Abstract
Since the initial suggestion that rapamycin, an inhibitor of target of rapamycin (TOR) nutrient signaling, increased lifespan comparable to dietary restriction, investigators have viewed rapamycin as a potential dietary restriction mimetic. Both dietary restriction and rapamycin increase lifespan across a wide range of evolutionarily diverse species (including yeast, Caenorhabditis elegans, Drosophila, and mice) as well as reducing pathology and improving physiological functions that decline with age in mice. The purpose of this article is to review the research comparing the effect of dietary restriction and rapamycin in mice. The current data show that dietary restriction and rapamycin have different effects on many pathways and molecular processes. In addition, these interventions affect the lifespan of many genetically manipulated mouse models differently. In other words, while dietary restriction and rapamycin may have similar effects on some pathways and processes; overall, they affect many pathways/processes quite differently. Therefore, rapamycin is likely not a true dietary restriction mimetic. Rather dietary restriction and rapamycin appear to be increasing lifespan and retarding aging largely through different mechanisms/pathways, suggesting that a combination of dietary restriction and rapamycin will have a greater effect on lifespan than either manipulation alone.
Keywords: Dietary restriction, Rapamycin, Lifespan, Insulin sensitivity, TOR
Currently, the two most robust manipulations that increase lifespan in mice are dietary restriction (DR; also called caloric restriction) and rapamycin. McCay et al. (1) made the discovery that dramatically reducing food consumption increased the lifespan of rats over 80 years ago. Since this initial observation, numerous laboratories have confirmed these results and have shown that reducing food consumption 30% to 40% (without malnutrition) consistently increased both the mean and maximum lifespan of laboratory rats and mice. In addition, DR has been shown to increase the lifespan of a variety of organisms, ranging from invertebrates (yeast, Caenorhabditis elegans, and Drosophila) to dogs and non-human primates (2). Studies in the 1980s, primarily by Ed Masoro’s group at the University of Texas Health Science Center in San Antonio (UTHSCSA) and Roy Walford’s group at the University of California, conclusively demonstrated that the increase in the lifespan of rats and mice by DR occurred because the animals were aging more slowly because DR improves physiological functions and reduces age-related pathology.
In 2004, Dave Sharp at UTHSCSA proposed that the NIA funded Intervention Testing Program (https://www.nia.nih.gov/research/dab/interventions-testing-program-itp) test the effect of rapamycin on the lifespan of mice. Dave’s interest in aging came about when he was part of a program project studying DR at UTHSCSA from 1998 to 2003. In trying to understand how DR had such a profound effect on the lifespan and health span of mice, Dave became interested in the potential role TOR (target of rapamycin) might play in the effects of DR because TOR was a nutrient sensor. In addition to that, data from Michael Hall’s group (3) showed that rapamycin mimicked the starvation phenotype in yeast by inhibiting TOR. Dave hypothesized that the reduced consumption of food by DR in mice and rats reduced mTOR signaling, which leads to increased lifespan. The report by Vellai et al. (4) showing that TOR regulated the lifespan of C. elegans provided additional support for TOR playing a role in the lifespan extension by DR. With Andy Bartke, Dave reported that long-lived Ames dwarf mice showed evidence of reduced mTORC1, suggesting that TOR complexes somehow coordinated nutrient and growth factor signaling in regulation of aging (5). Therefore, he proposed to the Intervention Testing Program that feeding mice rapamycin would reduce mTOR signaling, mimicking DR and/or the Ames dwarf mouse phenotypes of increased lifespan. In 2006, the rapamycin study was initiated, and in 2009, Harrison et al. (6) reported the first data showing that rapamycin increased the lifespan of both male and female mice. In the News and Views section of Nature, in which the effect of rapamycin on the lifespan of mice was first reported, Kaeberlein and Kennedy (7) wrote, “. . . it is tempting to speculate that rapamycin may be functioning as a dietary-restriction mimetic”. Thus, from the onset, the concept was that rapamycin and DR were likely to be increasing lifespan through similar mechanisms/pathways. Since the initial report in 2009, there have been nine additional studies showing that rapamycin increased the lifespan of a variety of strains of male or female mice, and these studies have been described by Richardson et al. (8) and Aurriola Apelo and Lamming (9). Although less well studied than DR, rapamycin also reduces many pathologies that increase with age and improves many (but not all) physiological functions that decline with age (10–12).
