Abstract
Rationale: Biomarker signatures are needed in children with children’s interstitial and diffuse lung disease (chILD) to improve diagnostic approaches, increase our understanding of disease pathogenesis, monitor disease progression, and develop new treatment strategies. Proteomic technology using SOMAmer (Slow Off-rate Modified Aptamer) nucleic acid–based protein-binding reagents allows for biomarker discovery.
Objectives: We hypothesized that proteins and protein pathways in BAL fluid (BALF) would distinguish children with neuroendocrine cell hyperplasia of infancy (NEHI), surfactant dysfunction mutations, and other chILD diagnoses and control subjects.
Methods: BALF was collected for clinical indications and banked in patients with chILD and disease control subjects using standardized protocols over 10 years. BALF supernatant was analyzed using an aptamer assay to measure 1,129 protein levels. Protein levels were compared between groups using an ANOVA and adjusted for multiple comparisons using false discovery rate. Proteins were classified into pathways. Hierarchical clustering was used to define endotypes in the group of children with NEHI.
Measurements and Main Results: After correcting for multiple testing, children with NEHI (n = 22) had 202 aptamers that were significantly different (P < 0.05) in BALF compared with control subjects (n = 9). Children with surfactant mutation (n = 8) had 51 aptamers significantly different (P < 0.05) in BALF compared with control subjects (n = 9). Proteins associated with pulmonary fibrosis and inflammation were associated with the surfactant dysfunction group but not the NEHI group. Using hierarchical clustering analysis, two distinct NEHI endotypes were identified.
Conclusions: Distinct proteins and protein pathways can be determined from BALF of children with chILD, and these hold promise to further our understanding of chILD.
Keywords: pediatric, surfactant, neuroendocrine
At a Glance Commentary
Scientific Knowledge on the Subject
The name children’s interstitial and diffuse lung disease (chILD) incorporates a large group of rare lung diseases found in children. Some of these diseases, such as neuroendocrine cell hyperplasia of infancy and surfactant dysfunction mutations, are more frequently found in infants and not adults. Limited biomarker data are available to understand molecular mechanisms in chILD.
What This Study Adds to the Field
Using advanced proteomic techniques, our study found distinct protein BAL fluid biomarkers for neuroendocrine cell hyperplasia of infancy, SFTPC (surfactant protein C), and ABCA3 (ATP-binding cassette transporter A3) in children. These data may provide insights into molecular mechanism and new treatment approaches.
Children’s interstitial and diffuse lung disease (chILD) is an umbrella term used to describe a heterogeneous group of rare diseases with varying prognosis, characterized by hypoxia, tachypnea, crackles, or poor somatic growth. Other findings include diffuse infiltrates found on radiographic imaging of the chest and abnormal gas exchange (1). After known causes of lung disease have been ruled out, this constellation of signs and symptoms has been labeled chILD syndrome (2). chILD is much less common than adult interstitial lung disease, and unlike adult interstitial lung disease and idiopathic pulmonary fibrosis (IPF) there is no predominant type of chILD syndrome (1). Children have unique diseases not found in adults. chILD syndrome includes specific diagnoses, such as genetic abnormalities of surfactant function; specifically, mutations in the SFTPB (surfactant protein B), SFTPC (surfactant protein C), TTF1 (thyroid transcription factor 1; also known as NKX2.1), and ABCA3 (ATP-binding cassette transporter A3). Surfactant mutations result in alveolar type 2 cellular dysfunction and predominately involve a mixed inflammatory and fibrotic process in the interstitium. Neuroendocrine cell hyperplasia of infancy (NEHI) has an unknown etiology but increased numbers of neuroendocrine cells and limited inflammation pathologically.
Because these diseases are rare, present similarly in the clinic, and can often masquerade as other entities, they can lead to difficult diagnostic dilemmas for clinicians. Lung biopsy has been the gold standard for diagnosis, as many of the chILD disorders are characterized histologically (2). Although surgical techniques for lung biopsy have improved, with decreased complications and recovery time, the ability to diagnose chILD syndrome by clinical features, imaging findings, and genetics has led to a decreased need for pediatric lung biopsies (3). The development of a diagnostic biomarker is appealing, as the correct identification of specific types of chILD, including NEHI, surfactant dysfunction mutations, and others, has genetic, prognostic, and therapeutic importance (4).
Recognizing the hurdles that children with chILD and their families face and the urgent need for more specific and new treatment options based on disease mechanism, the NHLBI convened a workshop in 2015 that called for the identification of pathogenic mechanisms, biomarkers, and pharmacotherapeutic targets (5). Powerful high-throughput aptamer arrays now exist to study complex biological samples, elucidate molecular mechanisms, and identify new biomarker signatures (6, 7). Using this novel technology, our goal was to identify aptamer signatures and disease pathways in BAL fluid (BALF) of children with chILD syndrome. We hypothesize that BALF protein profiles and disease pathways in chILD would differ from each other and from children without airway disease. Pilot results from this study were previously presented in the form of an abstract (8–10). A small number of cytokine results from a subset of our BALF samples with SFTPC mutations were included in a paper focused on a mouse model of SFTPC mutations and fibrosis (11).
Methods
Study Design and Patient Population
BALF samples were collected and banked for children with NEHI, surfactant protein dysfunction, including SFTPC and ABCA3, other chILD disorders, and disease control children at the Children’s Hospital Colorado between 2000 and 2010. BALF was collected from otherwise healthy children who had clinically indicated bronchoscopy for evaluation of cough and similar symptoms as disease controls. Evaluation, including bronchoscopy, in these children did not reveal airway or pulmonary cause of cough. All BALF were collected for clinical purposes in a standard fashion according to international guidelines (12). Normalization of BALF was done as recommended by consensus guidelines to report the percentage of volume recovered compared with volume instilled. The BALF recovered was required to be at least 25% of the volume instilled (12–14). One aliquot of BALF was obtained before spinning, and the rest was spun, cells and supernatant separated, frozen at −80°C, and stored in the Pediatric Colorado Clinical Translational Science Core Laboratory at the Children’s Hospital Colorado (details provided in online supplement). Bacterial culture and viral culture, direct fluorescent antibody (until 2007), and PCR (after 2007) were performed on the collected BALF.
