Abstract
Background
Orthostatic hypotension (OH) is associated with increased risk of falls, cognitive impairment and death, as well as a reduced quality of life. Although it is presumed to be common in older people, estimates of its prevalence vary widely. This study aims to address this by pooling the results of epidemiological studies.
Methods
MEDLINE, EMBASE, PubMed, Web of Science, and ProQuest were searched. Studies were included if participants were more than 60 years, were set within the community or within long-term care and diagnosis was based on a postural drop in systolic blood pressure (BP) ≥20 mmHg or diastolic BP ≥10 mmHg. Data were extracted independently by two reviewers. Random and quality effects models were used for pooled analysis.
Results
Of 23,090 identified records, 20 studies were included for community-dwelling older people (n = 24,967) and six were included for older people in long-term settings (n = 2,694). There was substantial variation in methods used to identify OH with differing supine rest duration, frequency and timing of standing BP, measurement device, use of standing and tilt-tables and interpretation of the diagnostic drop in BP. The pooled prevalence of OH in community-dwelling older people was 22.2% (95% CI = 17, 28) and 23.9% (95% CI = 18.2, 30.1) in long-term settings. There was significant heterogeneity in both pooled results (I2 > 90%).
Conclusions
OH is very common, affecting one in five community-dwelling older people and almost one in four older people in long-term care. There is great variability in methods used to identify OH.
Keywords: Postural hypotension, Epidemiology, Syncope
Orthostatic hypotension (OH) is a disabling condition, characterized by a sustained reduction in blood pressure (BP) upon standing upright (1). It results in a broad range of symptoms, leading to reduced quality of life and impairment basic activities of daily living (2). Recent studies have also identified an association between OH with cognitive impairment, stroke, and mortality (3,4).
While, OH is generally considered as a common finding in older populations, estimates of its prevalence vary widely (5,6). With the rapidly ageing population and the growing demand for health care resources in the older population, robust estimates of prevalence are required to justify and plan service design and provision. Furthermore, as good quality evidence for the treatment of OH is lacking, reliable estimates of its prevalence will be useful to draw more research funding to this area.
The aim of this study was, therefore, to provide pooled estimates of the prevalence of OH in older people living in the community and within long-term care facilities.
Methods
Eligibility Criteria
To be eligible for inclusion, all studies were required to meet the following criteria:
Participants: All study participants (or subgroup) had to be aged at least 60 years. If the lower age limit of participants was not reported, it was estimated from the mean and standard deviation [mean − (2 × standard deviation)] of the overall study population. Studies relating to OH secondary to acute illness (such as hemorrhage or sepsis) or hemodialysis were excluded. Studies reporting the prevalence of OH in specific populations (such as Parkinson’s disease or hypertension) were excluded unless they included a population-based control group.
Setting: Studies were included if participants were identified on a population level or an appropriate register (e.g. electoral roll, primary care database) and were excluded if recruitment occurred in specific settings such as a clinic. Participants were considered community-dwelling if they lived in their own home, regardless of whether they receive additional health or social care in their home. Studies in long-term care settings such as rehabilitation units, residential and nursing homes were included and reported as a specific group.
Diagnosis: To be included, studies were required to be published after 1996 when the international consensus diagnostic criteria were published. Where studies were published after this date, but were performed before this date, they were still eligible if the diagnostic criteria were equivalent to the 1996 consensus. Studies published since 1996 which had minor variations in diagnostic criteria were included, with these being reflected in the quality assessment.
Studies: Only studies written in the English language were eligible. Prospective, cross-sectional, and retrospective studies were considered if they reported the number or proportion of older people with OH. Case studies, case series, and case–control studies were excluded.
Data Sources and Searches
A search for published articles was performed using MEDLINE (1946 to 9th December 2016), EMBASE (1974 to 13th December 2016), and PubMed (to 13th December 2016). To reduce the risk of publication bias, conference proceedings and theses were searched for using Web of Science (1970 to 13th December 2016) and ProQuest (1970 to 15th December 2016). In addition, researchers searched reference lists when reviewing full-text articles, to identify additional studies. A comprehensive list of search terms for each database is included in Supplementary Table 1.
