Abstract
Background
To investigate trends over age by comorbidity status for the risk of limitations in individual activities of daily living for community-living older persons.
Methods
A longitudinal population-based study was conducted in 9,319 community-living Dutch persons aged 60 years and older. Self-reported multiple chronic conditions (MCC) and nine instrumental activities of daily livings (IADLs) were assessed in 15 studies of the Dutch National Care for the Elderly Program (TOPICS-MDS). Risks of limitations in IADLs, odds ratios (per 5 years), and rate ratios (per 5 years) were calculated with mixed logistic and negative binomial regression models with age as the underlying timescale, stratified by number of MCC (no, 1–2, ≥ 3 MCC), and corrected for confounders.
Results
At inclusion, the number of IADL limitations was highest for the “≥3 MCC” group (2.00 interquartile range [1.00–4.00]) and equal for “no MCC” or “1–2 MCC” (1.00 interquartile range [0.00–2.00]). Trends of individual IADLs depicted a higher risk in IADL limitation with increasing age over 2 years of follow-up, except for handling finances for the “no MCC” group. The longitudinal age effect on IADL limitations varied subject to MCC, being strongest for the “no MCC” group for most IADLs; grooming and telephone use were almost unaltered by age and MCC.
Conclusion
We observed a decline in IADL functioning with increasing age over 2 years of follow-up in persons with and without MCC. The impact of MCC on IADL decline varied per IADL activity.
Keywords: Trends, Multimorbidity, Aging, Community-living, Seniors
In an aging population and because of advances in health care, more individuals will live longer in the presence of chronic conditions. The prevalence of multiple chronic conditions (MCC) varies between 55% and 98% dependent on the definition and study population (1–6). The consequences and impact of MCC on individuals are significant as it has been associated with adverse health outcomes, lower quality of life, functional decline, increased health care utilization, and mortality (3,4,6). The co-occurrence of two or more chronic medical conditions, that is multimorbidity, is common among seniors, and is expected to rise because of the increase in life expectancy (1–3,7).
Living independently in the community and being engaged in daily life activities has been rated the most important priority among older adults (8). Instrumental activities of daily living (IADL) such as traveling, grocery shopping, financial handling, medication management, and walking are complex integrative measures of functioning and essential for a good quality of life and independency (9,10). Recent studies suggest a deterioration in IADL activities with age in the presence of MCC (5,11,12). Functional trajectories have been studied to describe this complex process of decline by measuring changes in functional status at different time points (13,14). By studying trajectories of decline, the best time to prevent long-term disability among community-living seniors may be identified. However, in most studies IADL functioning was either scored as present or absent, or the number of activities unable to perform was considered (5,11,12). Despite the importance of the individual domains of IADL functioning very little is known on the effects of MCC on difficulty with individual IADL activities. So far, limitations dependent on age in the individual IADLs in senior adults with MCC are unknown. Insight at activity level will help to generate insight in individual and societal impact of these losses and subsequent development and implementation of preventive strategies. Therefore, the aim of this study is to investigate trends over age by comorbidity status for the risk of individual IADL limitations for community-living older persons. In addition, summarized scores of IADL limitations will be presented.
Methods
Design and Setting
Data of the Dutch “The Older Persons and Informal Caregivers Survey–Minimum Dataset” (TOPICS-MDS), a dynamic public access database of 42 research studies conducted in the Netherlands, was used (15). These studies made use of the same self-report questionnaire, the TOPICS-MDS. Detailed information on the TOPIC-MDS has been described elsewhere (16).
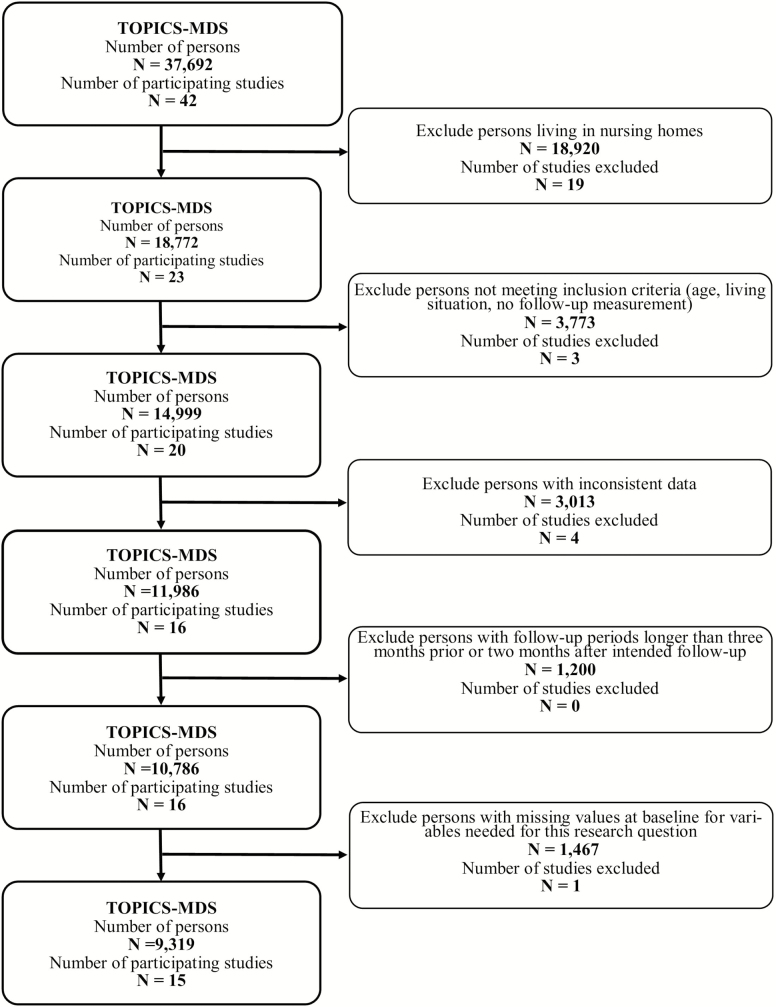
For this study, studies targeted at noninstitutionalized persons living independently (ie, independently or independently living with others) in the community with data of participants with at least one follow-up assessment at either 6, 12, 18, or 24 months were included. These studies (data collection between December 2009 and May 2014) included persons of different birth cohorts with and without frailty based on individual study inclusion criteria (see Supplementary Table 1). Frailty was based on the accumulation of deficits in health (ie, symptoms, morbidities, and/or functional limitations) according to TOPICS-MDS protocol (15). Participants were eligible if they were 60 years of age or older and had complete data at inclusion on all relevant variables. This resulted in a final data set (derived January 2016) consisting of 15 studies and data of 9,319 older persons (Figure 1 and Supplementary Table 1).