Diagnoses were established on the basis of chILD pathologic classification or accepted clinical presentation and computed tomography findings for NEHI (2, 15, 16). Within the NEHI group, biopsy-proven NEHI (n = 9), as well as NEHI syndrome (n = 13), were combined. Children with NEHI syndrome were those who had not undergone lung biopsy, but had symptoms consistent with NEHI, including tachypnea, crackles, and desaturation. All children with NEHI syndrome underwent computed tomography, with images reviewed by a radiologist experienced in recognizing NEHI, and all had findings consistent with NEHI, including ground glass opacities and air trapping (17). Children with SFTPC and ABCA3 were diagnosed with genetic testing or lung biopsy. Children with other chILD diagnoses had lung tissue review by a pediatric pathologist experienced in chILD as well as clinical context. Disease control subjects were confirmed by appropriate clinical presentation and confirmatory testing. Disease control subjects were defined as children with cough or suspected airway lesions but who had normal-appearing bronchoscopy. Published values for cell counts and cytology differentials in the BALF of healthy, nonwheezing children were also used as normal control reference ranges (13).
The study was approved by the Colorado Multiple Institutional Review Board. Informed consent was obtained from all patients over 17 years of age, or, if the child was a minor, from the legal guardian. In children aged 12–17 years, informed assent was obtained.
SomaScan Proteomics Platform
The SomaScan platform using SOMAmer (Slow Off-rate Modified Aptamer) reagents can sensitively screen over 1,000 proteins (18). BALF was diluted with buffer to a standard protein concentration of 20 mg/ml. One hundred microliters of diluted BALF was equilibrated with SOMAmer reagents. This allows for the detection and quantitation of 1,129 protein aptamers simultaneously in each sample, recorded as relative fluorescence units (RFU) (18). A description of the SomaScan platform and validation in BALF has been previously published and can be found in the online supplement (19, 20).
Statistical Analysis
Descriptive statistics were calculated using medians, ranges, and interquartile ranges where specified for continuous variables and percentages for categorical variables. Raw RFUs were adjusted on the basis of the individual dilution factor for each sample to reflect the initial protein amounts. These adjusted RFUs were log2 transformed, and quantile normalization (21) was used to obtain final values for analysis. RFUs for each of 1,129 protein aptamers were compared univariately between groups using ANOVA, and linear contrasts were used to test pairwise comparisons. P values were adjusted for multiple comparisons using the false discovery rate as described by Benjamini and Hochberg (22). Proteins were classified into pathways using Reactome (23, 24). A functional class scoring approach using the P values as the protein-level statistics was used to rank pathways (25). Statistical significance for the pathways was calculated by permuting group labels using 1,000 permutations. Random forests discriminating NEHI and surfactant dysfunction from the other chILD diagnoses and control subjects were used to identify the aptamers to include in a canonical discriminant analysis, a multivariate method similar to principal component analysis but that differs in that it uses the group information in assigning weights to predictor variables (see online supplement for additional details).
Results
Patient Characteristics
BALF samples from 47 patients were included in this investigation. The disease groups consisted of the following: 22 patients with NEHI, 8 patients with surfactant dysfunction mutations (5 SFTPC and 3 ABCA3), 8 patients with other chILD diseases (3 follicular bronchiolitis, 2 graft vs. host disease, 1 autoimmune lymphoproliferative syndrome, and 2 autoimmune polyendocrinopathy–candidiasis–ectodermal dystrophy [or APS 1]), and 9 non-chILD disease control subjects (nonspecific cough). The median age for the children at the time of BALF collection was 1.6 years, ranging between 1 month and 19 years old. BALF volume percentage returned was similar across groups. Forty-four percent of BALF samples were bacteria culture positive. Bacterial culture results were primarily associated with the NEHI group, although the absolute number of BALF white blood cells in patients with NEHI was significantly lower than in patients with surfactant dysfunction and more comparable to that in control subjects (Table 1). The percentage of BALF neutrophils in patients with surfactant dysfunction was significantly higher than in patients with NEHI and control subjects (Table 1).
Table 1.
Demographics and BAL Fluid Sample Characteristics
| Disease Controls (n = 9) | NEHI (n = 22) | Surfactant Dysfunction (n = 8) | Other chILD (n = 8) | |
|---|---|---|---|---|
| Age, yr | 1.8 (0.5–7) | 1.1 (0.1–5.7) | 1.4 (0.1–10.8) | 10.1 (2.1–18.8) |
| Sex, F:M | 5:4 | 7:15 | 5:3 | 5:3 |
| Weight, percentile | 54 (8.2–99.9) | 19.2 (0.1–99.2) | 5.5 (0.1–50) | 5.7 (0.1–57.3) |
| Fluid recovered, % | 44 (32–67) | 60 (33–78) | 71 (25–80) | 57 (33–76) |
| WBCs, cells/ml × 103* | 178 (59–358) | 181 (46–676) | 365 (141–950) | 297 (64–847) |
| Neutrophils, %* | 2 (0–18) | 3 (0–28) | 12 (7–74) | 7.5 (0–43) |
| Lymphs, %* | 9 (0–35) | 8 (0–45) | 3 (0–20) | 8 (1–23) |
| Macs/Monos, %* | 82 (58–97) | 83 (17–96) | 67 (22–89) | 77 (37–94) |
| Eosinophils, %* | 0 (0–0) | 0 (0–2) | 2 (0–15) | 0 (0–1) |
| Epithelium, %* | 4 (1–11) | 5 (0–56) | 0 (0–7) | 0.5 (0–3) |
| Culture positive, pathogens, virus | 2, 1 | 11†, 1 | 2, 1 | 3, 3 |
Definition of abbreviations: chILD = children’s interstitial and diffuse lung disease; Lymphs = lymphocytes; Macs = macrophages; Monos = monocytes; NEHI = neuroendocrine cell hyperplasia of infancy; WBC = white blood cell.
