Abstract
The mechanistic target of rapamycin (mTOR) is an essential nutrient-sensing kinase that integrates and regulates a number of fundamental cellular processes required for cell growth, cell motility, translation, metabolism, and autophagy. mTOR signaling has been implicated in the progression of many human diseases, and its dysregulation has been reported in several pathological processes, especially in age-related human diseases and mouse models of accelerated aging. In addition, many studies have demonstrated that the regulation of mTOR activity has a beneficial effect on longevity in several mouse models of aging. However, not all mouse models of accelerated aging show positive effects on aging-associated phenotypes in response to targeting mTOR signaling. Here, we review the effects of interventions that modulate mTOR signaling on aging-related phenotypes in different mouse models of accelerated aging and discuss their implications with respect to aging and aging-related disorders.
Keywords: mTORC1, Age-associated disease, Rapamycin, Dietary restriction, Mice
Aging is by far the biggest independent risk factor for a wide range of intrinsic diseases, including most types of cancer, cardiovascular disease, atherosclerosis, inflammation, and neurodegeneration (1). It affects different biological pathways and the underlying molecular and cellular mechanisms are complex. In view of the growing disease burden of aging populations, increasing efforts are being invested in understanding the pathways and mechanisms of aging (2). Since its discovery, evidence has accumulated that the mechanistic target of rapamycin (mTOR) pathway is the major nutrient-sensitive regulator of growth at the cellular level and within the organism as a whole playing a central role in physiology, energy metabolism, the aging process, and common age-related diseases (3–5). Consistently, dysregulated mTOR activity is observed in multiple types of human age-related disorders, such as cancers, diabetes, cardiovascular diseases, and neurodegenerative diseases, and is closely linked to the normal aging process (3).
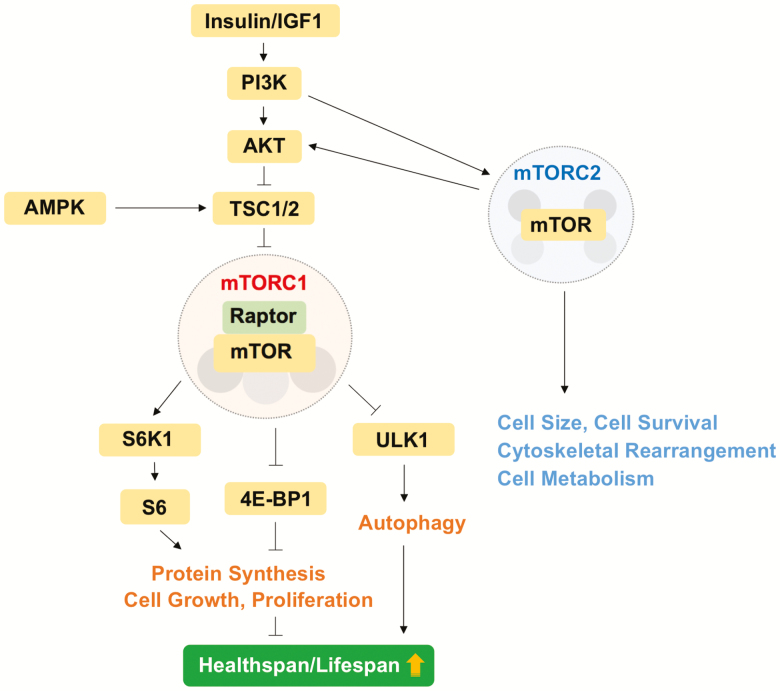
Cells respond to the availability of nutrients such as glucose and amino acids by triggering energy-consuming anabolic pathways under a state of nutrient sufficiency (6). In this setting, mTOR signaling is activated, whereas it is blocked under nutrient-limited conditions, such as dietary restriction (DR). The mTOR kinase exists in two structurally and functionally distinct multiprotein complexes, which are referred to as mTOR complex 1 (mTORC1) and mTORC2 (Figure 1). These two complexes regulate a variety of physiological cellular processes, including protein, lipid, and nucleotide synthesis, as well as autophagy in response to external cues. The main function of mTORC1 is the activation of anabolic processes including cell growth and proliferation, in part through control of protein synthesis, whereas mTORC2 participates in the control of cytoskeletal dynamics, cell size, and cell metabolism (5). The best-defined substrates of the mTORC1 complex are S6K1 (ribosomal protein S6 protein kinase 1) and 4E-BP1 (eIF4E [eukaryotic translation initiation factor 4E]-binding protein 1), both of which are important in the control of translation initiation (5). Activation of the mTORC1 signaling cascade results in the phosphorylation of downstream substrates such as S6K1 and 4E-BP1, which in turn affect protein synthesis (Figure 1). Specifically, phosphorylation of S6K1 results in its activation and the subsequent phosphorylation of ribosomal protein S6 (rpS6), as well as other components of the translation machinery, whereas phosphorylation of 4E-BP1 disrupts its binding to eIF4E, freeing this initiation factor to promote cap-dependent translation. Autophagy, an intracellular process in which damaged organelles and proteins are degraded and recycled for maintaining normal cellular homeostasis, is also regulated by mTORC1 activity (7) (Figure 1).
Figure 1.
Overview of mechanistic target of rapamycin complex 1 (mTORC1) and mTORC2 signaling pathway and function.
Several genetic, environmental, and pharmacologic prolongevity interventions modulate mTOR signaling (8). These interventions, including but not limited to DR or calorie restriction, rapamycin, genetically modified mTOR components, or mTORC1 downstream (such as S6K1), suppress mTORC1 activity, ameliorate age-related diseases, and extend life span in normal mice (6,9). In accelerated aging or human disease-bearing mouse models, the application of these mTOR-suppressing therapeutic strategies to ameliorate diseases stems from the assertions that these mouse models represent either accelerated normal aging processes or age-associated diseases in humans (10), but many also display dysregulation of mTORC1 activity in the pathological tissues (11).
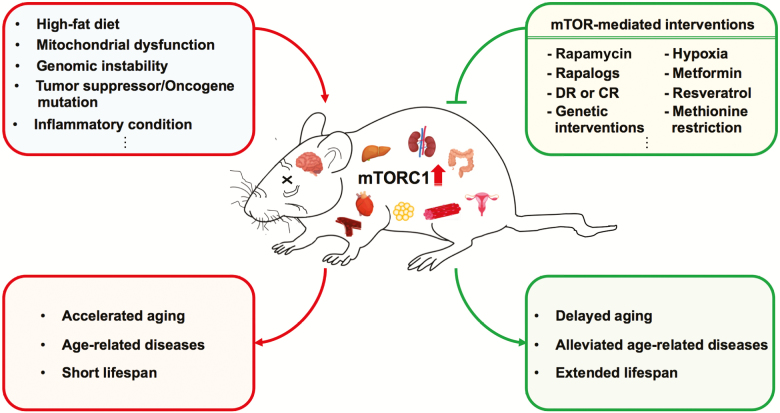
Here, we discuss studies to test the hypothesis that mouse models bearing human age-related diseases associated with mTORC1 dysregulation could benefit from pharmacologic or genetic suppression of mTORC1 (Figure 2). We scrutinize the published mouse models commonly associated with accelerated aging and/or dysregulation of mTORC1 activity and highlight the beneficial effects by interventions in pathological tissues of mouse models, especially with regard to longevity. Discrepant results from different models, therapeutic uses, as well as further studies are also described. A summary of rodent models of accelerated aging demonstrating elevated mTORC1 activity, and the potential benefits of targeting mTOR are also included (Table 1).