Study Selection
All identified studies were collated into the Endnote X7 software (Thomson Reuters) where duplicates were removed. Titles and abstracts of the remaining studies were screened for eligibility by two researchers (J.F., N.I.S.); full-text articles were reviewed where there was doubt about eligibility for inclusion. Following the screening process, full-text articles were retrieved for all selected articles.
Data Extraction
Data were extracted by two researchers (J.F., N.I.S.) independently onto data extraction forms specifically adapted for the topic of this review. Data collected included study design and methods, recruitment, sampling and setting, participant characteristics, BP measurement, diagnosis criteria, and prevalence of OH. Where prevalence was reported at more than one time point, the first time point was used. Disagreement between researchers was resolved by a third researcher (M.P.T.).
Quality Assessment
An existing tool for assessing the risk of bias in prevalence studies was completed by two reviewers (J.F., N.I.S.) (7). A score of 0 to 10 is assigned to each selected article, where 7–10 implies further research is unlikely to change the confidence in the estimate of prevalence, 3–6 suggests further research is likely to change confidence, and 0–2 implies that further research is likely to change the estimate of prevalence. Although funnel plots may overestimate publication bias in prevalence studies, they were constructed to explore the possibility of bias for analyses that contained more than 10 studies (8). These are presented in Supplementary Figure 1.
Data Synthesis and Analysis
As population-based studies are likely to be heterogeneous, a random-effects model was chosen. Estimates of prevalence were transformed using the double arcsine method, which addresses the problem of reduced variance, and therefore excessive weighting, of estimates close to 0 or 100%. Analysis was performed using MetaXL (www.epigear.com). Given the anticipated range of quality between studies, a quality effects model is also reported, which assigns greater weighting to studies of higher quality. Forest plots were visually inspected to identify obvious heterogeneity. The I2 method of calculating heterogeneity was used to identify where inconsistency may have been due to chance. An I2 estimate between 50 and 75% was considered moderate heterogeneity and greater than 75% was considered as high. Meta-regression data were created using MetaXL and exported to Stata for analysis.
Protocol and Registration
A protocol of the methodology used for this review was registered and published prospectively, protocol ID CRD42017070877 (http://www.crd.york.ac.uk/prospero/).
Results
Study Selection
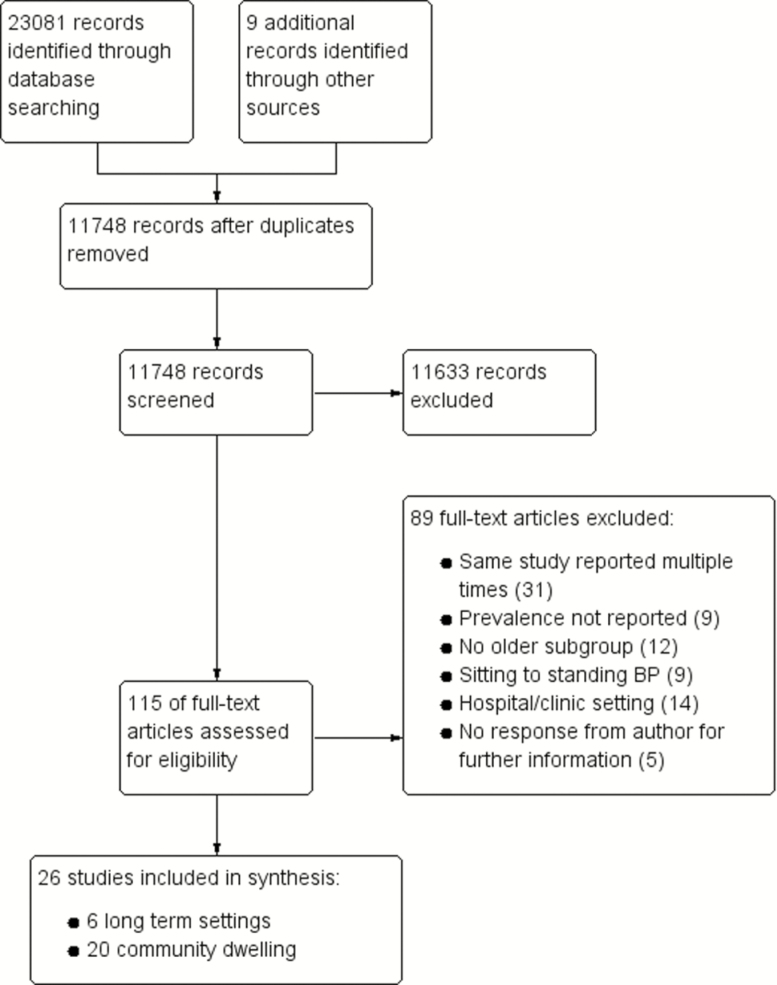
Twenty-six studies in total were included for both the review and analysis. The identification, screening, and selection of records are illustrated in Figure 1. Two studies provided data for an older subgroup, via personal communication with the corresponding author (9,10).