Initial studies in yeast (13) and C. elegans (14) showing that life extension by TOR mutations was not increased by DR supported the concept that DR and rapamycin increased lifespan through similar mechanisms. Kapahi et al. (15) compared the effect of inhibiting TOR signaling by overexpressing dTsc2 on the lifespan of Drosophila over a wide range of yeast concentrations and found a greater extension of lifespan by dTsc2 at high yeast concentrations than at low yeast concentrations, when the lifespan was maximal. In 2010, Bjedov et al. (16) reported that rapamycin significantly increased the lifespan of Drosophila over a wide range of yeast concentrations: both low yeast concentrations that maximized lifespan as well as at high yeast concentrations that reduced lifespan. Bjedov et al. (16) argued that rapamycin increased lifespan by “additional mechanisms” compared to DR. It is possible that the differences observed in the invertebrate studies are due to rapamycin having a more diverse effect on lifespan than when TOR signaling is genetically targeted, that is, rapamycin affects pathways other than those regulated by TOR.
The focus of this article is to review the data over the past 8 years that have compared the effect of rapamycin and DR on various pathways and functions in mice with the goal of providing the research community insight into whether rapamycin is a DR mimetic and increases lifespan through a similar mechanism(s) as DR.
Comparison of the Effect of Rapamycin and DR on Lifespan of Male and Female Mice
Although both rapamycin and DR increase the lifespan of various strains of mice, it appears that they might show differences in male and female mice. As given in Table 1, most of the studies that have compared the effect of various doses of rapamycin on the lifespan of male and female mice have found rapamycin to show a more robust effect on enhancing the lifespan of female mice than male mice starting by the initial study by Harrison et al. (6) when they gave the mice 14 ppm in their food. This difference is especially prominent at the lower doses of rapamycin. When the data from all the studies conducted to date are combined, the average effect of rapamycin on the lifespan of female mice is approximately 19% compared to 10% for males.
Table 1.
Comparison of the Effect of Rapamycin and Dietary Restriction on the Lifespan of Male and Female Mice
| Reference | Mouse Strain | Age Initiated | Dose | Increase in Lifespan | |
|---|---|---|---|---|---|
| Male | Female | ||||
| Rapamycin | |||||
| Harrison et al. (6) | UM-HET3 | 19 mo | 14 ppm | 9% | 14% |
| Miller et al. (17) | UM-HET3 | 9 mo | 14 ppm | 10% | 18% |
| Miller et al. (18) | UM-HET3 | 9 mo | 4.2 ppm | NS | 16% |
| Miller et al. (18) | UM-HET3 | 9 mo | 14 ppm | 13% | 21% |
| Miller et al. (18) | UM-HET3 | 9 mo | 42 ppm | 23% | 26% |
| Zhang et al. (19) | C56BL/6 | 19 mo | 14 ppm | NS | 6% |
| Fok et al. (20) | C56BL/6 | 4 mo | 14 ppm | 11% | 16% |
| Bitto et al. (21) | C56BL/6 | 20 mo | 126 ppm | 21% | 37% |
| Dietary restriction | |||||
| Turturro et al. (22) | C57BL/6 | 14 wk | 60% AL | ~24%a | ~27%a |
| Turturro et al. (22) | B6C3F1 | 14 wk | 60% AL | ~35%a | ~33%a |
| Turturro et al. (22) | DBA/2 | 14 wk | 60% AL | ~10%a | ~25%a |
| Bonkowski et al. (23) | unknown | 8 wk | 70% AL | 19% | 28% |
| Flurkey et al. (24) | UM-HET3 | 4–5 wk | 66%–70% AL | 32% | 40% |
| Flurkey et al. (24) | CByB6F1 | 4–5 wk | 66%–70% AL | 38% | 49% |
| Mitchell et al. (25) | C57BL/6 | 6 mo | 60% AL | 19% | NS |
| Mitchell et al. (25) | DBA/2 | 6 mo | 60% AL | 16% | 36% |
Notes: NS = not statistically significant. The lifespan data represent the percent increase in either median or mean lifespan of the dietary restricted mice compared to mice fed ad libitum.
aData calculated from lifespan curves in the publication.