Figure 1.
Flow of persons and study inclusion. N = number; TOPICS-MDS = The Older Persons and Informal Caregivers Survey–Minimum Dataset.
Ethical Considerations
Ethical approval for the fully anonymized data set was integrated in the individual studies within the data set. Studies using TOPICS-MDS data were exempted from ethical review (Radboud University Medical Center Ethical Committee review reference number CMO: 2012/120).
Measurements
Demographics and determinants
Demographic information on age, gender, marital status, education, and socioeconomic status was obtained with self-report questionnaires at inclusion. For details, see Supplementary Material.
Chronic conditions were also assessed with self-report questionnaires. At inclusion, participants were asked: “Place a tick next to the illnesses and conditions that you have at the moment or have had in the past 12 months. You can select more than one answer”: diabetes, stroke (brain hemorrhage, cerebral infarction, or transient ischemic attack), heart failure, cancer, pulmonary disease (asthma, chronic bronchitis, pulmonary emphysema, chronic obstructive pulmonary disease), involuntary urinary loss, joint damage of hips or knees, osteoporosis (females only), hip fracture, fractures other than hip, dizziness with falling, prostatism (males only), depression, anxiety or panic disorder, dementia, hearing problems, and vision problems (16). All items were scored with two answer categories “absent” (0) and “present” (1). Subsequently, the number of self-reported chronic conditions was calculated for each participant (range 0–15), and categorized into three MCC-groups: “no MCC” (0), “1–2 MCC” (1), and “≥3 MCC” (2).
Outcome
Ability to perform IADL: At inclusion and follow-up, self-reported activities of daily living (ADL) and IADL was assessed with the modified KATZ-15 ADL and IADL questionnaire (17). Nine IADL activities were assessed: grooming, handling finances, household tasks, preparation of a meal, taking medications as prescribed, grocery shopping, using the telephone, traveling, and walking. Participants were asked “Do you need help with [activity]?” Each activity had two answer categories: “no” (0) and “yes” (1) (17).
Statistical Analysis
Characteristics of the participants were reported as frequencies and percentages for categorical variables and means and standard deviations (SDs) or medians and interquartile ranges (IQRs) for continuous variables where applicable.
TOPICS-MDS is a pooled data set of individual-level information incorporating data of studies with different study designs and sampling frameworks. For our analysis, a one-stage analysis approach for the analysis of individual patient data was used (18,19). Mixed logistic regression models for dichotomous outcomes and mixed negative binomial models for the total IADL score were used to estimate the association between age and each MCC group separately and each of the IADL activities. To account for differences between studies, a random intercept was included, and a residual (ie, GEE type) covariance matrix to account for dependencies because of the repeated measurements (20,21). The models were fitted on an age scale (per 5 years), corrected for determinants reported to be associated with disability, such as gender, education, socioeconomic status, marital status at inclusion, and living situation at inclusion (22). Furthermore, predicted probabilities for the individual IADLs were estimated for ages 60, 65, 70, 75, 80, 85, 90, 95, and 100 years and each MCC group separately. Results of these analyses were reported as odds ratios (ORs; with 95% confidence intervals [CIs]) for binary outcomes and rate ratios (RRs; with 95% CIs) for score outcomes. The ORs are from the coefficients of the interaction between time-varying age variable and a dummy variable generated from the chronic condition variable. For details, see Supplementary Material. To produce the graphical presentation of the trends, predicted probabilities of the mixed logistic regression models and the number of IADLs from the mixed negative binomial regression were calculated and plotted against age. We restricted ourselves to the graphical presentation per MCC group and refrained from calculating p values comparing the trends of the different MCC groups because of constraints of the statistical models used (see Supplementary Material, Interpretation of Results section).