Figure 1.
Study screening and selection.
Study Characteristics
The majority of studies were performed in Europe (11), seven were conducted in North America, two were in Asia, and two were in Australia. Four studies included participants under the age of 60 years, but presented data for an older subgroup, and were therefore included (9,12,13). Detailed characteristics of included studies are displayed in Supplementary Table 2.
Participant Characteristics
Twenty studies were performed in community-dwelling individuals, with the remaining six studies occurring in long-term residential facilities (Supplementary Table 2). Inclusion and exclusion criteria were heterogeneous, as was the reporting of participant characteristics (Supplementary Table 2).
Diagnosis
Methods utilized for BP measurement varied between studies. Seven studies report using a manual device (11,14–19), 10 report using an automated device (9,10,12,20–26), two studies used noninvasive, continuous BP monitoring (5,27), five did not report their device (28–32), and two studies used other methods (random-zero sphygmomanometer (33); automatic BP cuff with a heart sound microphone (13)). Two studies used a tilt-table to induce orthostasis (5,24).
The majority of studies rested participants supine for 5 minutes prior to standing (5,10,14,16,19,22,24,26,28,29,31). Other supine durations included 10 minutes (11,27), 15 minutes (12,17), 20 minutes (18,23), and 25 minutes (9). One study measured the supine BP before the participants arose from bed in the morning (20) and the remaining seven studies did not provide sufficient information (13,15,21,25,30,32,33).
There was wide variation in the timing of standing BP measurement. The most common method was to record BP at 1 and 3 minutes of standing (11,12,16,19,20,22,23,25,26,28,33). Other timings included after 3 minutes (17,18), after 1 minute (14,29,31), at 1 and 2 minutes (15), every minute for 7 minutes (13), every minute for 5 minutes (24), at 1, 2 and 3 minutes (10), and every 30 seconds for 2 minutes (9). Three studies did not report the timing of standing BP measurement (21,30,32).
Diagnosis of OH was based on a drop in systolic BP of ≥20 mmHg, or of ≥10 mmHg for diastolic BP, upon standing, in most studies (5,9–13,16,17,19,22,24–32). One study also used these criteria but required the BP drop to be present at two time points during standing (15). In addition to this criteria, one study also diagnosed OH if there was a ≥10 mmHg drop in mean arterial pressure (20); another study also diagnosed OH if postural symptoms were present (even in the absence of a BP drop) (18). The four remaining studies focussed only on systolic BP (postural drop ≥20 mmHg) (14,21,23,33).
Quality Assessment
The quality of included studies is summarized in Supplementary Table 3. Sixteen of the 20 community-based studies yielded a quality assessment score of 7–10 indicating that further research is unlikely to change the confidence of estimated prevalence, with the remaining four studies yielding a score of 4–6 suggesting that further studies are likely to change the confidence of the studies. Of the six studies conducted in residential settings, the risk of bias score for four studies fell between 7 and 10, while the remaining two studies scored between 4 and 5. A funnel plot consisting of results from community-based studies is presented in Supplementary Figure 1. Asymmetry is observed suggesting there is a risk of publication bias. We were unable to obtain a funnel plot from long-term residential care studies due to the low number of studies in this category.