Although the effect of DR on the lifespan of rodents has been studied for many decades, there are limited studies comparing the effect of DR on male and female mice, and these are listed in Table 1. In contrast to rapamycin, the effect of DR on lifespan is similar for male and female mice, except for DBA/2 mice where the effect of DR on the lifespan is much greater for female mice (25%–36%) compared to male mice (10%–16%). Combining the DR data from all the mice except DBA/2 mice, the average effect of DR on the lifespan of female mice is 30% compared to 28% for male mice. Thus, the current data indicate that while DR and rapamycin increase the lifespan all strains of wild type mice tested; however, there are sex differences in the effect of these two manipulations on lifespan. Except for DBA/2 mice, DR increases lifespan of male and female mice to a similar extent, while rapamycin has a greater effect on longevity of female mice than male mice.
Effect of Rapamycin and DR on Mouse Models of Disease
The possibility that DR and rapamycin might affect lifespan through different mechanisms initially came from studies of mouse models of diseases. Several studies show that rapamycin increases the lifespan of mouse models of various diseases, especially cancer-prone mouse models (for reviews see Richardson et al. (8) and Aurriola Apelo and Lamming (9)). Although DR also has an effect on the lifespan of various mouse models of disease, there are several examples of mouse models where DR and rapamycin have different effects, and these studies are listed in Table 2. In 2012, our group reported that DR (60% ad libitum [AL]) but not rapamycin (14 ppm) increased the lifespan of a mouse model of amyotrophic lateral sclerosis (26). Sharp’s group (27) reported that rapamycin (14 ppm) increased lifespan of male and female Rb1+/- mice; a cancer-prone mouse model. The same group had earlier reported that DR (60% AL) had no effect of the lifespan of male Rb1+/- mice (28). More recently, Christy et al. (29) reported that rapamycin (14 ppm) had no effect of the lifespan of p53-/- mice even though DR was shown to increase the lifespan of p53-/- mice over 70% (30). However, Comas et al. (36) reported that Rapatar (a polymeric formulation of rapamycin given at 0.5 mg/kg via gavage) increased the lifespan of p53-/- mice. Both DR and rapamycin increase the lifespan of p53+/- mice (29,30,37).
Table 2.
Effect of Rapamycin and Dietary Restriction on the Lifespan of Various Genetic Mouse Models
| Mouse Model | Effect on Lifespan | Reference | |
|---|---|---|---|
| Rapa | DR | ||
| Amyotrophic lateral sclerosis (H46R/H48Q) | No effect | 14% increase | (26) |
| Cancer prone (Rb+/-) | 9%–14% increase | No effect | (27,28) |
| Cancer prone (p53-/-) | No effect | >70% increase | (29,30) |
| Genetically obese (ob/ob & db/db) | 18%–23% decrease | >50% increase | (31,32) |
| Disruption of Circadian clock (Bmal1-/-) | 50% increase | 25% decrease | (33,34) |
| Growth hormone receptor knockout | 5%–8% decrease | No effect | (23,35) |
Particularly impressive is the difference in the effect of rapamycin and DR on the lifespan of mouse models of obesity and the disruption of circadian rhythm. The two mouse models of obesity and type 2 diabetes have been studied extensively: leptin-deficient ob/ob mice and leptin-receptor deficient db/db mice. Harrison et al. (31) reported that DR (~66% AL) increased the lifespan of ob/ob mice over 50%. However, we found that rapamycin (14 ppm) reduced the lifespan of male and female db/db mice 18% to 23% (32). Rapamycin and DR have also been shown to have dramatically different effects on the lifespan of mice with a disruption in the in the circadian clock (ie, Bmal1-/- mice). Bmal1-/- mice are short lived and exhibit premature aging (38). Khapre et al. (33) reported that treatment with Rapatar (given at 125 mg/L in drinking water) resulted in a 50% increase in the lifespan of Bmal1-/- mice. However, Patel et al. (34) found that DR (70% of AL) reduced the lifespan of both male and female Bmal1-/- mice approximately 25%.