Figure 2.
Schematic model for the beneficial effects of mechanistic target of rapamycin complex 1 (mTORC1)-mediated interventions in age-related disease mouse models.
Table 1.
List of the Favorable Effects on Health Span or Life Span by Suppression of Dysregulated mTORC1 in Rodent Models of Accelerated Aging
| Mouse Models | Aging-Associated Diseases | mTOR- Upregulated Tissues | Interventions | Effects on Health Span or Life Span | Reference |
|---|---|---|---|---|---|
| Ndufs4 −/− | Leigh syndrome | Brain | Rapamycin | Delays onset of neurological symptoms and extends life span | (12) |
| Genetically whole-body as well as liver-specific S6K1 deletion | Prolongs survival and delays onset of neurological symptoms | (13) | |||
| Hypoxia | Improves survival and neuropathology | (14) | |||
| 3xTg-AD | Alzheimer’s disease | Cortex and hippocampus | Rapamycin | Reduces plaques, tangles, and cognitive deficits | (23) |
| Dietary restriction | Ameliorates age-related cognitive deficits | (24) | |||
| Tg2576 | Alzheimer’s disease | Hippocampus | APP/mTOR+/− mice (genetically reduced mTOR signaling in the brains) | Reduces Aβ deposits and rescues cognitive deficits | (25) |
| Dietary restriction | Prevents Aβ peptides generation and neuritic plaque deposition | (26) | |||
| Senescence-accelerated mouse-prone 8 (SAMP8) | Alzheimer’s disease | Cerebral cortex and hippocampus | Rapamycin | Promotes cell morphology and alleviates Tau phosphorylation of neurons | (36) |
| 6-OHDA-treated mice | Parkinson’s disease | Spiny neurons | Rapamycin | Attenuates levodopa-induced dyskinesia | (41) |
| Rapamycin | Reverts cognitive and affective deficits | (42) | |||
| Ts1Cje | Down syndrome | Hippocampus | Rapamycin | Restores memory deficits | (45,46) |
| Ts65Dn | Down syndrome | Hippocampus | Rapamycin | Reduces accumulation of toxic metabolites in aged mice | (47) |
| p53 +/− or p53−/− | Cancer | Heart | Rapamycin | Extends life span and delays tumorigenesis | (48–50) |
| Dietary restriction | Delays spontaneous tumorigenesis and extends life span | (53) | |||
| Metformin | Shows selective ability to inhibit tumor growth | (54) | |||
| Apc Delta716 | Colon cancer, FAP | Intestinal polyps | Everolimus | Inhibits intestinal polyp formation, tumor angiogenesis and extends life span | (62) |
| Pten +/− or Tagln-cre/Pten loxP/loxP | Cancer | Uterus | Everolimus | Decelerates tumor growth and prolongs life span | (64,65) |
| Lmna −/− | Dilated cardiomyopathy, EDMD | Cardiac and skeleton muscle | Rapamycin | Rescues cardiac and skeletal muscle function, reverses metabolic deficits, and elongates life span | (70,71) |
| Lmna −/− S6K1 +/− mice (genetically knockdown of S6K1 ubiquitously or muscle specifically) | Extends life span by improving skeletal muscle function | (72) | |||
| Lmna H222P/H222P | AD-EDMD | Heart | Temsirolimus | Ameliorates cardiomyopathy | (73) |
| Bmal1 −/− | Dilated cardiomyopathy | Cerebellum, frontal cortex, liver, heart | Rapamycin | Increases life span | (92) |
| CRF (rat) | Chronic renal failure | Aortic wall | Rapamycin | Ameliorates vascular calcification | (96) |
Notes: AD-EDMD = Autosomal Dominant Emery–Dreifuss muscular dystrophy; CRF = chronic renal failure; EDMD = Emery–Dreifuss muscular dystrophy; FAP = familial adenomatous polyposis; mTORC1 = mechanistic target of rapamycin complex 1; 6-OHDA = 6-hydroxydopamine.
mTORC1 in Mouse Models With Neurodegeneration
Rapamycin has been shown to ameliorate morbidity and mortality in mouse models of several neurological diseases, most notably a model of mitochondrial disease caused by ablation of the nucleus-encoded gene specifying the Ndufs4 (NADH dehydrogenase [ubiquinone] Fe-S protein 4) subunit of oxidative phosphorylation complex I (12). Mice deficient for the Ndufs4 protein (Ndufs4−/− mice) are models for Leigh syndrome, an inherited mitochondrial encephalopathy that leads to early disability and death in affected young children. Because mTORC1 activity is elevated in pathological tissues, such as brain tissue, of Ndufs4−/− mice, they were treated with rapamycin, which was found to extend the survival of Ndufs4−/− mice. Follow-up studies by the same group found that whole-body, as well as liver-specific S6K1 knockout, improves the survival of Ndufs4−/− mice (13). However, genetically suppressing S6K1 in the brain—the most affected organ in this mouse—did not improve Ndufs4−/− mice. This study also highlights the importance of considering potential noncell-autonomous effects of mTOR modulation.
More recent work on Ndufs4−/− mice has shown that hypoxia dramatically increases life span of Ndufs4−/− mice even more robustly than rapamycin (14). Similar to rapamycin, hypoxia suppresses mTORC1 (15), possibly explaining some of its benefits. However, the molecular mechanisms underlying the life-span extension by these two distinct interventions, namely rapamycin and hypoxia, may not completely overlap, given the fact that life extension by rapamycin was accompanied by overt weight loss in Ndufs4−/− mice (12), hypoxia treatment was associated with increased body weight (14). Together, these observations suggest that the short-lived Ndufs4−/− mouse model of severe mitochondrial disease can be used as relatively rapid discovery platform for interventions likely to extend life span in wild-type mice and perhaps humans. In line with this, a recent and promising clinically study showed rapamycin indeed improves mitochondrial function in Leigh syndrome-like patients (16).
A mouse model with a homozygous knock-in mutation in the mitochondrial nucleotide salvage enzyme thymidine kinase 2 (Tk2Kl/Kl mice) also benefits from low-dose oral rapamycin treatment (17): rapamycin almost doubled the survival of these extremely short-lived Tk2Kl/Kl mice. This is the first evidence of the therapeutic benefit of rapamycin in a mouse model of mitochondrial DNA-driven disease. This life extension by rapamycin in Tk2Kl/Kl mice is intriguing given the fact that there was no apparent improvement in the brain—the most affected tissue in this mouse—at least in canonical rapamycin-mediated pathways. Despite the fact that Tk2Kl/Kl mice are cachexic, rapamycin further decreased body weight in Tk2Kl/Kl mice, probably due to the depletion of fat stores. Thus, it is possible that rapamycin exerts its effects noncell-autonomously or through noncanonical substrates.