Prevalence of OH in Community-Dwelling Older People
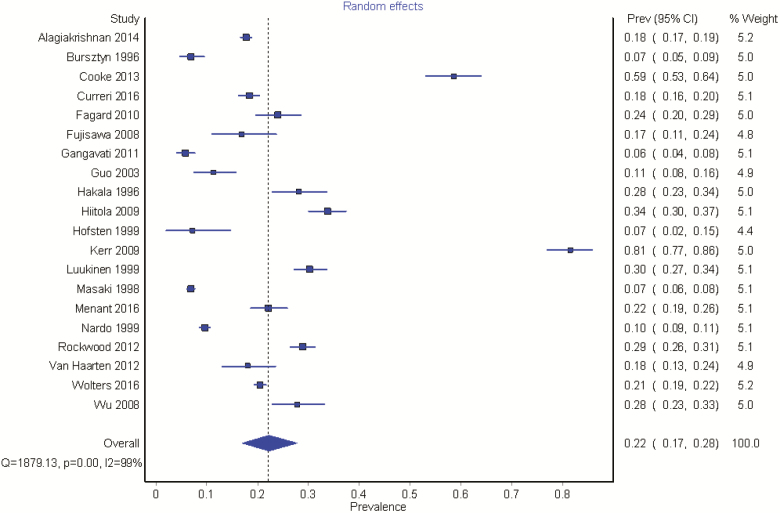
The 20 included studies resulted in a pooled sample size of 24,967. Study estimates of prevalence varied from 6% to 81%, with the two highest estimates arising from continuous BP monitoring. Pooled analysis revealed a prevalence of 22.2% (95% CI = 17, 28). Substantial levels of heterogeneity were evident between studies (I2 = 99%), Figure 2.
Figure 2.
The prevalence of OH in community-dwelling older people. The highest prevalence rates (Cooke et al. (5), Kerr (27)) are seen in studies using continuous BP monitoring.
Incorporating the quality of the included studies into the pooled analysis, giving greater weighting to those of higher quality, resulted in a slightly lower prevalence, 18.4% (95% CI = 11.4, 26.6), but heterogeneity remained considerable (I2 = 99%).
A subgroup analysis, based on whether intermittent or continuous BP recordings were measured was performed. As only two studies used continuous BP measurement, with a pooled sample size of 623, the result may not be considered as reliable [70% (95% CI = 46, 92), I2 = 97%]. However, focussing on intermittent BP measurements, the pooled estimates are very similar to those described above [18% (95% CI = 11, 27), I2 = 99%].
Prevalence of OH in Long-Term Residential Settings
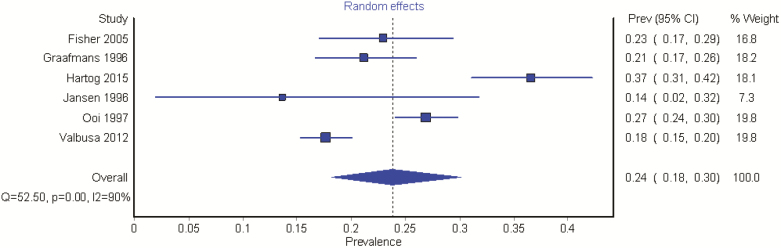
Six studies were included, with a sample size of 2,694. All studies used intermittent BP measurement. The prevalence of OH in the included studies ranged from 13.6% to 36.6%. The pooled prevalence was 23.9% (95% CI = 18.2, 30.1). Once again levels of heterogeneity were substantial (I2 = 90%), Figure 3.
Figure 3.
The prevalence of OH in long-term care.
Incorporating the quality of the studies into the pooled analysis revealed a prevalence of 22.2% (95% CI = 15.7, 29.6), with considerable heterogeneity (I2 = 90%).