Rapamycin and DR also have different effects on growth hormone receptor knockout (GHR-KO) mice. It is well documented that knocking out growth hormone receptor results in a 20% to 50% increase in the lifespan of male and female mice (23,39,40). In 2006, Bonkowski et al. (23) reported that DR (70% of AL) had no effect on the lifespan of male GHR-KO mice and slightly increased maximum survival in female GHR-KO mice. In contrast, Fang et al. (35) recently reported that rapamycin treatment (i.p. injection of 4 mg/kg every other day) reduced the lifespan of both male and female GHR-KO mice.
The effect of DR and rapamycin on neural stem cells has also been studied. Park et al. (41) compared the effect of 12 months of DR (60% AL) and rapamycin (14ppm) on hippocampal neural stem and progenitor cell proliferation in 18-month-old male and female C57BL/6 mice. They found that DR increased the number of dividing cells, including dividing neural stem cells in the dentate gyrus of female mice, but not in male mice. However, rapamycin had no effect on the basal level of progenitor cell division or the size of the stem cell pool in either male or female mice.
Effect of Rapamycin and DR on mTOR Signaling and Autophagy
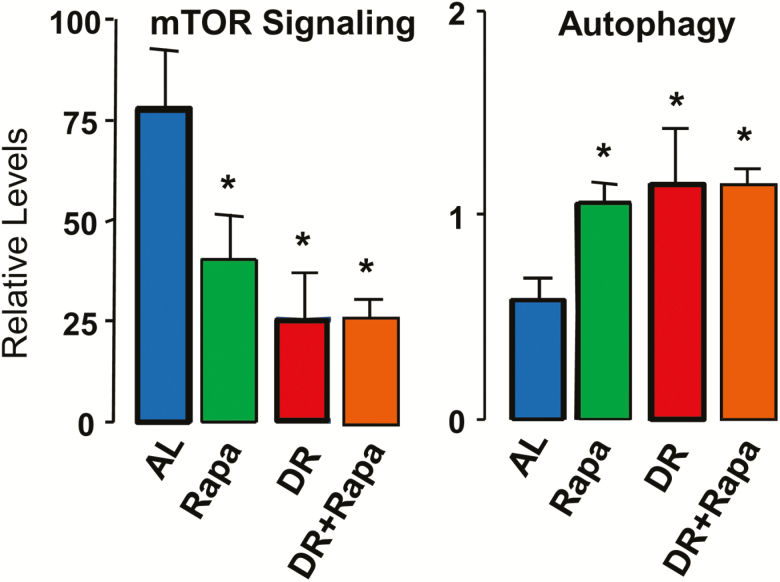
To our knowledge the first report to show that DR reduced mTOR signaling came from a study on the mechanism of action of DR in preventing mammary tumors in MMTV-TGF-α mice, which showed that 7 to 16 months of DR (50% reduction in food consumption) reduced mTOR signaling in mammary tissue (42). Fok et al. (43) was the first group to compare the effect of DR and rapamycin on a variety of parameters. They found that 6 months of DR (ie, 60% AL) or rapamycin treatment (14 ppm) reduced mTOR signaling to the same extent in liver (Figure 1). Figure 1 also shows that combining DR and rapamycin did not reduce mTOR signaling further. Fok et al. (43) also measured autophagy in the liver of the mice because autophagy is one of the major pathways regulated by mTOR, for example, reduced mTOR signaling results in increased autophagy and increased mTOR signaling results in reduced autophagy (44). As shown in Figure 1, autophagy was induced to a similar extent by DR and rapamycin in mouse liver and that combining DR and rapamycin did not result in a significant additional increase in autophagy. Subsequently, several reports have shown that DR reduces mTOR signaling in brain (45,46), adipose tissue (42), intestine (47), and skeletal muscle (48). These data are consistent with the original concept that DR and rapamycin are acting through similar mechanisms.