Data are shown as median (range) unless otherwise indicated.
Values missing for one child with NEHI.
Culture results are presented in Table E5.
Comparison of Proteins between Patients with NEHI and Surfactant Dysfunction and Control Subjects
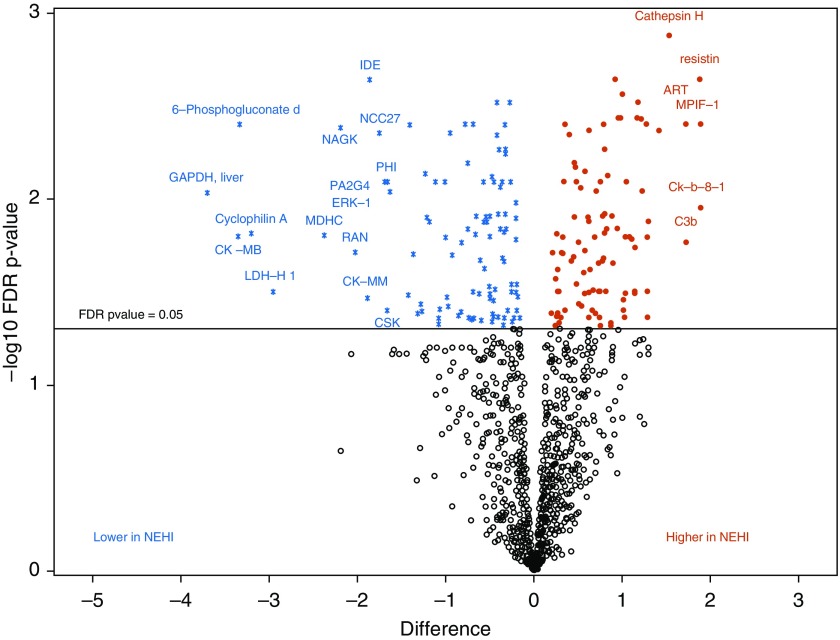
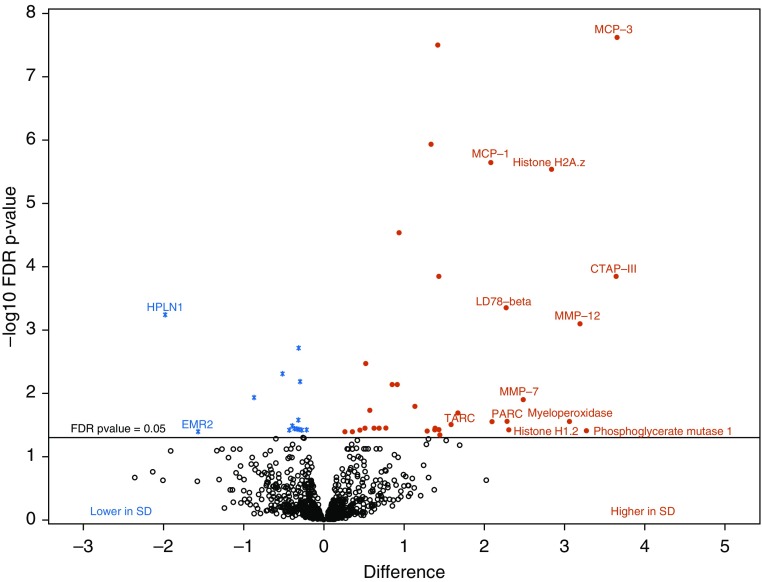
When comparing each protein separately, 202 proteins differed between patients with NEHI and control subjects (Figure 1 and Table E1 in the online supplement), including cathespsin H, resistin, 6-phosphogluconate dehydrogenase, liver GAPDH, and DDL4 (delta-like canonical notch ligand). There were 51 aptamers that differed between surfactant dysfunction and controls (Figure 2 and Table E2), including MCP-1, MCP3, and TARC cytokines, DKK1 (Dickkopf-related protein-1) and DKK4, and extracellular matrix proteins TSP2 (thrombospondin) and multiple matrix metalloproteinases (MMP12, MMP3, and MMP7). The degradation of the extracellular matrix pathway was a top-ranked pathway differentiating both NEHI and surfactant dysfunction (Table 2). PI3K/AKT network regulation and signaling, links to neuronal systems, protein–protein interactions at synapses, and NGF (nerve growth factor) were pathways associated with NEHI. The WNT, IL, and chemokine signaling pathways were associated with surfactant dysfunction (Table 2).
Figure 1.
Volcano plot shows a large magnitude of significance (y-axis) and difference (x-axis) for proteins higher in neuroendocrine cell hyperplasia of infancy (NEHI) (solid red circles) and lower in NEHI (blue asterisks) versus controls. Black open circles are proteins with a false discovery rate (FDR) P value greater than 0.05. Differences between group means in NEHI compared with controls, where a difference of 1 on log2 scale corresponds to a fold change of 2. 6-Phosphogluconate d = 6-Phosphogluconate dehydrogenase.
