dysfunction was identified on echocardiography in 39% of the infants (15% with isolated right ventricular dysfunction, 5% with LV dysfunction, and 19% with biventricular dysfunction). Both LV and biventricular dysfunction were independently associated with mortality and extracorporeal membrane oxygenation use, with biventricular dysfunction conferring the highest risk of mortality (49%). Importantly, the mortality risks associated with LV/biventricular dysfunction were comparable to established prognosticating factors, specifically the diaphragmatic defect size, presence of liver in the chest, and need for a surgical patch (11). This study provides the first large population signal to support the complex but critical interplay between cardiac function and outcomes for infants born with CDH.
The most important finding of this study is the impact of early LV dysfunction on CDH outcomes. Pulmonary hypertension is established as a hallmark of CDH, and resultant right ventricular systolic and diastolic dysfunction well described (1). The finding that biventricular dysfunction is common in CDH (48% of all infants with any cardiac dysfunction) is thus not surprising. Patel and colleagues show that the left ventricle may also be the primary cause of cardiac dysfunction, whether from hypoplasia and a reduced ability to manage the acute increase in afterload after birth, or by directly elevating pulmonary venous pressure and pulmonary vascular resistance. An understanding of the interplay between right ventricular and LV function will allow the development of targeted therapies. Pulmonary vasodilatation to reduce right-heart afterload is often advocated (3), but in the presence of LV dysfunction this may worsen LV diastolic function and increase systemic hypoxemia and acidosis. In such situations, inotropic and lusitropic support (for example, with the use of milrinone) may be more appropriate; however, there are insufficient data to warrant routine use (12, 13).
The authors provide a strong argument for routine, early assessments of cardiac function. Their study provides the first step toward meaningful translation rather than the definitive answer. In their study, echocardiography was performed in the first 48 hours of life, and only 37.5% of the infants had a second assessment within 14 days, so conclusions regarding balancing LV dysfunction and pulmonary hypertension are limited to the period of immediate preoperative CDH stabilization. Furthermore, echocardiography was not performed on 29% of eligible infants in the registry, highlighting the fact that it is difficult to assess cardiac function. It is also difficult to define “function.” In the study by Patel and colleagues, ventricular function was categorized semiqualitatively by experienced operators. Measures of diastolic and systolic right-sided function were used, but specific parameters for LV dysfunction were not recorded. Although quantitative methods of LV function have been suggested (1, 14), validation of these methods is lacking, and there is a pressing need for international agreement on parameters to define LV function.
In this study, large diaphragmatic defects were common in infants with ventricular dysfunction; however, it is more interesting that early ventricular dysfunction was also present in at least 25% of infants with smaller diaphragmatic defects. Clinicians are often confronted with an infant with relatively favorable prenatal measures of lung growth but a severe postnatal disease course. This highlights the limitation of prognosticating outcomes purely on the basis of antenatal markers (15).
The management of CDH is complicated by structural and functional changes in the heart, pulmonary vasculature, and lung; consequently, it is challenging to determine the optimal management strategies. The study by Patel and colleagues reinforces the need for vigilant management of cardiac function in early life and recognition of the important cardiopulmonary interactions that characterize CDH if outcomes are to improve.
Footnotes
D.G.T. is supported by a National Health and Medical Research Council Clinical Career Development Fellowship (1053889) and the Victorian Government Operational Infrastructure Support Program (Melbourne, Australia). J.P.K. is supported by the NIH/National Center for Advancing Translational Sciences Colorado Clinical and Translational Science Awards Program (UL1 TR002535).