Statistical significance levels were set at .05 for all analyses. All analyses were performed using the statistical program IBM SPSS Statistics for Windows (version 24.0; IBM Corp.) and SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
Results
Most of the 9,319 participants reported three or more MCC (n = 4,690, 49.5%) whereas 1,044 (11.2%) participants reported no MCC. With increasing age, a higher number of chronic conditions was reported (see Table 1). The number of reported limitations in IADL functioning was the highest for participants with “≥3 MCC” (2.00 IQR [1.00–4.00]) and equal for those with “no MCC” or “1–2 MCC” (1.00 IQR [0.00–2.00]).
Table 1.
Characteristics of Participants at Inclusion Stratified by Chronic Conditions
| All Participants (N = 9,319) | No Chronic Conditions (n = 1,044) | 1 or 2 Chronic Conditions (n = 3,666) | ≥3 Chronic Conditions (n = 4,609) | |
|---|---|---|---|---|
| Age (mean ± SD) | 78.58 ± 6.57 | 76,48 ± 5.64 | 77,85 ± 6.37 | 79,64 ± 6.73 |
| Gender, N (%) | ||||
| Male | 3,802 (40.8) | 440 (42.1) | 1,669 (45.5) | 1,693 (36.7) |
| Female | 5,517 (59.2) | 606 (57.9) | 1,997 (54.5) | 2,916 (63.3) |
| Education, N (%) | ||||
| Low* | 2,993 (32.1) | 289 (25.8) | 1,103 (30.0) | 1,621 (35.2) |
| Medium† | 5,301 (56.9) | 643 (61.6) | 2,088 (57.0) | 2,570 (55.8) |
| High‡ | 1,025 (11.0) | 132 (12.6) | 475 (13.0) | 418 (9.1) |
| Marital status, N (%) | ||||
| Married | 4,675 (50.2) | 597 (57.2) | 2,012 (54.9) | 2,066 (44.8) |
| Divorced | 609 (6.5) | 62 (5.9) | 186 (5.1) | 361 (7.8) |
| Widow/widower/partner deceased | 3,403 (36.5) | 319 (30.6) | 1,206 (32.9) | 1,878 (40.7) |
| Single | 475 (5.1) | 47 (4.5) | 195 (5.3) | 233 (5.1) |
| Sustainable living/unmarried | 157 (1.7) | 19 (1.8) | 67 (1.8) | 71 (1.5) |
| Living situation, N (%) | ||||
| Independent alone | 4,405 (47.3) | 411 (39.4) | 1,560 (42.6) | 2,434 (52.8) |
| Independent with others | 4,914 (52.7) | 633 (60.6) | 2,106 (57.4) | 2,175 (47.2) |
| Socioeconomic status 2010 (mean ± SD) | 0.07 ± 1.01 | 0.11 ± 1.01 | 0.10 ± 1.03 | 0.02 ± 1.00 |
| Chronic conditions (median ± IQR) | 2.00 [1.00–3.00] | 0.00 [0.00–0.00] | 2.00 [1.00–2.00] | 2.00 [2.00–4.00] |
| Frailty§ [0.00–35.6] (median [IQR]) | 8.15 [4.95–12.6] | 3.00 [1.80–4.90] | 5.75 [4.00–8.40] | 11.75 [8.50–15.40] |
| Number of IADL limitations [total 0–9] (median [IQR]) | 1.00 [0.00–3.00] | 1.00 [0.00–2.00] | 1.00 [0.00–2.00] | 2.00 [1.00–4.00] |
| Difficulties grooming YES, N (%) | 215 (2.3) | 10 (1.0) | 53 (1.4) | 152 (3.3) |
| Difficulties handling finances YES, N (%) | 2,120 (22.7) | 360 (34.5) | 866 (23.6) | 894 (19.4) |
| Difficulties with household tasks YES, N (%) | 5,022 (53.9) | 291 (27.9) | 1,568 (42.8) | 3,163 (68.6) |
| Difficulties preparing a meal YES, N (%) | 1,724 (18.5) | 103 (9.9) | 547 (14.9) | 1,074 (23.3) |
| Difficulties handling medication YES, N (%) | 764 (8.2) | 40 (3.8) | 223 (6.1) | 501 (10.9) |
| Difficulties with shopping YES, N (%) | 2,490 (26.7) | 103 (9.9) | 697 (19.0) | 1,690 (36.7) |
| Difficulties using phone YES, N (%) | 379 (4.1) | 23 (2.2) | 108 (2.9) | 248 (5.4) |
| Difficulties with traveling YES, N (%) | 2,830 (30.4) | 146 (14.0) | 780 (21.3) | 1,904 (41.3) |
| Difficulties with walking YES, N (%) | 2,614 (28.1) | 121 (11.6) | 735 (20.0) | 1,758 (38.1) |
Notes: IADL = instrumental activities of daily living; IQR = interquartile range; N = number.
*Less than six classes of primary school, six primary school classes, more than primary school/primary school with uncompleted further education.
†Practical training, Secondary vocational education.
‡Preuniversity education, university/higher professional education.