Meta-Regression
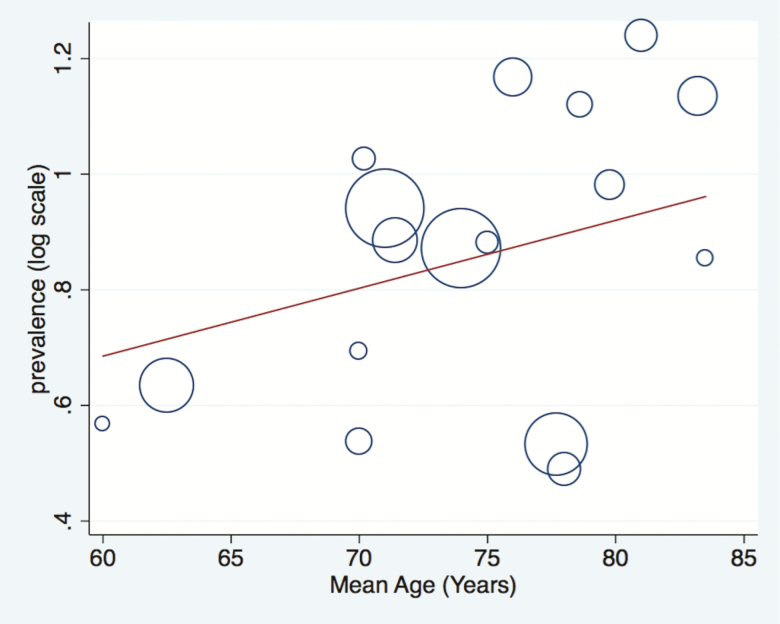
The relationship between age and the prevalence of OH was explored in a meta-regression model, using the mean age of the included studies. Figure 4 demonstrates that there was a trend for increasing age to be associated with an increasing prevalence of OH. However, this trend was not statistically significant and the difference in the mean age of sample populations in included studies does not explain the high level of heterogeneity.
Figure 4.
Meta-regression bubble graph demonstrating the relationship between the age of the included cohorts and the prevalence of OH. Slope coefficient (s.e.) = 0.012 (0.01), p = 0.264. The area of each circle is inversely proportional to the variance of the log-prevalence. The superimposed line is obtained by inverse variance heterogeneity weighted regression. The plot depicts the estimated prevalence of orthostatic hypotension in included studies according to the mean age of sample populations in years.
Discussion
This meta-analysis demonstrates that OH affects nearly one in five older persons living in the community, and almost one in four persons living in long-term residential care facilities. These pooled analyses need to be interpreted with caution in view of the significant heterogeneity between studies.
Despite the availability of consensus guidelines on the magnitude of BP drop required to diagnose OH, this study demonstrates huge variations in assessment methods used to measure postural BP. These included differing supine rest periods, the use of active standing or tilt-table measurements, frequency and timing of BP measurements, and duration of orthostasis. Such was the degree of variation between studies, it was not possible to perform post-hoc sensitivity analyses to explore the impact of different methods on the prevalence of OH. This is an important finding and suggests there is a need for more structured guidance on the methods to measure postural BP. Such guidance would provide a potential solution to the substantial qualitative and statistical heterogeneity found in this study.
The significant heterogeneity is further compounded by inclusion of studies derived from diverse populations of different ages, with different co-morbidities and in different settings. But perhaps this is unsurprising as the population in this study focussed on ‘community-dwelling older people’, which is a rather general and undefined population to study. While more precise estimates may be achieved by focussing on specific populations such as those with hypertension, diabetes or Parkinson’s disease, this leads to a common paradox in ageing research. Increasing levels of homogeneity of the study population, leads to reduced external validity and loss of relevance to the real-world.
None of the included studies in this review used the 2011 update to the diagnostic criteria, in particular the criteria for a sustained drop in BP (1). The two clear outliers in Figure 2 used continuous BP monitoring to measure postural BP. They report a fourfold greater prevalence of OH than with intermittent readings. Although the 2011 updated criteria may have attenuated their estimate of prevalence, the update does not define what constitutes a sustained drop, thus it is difficult for clinicians and academics to adopt this rationalized criteria.
The current method to measure postural BP is focussed on the magnitude of drop and may represent an over simplification of the approach. Although a recent meta-analysis has suggested that the presence of OH is associated with time to first fall (34), other studies have suggested that actual standing BP may have more relevance to clinical outcomes than the reduction in BP with standing (35). Furthermore, the presence of a significant BP drop may not necessarily be symptomatic or pathological, and little is currently understood about the underlying mechanisms that would determine the presence of symptoms in those with OH.
Although these pooled results demonstrate a high overall prevalence of OH, they do not reflect the severity of the condition, particularly whether individuals are symptomatic or disabled by their OH. If the inclusion of symptoms were to be considered as a diagnostic criteria, it is possible that these estimates would be reduced. However, there is no standardized method of quantifying symptoms associated with OH, nor an agreement of how severe symptoms would need to be for a positive diagnosis. Furthermore, a lack of awareness of hypotensive symptoms may be present among individuals with OH who may then sustain a fall. Despite the clinical plausibility for this conjecture which could be extrapolated from the accepted wisdom of the lack of awareness of hypoglycaemic symptoms among older persons, limited evidence currently exists to support this hypothesis. However, previous studies have documented the presence of amnesia for loss of consciousness in the related hypotensive disorders or carotid sinus hypersensitivity and vasovagal syncope (36,37).