Familial amyotrophic lateral sclerosis (ALS) is an adult-onset neurodegenerative disorder whose incidence increases with age. Two mouse models bearing increased mitochondrial oxidative stress induced by mutant manganese superoxide dismutase (SOD), SOD1G93A mice and SOD1H46R/H48Q mice, both exhibit ALS-like syndromes. Interestingly, rapamycin shortens the survival of SOD1G93A mouse model of ALS (18). Consistent with the canonical mechanism that mTORC1 is a negative regulator of autophagy, mTORC1 activity was significantly reduced, whereas autophagosomal markers were increased in spinal cord motor neurons in SOD1G93A mouse. Nevertheless, autophagic flux was impaired as indicated by the accumulation of the p62 protein. Rapamycin further increased the p62 accumulation probably increasing mitochondrial impairment. This intriguing results may reflect the fact that mTOR is still required in the neuroprotection mechanism in this mouse model (19). In agreement, the other mTORC1 suppression intervention, DR, also shows no beneficial (and in some instances, detrimental) effects in SOD1G93A mice (20).
However, it is quite a different story for the SOD1H46R/H48Q mouse model of ALS: DR but not rapamycin delays disease onset and extends survival in this context (21). The mTORC1 activity, especially autophagy, was not evaluated in the DR study. The implications of these different data are twofold: (i) DR and rapamycin may work in overlapping pathways, that is, the effects of DR are not completely mediated through inhibition of mTORC1, and (ii) mTORC1 signaling may not globally affect ALS disease pathology caused by different SOD1 mutations. Also, these findings point to the value of using multiple mouse models of a disease whenever feasible.
Alzheimer’s disease (AD) is a neurodegenerative disorder characterized by numerous pathological features, including the presence of intracellular neurofibrillary tangles and extracellular β–amyloid plaques (Aβ). Numerous studies using AD mouse models were conducted to investigate the effects of interventions on cognition and the pathological characteristics of AD, including tests with rapamycin and DR (22). In the widely used triple-transgenic mouse model of AD (3xTg-AD mice), mTORC1 signaling is increased in the cortex and hippocampus and rapamycin significantly reduces mTORC1 signaling, rescues cognitive deficits, and ameliorates Aβ and tau pathology by increasing autophagy (23). In the same mouse model, DR also ameliorates age-related behavioral deficits and tau pathology (24). In line with mTORC1 suppression by rapamycin, genetically reduction of mTOR signaling (ie, deletion of one copy of mTOR) in the forebrain of Tg2576 AD mice suppressed the elevated mTOR signaling and reduced Aβ deposits and rescued memory deficits (25). Tg2576 AD mice also benefit from DR by lowering Aβ neuropathology (26). Studies using mice overexpressing mutant human tau (P301S mice) further support the role of mTORC1 activity in AD pathology: rapamycin ameliorates tau pathology and the associated behavioral deficits (27). Despite the improvement of “health span” by rapamycin or DR on these AD mouse models, a survival study by these promising interventions should be conducted on these models as well as experiments to address the molecular basis underlying the beneficial effects of reduced mTOR signaling.
Apolipoprotein E-deficient (ApoE−/−) mice serve as a well-established neurodegenerative model of AD displaying hyperphosphorylation of tau protein (28). Inhibition of the mTOR pathway with rapamycin protects the entorhinal cortex from tau-mediated neurodegeneration (29). This neuroprotection upon rapamycin was further supported by a study showing DR exerts neuroprotective effects in brain of ApoE−/− mice by inhibiting phosphorylation of mTOR and tau protein, potentially due to the enhanced AMP-activated protein kinase (AMPK) activity (30). In addition to neurodegeneration, ApoE−/− mice also served as a good model for one of age-associated diseases, atherosclerosis. Rapamycin slows progression of aortic atherosclerosis in ApoE−/− mice (31). An unanswered question, however, is whether rapamycin or DR improve the survival of ApoE−/− mice by suppressing the potential elevated mTORC1 activity. Of note, spermidine, a natural compound that extends life span in normal mice by inducing autophagy (32), reduces lipid accumulation and necrotic core formation in atherosclerotic plaques of ApoE−/− mice (33). This study further enforces the hypothesis that targeting mTORC1 downstream along is a potentially useful route for slowing aging in different diseases.
Although the genetic basis of AD mouse models has been well established, the nongenetically modified senescence-accelerated mouse-prone 8 mice (SAMP8) are also widely used as mouse model for AD (34), given the fact that SAMP8 mice exhibit age-related learning impairment and cognitive decline (34). These abnormalities are probably associated with reduced autophagic activity since there is lower Beclin-1 expression in the hippocampus and cortex of aged SAMP8 mice (35). In line with this, both upregulated mTORC1 activity and hyperphosphorylation of Tau were also observed in the cerebral cortex and hippocampus of SAMP8 mice and rapamycin administration significantly reduced phosphorylation of Tau protein (36). Given these promising results, more in vivo studies in this model are warranted, especially behavioral and survival studies.
Huntington’s disease (HD) is a neurodegenerative disorder where a genetic mutation causes an expansion of the polyglutamine tract within the Huntingtin protein (HTT), resulting in protein aggregation. As mTORC1 signaling suppresses autophagy, which is responsible for recycling protein aggregates, it has been implicated in HD pathology. Interestingly and unlikely that observed in other neurodegenerative conditions, mTORC1 activity was intrinsically decreased in the striatum of both affected individuals and the N171-82Q HD mouse model (37). Introducing the constitutively active form of mTORC1 regulator, Rheb, activated basal autophagy, improved mitochondrial function, and decreased HTT abundance in mouse brain, coincident with improved motor performance (37). Thus, mTORC1 activation may actually be beneficial and required in natural course for HD and mTORC1 suppression by rapamycin or rapalog may be contraindicative for this disease. In the same mouse model; however, DR slows down the disease progression and extends life span (38). In this setting, it is of critical to evaluate the mTORC1 signaling as well as autophagy activity in the brain tissues to further disentangle this complicated mechanism underlying HD progression.
Parkinson’s disease (PD) is also a progressive age-associated neurodegenerative disorder associated with the death of neurons in the substantia nigra. Though a number of genes are associated with PD, much of the disease etiology remains to be unraveled. Levels of mTORC1 activity in PD are controversial, as both neuroprotective and neurotoxic roles have been proposed (39). First, mTORC1 activity has been suggested to be neuroprotective in PD given that suppression of mTORC1 signaling by several routes (such as AMPK and phosphatase and tensin homolog [PTEN] activation) leads to neuronal cell death in in vitro models of PD (40). On the other hand, a number of recent studies have also demonstrated the benefits of rapamycin in PD (39). For example, in a 6-hydroxydopamine–induced mouse model of PD, inhibition of mTOR signaling by rapamycin prevents L-DOPA-induced dyskinesia (41). This study shows that L-DOPA-mediated activation of mTORC1 persisted in mice that developed dyskinesia. Interestingly, rapamycin prevented the development of dyskinesia without affecting the therapeutic efficacy of L-DOPA. In the same mouse model, rapamycin reverted cognitive and affective deficits via largely unexplored mechanisms (42). More mechanistic studies are required to elucidate how mTOR activity is perturbed in cellular and animal models of PD and survival studies with interventions that target mTOR may be informative.