§Operational definitions for frailty differed across studies: doi:10.1371/journal.pone.0081673
Trends of Risk of IADL Limitations
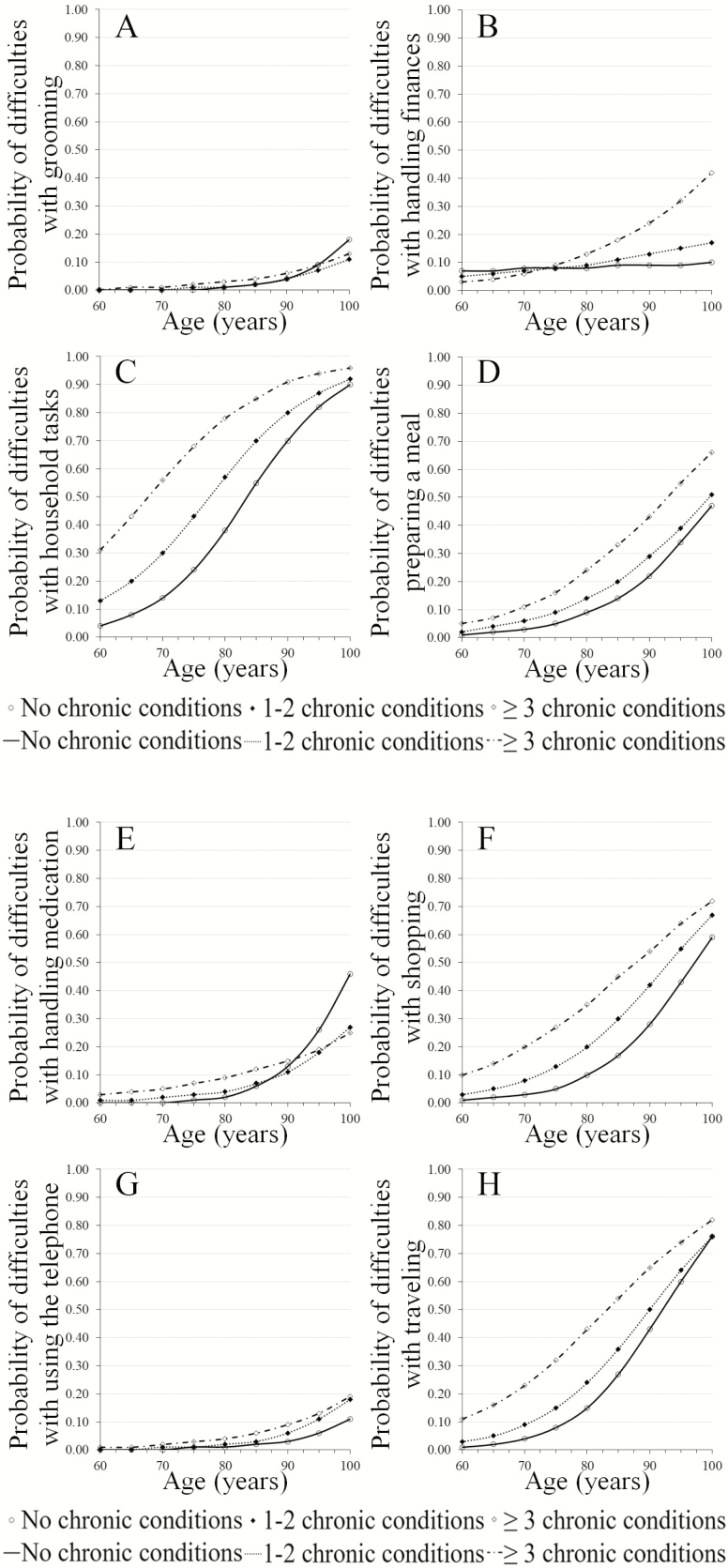
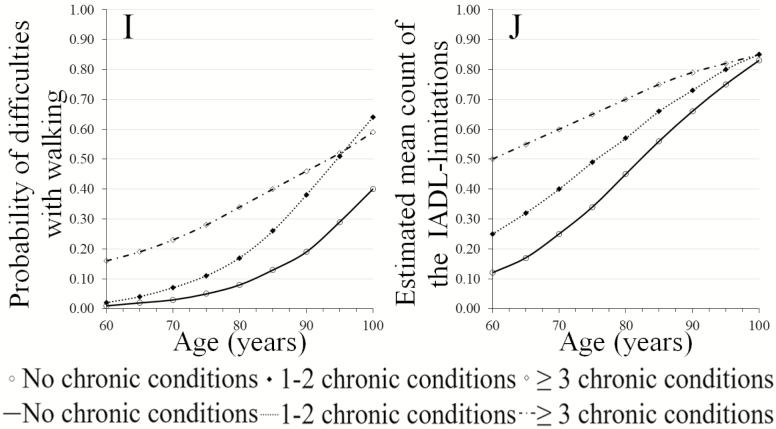
Supplementary Table 2 provides an overview of the number of participants in the analysis per IADL across MCC groups per time of measurement. The duration of follow-up varied between studies with a minimum of 6 months to a maximum of 2 years. The number of measurements varied between two and five follow-up measurements, see Supplementary Table 1 for details. Figure 2A–J show the trends in risk of IADL limitations by the number of chronic conditions over ages 60–100 years as observed over a 2-year timeframe.
Figure 2.
(A–I) Limitations in individual instrumental activities of daily living (IADL) by the number of chronic conditions over ages 60–100 years as observed over a 2-year timeframe. (J) Limitations in the number of IADL by the number of chronic conditions over ages 60–100 years as observed over a 2-year timeframe.
Trends of the individual IADLs depict a higher risk in IADL limitation with age with the exception of handling finances; participants with “no MCC” did not have a higher risk of decline in handling finances over the entire age range (Figure 2B) and those with “≥3 MCC” showed a higher pace of decline after age 75 years. Furthermore, difficulties with grooming (Figure 2A) and using the telephone (Figure 2G) appear to be almost unaltered in all MCC groups up to age 85 years, with more than 80% capable of performing these activities at age 100 years.