Figure 1.
Effect of DR and rapamycin on mTOR signaling and autophagy. The mTOR pathway was assessed in liver samples by measuring levels of phosphorylated S6 respect to the total S6 and autophagy by measuring levels of LC3II and LC3I (LC3II/LC3I ratio). At 2 months of age, male C57BL/6 mice were fed either ad libitum (AL, blue), 60% of AL (DR, red), 14 ppm rapamycin (Rapa, green), or DR and rapamycin (DR+Rapa, orange) for 6 months. The mice were fasted for 16 hours before they were used in this study. The data were obtained from 10 mice per group and expressed as mean ± SD; an asterisk denotes those values that are significantly different from AL mice at the p ≤ .05 level. There was no significant difference between the DR, Rapa, or DR+Rapa groups. The data for the AL, DR, and Rapa mice taken from Fok et al. (43) while the data for the DR+Rapa mice were generated at the same time but not published.
Effect of Rapamycin and DR on Insulin Sensitivity
It is well documented that DR has a dramatic effect on insulin sensitivity, and it has been argued that improved insulin sensitivity plays a role in the life-extending action of DR (49). In 1992, Masoro et al. (50) showed that DR significantly reduced plasma glucose and insulin at low levels throughout the lifespan of male F344 rats. Similar results were observed in male C57BL/6 mice (51). Several studies subsequently showed that both short- and long-term DR significantly improved glucose and insulin tolerance in laboratory rodents (25,52–54). DR has also been shown to have a similar effect in non-human primates; Gresl et al. (55), showed that DR increased insulin sensitivity and plasma glucose disappearance rate in Rhesus monkeys. Studies from humans have also shown that DR improves insulin sensitivity (56,57). In contrast, Lamming et al. (58) reported in 2012 that chronic rapamycin treatment (~2mg/kg/day) lead to glucose intolerance and insulin resistance in both male and female C57BL/6 mice as measured by the hyperinsulinemic-euglycemic clamp. The apparent dichotomy of effect on glucose metabolism suggests that rapamycin may not be a DR mimetic. Subsequently, there have been a large number of reports studying the effect of rapamycin on glucose and insulin tolerance in mice.
We directly compared the effects of 6 months of DR or rapamycin on insulin sensitivity (43). As shown in Figure 2, DR and rapamycin had quite different effects on glucose and insulin tolerance in male C57BL/6 mice. While DR improved insulin sensitivity as shown by increased glucose and insulin tolerance, rapamycin resulted in glucose intolerance. Two subsequent studies by our group further pointed towards an uncoupling of rapamycin-mediated effects on longevity and glucose metabolism. Miller et al. (18) measured glucose tolerance in male and female UM-HET3 mice fed three levels of rapamycin (4.7, 14, and 42 ppm) for 1 month. Rapamycin significantly increased glucose intolerance, which increased with increasing rapamycin levels, and this effect was more pronounced in male mice. Interestingly, the increased glucose intolerance was associated with increased lifespan of the mice; mice on 42 ppm rapamycin lived longer and were more glucose intolerant. Liu et al. (59) reported that feeding rapamycin (14 ppm) for 2 or 4 months induced glucose intolerance and insulin resistance in both male C57BL/6 and UM-HET3 mice fed either low- or high-fat diets. Importantly, they showed that the effect of rapamycin on glucose and insulin tolerance was lost within weeks after stopping rapamycin treatment. Other laboratories have also reported that rapamycin resulted in insulin resistance in mice as shown by the reduction in either glucose and/or insulin tolerance (60–62). Fang et al. (35) studied the effect of rapamycin on the GHR-KO mice, which are hypoinsulinemic and extremely insulin sensitive (63). They found that rapamycin-induced insulin resistance in GHR-KO mice (35).
Figure 2.
Effect of DR and rapamycin on glucose and insulin tolerance. The data were obtained from male mice described in Figure 1, and data are expressed as mean ±SEM for 10 mice per group. The values that are significantly different (p ≤ .05) from AL mice is shown by the asterisks. The data for the AL (blue), DR (red), and Rapa (green) mice taken from Fok et al. (43) while the data for the mice treated with both rapamycin and DR (orange) were generated at the same time but not published.