Excessive activation of mTORC1 in neurons is also linked to intellectual disabilities, including Down syndrome (DS) (43), which has recently been speculated to be associated with aspects of accelerated aging (44). In Ts1Cje mouse model for DS, elevated level of mTORC1 signaling was observed in the hippocampal neurons, and rapamycin restored normal mTORC1 activity (45) and reversed memory deficits (46). In agreement, rapamycin also ameliorates age-related accumulation of toxic brain metabolites in another mouse model for DS, the Ts65Dn mice (47). Given that DS in patients is caused by trisomy of chromosome 21, a key question moving forward is how this chromosome anomaly results in mTORC1 activation.
mTORC1 in Cancer-Prone Mouse Models and Mouse Models With Genomic Instability
Consistent with its role as a central regulator of cell growth, proliferation, and angiogenesis, many oncogenic mutations activate mTOR signaling (3), making the pathway a key target in anti-cancer therapy. mTOR activity is also frequently deregulated in cancer, where it plays a key oncogenetic role driving tumor cell proliferation, survival, metabolic transformation, and metastatic potential. Promising preclinical studies using TORC1 inhibitors have demonstrated efficacy in many human cancer types.
Mutations of the p53 tumor suppressor constitute one of the most frequent genetic changes across the cancer spectrum. There is an extensive cross-talk between p53 and mTORC1. For instance, in p53−/− mice, mTORC1 activity, indicated by phosphorylated S6 (p-S6), is increased predominantly in heart tissue of p53−/− mice (48). Rapamycin, which acutely suppresses mTORC1 activity, extended the survival of p53−/− mice by delaying carcinogenesis (49). From the same research group, heterozygous p53+/− mice also benefit from rapamycin treatment (50). Because p53 and rapamycin are tumor suppressive, studies were performed to see how they interact. Interestingly, life-span extension by rapamycin was greater with increasing p53 copy number (51); p53+/+ mice benefit life extension from rapamycin better than p53+/− mice. This is in contrast to dietary restriction (DR), a longevity intervention, and well-documented inhibitor of tumors in several species and a multiplicity of tumor types (52), which markedly extends life span in this cancer-prone p53−/− mice probably by delaying the onset of tumor development (53). Similarly, metformin, another longevity intervention clinically used in metabolic diseases and indirect activator of AMPK (8), suppresses p53−/− tumor cell-implanted tumor growth by activating autophagy (54). One interpretation of this collection of data is the unlike DR and metformin, the tumor suppressive effects of mTOR inhibition by rapamycin require an intact p53 response. However, the situation is more complex because both DR and metformin are known to reduce mTOR activity as well (55). In fact, all three interventions probably evoke overlapping cellular metabolic responses, and the relative contribution of mTOR inhibition in each context remains to be fully determined.
Other mice are protected by rapamycin treatment, including the HER-2/neu cancer-prone model (56) where rapamycin extends survival probably by delaying tumor onset, decreasing tumor number and/or tumor size. Even at low dose, rapamycin still significantly extends life span in this model (57).
Immunosuppression is one of the major concerns with long-term mTOR inhibition by rapamycin; however, as a monotherapy it acts might be described as immunomodulatory, Somewhat unexpectedly, however, rapamycin increases life span of two different immunocompromised cancer-prone mice, recombinase-activating gene 2-deficient (Rag2−/−) mice, and interferon γ-deficient (IFN-γ−/−) mice (58). However, the molecular mechanisms underlying the benefits of mTOR inhibition remain to be ascertained.
Mice with single copy of the gene encoding the retinoblastoma tumor suppressor protein (Rb1; Rb1+/− mice) are highly predisposed to cancer of neuroendocrine origin. DR has a long history of retarding most types of cancers and age-associated diseases (52). Previous results showed that DR had minimal or no effect on the life span as well as growth and progression of neuroendocrine tumors of Rb1+/− mice (59). However, rapamycin feeding dramatically extended life span of Rb1+/− mice concomitant with decreased incidence of thyroid tumors (60). In contrast to DR, chronic rapamycin treatment did not reduce body weight of Rb1+/− mice. Thus, rapamycin appears to act differently than DR in Rb1+/− mice, suggesting diverse mechanisms of action on survival and tumor suppression, again even both interventions chronically inhibit mTORC1 activity (3).
Interestingly, high concentrations of rapamycin restore a normal life span in a familial adenomatous polyposis mouse model (ApcMin/+ mice) with median life span ~500 days (61). Familial adenomatous polyposis is often due to adenomatous polyposis coli gene germline mutations. Somatic adenomatous polyposis coli defects are found in about 80% of adenomas and colorectal cancers. This substantial life extension by rapamycin in ApcMin/+ mice in part due to the dose-dependent effects of rapamycin in inhibiting mTOR protein in the intestine. In separate mouse model, ApcDelta716 mice, inhibition of the mTORC1 activity by the rapamycin derivative everolimus also suppresses intestinal polyp formation and markedly extends life span (62). Although mTORC1 activity was substantially dysregulated in pathological tissue of this mouse model, the role of mTORC1 in polyposis and colon cancer remains to be fully determined.
The PTEN (deleted on chromosome 10) tumor suppressor gene is one of the most frequently mutated/deleted genes in various human cancers (63). Genetic inactivation of Pten specifically in the smooth muscle lineage results in induced mTORC1 activity, leiomyosarcomas, and short life span (64). Here, the rapamycin derivative everolimus substantially decelerated tumor growth and prolonged life span. The critical role for tumor suppressor PTEN is also manifested in human and mouse endometrial hyperplasia (65) showing mTOR inhibitors (everolimus and BEZ235) suppressed elevated mTORC1 activity in PTEN-mutant endometrial cancer cells. Of note, Pten overexpressing mice are long-lived, possibly due to PTEN protection from many sorts of cancers (66).
Mice deficient in the DNA excision-repair gene Ercc1 (Ercc1−/Δ mice, harboring one knockout and one hypomorphic allele of Ercc1) show numerous accelerated aging features that together limit their median life span to 4 months. Interestingly, DR substantially extends the health span and life span of Ercc1−/Δ and Ercc5−/− mice (also known as Xpg−/−, a model of Cockayne syndrome) by preserving genome function (67). However, mTORC1 activity is intriguingly unaffected by DR in liver of Ercc1−/Δ mice. Interestingly, mTORC1 signaling pathways are activated in muscle-derived stem/progenitor cells derived from Ercc1−/Δ mice compared with wild-type muscle-derived stem/progenitor cells (68). Inhibition of mTORC1 activity by rapamycin restored adult stem cell function by promoting autophagy and the myogenic differentiation capacity of the Ercc1−/Δ muscle-derived stem/progenitor cells, accompanied by decreases in apoptotic and senescent cells (68). A survival study was not reported, and studies to compare the effects of rapamycin and DR in Ercc1−/Δ mice would be of interest.
mTORC1 in Mouse Models With Nuclear Mechanical Properties
The Lmna gene encodes lamin A and C, constituents of the nuclear envelope that form intermediate filaments in the nucleus and are involved in the maintenance of nuclear mechanical properties, nuclear-cytoskeletal interactions, and the regulation of gene expression. Over 450 distinct mutations in LMNA in a range of diseases termed laminopathies have been identified (69). Laminopathies include cardiac and skeletal muscle syndromes as well as Hutchinson–Gilford progeria syndrome (HGPS) and related progeria syndromes, and several are associated with aberrant mTOR regulation.