Figure 2.
Volcano plot shows a large magnitude of significance (y-axis) and difference (x-axis) for proteins higher in surfactant dysfunction (SD) (solid red circles) and lower in surfactant dysfunction (blue asterisks) versus controls. Black open circles are proteins with a false discovery rate P value greater than 0.05. Differences between group means in surfactant dysfunction compared with controls, where a difference of 1 on log2 scale corresponds to a fold change of 2. FDR = false discovery rate.
Table 2.
Top-ranked Pathways for Neuroendocrine Cell Hyperplasia of Infancy and Surfactant Dysfunction Compared with Controls
| Rank* | Aptamers† | Pathway | P Value |
|---|---|---|---|
| Top-ranked pathways for NEHI compared with controls | |||
| 1 | 48 | Degradation of the extracellular matrix | 0.157 |
| 2 | 33 | Metabolism of carbohydrates | 0.157 |
| 3 | 49 | Negative regulation of the PI3K/AKT network | 0.157 |
| 4 | 48 | PI5P, PP2A, and IER3 regulate PI3K/AKT signaling | 0.157 |
| 5 | 57 | Platelet degranulation | 0.157 |
| 6 | 60 | Response to elevated platelet cytosolic Ca2+ | 0.157 |
| 7 | 23 | IL-12 family signaling | 0.161 |
| 8 | 45 | PI3K/AKT signaling in cancer | 0.163 |
| 9 | 41 | Constitutive signaling by aberrant PI3K in cancer | 0.180 |
| 10 | 41 | Infectious disease | 0.183 |
| 11 | 27 | Neuronal system | 0.189 |
| 12 | 24 | Activation of matrix metalloproteinases | 0.190 |
| 13 | 13 | Protein–protein interactions at synapses | 0.191 |
| 14 | 18 | Metabolism of vitamins and cofactors | 0.199 |
| 15 | 29 | Signaling by NGF | 0.200 |
| Top-ranked pathways for surfactant dysfunction compared with controls | |||
| 1 | 55 | IL-4 and -13 signaling | 0.157 |
| 2 | 17 | TCF-dependent signaling in response to WNT | 0.163 |
| 3 | 48 | Degradation of the extracellular matrix | 0.183 |
| 4 | 31 | IL-10 signaling | 0.194 |
| 5 | 63 | GPCR ligand binding | 0.200 |
| 6 | 26 | Signaling by WNT | 0.213 |
| 7 | 12 | Diseases associated with O-glycosylation of proteins | 0.241 |
| 8 | 26 | Chemokine receptors bind chemokines | 0.241 |
| 9 | 21 | Diseases of glycosylation | 0.245 |
| 10 | 57 | Platelet degranulation | 0.248 |
| 11 | 50 | Peptide ligand-binding receptors | 0.250 |
| 12 | 18 | Collagen degradation | 0.266 |
| 13 | 15 | Amyloid fiber formation | 0.273 |
| 14 | 9 | O-linked glycosylation | 0.293 |
| 15 | 24 | Activation of matrix metalloproteinases | 0.318 |
Definition of abbreviations: GPCR = G protein–coupled receptor; NEHI = neuroendocrine cell hyperplasia of infancy; NGF = nerve growth factor; TCF = T-cell factor.
Limited to the top 15 ranked pathways.
Refers to the number of measured aptamers included in the pathway.
Discriminating between Disease Groups
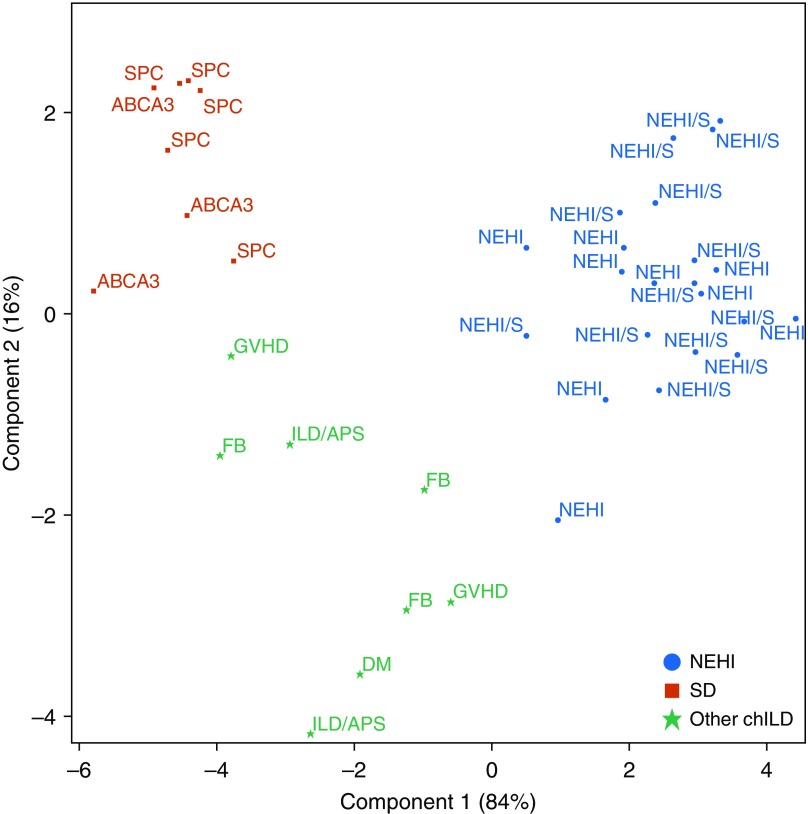
Random forests were used to identify the top 20 proteins that best discriminate NEHI and surfactant dysfunction (Table 3). These aptamers were then used in a canonical discriminatory analysis to illustrate the variability between and within groups; the first component distinguishes NEHI from the other groups, whereas the second component distinguishes surfactant dysfunction (Figure 3). The top 20 proteins in this analysis overlap with the proteins that differed significantly to distinguish surfactant dysfunction and NEHI from controls. The NEHI and NEHI syndrome samples are intermingled together in their respective clusters, indicating their similarity, and are distinct from the other two groups on the basis of the first component. There is more variability in the samples from the other chILD diagnoses.