Prevalence studies may be less likely to suffer from publication bias than interventional studies. In an attempt to negate publication bias, the grey literate was included in the search strategies and did identify abstracts and PhD theses which were included in the analysis. However, this review does exclude studies published in non-English language and is therefore open to reporting bias. This limitation should be considered when interpreting the results.
It is important to accumulate evidence for the high prevalence of OH among the older population so that service providers and commissioners will recognize its importance as an age-related condition and hence plan appropriately for the populations’ needs. This is particularly relevant in the context of the rapidly ageing population. OH is associated with significant morbidity, such as falls, a reduced quality of life and an increased risk of dementia, cardiovascular disease, and mortality (10,34,38). Given the high prevalence of OH, and the rapidly expanding older population, this is an area where further research is urgently needed. Further evidence-based recommendations are also needed to standardize the diagnosis of OH, for both intermittent and continuous BP monitoring.
Funding
This report is independent research arising from a Clinician Scientist award (CS-2014-002) supported by National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the UK NHS, National Institute for Health Research or the Department of Health.
Supplementary Material
Acknowledgements
The Research was supported by the National Institute for Health Research Newcastle Biomedical Research Centre based at Newcastle Hospitals NHS Foundation Trust and Newcastle University. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR, or the Department of Health’.
Conflict of interest statement
None declared.
References
- 1. Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5 [DOI] [PubMed] [Google Scholar]
- 2. Low PA, Opfer-Gehrking TL, McPhee BR, et al. Prospective evaluation of clinical characteristics of orthostatic hypotension. Mayo Clin Proc. 1995;70:617–622. doi: 10.1016/S0025-6196(11)63911-6 [DOI] [PubMed] [Google Scholar]
- 3. Angelousi A, Girerd N, Benetos A, et al. Association between orthostatic hypotension and cardiovascular risk, cerebrovascular risk, cognitive decline and falls as well as overall mortality: a systematic review and meta-analysis. J Hypertens. 2014;32:1562–1571; discussion 1571. doi: 10.1097/HJH.0000000000000235 [DOI] [PubMed] [Google Scholar]
- 4. Ricci F, Fedorowski A, Radico F, et al. Cardiovascular morbidity and mortality related to orthostatic hypotension: a meta-analysis of prospective observational studies. Eur Heart J. 2015;36:1609–1617. doi: 10.1093/eurheartj/ehv093 [DOI] [PubMed] [Google Scholar]
- 5. Cooke J, Carew S, Quinn C, et al. The prevalence and pathological correlates of orthostatic hypotension and its subtypes when measured using beat-to-beat technology in a sample of older adults living in the community. Age Ageing. 2013;42:709–714. doi: 10.1093/ageing/aft112 [DOI] [PubMed] [Google Scholar]
- 6. Mader SL, Josephson KR, Rubenstein LZ. Low prevalence of postural hypotension among community-dwelling elderly. JAMA. 1987;258:1511–1514. doi: 10.1001/jama.1987.03400110093033 [PubMed] [Google Scholar]
- 7. Hoy D, Brooks P, Woolf A, et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65:934–939. doi: 10.1016/j.jclinepi. 2011.11.014 [DOI] [PubMed] [Google Scholar]
- 8. Hunter JP, Saratzis A, Sutton AJ, Boucher RH, Sayers RD, Bown MJ. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 9. Nardo CJ, Chambless LE, Light KC, et al. Descriptive epidemiology of blood pressure response to change in body position. The ARIC study. Hypertension. 1999;33:1123–1129. doi: 10.1161/01.HYP.33.5.1123 [DOI] [PubMed] [Google Scholar]