IADLs with the highest risk of decline over the entire age range and all for MCC groups were limitations in household tasks (Figure 2C) and traveling (Figure 2H), followed by limitations in shopping (Figure 2F) and preparing a meal (Figure 2D).
A higher risk of decline was observed for those with increased number of chronic conditions for six IADL activities; limitations in grooming (Figure 2A) and handling medications (Figure 2E) appear to have a relatively small decline for all MCC groups up to the age of 85 years. Thereafter, a higher risk of decline was observed for “no MCC” compared to “1–2 MCC” or “≥3 MCC.” Concerning limitations in walking (Figure 2I), participants with “1–2 MCC” appear to have a higher risk of decline after age 75 years compared to those with “no MCC” or “≥3 MCC.”
Trends in the total number of IADL limitations (Figure 2J) show a higher risk in the number of IADL limitations at younger-old age for those of with “≥3 MCC.” The change in risk for acquiring more IADL limitations is higher for those with “1–2 MCC” or “no MCC” compared to those with “≥3 MCC” indicating an accelerated transition into disability for the “1–2 MCC” or “no MCC” groups.
Age Effect on Individual IADL Limitations Across Groups of MCC
Table 2 shows the effect of the interaction between age (per 5 year) and chronic conditions for each group of chronic conditions. The presented ORs and RRs represent the estimated effect of age on the odds of limitations per 5 years increase in age (centered at 60 years) as observed over a 2-year timeframe.
Table 2.
Estimated Odds Ratios and Rate Ratios for IADL Limitations Associated With 5 Year Increase of Age*, for Each Group of Chronic Conditions
| Chronic Conditions | OR† | CI | |
|---|---|---|---|
| Grooming‡ | No chronic conditions | 2.26 | (1.55; 3.29) |
| 1–2 chronic conditions | 1.80 | (1.54; 2.11) | |
| ≥3 chronic conditions | 1.54 | (1.40; 1.70) | |
| Managing finances‡ | No chronic conditions | 1.04 | (0.92; 1.17) |
| 1–2 chronic conditions | 1.19 | (1.12; 1.27) | |
| ≥3 chronic conditions | 1.49 | (1.42; 1.55) | |
| Household tasks‡ | No chronic conditions | 1.94 | (1.74; 2.17) |
| 1–2 chronic conditions | 1.74 | (1.66; 1.82) | |
| ≥3 chronic conditions | 1.66 | (1.60; 1.72) | |
| Preparing a meal‡ | No chronic conditions | 1.77 | (1.53; 2.04) |
| 1–2 chronic conditions | 1.60 | (1.51; 1.69) | |
| ≥3 chronic conditions | 1.58 | (1.52; 1.64) | |
| Handling medication‡ | No chronic conditions | 2.41 | (1.92; 3.04) |
| 1–2 chronic conditions | 1.70 | (1.56; 1.86) | |
| ≥3 chronic conditions | 1.36 | (1.30; 1.44) | |
| Grocery shopping‡ | No chronic conditions | 1.91 | (1.65; 2.21) |
| 1–2 chronic conditions | 1.70 | (1.61; 1.79) | |
| ≥3 chronic conditions | 1.48 | (1.43; 1.54) | |
| Using the telephone‡ | No chronic conditions | 1.88 | (1.38; 2.55) |
| 1–2 chronic conditions | 1.84 | (1.63; 2.06) | |
| ≥3 chronic conditions | 1.49 | (1.38; 1.61) | |
| Traveling‡ | No chronic conditions | 2.05 | (1.80; 2.34) |
| 1–2 chronic conditions | 1.78 | (1.69; 1.88) | |
| ≥3 chronic conditions | 1.57 | (1.52; 1.63) | |
| Walking‡ | No chronic conditions | 1.67 | (1.46; 1.92) |
| 1–2 chronic conditions | 1.71 | (1.62; 1.81) | |
| ≥3 chronic conditions | 1.29 | (1.24; 1.34) | |
| Chronic Conditions | RR† | CI | |
| Number of IADL limitations (0–9)‡ | No chronic conditions | 1.56 | (1.37; 1.77) |
| 1–2 chronic conditions | 1.42 | (1.35; 1.51) | |
| ≥3 chronic conditions | 1.25 | (1.19; 1.30) |
Notes: CI = confidence interval; OR = odds ratio between age (per 5 years) and the interaction with each group of multiple chronic conditions; RR = rate ratio between age (per 5 years) and the interaction with each group of multiple chronic conditions.
*Age was centered at 60 years (ie, subtract 60 from each patients age) and divided by 5. Centering was used to obtain corrected risks at age 60 years; the purpose of the division was to obtain an increase in risk (odds ratio) per 5 years to enhance interpretation.
†Adjusted for gender, education, socioeconomic status, marital status at inclusion, and living situation at inclusion.
‡No p values comparing multiple chronic condition (MCC) groups were produced because of the constraints of models used. Age effects may be compared by interpreting confidence intervals, with more overlap of intervals indicating less distinctive effects.
The age effect on difficulties handling finances was higher for groups with MCC compared to the group without MCC: “≥3 MCC” (OR = 1.49 [95% CI 1.42; 1.55]), “1–2 MCC” (OR = 1.19 [95% CI 1.12; 1.27]) versus “no MCC” (OR = 1.04 [95% CI 0.92; 1.17]).