Table 3.
Weights for Each of the Top 20 Proteins from the Canonical Discriminant Analysis
| Target | Uniprot | Weights for Component 1* | Weights for Component 2* |
|---|---|---|---|
| sRAGE | Q15109 | −0.194 | 0.806 |
| PDGF_AA | P04085 | 0.950 | 1.068 |
| HistoneH2A_z | P0C0S5 | −1.018 | 0.480 |
| FGF-10 | O15520 | −0.032 | −0.198 |
| MCP-3 | P80098 | −0.572 | 0.187 |
| WIF-1 | Q9Y5W5 | 0.636 | −0.472 |
| DKK1 | O94907 | −0.895 | 0.256 |
| NKp30 | O14931 | −0.903 | 3.131 |
| Lysozyme | P61626 | 0.779 | −0.770 |
| G-CSF-R | Q99062 | −0.965 | −2.537 |
| ERBB3 | P21860 | 0.054 | −2.749 |
| Contactin_4 | Q8IWV2 | 1.058 | 0.372 |
| MMP-17 | Q9ULZ9 | 0.732 | 1.187 |
| SLAF5 | Q9UIB8 | 1.381 | −0.362 |
| HPLN1 | P10915 | 0.030 | −0.398 |
| Met | P08581 | 1.859 | −0.569 |
| ON | P09486 | 0.684 | −0.180 |
| RGM-C | Q6ZVN8 | −0.561 | 1.151 |
| Nucleoside diphosphate kinase A | P15531 | −0.370 | −0.502 |
| gp130, soluble | P40189 | −1.675 | 1.107 |
Corresponds to the weights applied to each protein in order to calculate the components displayed in Figure 3. Proteins given higher weights for component 1 are more important for distinguishing neuroendocrine cell hyperplasia of infancy from the other groups, whereas proteins with lower weights for component 1 and higher weights for component 2 distinguish surfactant dysfunction.
Figure 3.
Discrimination of groups from canonical discriminant analysis using the top 20 proteins identified from the random forest fit to the three disease groups. Component 1 distinguishes neuroendocrine cell hyperplasia of infancy from the other groups, and component 2 distinguishes surfactant dysfunction from other children’s interstitial and diffuse lung disease diagnoses. Corresponding weights for each aptamer are displayed in Table 3. ILD = interstitial lung disease; NEHI = neuroendocrine cell hyperplasia of infancy; SD = surfactant dysfunction.
NEHI Endotypes
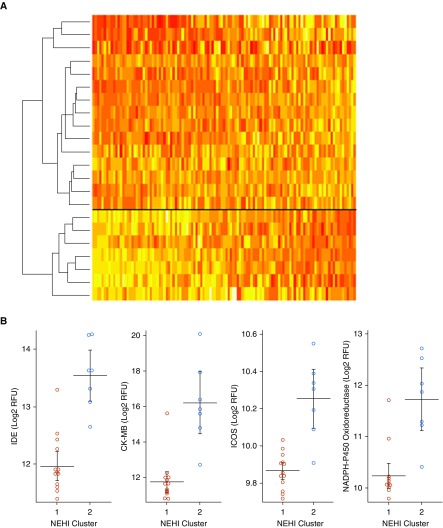
Using hierarchical Ward clustering on all proteins for the NEHI group, two distinct clusters are identified with different protein profiles (Figure 4). Cluster 1 (n = 15) includes 10 (67%) NEHI syndrome, and cluster 2 (n = 7) includes 3 (43%) NEHI syndrome. Thus, NEHI syndrome versus NEHI diagnostic group did not explain the two groups (P = 0.38). There were no differences in any of the clinical factors between the clusters (Table E3), including bacterial culture results. There were 47 proteins that differed between the two groups (Table E4), including IDE, CK-MB, ICOS, and NADPH-P450 oxidoreductase.
Figure 4.
There are two neuroendocrine cell hyperplasia of infancy (NEHI) endotypes based on protein profiles. (A) Heatmap showing two endotypes for the NEHI samples across all measured aptamers. (B) The distributions of the top four aptamers that differ between the two NEHI clusters are plotted. CK-MD = creatine kinase-MB; ICOS = inducible T-cell costimulator; IDE = insulin-degrading enzyme; RFU = relative fluorescence units.
Discussion
We have shown that specific proteins and pathways can be found in the BALF of children with different types of chILD and may distinguish different disease mechanisms. The proteomes of NEHI, with limited inflammation but increased pulmonary neuroendocrine cell (PNEC) hyperplasia, and surfactant dysfunction, with increased fibrosis on histopathology, segregated from each other (Figure 3) and can be distinguished by a panel of 20 proteins (Table 3). NEHI and surfactant dysfunction can also be distinguished from other chILD (predominately with immune-mediated mechanisms) and controls. With validation, this could be useful in diagnosis, especially in chILD diseases that have overlapping clinic presentations, such as follicular bronchiolitis and NEHI, potentially avoiding the need for lung biopsy.