Lamin A/C-deficient (Lmna−/−) mice, which display dilated cardiomyopathy, skeletal muscle dystrophy, neuropathy, and lipodystrophy, die between 6 and 8 weeks of age. Previously, we have reported that dilated cardiomyopathy, skeletal muscle dystrophy, and lipodystrophy are all associated with aberrantly elevated mTORC1 signaling. Consistently, rapamycin-mediated mTORC1 inhibition restores function in affected tissues and robustly enhances survival in Lmna−/− mice (70,71). Rapamycin may largely extend survival in Lmna−/− mice by suppressing S6K1 signaling because genetically knockdown S6K1 (ubiquitously or specifically in muscle) significantly extends the survival of Lmna−/− mice as well (72). In contrast, overexpression 4E-BP1 protein, the other targets suppressed by mTORC1, reduced the survival of Lmna−/− mice. A related finding from another lamin-associated model of dilated cardiomyopathy, LmnaH222P/H222P mice, also demonstrated that temsirolimus, a derivative of the mTORC1 inhibitor rapamycin, reduced mTORC1 activation and prevented cardiac defects by reactivating autophagy (73). Thus, rapamycin or rapamycin-related compounds might be effective treatment for this Lmna-based laminopathies associated with muscle defects.
Knock-in LmnaG609G/G609G mice carry the most common HGPS-associated mutation, p.Gly609Gly (equivalent of p.Gly608Gly in human), which is genetically dominant implying that the allele acts neomorphically or hypermorphically (74). The mutation does not alter coding but rather activates a cryptic splice site that results in enhanced expression of an altered splice form of LMNA, termed progerin (75). Since this mouse model recapitulates most alterations observed in children with HGPS (median life expectancy 13 years), LmnaG609G/G609G mice have been intensely studied and become the model of choice to find the therapeutic interventions for HGPS patients. The importance of mTORC1 in aging and age-related diseases is mostly related to its function as a master regulator of autophagy (2). Given that toxic progerin is the culprit for the pathological dystrophy in HGPS, removing and/or reducing this accumulated protein could be a promising therapeutic target. In support of this hypothesis, one provocative report showed that mTORC1 inhibitor rapamycin reduces progerin protein accumulation in HGPS fibroblast by activating autophagy in vitro (76). Since then, rapamycin has been touted as an autophagy activator for treating in HGPS, age-related disorders, as well as normal aging (77). If aberrant mTORC1 activity is the culprit for the pathological phenotypes, we would expect that mTORC1 suppressor, such as rapamycin, would benefit LmnaG609G/G609G mice. To this point, however, there is no compelling in vivo data showing aberrantly elevated mTORC1 or suppressed autophagy activity in tissues of LmnaG609G/G609G mice. Actually, reduced mTORC1 activity, and probably enhanced autophagy activity, was observed in liver of LmnaG609G/G609G mice (78). Interestingly, the same observations have been reported in HGPS fibroblasts (79). It remains an unanswered question whether administration of mTOR inhibitors or mTOR-independent autophagy activators can rescue the survival of LmnaG609G/G609G mice. Indeed, methionine restriction, one of the interventions that can suppress mTORC1 activity (80), extends the survival of LmnnG609G/G609G mice (78). However, this rescue is probably not mediated through mTORC1 pathway since methionine restriction did not affect intrinsically low mTORC1 activity in the liver of LmnnG609G/G609G mice (78).
HGPS can also be mimicked by knocking out the gene coding for the lamin A processing metalloproteinase ZMPSTE24 enzyme. Genetically engineered mice deficient in ZMPSTE24 (Zmpste24−/− mice), which is responsible for its accurate proteolytic processing, are more likely to accumulate prelamin A, which acts similarly to progerin, leading to dramatic changes in nuclear morphology and function. Zmpste24−/− mice exhibit reduced mTOR activity and an extensive basal activation of autophagy in skeletal muscle, liver, and heart tissues (81). Resveratrol, a potential DR mimetic and indirect mTORC1 suppressor (82), was shown to extend life span in Zmpste24−/− mice by rescuing the decline in bone marrow-derived stem cells (83). Methionine restriction also enhances life span in Zmpste24−/− mice via an unknown mechanism (78). Unlike methionine restriction (78), however, resveratrol did not reduce the growth rate of the mice and instead led to better body weight maintenance.
Of note, metformin is reported to decrease progerin expression in mesenchymal stem cells (84) and dermal fibroblasts (85) from HGPS patients. Together, data from mouse models regarding the use of longevity drugs to ameliorate the phenotypes have been mixed at best, and further studies need to be performed. Nonetheless, a phase I/II monocentric trial (http://clinicaltrials.gov; NCT02579044) of rapamycin derivative everolimus in combination with lonafarnib in HGPS/progeria patients is currently conducted at the Clinical and Translational Study Unit (CTSU) at Boston Children’s Hospital.
mTORC1 in Mouse Models With Metabolic Disorders
Overnutrition induced by high-fat diet feeding is a well-established approach to promote obesity and type 2 diabetes (86) and shortens life span in animals. Genetically diabetic (db/db) mice also have a reduced life span. Rapamycin improves survival in obese male mice under high-fat diet (87). Both during high-fat diet feeding and in db/db mice, mTORC1 activity is elevated. In contrast to high-fat diet–treated mice, however, rapamycin increases mortality in db/db mice (88), in spite of the fact that mTORC1 inhibition improves cardiac function in the same mouse model (89). The discrepancy between these two obese mouse models by rapamycin treatment is still unexplained and warrants further study.
mTORC1 in Mouse Models With Dysregulated Signaling
The circadian clock, an internal time-keeping system, regulates physiological processes through generation of rhythms in gene expression. Disruption of the circadian clock contributes to many pathological conditions, including metabolic syndromes, cardiovascular diseases and inflammation, and deregulation of mTOR signaling is closely linked to circadian clock dysfunction (90). Mice deficient for the core clock-transcriptional protein BMAL1 (Bmal1−/− mice) develop not only a loss of circadian rhythms but also severe premature aging phenotype (91). Furthermore, mTORC1 activity is increased in multiple tissues, such as liver, heart, and brain, of Bmal1−/− mice (92). In line with this mechanism, treatment with rapamycin extended the survival of Bmal1−/− mice (92). DR, in contrast, shortened the life span of Bmal1−/− mice (93). Circadian rhythms decline during the normal aging process, and one area of future research will be to understand the links between altered rhythms and mTOR dysregulation in aging animals.
Previously, we discussed the molecular links between long-lived and short-lived mouse models (9), and Klotho stands out as one of the few genes that can go both ways: deletion of klotho leads to a syndrome resembling accelerated aging, whereas mice overexpressing Klotho are long-lived. Klotho is a transmembrane protein expressed mainly in the distal convoluted tubules in the kidney and the choroid plexus in the brain. Interestingly, secreted Klotho functions as a circulating hormone that binds to a cell-surface receptor and represses intracellular insulin/insulin-like growth factor-1 signals (94). However, the molecular link between mTORC1 activity and Klotho functions has just emerged. Diabetic mice have significantly higher levels of mTORC1 activity in kidneys, and Klotho heterozygosity further enhances kidney mTORC1 activity in this context (95). This may contribute to the exacerbation of diabetic nephropathy in Klotho+/− mice.
The link between mTOR signaling and Klotho function was further strengthened in a rat model of chronic renal failure treated with rapamycin (96). This study reports that mTOR signaling pathway is upregulated in the aortic wall of chronic renal failure rats, partly through exposure to high phosphate levels, and that mTOR activation plays a role in the pathogenesis of medial arterial calcification involving the suppression of Klotho expression in the vasculature. As predicted, oral rapamycin administration significantly reduced vascular calcification in rats with chronic renal failure. Interestingly, the expression of Klotho, which suppresses vascular calcification, was reduced in calcified vasculature and reversed by rapamycin treatment. This effect is blunted in the absence of Klotho in Klotho−/− mice, supporting a requirement of Klotho for the therapeutic potential of mTOR inhibition in this setting. As chronic kidney disease is a state of severe renal and systemic Klotho deficiency, restoration of Klotho, by any means, could be of great value.