Concerning difficulties in handling medication, the age effect was present for all MCC groups, however the effect was strongest for those with “no MCC”: “no MCC” (OR = 2.41 [1.92; 3.04]) versus “1–2 MCC” (OR = 1.70 [1.56; 1.86]) versus “≥3 MCC” (OR = 1.36 [1.30; 1.44]).
The age effect on difficulties with household tasks, traveling, shopping, preparing a meal, grooming, and telephone use was highest for “no MCC” and lowest for “≥3 MCC” (see Table 2). Point estimates of “1–2 MCC” lay between these groups.
The age effect on difficulties walking was highest for “1–2 MCC” (OR = 1.71 [95% CI 1.62; 1.81]) and lowest for “≥3 MCC” (OR = 1.29 [1.24; 1.34]). Point estimates of “no MCC” lay between these groups. Concerning the limitations in the total number of IADLs, the age effect for “no MCC,” “1–2 MCC,” and “≥3 MCC” were RR = 1.56 [1.37; 1.77], RR = 1.42 [1.35; 1.51], and RR = 1.25 [1.19; 1.30], respectively.
Discussion
The aim of this study was to investigate trends of age by comorbidity status for the risk of activity-specific IADL decline for community-living older persons.
We observed that older persons with more self-reported chronic conditions at inclusion had higher levels of self-reported functional limitations at inclusion. The age effect on limitations in IADL functioning was highest for those with “no MCC” at inclusion and lowest for those with “≥3 MCC” at inclusion, except for difficulties in handling finances and walking. Using the telephone and grooming appear to be least effected by MCC and age; whereas difficulties with household tasks showed the highest risk of decline throughout the entire age range with or without MCC. The latter may be the effect of the Dutch Health Care System and Social Support Act that offers financial support for housekeeping service to persons if needed (23), whereas telephone use may be facilitated by recent technological advances.
The higher age effect of decline in functioning in almost all IADL activities in those with “no MCC” compared to those with “≥3 MCC” was unexpected. We hypothesize that older adults with MCC at younger-old age were perhaps more able to adapt to the situation by various coping strategies and acceptance, which has been described in the literature (24–26). Whereas persons with new onset of IADL difficulty at older age may be troubled by the new situation. This hypothesis is supported by other studies suggesting that older persons with multimorbidity apply different coping strategies (24–26) depending on available resources, like anticipating more chronic conditions or disability and prepare for it, or acceptance of the situation to manage daily functioning (24–26). Furthermore, adaptation of their daily routine to levels in accordance with their potentials in light of multimorbidity may play a role (25,26).
In line with other studies, age was identified as a major risk factor for a decline in IADL functioning (27–29). Even though we noticed a more prominent age effect on the decline in several IADL activities for persons with “no MCC” at inclusion, we were able to confirm the findings of other studies that multimorbidity imposes a risk for decline in IADL functioning (5,7,28,29). The trends illustrated that persons with “≥3 MCC” and “1–2 MCC” had higher levels of disability at inclusion that progressed over time with higher risks of disability in late life for the majority of activities.
In our study, the observed risks of difficulties in handling finances and medication were lower over the entire age range than the age-related risks reported in other studies (14). Moreover, the ability to handle finances was unaltered over age in those that reported no MCC.
Some methodological aspects need to be considered to interpret our results. First, we used self-reported measures to assess the number of chronic conditions and IADL functioning. However, a recent study on the comparison of patient reported comorbidities and those retrieved from medical records provided similar information in patients between 50 and 80 years of age (30). Second, we had no information on the severity of or changes in health status or MCC. Furthermore, nuances in daily functioning and health status could not be accounted for by limiting answer categories to yes and no, lacking discriminative ability (31). This may have led to an underestimation of trends for persons with severe disease status or change in MCC status. Third, the selection of chronic conditions and categorization may have influenced our results as a selection of 16 conditions were assessed. These conditions, including physical and mental health status, were considered as having equal impact on functioning whereas other studies considered between 9 and 40 different conditions or examined particular patterns of diseases (1–7,11). Fourth, the heterogeneity within TOPICS-MDS as a pooled data set of different studies varying in population size and reporting level may be of concern. To address this issue, statistical strategies frequently used for the analysis of individual patient data from various data sources were applied and random effects by research project were used to account for differences between studies (18–21). Yet, differences in results due to choice of statistical models and modeling approaches have been described (18,19). Fifth, survival and selection bias may have let to biased estimates. Participants with, for example, dementia or language barriers may have refrained from study participation. Follow-up data may have been unavailable due to study dropout after death or change in living situation due to health problems. Even though we used statistical models robust for missing-at-random and included age and comorbidity as prognostic factors for deteriorating health, future comparison with other studies are needed to provide insight in any bias (20). Sixth, restriction of data collection to 2 years did not allow a distinction of effects between birth cohorts. Seventh, we restricted our results to graphical presentations of the MCC groups as constraints of the statistical models, that is, unavailability of likelihood ratio tests for mixed models, prohibited statistical comparison between MCC groups. The strengths of our study are the investigation of the longitudinal impact of MCC combined with the long-term impact of age on individual IADL activities. Moreover, the broad age range of 60–100 years and the large number of community-living older adults with and without frailty included in the study enable an in-depth understanding of the aging process in this population. The evaluation of IADL functioning at activity-level is the most important contribution to current research as it provides new insight into the activity-specific decline. It clearly displayed the necessity for a closer look at the individual activity to successfully develop and implement patient-tailored interventions.