While most of the studies show that rapamycin results in insulin resistance/glucose intolerance in mice, there are studies reporting that rapamycin has no effect or improves glucose tolerance or insulin sensitivity. In 2013, Fang et al. (64) reported that rapamycin treatment (injected i.p.) of a genetically heterozygous strain of male mice resulted in insulin resistance after 2 to 6 weeks of treatment; however, insulin sensitivity was improved after 5 months of rapamycin treatment. Lesneiwski et al. (65) reported that while ~4 months of rapamycin (14 ppm) lead to glucose intolerance in young male B6D2F1 mice; rapamycin improved glucose tolerance in old (~30 months) male mice. Lamming et al. (66) reported that feeding UM-HET3 mice rapamycin (14 ppm for 3 or 12 weeks) leads to glucose intolerance in both young (6 months) and old (21 months) mice. However, in contrast to their previous study in C57BL/6 mice, rapamycin treatment had no effect on insulin tolerance in UM-HET3 mice. Reifsnyder et al. (67) reported that rapamycin treatment (14 ppm) did not exacerbate impaired glucose or insulin tolerance in five classic mouse strains of type 2 diabetes and increased insulin sensitivity in three of the strains. On the other hand, Rapatar was reported to improved glucose tolerance and insulin sensitivity in db/db mice (68). More recently, den Hartigh et al. (69) reported that rapamycin (14 ppm) improved insulin sensitivity in male C57BL/6 male mice fed a high-fat, high-sucrose diet and for 20 weeks.
To directly address shared mechanistic pathways between DR and rapamycin, we tested the effect of combined DR and rapamycin intervention in mice (70). As shown in Figure 3, mice given a combination of DR and rapamycin showed improved glucose and insulin tolerance compared to mice fed AL or rapamycin alone. These data indicate that DR can over-ride the negative effects of rapamycin on insulin sensitivity. Therefore, would a manipulation that can negate rapamycin’s harmful effect on insulin sensitivity have a beneficial effect on lifespan when combined with rapamycin? The Intervention Testing Program recently studied the effect of combining metformin with rapamycin on lifespan because metformin, an anti-diabetic drug, might help alleviate insulin resistance induced by rapamycin (71). When male and female UM-HET3 mice were fed a combination of metformin (0.1%) and rapamycin (14 ppm), lifespan was significantly increased, and this increase in lifespan appeared to be greater than previous studies in which rapamycin was given alone (72). Weiss et al. (73) reported that glucose intolerance induced in female UM-HET3 mice by rapamycin (14 ppm) was reversed by treatment with metformin (0.1%) but not in male mice even though lifespan was increased in both male and female mice (72). These results again suggest that in mice, the effects of rapamycin on longevity and glucose metabolism can be at least partially uncoupled.
Figure 3.
Effect of DR and rapamycin on the liver transcriptome. The principle component analysis of transcriptome data obtained from the livers of AL (blue), DR (red), rapamycin (green) and DR + rapamycin (orange) mice are shown for the top three principle components. Using linear discrimination predictor and the quadratic discriminant analysis, the PCA data were statistically analyzed. The DR group showed perfect separation of 1 between either the AL group or the rapamycin group. The rapamycin group showed a separation of 0.8 from the AL group, which indicates separation but some overlap between the two groups. Similarly, when the DR group was compared with the DR + rapamycin group a separation of 0.8 was observed. However, when the DR + rapamycin group was compared to either the rapamycin group or AL group, a perfect separation of 1 was observed. Data were taken from Fok et al. (70)
In summary, the current data consistently show that DR improves glucose tolerance and insulin sensitivity in mice. However, the effect of rapamycin on glucose tolerance and insulin sensitivity is less clear as given in Table 3. Almost all the studies with wild type strains of mice show reduced glucose tolerance when treated with rapamycin. However, the reports on the effect of rapamycin on insulin sensitivity are mixed. While several studies show that rapamycin induces insulin resistance, many studies show no change, and a few studies show that rapamycin improves insulin sensitivity. In contrast to wild type strains of mice, many studies with mice that have insulin resistance, that is, fed high-fat diets or were genetic models of diabetes, report that rapamycin treatment improves insulin sensitivity. It is not clear at this time why different results are found for the effect of rapamycin on insulin sensitivity; however, the differences could be related to the duration of rapamycin treatment, the mode of treating mice with rapamycin, or the strain of mice studied. It has been suggested that rapamycin induces insulin resistance at least partially through inhibition of mTORC2 complex, and chronic administration of rapamycin may also impair insulin action via inhibition of mTORC1 (58,74).