Conclusion
It is a general consensus that aging and age-associated diseases that arise from hyperactive mTORC1 signaling may benefit from the application of mTORC1 inhibitors. Here we have briefly touched on a number of animal models that represent a variety of human age-associated diseases and the interventions that bring dysregulated mTORC1 back to balance (Table 1). These findings establish elevated mTOR as a contributor to a wide range of age-related pathologies and point to the need for clinical trials in corresponding human conditions. Moreover, they further the possibility that effective mTOR inhibition may extend human health span and life span.
Funding
This work was funded by the National Institute on Aging (RO1 AG050441 to B.K.K. and 1R56 AG057620-01 to J.Y.L.).
Author Contributions
C.-Y.L. and J.Y.L. both researched the articles. C.-Y.L. wrote the manuscript with input from the coauthors.
Conflict of Interest
None reported.
Acknowledgments
We apologize to those not cited due to space limitations. We sincerely thank Dr. Arjun N. Sasikumar for proofreading of the manuscript.
References
- 1. Kennedy BK, Berger SL, Brunet A, et al. Geroscience: linking aging to chronic disease. Cell. 2014;159:709–713. doi: 10.1016/j.cell.2014.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sabatini DM. Twenty-five years of mTOR: uncovering the link from nutrients to growth. Proc Natl Acad Sci USA. 2017;114:11818–11825. doi: 10.1073/pnas.1716173114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kennedy BK, Lamming DW. The mechanistic target of rapamycin: the grand conducTOR of metabolism and aging. Cell Metab. 2016;23:990–1003. doi: 10.1016/j.cmet.2016.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cummings NE, Lamming DW. Regulation of metabolic health and aging by nutrient-sensitive signaling pathways. Mol Cell Endocrinol. 2017;455:13–22. doi: 10.1016/j.mce.2016.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Parzych KR, Klionsky DJ. An overview of autophagy: morphology, mechanism, and regulation. Antioxid Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Barzilai N, Huffman DM, Muzumdar RH, Bartke A. The critical role of metabolic pathways in aging. Diabetes. 2012;61:1315–1322. doi: 10.2337/db11-1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liao CY, Kennedy BK. Mouse models and aging: longevity and progeria. Curr Top Dev Biol. 2014;109:249–285. doi: 10.1016/B978-0-12-397920-9.00003-2 [DOI] [PubMed] [Google Scholar]
- 10. Folgueras AR, Freitas-Rodríguez S, Velasco G, López-Otín C. Mouse models to disentangle the hallmarks of human aging. Circ Res. 2018;123:905–924. doi: 10.1161/CIRCRESAHA.118.312204 [DOI] [PubMed] [Google Scholar]
- 11. Walters HE, Cox LS. mTORC inhibitors as broad-spectrum therapeutics for age-related diseases. Int J Mol Sci. 2018;19:2325–2357. doi: 10.3390/ijms19082325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson SC, Yanos ME, Kayser EB, et al. mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science. 2013;342:1524–1528. doi: 10.1126/science.1244360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ito TK, Lu C, Khan J, et al. Hepatic S6K1 partially regulates lifespan of mice with mitochondrial complex I deficiency. Front Genet. 2017;8:113. doi: 10.3389/fgene.2017.00113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain IH, Zazzeron L, Goli R, et al. Hypoxia as a therapy for mitochondrial disease. Science. 2016;352:54–61. doi: 10.1126/science.aad9642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brugarolas J, Lei K, Hurley RL, et al. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Johnson SC, Martinez F, Bitto A, et al. mTOR inhibitors may benefit kidney transplant recipients with mitochondrial diseases. Kidney Int. 2019;95:455–466. doi: 10.1016/j.kint.2018.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Siegmund SE, Yang H, Sharma R, et al. Low-dose rapamycin extends lifespan in a mouse model of mtDNA depletion syndrome. Hum Mol Genet. 2017;26:4588–4605. doi: 10.1093/hmg/ddx341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang X, Li L, Chen S, et al. Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy. 2011;7:412–425. doi: 10.4161/auto.7.4.14541 [DOI] [PubMed] [Google Scholar]
- 19. Saxena S, Roselli F, Singh K, et al. Neuroprotection through excitability and mTOR required in ALS motoneurons to delay disease and extend survival. Neuron. 2013;80:80–96. doi: 10.1016/j.neuron.2013.07.027 [DOI] [PubMed] [Google Scholar]
- 20. Hamadeh MJ, Tarnopolsky MA. Transient caloric restriction in early adulthood hastens disease endpoint in male, but not female, Cu/Zn-SOD mutant G93A mice. Muscle Nerve. 2006;34:709–719. doi: 10.1002/mus.20630 [DOI] [PubMed] [Google Scholar]
- 21. Bhattacharya A, Bokov A, Muller FL, et al. Dietary restriction but not rapamycin extends disease onset and survival of the H46R/H48Q mouse model of ALS. Neurobiol Aging. 2012;33:1829–1832. doi: 10.1016/j.neurobiolaging.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 22. Van Cauwenberghe C, Vandendriessche C, Libert C, Vandenbroucke RE. Caloric restriction: beneficial effects on brain aging and Alzheimer’s disease. Mamm Genome. 2016;27:300–319. doi: 10.1007/s00335-016-9647-6 [DOI] [PubMed] [Google Scholar]
- 23. Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120. doi: 10.1074/jbc.M110.100420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Halagappa VK, Guo Z, Pearson M, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019 [DOI] [PubMed] [Google Scholar]
- 25. Caccamo A, De Pinto V, Messina A, Branca C, Oddo S. Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J Neurosci. 2014;34:7988–7998. doi: 10.1523/JNEUROSCI.0777-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Ho L, Qin W, et al. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje [DOI] [PubMed] [Google Scholar]
- 27. Caccamo A, Magrì A, Medina DX, et al. mTOR regulates tau phosphorylation and degradation: implications for Alzheimer’s disease and other tauopathies. Aging Cell. 2013;12:370–380. doi: 10.1111/acel.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Genis I, Gordon I, Sehayek E, Michaelson DM. Phosphorylation of tau in apolipoprotein E-deficient mice. Neurosci Lett. 1995;199:5–8. doi: 10.1016/0304-3940(95)12007-Q [DOI] [PubMed] [Google Scholar]
- 29. Siman R, Cocca R, Dong Y. The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early-stage Alzheimer-type tauopathy. PLoS One. 2015;10:e0142340. doi: 10.1371/journal.pone.0142340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rühlmann C, Wölk T, Blümel T, Stahn L, Vollmar B, Kuhla A. Long-term caloric restriction in ApoE-deficient mice results in neuroprotection via Fgf21-induced AMPK/mTOR pathway. Aging (Albany NY). 2016;8:2777–2789. doi: 10.18632/aging.101086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Elloso MM, Azrolan N, Sehgal SN, et al. Protective effect of the immunosuppressant sirolimus against aortic atherosclerosis in apo E-deficient mice. Am J Transplant. 2003;3:562–569. doi: 10.1034/j.1600-6143.2003.00094.x [DOI] [PubMed] [Google Scholar]