Our findings suggest that functional ability assessment should be an integral part of a geriatric assessment and a subsequent management plan instead of focusing on the single disease. Our findings are useful for clinical practitioners in counseling patients, family members, and social workers in the planning of late life needs. In line with other studies, the aging trends confine the age of 75 years as a boundary of IADL functioning (27). In light of our results, multidisciplinary teams of physicians, nurses, and social workers may be needed to provide practical advice to older persons and family members, especially to those persons aged 75 years or older. Special attention should be paid to those who may be diagnosed with their first chronic disease at older age. Reablement by regaining skills, building a social network, and education may lower the risk of decline or postpone age of progression (32,33).
Our results may be valuable to health policy makers and guideline developers. With older persons living in the community longer, budgeting financial resources for health care and support of older persons needing assistance is a challenge especially in light of demographic developments (33,34). The results of our study may contribute to tailored recommendations for the development and implementation of preventatives strategies for individuals as well as societal support planning.
Conclusion
We observed a decline in IADL functioning with increasing age in older persons with and without MCC. The impact of MCC on functional decline varied per individual IADL activity. With the exception of handling finances and walking, the age effect on the decline in IADL functioning was highest in the group with “no MCC” at inclusion. Furthermore, using the telephone and grooming were almost unaltered by age and MCC. Future longitudinal research should include severity of disease, as well as context and coping strategies and IADL functioning.
Funding
This work was supported by ZonMW, Organization for Health Research and Development, the Netherlands (grant number 60-63300-98-406).
Conflict of interest statement
None reported.
Supplementary Material
Acknowledgments
We thank all TOPICS-MDS Consortium members and all participants of the individual research studies contributing to the Dutch National Care for the Elderly Programme for their contribution this research. We particularly acknowledge the work of Noor Heim, PhD, in preparing the data.
References
- 1. Marengoni A, Angleman S, Melis R, et al. Aging with multimorbidity: a systematic review of the literature. Ageing Res Rev. 2011;10:430–439. doi: 10.1016/j.arr.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 2. Fortin M, Stewart M, Poitras ME, Almirall J, Maddocks H. A systematic review of prevalence studies on multimorbidity: toward a more uniform methodology. Ann Fam Med. 2012;10:142–151. doi: 10.1370/afm.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nunes BP, Flores TR, Mielke GI, Thumé E, Facchini LA. Multimorbidity and mortality in older adults: a systematic review and meta-analysis. Arch Gerontol Geriatr. 2016;67:130–138. doi: 10.1016/j.archger.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 4. Pefoyo AJ, Bronskill SE, Gruneir A, et al. The increasing burden and complexity of multimorbidity. BMC Public Health. 2015;15:415. doi: 10.1186/s12889-015-1733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bahat G, Tufan F, Bahat Z, et al. Comorbidities, polypharmacy, functionality and nutritional status in Turkish community-dwelling female elderly. Aging Clin Exp Res. 2014;26:255–259. doi: 10.1007/s40520-014-0229-8 [DOI] [PubMed] [Google Scholar]
- 6. Palladino R, Tayu Lee J, Ashworth M, Triassi M, Millett C. Associations between multimorbidity, healthcare utilisation and health status: evidence from 16 European countries. Age Ageing. 2016;45:431–435. doi: 10.1093/ageing/afw044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380:37–43. doi: 10.1016/S0140-6736(12)60240-2 [DOI] [PubMed] [Google Scholar]
- 8. Fried TR, Tinetti ME, Iannone L, O’Leary JR, Towle V, Van Ness PH. Health outcome prioritization as a tool for decision making among older persons with multiple chronic conditions. Arch Intern Med. 2011;171:1854–1856. doi: 10.1001/archinternmed.2011.424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Millán-Calenti JC, Tubío J, Pita-Fernández S, et al. Prevalence of functional disability in activities of daily living (ADL), instrumental activities of daily living (IADL) and associated factors, as predictors of morbidity and mortality. Arch Gerontol Geriatr. 2010;50:306–310. doi: 10.1016/j.archger.2009.04.017 [DOI] [PubMed] [Google Scholar]
- 10. Duda B, Puente AN, Miller LS. Cognitive reserve moderates relation between global cognition and functional status in older adults. J Clin Exp Neuropsychol. 2014;36:368–378. doi: 10.1080/13803395.2014.892916 [DOI] [PubMed] [Google Scholar]
- 11. Jackson CA, Jones M, Tooth L, Mishra GD, Byles J, Dobson A. Multimorbidity patterns are differentially associated with functional ability and decline in a longitudinal cohort of older women. Age Ageing. 2015;44:810–816. doi: 10.1093/ageing/afv095 [DOI] [PubMed] [Google Scholar]