- 10. Wolters FJ, Mattace-Raso FU, Koudstaal PJ, Hofman A, Ikram MA; Heart Brain Connection Collaborative Research Group Orthostatic hypotension and the long-term risk of dementia: a population-based study. PLoS Med. 2016;13:e1002143. doi: 10.1371/journal.pmed.1002143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hiitola P, Enlund H, Kettunen R, Sulkava R, Hartikainen S. Postural changes in blood pressure and the prevalence of orthostatic hypotension among home-dwelling elderly aged 75 years or older. J Hum Hypertens. 2009;23:33–39. doi: 10.1038/jhh.2008.81 [DOI] [PubMed] [Google Scholar]
- 12. Wu JS, Yang YC, Lu FH, Wu CH, Chang CJ. Population-based study on the prevalence and correlates of orthostatic hypotension/hypertension and orthostatic dizziness. Hypertens Res. 2008;31:897–904. doi: 10.1291/hypres.31.897 [DOI] [PubMed] [Google Scholar]
- 13. Hofsten A, Elmfeldt D, Svärdsudd K. Age-related differences in blood pressure and heart rate responses to changes in body position: results from a study with serial measurements in the supine and standing positions in 30-, 50- and 60-year-old men. Blood Press. 1999;8:220–226. doi: 10.1080/080370599439607 [DOI] [PubMed] [Google Scholar]
- 14. Bursztyn M, Shpilberg O, Ginsberg GM, Cohen A, Stessman J. Hypertension in the Jerusalem 70 year olds study population: prevalence, awareness, treatment and control. Isr J Med Sci. 1996;32:629–633. [PubMed] [Google Scholar]
- 15. Fagard RH, De Cort P. Orthostatic hypotension is a more robust predictor of cardiovascular events than nighttime reverse dipping in elderly. Hypertension. 2010;56:56–61. doi: 10.1161/HYPERTENSIONAHA.110.151654 [DOI] [PubMed] [Google Scholar]
- 16. Luukinen H, Koski K, Laippala P, Kivelä SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–280. doi: 10.1001/archinte.159.3.273 [DOI] [PubMed] [Google Scholar]
- 17. Masaki KH, Schatz IJ, Burchfiel CM, et al. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–2295. doi: 10.1161/01.CIR.98.21.2290 [DOI] [PubMed] [Google Scholar]
- 18. Alagiakrishnan K, Patel K, Desai RV, et al. Orthostatic hypotension and incident heart failure in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2014;69:223–230. doi: 10.1093/gerona/glt086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Curreri C, Giantin V, Veronese N, et al. Orthostatic changes in blood pressure and cognitive status in the elderly: the Progetto Veneto Anziani study. Hypertension. 2016;68:427–435. doi: 10.1161/HYPERTENSIONAHA.116.07334 [DOI] [PubMed] [Google Scholar]
- 20. Fisher AA, Davis MW, Srikusalanukul W, Budge MM. Postprandial hypotension predicts all-cause mortality in older, low-level care residents. J Am Geriatr Soc. 2005;53:1313–1320. doi: 10.1111/j.1532-5415.2005.53415.x [DOI] [PubMed] [Google Scholar]
- 21. Fujisawa M, Okumiya K, Matsubayashi K, Hamada T, Endo H, Doi Y. Factors associated with carotid atherosclerosis in community-dwelling oldest elderly aged over 80 years. Geriatr Gerontol Int. 2008;8:12–18. doi: 10.1111/j.1447-0594.2008.00441.x [DOI] [PubMed] [Google Scholar]
- 22. Hartog LC, Cizmar-Sweelssen M, Knipscheer A, et al. The association between orthostatic hypotension, falling and successful rehabilitation in a nursing home population. Arch Gerontol Geriatr. 2015;61:190–196. doi: 10.1016/j.archger.2015.05.005 [DOI] [PubMed] [Google Scholar]
- 23. Jansen RW, Kelly-Gagnon MM, Lipsitz LA. Intraindividual reproducibility of postprandial and orthostatic blood pressure changes in older nursing-home patients: relationship with chronic use of cardiovascular medications. J Am Geriatr Soc. 1996;44:383–389. doi: 10.1111/j.1532-5415.1996.tb06406.x [DOI] [PubMed] [Google Scholar]