In addition to improving diagnostic accuracy, proteomic profiles could suggest new therapeutic targeted interventions. To develop mechanistic therapeutics and move away from empiric treatment, we need to understand pathophysiology of chILD disorders. Our data support the idea that abnormalities of surfactant, including SFTPC and ABCA3, group with each other on the basis of their proteomic profile. Treatment could be proposed for both of these conditions based on the disease mechanisms suggested by their proteomic profiles, for example, using antifibrotic medication for fibrosis. This would be a similar approach to the non-IPF fibrosis studies in adults in which subjects with different clinical diagnoses like collagen vascular disease or hypersensitivity pneumonitis demonstrating progressive fibrosing interstitial lung disease are combined into one treatment study (26).
As advanced translational techniques are improving our understanding of mechanisms for chILD, protein biomarkers from children with chILD are important to establish human/mouse correlation for animal models and to suggest potential therapeutic outcome measures in both mouse models and pluripotent stem cell systems. In addition to mouse models for surfactant dysfunction and evolving mouse models for NEHI, inducible pluripotent stem cell systems using gene correction for surfactant dysfunction have been developed (11, 27). Katzen and colleagues (11) found cytokines from alveolar type 2 cells (CCL2/MCP-1, CCL17/TARC, CCL7/MCP3, and MMP7) in BALF of children with abnormal SFTPC and in a SFTPC mouse model of pulmonary fibrosis. Investigations in these model systems are needed along with analysis of human samples to further our understanding of PNEC biology, pulmonary fibrosis, and inflammatory pathways in the lung.
In our results, the protein signature in NEHI differs from the other groups and, as has been shown before, displays little evidence of inflammation (2, 28, 29). Top-ranked pathways for NEHI compared with controls may provide intriguing clues, such as the PI3K/AKT network and links to neuronal systems, protein–protein interactions at synapses, and NGF. The highest increased NEHI protein was cathespsin H, which functions as an aminopeptidase in secretory vesicles to produce enkephalin and galanin peptide neurotransmitters (30). The second-highest protein, resistin, is an adipokine that modulates food intake and energy expenditures. Impacts on metabolism are also suggested by decreases in aptamer proteins 6-phosphogluconate dehydrogenase and GAPDH in the liver and a top-ranked pathway for metabolism of carbohydrates. This is interesting, given patients with NEHI have significant issues with somatic growth (31). DLL4, another top increased protein, is involved in notch signaling for angiogenesis. Other groups have shown that notch pathways are implicated in PNEC regulation and airway cell fate decisions (32). It remains unknown if PNECs and notch pathways are markers of NEHI or involved in disease pathogenesis. Finally, although the clinical presentation of NEHI may be quite similar across patients, there appear to be two distinct NEHI endotypes, as shown using clustering (Figure 4). This may relate to differences in specific underlying genetic or environmental mechanisms. More work is needed by cellular biologists, experts in neuroendocrine cell biology, and translational scientists, using NEHI animal models to understand the importance of these findings.
Though small in numbers, the proteome of NEHI and NEHI syndrome were similar, as indicated by two different statistical methods (hierarchical ward clustering and canonical discriminatory analysis). This is important, as current NEHI diagnostic practice relies on classic imaging findings and clinical history; biopsies are not routinely done, unless the diagnosis is unclear. These data are reassuring of this practice, supporting continued avoidance of lung biopsy in the correct clinical setting for NEHI. These data support grouping NEHI and NEHI syndrome for research purposes on this rare disease.
Surfactant mutations in the SFTPC and ABCA3 genes both create epithelial dysfunction in the alveolar type 2 cell. SFTPC mutations can be classified as BRICHOS (distal C-terminal pro-SPC domain) or non-BRICHOS mutations, each with its own pathogenesis: BRICHOS mutations results in induction of endoplasmic reticulum (ER) stress, and non-BRICHOS mutations, which include the most common mutation, I73T, block macroautophagy (11, 33, 34). ABCA3 mutations have a recessive inheritance pattern and disrupt the transport of lipid required for the formation of lamellar bodies in alveolar type 2 cells. Mortality in infants is based on the presence of null mutations (35). Despite different individual mechanisms of disease, both SFTPC and ABCA3 present with similar clinical features and result in an early inflammatory disease state that progresses to fibrosis.
We combined children with SFTPC and ABCA3 genetic abnormalities into a surfactant dysfunction group to examine potential biomarkers of fibrosis. Top-ranked pathways for surfactant dysfunction compared with controls included WNT signaling (36), IL, cytokine and chemokines, and extracellular matrix, all pathways implicated in fibrosis (37). Cytokines CCL2/MCP-1, CCL17/TARC, and CCL7/MCP3 (Table E2) are some of the dominant proteins in this cohort and have been reported in SFTPC mouse models of fibrosis and IPF (11). Both WNT signaling pathways and related proteins DKK1 and DKK4 have increased expression in IPF (38), as well as other proteins that have been identified as biomarkers in IPF (39–41). Extracellular matrix proteins, including TSP2 and multiple matrix metalloproteinases (MMP12, MMP3, and MMP7), are represented in the top 20 increased proteins for surfactant dysfunction. Although single-center, these results suggest that antifibrotic therapeutic approaches may have relevance to treating surfactant dysfunction (26). Further study is needed to understand if these proteins could also serve as therapeutic outcome measures.
This study is not without limitations that could introduce bias and may limit broader generalization. It is a single-center study using convenience samples. Bronchoscopy and BALF were obtained for clinical purposes as determined by the clinician, although most procedures were done early in the disease process for diagnostic purposes and not during exacerbations. Treatment decisions were made by the clinical team, and different treatment approaches may have occurred even in similar patients. This study evaluates rare diseases, and the number of samples per disease is thus very limited. Our findings are also limited to the BALF. Blood-based biomarkers may be different and should be studied. Despite these limitations, we believe the results provide valuable data that should encourage prospective multiinstitutional studies in chILD.