- 32. Eisenberg T, Abdellatif M, Schroeder S, et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat Med. 2016;22:1428–1438. doi: 10.1038/nm.4222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Michiels CF, Kurdi A, Timmermans JP, De Meyer GRY, Martinet W. Spermidine reduces lipid accumulation and necrotic core formation in atherosclerotic plaques via induction of autophagy. Atherosclerosis. 2016;251:319–327. doi: 10.1016/j.atherosclerosis.2016.07.899 [DOI] [PubMed] [Google Scholar]
- 34. Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp Gerontol. 2005;40:774–783. doi: 10.1016/j.exger.2005.05.007 [DOI] [PubMed] [Google Scholar]
- 35. Ma Q, Qiang J, Gu P, Wang Y, Geng Y, Wang M. Age-related autophagy alterations in the brain of senescence accelerated mouse prone 8 (SAMP8) mice. Exp Gerontol. 2011;46:533–541. doi: 10.1016/j.exger.2011.02.006 [DOI] [PubMed] [Google Scholar]
- 36. Wang Y, Ma Q, Ma X, Zhang Z, Liu N, Wang M. Role of mammalian target of rapamycin signaling in autophagy and the neurodegenerative process using a senescence accelerated mouse-prone 8 model. Exp Ther Med. 2017;14:1051–1057. doi: 10.3892/etm.2017.4618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee JH, Tecedor L, Chen YH, et al. Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron. 2015;85:303–315. doi: 10.1016/j.neuron.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Duan W, Guo Z, Jiang H, Ware M, Li XJ, Mattson MP. Dietary restriction normalizes glucose metabolism and BDNF levels, slows disease progression, and increases survival in huntingtin mutant mice. Proc Natl Acad Sci USA. 2003;100:2911–2916. doi: 10.1073/pnas.0536856100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lan AP, Chen J, Zhao Y, Chai Z, Hu Y. mTOR signaling in Parkinson’s disease. Neuromolecular Med. 2017;19:1–10. doi: 10.1007/s12017-016-8417-7 [DOI] [PubMed] [Google Scholar]
- 40. Xu Y, Liu C, Chen S, et al. Activation of AMPK and inactivation of Akt result in suppression of mTOR-mediated S6K1 and 4E-BP1 pathways leading to neuronal cell death in in vitro models of Parkinson’s disease. Cell Signal. 2014;26:1680–1689. doi: 10.1016/j.cellsig.2014.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Santini E, Heiman M, Greengard P, Valjent E, Fisone G. Inhibition of mTOR signaling in Parkinson’s disease prevents L-DOPA-induced dyskinesia. Sci Signal. 2009;2:ra36. doi: 10.1126/scisignal.2000308 [DOI] [PubMed] [Google Scholar]
- 42. Masini D, Bonito-Oliva A, Bertho M, Fisone G. Inhibition of mTORC1 signaling reverts cognitive and affective deficits in a mouse model of Parkinson’s disease. Front Neurol. 2018;9:208. doi: 10.3389/fneur.2018.00208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Troca-Marín JA, Casañas JJ, Benito I, Montesinos ML. The Akt-mTOR pathway in Down’s syndrome: the potential use of rapamycin/rapalogs for treating cognitive deficits. CNS Neurol Disord Drug Targets. 2014;13:34–40. doi: 10.2174/18715273113126660184 [DOI] [PubMed] [Google Scholar]
- 44. Horvath S, Garagnani P, Bacalini MG, et al. Accelerated epigenetic aging in Down syndrome. Aging Cell. 2015;14:491–495. doi: 10.1111/acel.12325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Troca-Marín JA, Alves-Sampaio A, Montesinos ML. An increase in basal BDNF provokes hyperactivation of the Akt-mammalian target of rapamycin pathway and deregulation of local dendritic translation in a mouse model of Down’s syndrome. J Neurosci. 2011;31:9445–9455. doi: 10.1523/JNEUROSCI.0011-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Andrade-Talavera Y, Benito I, Casañas JJ, Rodríguez-Moreno A, Montesinos ML. Rapamycin restores BDNF-LTP and the persistence of long-term memory in a model of Down’s syndrome. Neurobiol Dis. 2015;82:516–525. doi: 10.1016/j.nbd.2015.09.005 [DOI] [PubMed] [Google Scholar]
- 47. Duval N, Vacano GN, Patterson D. Rapamycin treatment ameliorates age-related accumulation of toxic metabolic intermediates in brains of the Ts65Dn mouse model of Down syndrome and aging. Front Aging Neurosci. 2018;10:263. doi: 10.3389/fnagi.2018.00263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Leontieva OV, Novototskaya LR, Paszkiewicz GM, Komarova EA, Gudkov AV, Blagosklonny MV. Dysregulation of the mTOR pathway in p53-deficient mice. Cancer Biol Ther. 2013;14:1182–1188. doi: 10.4161/cbt.26947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Comas M, Toshkov I, Kuropatwinski KK, et al. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53−/− mice by delaying carcinogenesis. Aging (Albany NY). 2012;4:715–722. doi: 10.18632/aging.100496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Komarova EA, Antoch MP, Novototskaya LR, et al. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/− mice. Aging (Albany NY). 2012;4:709–714. doi: 10.18632/aging.100498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Christy B, Demaria M, Campisi J, et al. p53 and rapamycin are additive. Oncotarget. 2015;6:15802–15813. doi: 10.18632/oncotarget.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weindruch R, Walford LR.. The Retardation of Aging and Disease by Dietary Restriction. Springfield, IL: Charles C Thomas Publisher; 1988. [Google Scholar]
- 53. Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci USA. 1994;91:7036–7040. doi: 10.1073/pnas.91.15.7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buzzai M, Jones RG, Amaravadi RK, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–6752. doi: 10.1158/0008-5472.CAN-06-4447 [DOI] [PubMed] [Google Scholar]
- 55. Howell JJ, Hellberg K, Turner M, et al. Metformin inhibits hepatic mTORC1 signaling via dose-dependent mechanisms involving AMPK and the TSC complex. Cell Metab. 2017;25:463–471. doi: 10.1016/j.cmet.2016.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Anisimov VN, Zabezhinski MA, Popovich IG, et al. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol. 2010;176:2092–2097. doi: 10.2353/ajpath.2010.091050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Popovich IG, Anisimov VN, Zabezhinski MA, et al. Lifespan extension and cancer prevention in HER-2/neu transgenic mice treated with low intermittent doses of rapamycin. Cancer Biol Ther. 2014;15:586–592. doi: 10.4161/cbt.28164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hurez V, Dao V, Liu A, et al. Chronic mTOR inhibition in mice with rapamycin alters T, B, myeloid, and innate lymphoid cells and gut flora and prolongs life of immune-deficient mice. Aging Cell. 2015;14:945–956. doi: 10.1111/acel.12380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sharp ZD, Lee WH, Nikitin AY, et al. Minimal effects of dietary restriction on neuroendocrine carcinogenesis in Rb+/− mice. Carcinogenesis. 2003;24:179–183. doi: 10.1093/carcin/24.2.179 [DOI] [PubMed] [Google Scholar]