Originally Published in Press as DOI: 10.1164/rccm.201909-1737ED on October 10, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kinsella JP, Steinhorn RH, Mullen MP, Hopper RK, Keller RL, Ivy DD, et al. Pediatric Pulmonary Hypertension Network (PPHNet) The left ventricle in congenital diaphragmatic hernia: implications for the management of pulmonary hypertension. J Pediatr. 2018;197:17–22. doi: 10.1016/j.jpeds.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Bohn D. Congenital diaphragmatic hernia. Am J Respir Crit Care Med. 2002;166:911–915. doi: 10.1164/rccm.200204-304CC. [DOI] [PubMed] [Google Scholar]
- 3.Snoek KG, Reiss IK, Greenough A, Capolupo I, Urlesberger B, Wessel L, et al. CDH EURO Consortium. Standardized postnatal management of infants with congenital diaphragmatic hernia in europe: the CDH EURO Consortium Consensus. 2015 update. Neonatology. 2016;110:66–74. doi: 10.1159/000444210. [DOI] [PubMed] [Google Scholar]
- 4.Tracy ET, Mears SE, Smith PB, Danko ME, Diesen DL, Fisher KA, et al. Protocolized approach to the management of congenital diaphragmatic hernia: benefits of reducing variability in care. J Pediatr Surg. 2010;45:1343–1348. doi: 10.1016/j.jpedsurg.2010.02.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Putnam LR, Harting MT, Tsao K, Morini F, Yoder BA, Luco M, et al. Diaphragmatic Hernia Study Group. Congenital diaphragmatic hernia defect size and infant morbidity at discharge. Pediatrics. 2016;138:e20162043. doi: 10.1542/peds.2016-2043. [DOI] [PubMed] [Google Scholar]
- 6.Siebert JR, Haas JE, Beckwith JB. Left ventricular hypoplasia in congenital diaphragmatic hernia. J Pediatr Surg. 1984;19:567–571. doi: 10.1016/s0022-3468(84)80105-0. [DOI] [PubMed] [Google Scholar]
- 7.Patel N, Kipfmueller F. Cardiac dysfunction in congenital diaphragmatic hernia: pathophysiology, clinical assessment, and management. Semin Pediatr Surg. 2017;26:154–158. doi: 10.1053/j.sempedsurg.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Altit G, Bhombal S, Van Meurs K, Tacy TA. Ventricular performance is associated with need for extracorporeal membrane oxygenation in newborns with congenital diaphragmatic hernia. J Pediatr. 2017;191:28–34.e1. doi: 10.1016/j.jpeds.2017.08.060. [DOI] [PubMed] [Google Scholar]
- 9.Patel N, Massolo AC, Paria A, Stenhouse EJ, Hunter L, Finlay E, et al. Early postnatal ventricular dysfunction is associated with disease severity in patients with congenital diaphragmatic hernia. J Pediatr. 2018;203:400–407.e1. doi: 10.1016/j.jpeds.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 10.Patel N, Lally PA, Kipfmueller F, Massolo AC, Luco M, Van Meurs KP, et al. Congenital Diaphragmatic Hernia Study Group. Ventricular dysfunction is a critical determinant of mortality in congenital diaphragmatic hernia. Am J Respir Crit Care Med. 2019;200:1522–1530. doi: 10.1164/rccm.201904-0731OC. [DOI] [PubMed] [Google Scholar]
- 11.Lally KP, Lally PA, Lasky RE, Tibboel D, Jaksic T, Wilson JM, et al. Congenital Diaphragmatic Hernia Study Group. Defect size determines survival in infants with congenital diaphragmatic hernia. Pediatrics. 2007;120:e651–e657. doi: 10.1542/peds.2006-3040. [DOI] [PubMed] [Google Scholar]
- 12.Patel N. Use of milrinone to treat cardiac dysfunction in infants with pulmonary hypertension secondary to congenital diaphragmatic hernia: a review of six patients. Neonatology. 2012;102:130–136. doi: 10.1159/000339108. [DOI] [PubMed] [Google Scholar]
- 13.Lakshminrusimha S, Keszler M, Kirpalani H, Van Meurs K, Chess P, Ambalavanan N, et al. Milrinone in congenital diaphragmatic hernia: a randomized pilot trial. Study protocol, review of literature and survey of current practices. Matern Health Neonatol Perinatol. 2017;3:27. doi: 10.1186/s40748-017-0066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baptista MJ, Nogueira-Silva C, Areias JC, Correia-Pinto J. Perinatal profile of ventricular overload markers in congenital diaphragmatic hernia. J Pediatr Surg. 2008;43:627–633. doi: 10.1016/j.jpedsurg.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 15.Jani JC, Benachi A, Nicolaides KH, Allegaert K, Gratacós E, Mazkereth R, et al. Antenatal-CDH-Registry Group. Prenatal prediction of neonatal morbidity in survivors with congenital diaphragmatic hernia: a multicenter study. Ultrasound Obstet Gynecol. 2009;33:64–69. doi: 10.1002/uog.6141. [DOI] [PubMed] [Google Scholar]
- 16.Extracorporeal Life Support Organization North American summary data 2018. Available from: https://www.elso.org.