In conclusion, we have shown that protein signatures in BALF distinguish children with NEHI and surfactant dysfunction. With validation, these data could be used to develop diagnostics, targets for therapeutic intervention, and biomarkers for clinical trial outcome measures. More translational science and future prospective multiinstitutional sample collection and studies are needed worldwide through chILD networks to advance the field and deliver desperately needed solutions for children with chILD.
Acknowledgments
Acknowledgment
The authors thank Churee Pardee for her assistance and support in obtaining consent and samples and Frank Accurso for his encouragement and expertise on biomarkers. SomaLogic, Inc., provided technical support and development for using BAL fluid as a biological matrix for aptamer-based measurements.
Footnotes
Primary source of funding: American Thoracic Society Foundation/Children’s Interstitial Lung Disease (chILD) Foundation/La Fundación Pequeños Pulmones Research Award titled “Novel Proteomic Profiles in Neuroendocrine Cell Hyperplasia of Infancy (NEHI).” Also supported by NIH/National Center for Advancing Translational Science Colorado Clinical and Translational Science Award UL1 TR001082. The contents of this article are the authors’ sole responsibility and do not necessarily represent official NIH views. The funding agencies had no role in study decision, manuscript preparation, or submission.
Author Contributions: Conception and design: R.R.D. and J.K.H. Drafting of manuscript: R.R.D., B.D.W., and E.M.D. Review and revision of manuscript: All authors. Analysis, data acquisition, and data interpretation: R.R.D. and B.D.W.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201903-0547OC on August 13, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Fan LL, Deterding RR, Langston C. Pediatric interstitial lung disease revisited. Pediatr Pulmonol. 2004;38:369–378. doi: 10.1002/ppul.20114. [DOI] [PubMed] [Google Scholar]
- 2.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, et al. Pathology Cooperative Group; chILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nadlonek NA, Acker SN, Deterding RR, Partrick DA. Intraoperative chest tube removal following thoracoscopic lung biopsy results in improved outcomes. J Pediatr Surg. 2014;49:1573–1576. doi: 10.1016/j.jpedsurg.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, et al. American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Young LR, Trapnell BC, Mandl KD, Swarr DT, Wambach JA, Blaisdell CJ. Accelerating scientific advancement for pediatric rare lung disease research: report from a National Institutes of Health-NHLBI workshop, September 3 and 4, 2015. Ann Am Thorac Soc. 2016;13:385–393. doi: 10.1513/AnnalsATS.201605-402OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA. 2016;315:2532–2541. doi: 10.1001/jama.2016.5951. [DOI] [PubMed] [Google Scholar]
- 7.Hathout Y, Brody E, Clemens PR, Cripe L, DeLisle RK, Furlong P, et al. Large-scale serum protein biomarker discovery in Duchenne muscular dystrophy. Proc Natl Acad Sci USA. 2015;112:7153–7158. doi: 10.1073/pnas.1507719112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deterding RR, Wagner BD, Harris JK, Popler J, Smith BC, Katilius E, et al. Novel disease pathways and SOMAmer proteomic signatures in neuroendocrine cell hyperplasia of infancy and surfactant protein C bronchoalveolar lavage fluid [abstract] Am J Respir Crit Care Med. 2013;187:A3806. [Google Scholar]
- 9.Deterding RR, Wolfson A, Harris JK, Walker JJ, Accurso FJ. Aptamer proteomic analysis of bronchoalveolar lavage fluid yields different protein signatures from children with children’s interstitial lung disease, cystic fibrosis and disease controls [abstract] Am J Respir Crit Care Med. 2010;181:A6722. [Google Scholar]
- 10.Deterding RR, Wolfson A, Harris JK, Walker JJ, Wagner BD, Accurso FJ. Bronchoalveolar lavage protein biomarkers in children with surfactant dysfunction mutations: an aptamer proteomics approach. Proc Am Thorac Soc. 2011;8:210. [Google Scholar]
- 11.Katzen J, Wagner BD, Venosa A, Kopp M, Tomer Y, Russo SJ, et al. An SFTPC BRICHOS mutant links epithelial ER stress and spontaneous lung fibrosis. JCI Insight. 2019;4:e126125. doi: 10.1172/jci.insight.126125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Blic J, Midulla F, Barbato A, Clement A, Dab I, Eber E, et al. European Respiratory Society. Bronchoalveolar lavage in children: ERS task force on bronchoalveolar lavage in children. Eur Respir J. 2000;15:217–231. doi: 10.1183/09031936.00.15121700. [DOI] [PubMed] [Google Scholar]
- 13.Krawiec ME, Westcott JY, Chu HW, Balzar S, Trudeau JB, Schwartz LB, et al. Persistent wheezing in very young children is associated with lower respiratory inflammation. Am J Respir Crit Care Med. 2001;163:1338–1343. doi: 10.1164/ajrccm.163.6.2005116. [DOI] [PubMed] [Google Scholar]
- 14.Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, et al. American Thoracic Society Committee on BAL in Interstitial Lung Disease. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004–1014. doi: 10.1164/rccm.201202-0320ST. [DOI] [PubMed] [Google Scholar]
- 15.Deterding RR, Pye C, Fan LL, Langston C. Persistent tachypnea of infancy is associated with neuroendocrine cell hyperplasia. Pediatr Pulmonol. 2005;40:157–165. doi: 10.1002/ppul.20243. [DOI] [PubMed] [Google Scholar]