- 60. Livi CB, Hardman RL, Christy BA, et al. Rapamycin extends life span of Rb1+/− mice by inhibiting neuroendocrine tumors. Aging (Albany NY). 2013;5:100–110. doi: 10.18632/aging.100533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hasty P, Livi CB, Dodds SG, et al. eRapa restores a normal life span in a FAP mouse model. Cancer Prev Res (Phila). 2014;7:169–178. doi: 10.1158/1940-6207.CAPR-13-0299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fujishita T, Aoki K, Lane HA, Aoki M, Taketo MM. Inhibition of the mTORC1 pathway suppresses intestinal polyp formation and reduces mortality in ApcDelta716 mice. Proc Natl Acad Sci USA. 2008;105:13544–13549. doi: 10.1073/pnas.0800041105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stambolic V, Tsao MS, Macpherson D, Suzuki A, Chapman WB, Mak TW. High incidence of breast and endometrial neoplasia resembling human Cowden syndrome in pten+/− mice. Cancer Res. 2000;60:3605–3611. [PubMed] [Google Scholar]
- 64. Hernando E, Charytonowicz E, Dudas ME, et al. The AKT-mTOR pathway plays a critical role in the development of leiomyosarcomas. Nat Med. 2007;13:748–753. doi: 10.1038/nm1560 [DOI] [PubMed] [Google Scholar]
- 65. Bajwa P, Nielsen S, Lombard JM, et al. Overactive mTOR signaling leads to endometrial hyperplasia in aged women and mice. Oncotarget. 2017;8:7265–7275. doi: 10.18632/oncotarget.13919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, et al. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab. 2012;15:382–394. doi: 10.1016/j.cmet.2012.02.001 [DOI] [PubMed] [Google Scholar]
- 67. Vermeij WP, Dollé ME, Reiling E, et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature. 2016;537:427–431. doi: 10.1038/nature19329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Takayama K, Kawakami Y, Lavasani M, et al. mTOR signaling plays a critical role in the defects observed in muscle-derived stem/progenitor cells isolated from a murine model of accelerated aging. J Orthop Res. 2017;35:1375–1382. doi: 10.1002/jor.23409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schreiber KH, Kennedy BK. When lamins go bad: nuclear structure and disease. Cell. 2013;152:1365–1375. doi: 10.1016/j.cell.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liao CY, Anderson SS, Chicoine NH, et al. Rapamycin reverses metabolic deficits in lamin A/C-deficient mice. Cell Rep. 2016;17:2542–2552. doi: 10.1016/j.celrep.2016.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ramos FJ, Chen SC, Garelick MG, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liao CY, Anderson SS, Chicoine NH, et al. Evidence that S6K1, but not 4E-BP1, mediates skeletal muscle pathology associated with loss of A-type lamins. Cell Discov. 2017;3:17039. doi: 10.1038/celldisc.2017.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Choi JC, Muchir A, Wu W, et al. Temsirolimus activates autophagy and ameliorates cardiomyopathy caused by lamin A/C gene mutation. Sci Transl Med. 2012;4:144ra102. doi: 10.1126/scitranslmed.3003875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Osorio FG, Navarro CL, Cadiñanos J, et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med. 2011;3:106ra107. doi: 10.1126/scitranslmed.3002847 [DOI] [PubMed] [Google Scholar]
- 75. Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson–Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cao K, Graziotto JJ, Blair CD, et al. Rapamycin reverses cellular phenotypes and enhances mutant protein clearance in Hutchinson–Gilford progeria syndrome cells. Sci Transl Med. 2011;3:89ra58. doi: 10.1126/scitranslmed.3002346 [DOI] [PubMed] [Google Scholar]
- 77. Graziotto JJ, Cao K, Collins FS, Krainc D. Rapamycin activates autophagy in Hutchinson–Gilford progeria syndrome: implications for normal aging and age-dependent neurodegenerative disorders. Autophagy. 2012;8:147–151. doi: 10.4161/auto.8.1.18331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Bárcena C, Quirós PM, Durand S, et al. Methionine restriction extends lifespan in progeroid mice and alters lipid and bile acid metabolism. Cell Rep. 2018;24:2392–2403. doi: 10.1016/j.celrep.2018.07.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Evangelisti C, Cenni V, Lattanzi G. Potential therapeutic effects of the MTOR inhibitors for preventing ageing and progeria-related disorders. Br J Clin Pharmacol. 2016;82:1229–1244. doi: 10.1111/bcp.12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lees EK, Banks R, Cook C, et al. Direct comparison of methionine restriction with leucine restriction on the metabolic health of C57BL/6J mice. Sci Rep. 2017;7:9977. doi: 10.1038/s41598-017-10381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Mariño G, Ugalde AP, Salvador-Montoliu N, et al. Premature aging in mice activates a systemic metabolic response involving autophagy induction. Hum Mol Genet. 2008;17:2196–2211. doi: 10.1093/hmg/ddn120 [DOI] [PubMed] [Google Scholar]
- 82. Widlund AL, Baur JA, Vang O. mTOR: more targets of resveratrol? Expert Rev Mol Med. 2013;15:e10. doi: 10.1017/erm.2013.11 [DOI] [PubMed] [Google Scholar]
- 83. Liu B, Ghosh S, Yang X, et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012;16:738–750. doi: 10.1016/j.cmet.2012.11.007 [DOI] [PubMed] [Google Scholar]
- 84. Egesipe AL, Blondel S, Cicero AL, et al. Metformin decreases progerin expression and alleviates pathological defects of Hutchinson–Gilford progeria syndrome cells. Aging Mech Dis. 2016;2:16026. doi: 10.1038/npjamd.2016.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Park SK, Shin OS. Metformin alleviates ageing cellular phenotypes in Hutchinson-Gilford progeria syndrome dermal fibroblasts. Exp Dermatol. 2017;26:889–895. doi: 10.1111/exd.13323 [DOI] [PubMed] [Google Scholar]
- 86. Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53 (Suppl 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.S215 [DOI] [PubMed] [Google Scholar]
- 87. Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell. 2014;13:616–622. doi: 10.1111/acel.12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sataranatarajan K, Ikeno Y, Bokov A, et al. Rapamycin increases mortality in db/db mice, a mouse model of type 2 diabetes. J Gerontol A Biol Sci Med Sci. 2016;71:850–857. doi: 10.1093/gerona/glv170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289:4145–4160. doi: 10.1074/jbc.M113.521062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cao R. mTOR signaling, translational control, and the Circadian clock. Front Genet. 2018;9:367. doi: 10.3389/fgene.2018.00367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Khapre RV, Kondratova AA, Patel S, et al. BMAL1-dependent regulation of the mTOR signaling pathway delays aging. Aging (Albany NY). 2014;6:48–57. doi: 10.18632/aging.100633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Patel SA, Chaudhari A, Gupta R, Velingkaar N, Kondratov RV. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 2016;30:1634–1642. doi: 10.1096/fj.15-282475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kuro-o M. Klotho and the aging process. Korean J Intern Med. 2011;26:113–122. doi: 10.3904/kjim.2011.26.2.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lin Y, Kuro-o M, Sun Z. Genetic deficiency of anti-aging gene klotho exacerbates early nephropathy in STZ-induced diabetes in male mice. Endocrinology. 2013;154:3855–3863. doi: 10.1210/en.2013-1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhao Y, Zhao MM, Cai Y, et al. Mammalian target of rapamycin signaling inhibition ameliorates vascular calcification via Klotho upregulation. Kidney Int. 2015;88:711–721. doi: 10.1038/ki.2015.160 [DOI] [PubMed] [Google Scholar]