- 24. Menant JC, Wong AK, Trollor JN, Close JC, Lord SR. Depressive symptoms and orthostatic hypotension are risk factors for unexplained falls in community-living older people. J Am Geriatr Soc. 2016;64:1073–1078. doi: 10.1111/jgs.14104 [DOI] [PubMed] [Google Scholar]
- 25. Valbusa F, Labat C, Salvi P, Vivian ME, Hanon O, Benetos A; PARTAGE investigators Orthostatic hypotension in very old individuals living in nursing homes: the PARTAGE study. J Hypertens. 2012;30:53–60. doi: 10.1097/HJH.0b013e32834d3d73 [DOI] [PubMed] [Google Scholar]
- 26. van Hateren KJ, Kleefstra N, Blanker MH, et al. Orthostatic hypotension, diabetes, and falling in older patients: a cross-sectional study. Br J Gen Pract. 2012;62:e696–e702. doi: 10.3399/bjgp12X656838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerr SRJ. The Prevalence of Neurocardiovascular Instability and Its Clinical Associations in Community-Dwelling Older People [Ph.D. thesis]. Ann Arbor: University of Newcastle Upon Tyne (United Kingdom); 2009. [Google Scholar]
- 28. Gangavati A, Hajjar I, Quach L, et al. Hypertension, orthostatic hypotension, and the risk of falls in a community-dwelling elderly population: the maintenance of balance, independent living, intellect, and zest in the elderly of Boston study. J Am Geriatr Soc. 2011;59:383–389. doi: 10.1111/j.1532-5415.2011.03317.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Graafmans WC, Ooms ME, Hofstee HM, Bezemer PD, Bouter LM, Lips P. Falls in the elderly: a prospective study of risk factors and risk profiles. Am J Epidemiol. 1996;143:1129–1136. doi: 10.1093/oxfordjournals.aje.a008690 [DOI] [PubMed] [Google Scholar]
- 30. Guo XX, Matousek M, Sonn U, Skoog I, Bjorkelund C, Steen B. A longitudinal study on changes of movement performance and their relation to medical conditions in a female population followed from age 70 to 78. Arch Gerontol Geriatr. 2003;36:127–140. doi: 10.1016/s0167-4943(02)00083-3 [DOI] [PubMed] [Google Scholar]
- 31. Hakala SM, Tilvis RS. How stable is postural hypotension in the general aged population?Arch Gerontol Geriatr. 1996;23:129–138. doi: 10.1016/0167-4943(96)00713-3 [DOI] [PubMed] [Google Scholar]
- 32. Rockwood MR, Howlett SE, Rockwood K. Orthostatic hypotension (OH) and mortality in relation to age, blood pressure and frailty. Arch Gerontol Geriatr. 2012;54:e255–e260. doi: 10.1016/j.archger.2011.12.009 [DOI] [PubMed] [Google Scholar]
- 33. Ooi WL, Barrett S, Hossain M, Kelley-Gagnon M, Lipsitz LA. Patterns of orthostatic blood pressure change and their clinical correlates in a frail, elderly population. JAMA. 1997;277:1299–1304. doi: 10.1001/jama.277.16.1299 [PubMed] [Google Scholar]
- 34. Hartog LC, Schrijnders D, Landman GWD, et al. Is orthostatic hypotension related to falling? A meta-analysis of individual patient data of prospective observational studies. Age Ageing. 2017;46:568–575. doi: 10.1093/ageing/afx024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zia A, Kamaruzzaman SB, Myint PK, Tan MP. The association of antihypertensives with postural blood pressure and falls among seniors residing in the community: a case-control study. Eur J Clin Invest. 2015;45:1069–1076. doi: 10.1111/eci.12508 [DOI] [PubMed] [Google Scholar]
- 36. Parry SW, Steen IN, Baptist M, Kenny RA. Amnesia for loss of consciousness in carotid sinus syndrome: implications for presentation with falls. J Am Coll Cardiol. 2005;45:1840–1843. doi: 10.1016/j.jacc.2005.02.060 [DOI] [PubMed] [Google Scholar]
- 37. O’Dwyer C, Bennett K, Langan Y, Fan CW, Kenny RA. Amnesia for loss of consciousness is common in vasovagal syncope. Europace. 2011;13:1040–1045. doi: 10.1093/europace/eur069 [DOI] [PubMed] [Google Scholar]
- 38. Frith J. The association of orthostatic hypotension with falls-an end to the debate?Age Ageing. 2017;46:540–541. doi: 10.1093/ageing/afx053 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.