- 12. Lin SF, Beck AN, Finch BK. The dynamic contribution of chronic conditions to temporal trends in disability among U.S. adults. Disabil Health J. 2016;9:332–340. doi: 10.1016/j.dhjo.2015.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Covinsky KE, Palmer RM, Fortinsky RH, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51:451–458. [DOI] [PubMed] [Google Scholar]
- 14. Bleijenberg N, Smith AK, Lee SJ, Cenzer IS, Boscardin JW, Covinsky KE. Difficulty managing medications and finances in older adults: a 10-year cohort study. J Am Geriatr Soc. 2017;65:1455–1461. doi: 10.1111/jgs.14819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lutomski JE, Baars MA, Schalk BW, et al. ; TOPICS-MDS Consortium. The development of the Older Persons and Informal Caregivers Survey Minimum DataSet (TOPICS-MDS): a large-scale data sharing initiative. PLoS One. 2013;8:e81673. doi: 10.1371/journal.pone.0081673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lutomski JE, van Exel NJ, Kempen GI, et al. Validation of the Care-Related Quality of Life Instrument in different study settings: findings from The Older Persons and Informal Caregivers Survey Minimum Dataset (TOPICS-MDS). Qual Life Res. 2015;24:1281–1293. doi: 10.1007/s11136-014-0841-2 [DOI] [PubMed] [Google Scholar]
- 17. Weinberger M, Samsa GP, Schmader K, Greenberg SM, Carr DB, Wildman DS. Comparing proxy and patients’ perceptions of patients’ functional status: results from an outpatient geriatric clinic. J Am Geriatr Soc. 1992;40:585–588. [DOI] [PubMed] [Google Scholar]
- 18. Debray TP, Moons KG, Abo-Zaid GM, Koffijberg H, Riley RD. Individual participant data meta-analysis for a binary outcome: one-stage or two-stage? PLoS One. 2013;8:e60650. doi: 10.1371/journal.pone.0060650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Burke DL, Ensor J, Riley RD. Meta-analysis using individual participant data: one-stage and two-stage approaches, and why they may differ. Stat Med. 2017;36:855–875. doi: 10.1002/sim.7141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Twisk JWR. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge: Cambridge University Press; 2003. [Google Scholar]
- 21. Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 22. Bleijenberg N. Disability in the individual ADL, IADL, and mobility among older adults: a prospective cohort study. J Nutr Health Aging. 2017;21(8):897–903. doi: 10.1007/s12603-017-0891-6 [DOI] [PubMed] [Google Scholar]
- 23. Wet maatschappelijke ondersteuning 2015 (WMO 2015) The Netherlands; 2014:01-08-2016. http://wetten.overheid.nl/BWBR0035362/2016-08-01. AccessedNovember 26, 2017. [Google Scholar]
- 24. Bower P, Harkness E, Macdonald W, Coventry P, Bundy C, Moss-Morris R. Illness representations in patients with multimorbid long-term conditions: qualitative study. Psychol Health. 2012;27:1211–1226. doi: 10.1080/08870446.2012.662973 [DOI] [PubMed] [Google Scholar]
- 25. Löffler C, Kaduszkiewicz H, Stolzenbach CO, et al. Coping with multimorbidity in old age–a qualitative study. BMC Fam Pract. 2012;13:45. doi: 10.1186/1471-2296-13-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cottrell E, Yardley S. Lived experiences of multimorbidity: an interpretative meta-synthesis of patients’, general practitioners’ and trainees’ perceptions. Chronic Illn. 2015;11:279–303. doi: 10.1177/1742395315574764 [DOI] [PubMed] [Google Scholar]
- 27. Connolly D, Garvey J, McKee G. Factors associated with ADL/IADL disability in community dwelling older adults in the Irish longitudinal study on ageing (TILDA). Disabil Rehabil. 2017;39:809–816. doi: 10.3109/09638288.2016.1161848 [DOI] [PubMed] [Google Scholar]
- 28. Su P, Ding H, Zhang W, et al. The association of multimorbidity and disability in a community-based sample of elderly aged 80 or older in Shanghai, China. BMC Geriatr. 2016;16:178. doi: 10.1186/s12877-016-0352-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Qian J, Ren X. Association between comorbid conditions and BADL/IADL disability in hypertension patients over age 45: based on the China Health and Retirement Longitudinal Study (CHARLS). Medicine (Baltimore). 2016;95:e4536. doi: 10.1097/MD.0000000000004536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye F, Moon DH, Carpenter WR, et al. Comparison of patient report and medical records of comorbidities: results from a population-based cohort of patients with prostate cancer. JAMA Oncol. 2017;3:1035–1042. doi: 10.1001/jamaoncol.2016.6744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lutomski JE, Krabbe PF, den Elzen WP, et al. ; TOPICS Consortium. Rasch analysis reveals comparative analyses of activities of daily living/instrumental activities of daily living summary scores from different residential settings is inappropriate. J Clin Epidemiol. 2016;74:207–217. doi: 10.1016/j.jclinepi.2015.11.006 [DOI] [PubMed] [Google Scholar]
- 32. Aspinal F, Glasby J, Rostgaard T, Tuntland H, Westendorp RG. New horizons: Reablement-supporting older people towards independence. Age Ageing. 2016;45:572–576. doi: 10.1093/ageing/afw094 [DOI] [PubMed] [Google Scholar]
- 33. Marengoni A, Fratiglioni L, Onder G. Improving public awareness of multimorbidity. J Am Med Dir Assoc. 2017;18:372–373. doi: 10.1016/j.jamda.2017.01.010 [DOI] [PubMed] [Google Scholar]
- 34. Picco L, Achilla E, Abdin E, et al. Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res. 2016;16:173. doi: 10.1186/s12913-016-1421-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.