- 16.Brody AS, Guillerman RP, Hay TC, Wagner BD, Young LR, Deutsch GH, et al. Neuroendocrine cell hyperplasia of infancy: diagnosis with high-resolution CT. AJR Am J Roentgenol. 2010;194:238–244. doi: 10.2214/AJR.09.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kerby GS, Wagner BD, Popler J, Hay TC, Kopecky C, Wilcox SL, et al. Abnormal infant pulmonary function in young children with neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2013;48:1008–1015. doi: 10.1002/ppul.22718. [DOI] [PubMed] [Google Scholar]
- 18.Gold L, Ayers D, Bertino J, Bock C, Bock A, Brody EN, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS One. 2010;5:e15004. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeBoer EM, Kroehl ME, Wagner BD, Accurso FJ, Harris JK, Lynch DA, et al. Proteomic profiling identifies novel circulating markers associated with bronchiectasis in cystic fibrosis. Proteomics Clin Appl. 2017;11:1800085. doi: 10.1002/prca.201600147. [DOI] [PubMed] [Google Scholar]
- 20.DeBoer EM, Wagner BD, Popler J, Harris JK, Zemanick ET, Accurso FJ, et al. Novel application of aptamer proteomic analysis in cystic fibrosis bronchoalveolar lavage fluid. Proteomics Clin Appl. 2019;13:e1800085. doi: 10.1002/prca.201800085. [DOI] [PubMed] [Google Scholar]
- 21.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 22. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 23. Fabregat A, Sidiropoulos K, Garapati P, Gillespie M, Hausmann K, Haw R, et al. The reactome pathway knowledgebase. Nucleic Acids Res. 2016;44:D481–D487. doi: 10.1093/nar/gkv1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Milacic M, Haw R, Rothfels K, Wu G, Croft D, Hermjakob H, et al. Annotating cancer variants and anti-cancer therapeutics in reactome. Cancers (Basel) 2012;4:1180–1211. doi: 10.3390/cancers4041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: current approaches and outstanding challenges. PLoS Comput Biol. 2012;8:e1002375. doi: 10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Flaherty KR, Brown KK, Wells AU, Clerisme-Beaty E, Collard HR, Cottin V, et al. Design of the PF-ILD trial: a double-blind, randomised, placebo-controlled phase III trial of nintedanib in patients with progressive fibrosing interstitial lung disease. BMJ Open Respir Res. 2017;4:e000212. doi: 10.1136/bmjresp-2017-000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacob A, Morley M, Hawkins F, McCauley KB, Jean JC, Heins H, et al. Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell. 2017;21:472–488, e10. doi: 10.1016/j.stem.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Popler J, Wagner BD, Accurso FJ, Deterding RR. Airway cytokine profiles in children’s interstitial lung diseases [abstract] Am J Respir Crit Care Med. 2010;181:A3316. [Google Scholar]
- 29. Doan ML, Elidemir O, Dishop MK, Zhang H, Smith EO, Black PG, et al. Serum KL-6 differentiates neuroendocrine cell hyperplasia of infancy from the inborn errors of surfactant metabolism. Thorax. 2009;64:677–681. doi: 10.1136/thx.2008.107979. [DOI] [PubMed] [Google Scholar]
- 30. Lu WD, Funkelstein L, Toneff T, Reinheckel T, Peters C, Hook V. Cathepsin H functions as an aminopeptidase in secretory vesicles for production of enkephalin and galanin peptide neurotransmitters. J Neurochem. 2012;122:512–522. doi: 10.1111/j.1471-4159.2012.07788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nevel RJ, Garnett ET, Schaudies DA, Young LR. Growth trajectories and oxygen use in neuroendocrine cell hyperplasia of infancy. Pediatr Pulmonol. 2018;53:656–663. doi: 10.1002/ppul.23958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cutz E, Perrin DG, Pan J, Haas EA, Krous HF. Pulmonary neuroendocrine cells and neuroepithelial bodies in sudden infant death syndrome: potential markers of airway chemoreceptor dysfunction. Pediatr Dev Pathol. 2007;10:106–116. doi: 10.2350/06-06-0113.1. [DOI] [PubMed] [Google Scholar]
- 33. Hawkins A, Guttentag SH, Deterding R, Funkhouser WK, Goralski JL, Chatterjee S, et al. A non-BRICHOS SFTPC mutant (SP-CI73T) linked to interstitial lung disease promotes a late block in macroautophagy disrupting cellular proteostasis and mitophagy. Am J Physiol Lung Cell Mol Physiol. 2015;308:L33–L47. doi: 10.1152/ajplung.00217.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thurm T, Kaltenborn E, Kern S, Griese M, Zarbock R. SFTPC mutations cause SP-C degradation and aggregate formation without increasing ER stress. Eur J Clin Invest. 2013;43:791–800. doi: 10.1111/eci.12107. [DOI] [PubMed] [Google Scholar]
- 35. Wambach JA, Casey AM, Fishman MP, Wegner DJ, Wert SE, Cole FS, et al. Genotype-phenotype correlations for infants and children with ABCA3 deficiency. Am J Respir Crit Care Med. 2014;189:1538–1543. doi: 10.1164/rccm.201402-0342OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Königshoff M, Balsara N, Pfaff EM, Kramer M, Chrobak I, Seeger W, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3:e2142. doi: 10.1371/journal.pone.0002142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Morrisey EE. Wnt signaling and pulmonary fibrosis. Am J Pathol. 2003;162:1393–1397. doi: 10.1016/S0002-9440(10)64271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pfaff EM, Becker S, Günther A, Königshoff M. Dickkopf proteins influence lung epithelial cell proliferation in idiopathic pulmonary fibrosis. Eur Respir J. 2011;37:79–87. doi: 10.1183/09031936.00142409. [DOI] [PubMed] [Google Scholar]
- 39. Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Kaminski N. Biomarkers in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18:441–446. doi: 10.1097/MCP.0b013e328356d03c. [DOI] [PMC free article] [PubMed] [Google Scholar]