Table 3.
Effect of Rapamycin on Glucose Tolerance and Insulin Sensitivity in Mice
| Reference | Strain (Sex) and Age of Mice | Dose | Duration of Treatment | Glucose Tolerance | Insulin Sensitivity |
|---|---|---|---|---|---|
| Chang et al. (60) | KK/HIJ(M)a 4–6 wk |
2 mg/kg* | 42 d | Decrease | Decrease |
| Lamming et al. (58) | C57BL/6 (M&F) age? | 2 mg/kg* | 2 wk | Decrease | Decrease |
| Yang et al. (61) | B6D2F1(?) old | 14 ppm | 1 mo | Decrease | NC |
| Fok et al. (43) | C57BL/6 (M) 2 mo |
14 ppm | 6 mo | Decrease | NC |
| Laming et al. (66) | UM-HET3(M) 6 mo |
14 ppm | 3 and 12 wk | Decrease | NC |
| Laming et al. (66) | UM-HET3(M) 21 mo |
14 ppm | 3 and 12 wk | Decrease | NC |
| Fang et al. (64) | Mixed(M) 3 mo |
4 mg/kg* | 2 wk | Decrease | Decrease |
| Fang et al. (64) | Mixed(M) 3 mo |
4 mg/kg* | 6 wk | Decrease | NC |
| Fang et al. (64) | Mixed(M) 3 mo |
4 mg/kg* | 5 mo | Decrease | Increase |
| Miller et al. (18) | UM-HET3 (M&F) 4 mo |
4.7, 14, 42 ppm | 1 mo | Decrease | -- |
| Liu et al. (59) | UM-HET3(M)b 10–12 mo |
14 ppm | 2 and 4 mo | Decrease | Decrease |
| Liu et al. (59) | C57BL/6(M)b 2 mo |
14 ppm | 2 and 4 mo | Decrease | Decrease |
| Reifsnyder et al. (67) | KK(M) 8–11 wk |
14 ppm | 2–6 wk | NC | NC |
| Reifsnyder et al. (67) | KK-Ay(M) 8–11 wk |
14 ppm | 2–6 wk | NC | NC |
| Reifsnyder et al. (67) | NcZ10(M) 8–11 wk |
14 ppm | 2–6 wk | NC | Increase |
| Reifsnyder et al. (67) | BKS-db/db(M) 8–11 wk |
14 ppm | 2–6 wk | NC | Increase |
| Reifsnyder et al. (67) | NcZ10(M) 8–11 wk |
14 ppm | 2–6 wk | NC | Increase |
| Lesniewski et al. (65) | B6D2F1(M) ~4 mo old |
14 ppm | 6–8 wk | Decrease | -- |
| Lesniewski et al. (65) | B6D2F1(M) 30 mo old |
14 ppm | 6–8 wk | Increase | -- |
| Samidurai et al. (68) |
db/db(?) adult |
Rapatarc | 10 wk | Increase | Increase |
| Fang et al. (35) | GHR-KO(M) 3 mo |
4 mg/kg* | 5 mo | NC | Decrease |
| den Hartigh et al. (69) | C57BL/6(M)a 10 wk |
14 ppm | 5 mo | Increase | Increase |
| Weiss et al. (73) | UM-HET3 (M&F) 4 mo | 14 ppm | 1, 2, 3, and 9 mo | Decrease | Decrease |
Notes: NC = no change. Unless otherwise indicated, rapamycin was given in the food. The asterisk shows those studies where rapamycin was given by i.p. injection.
aMice fed a high-fat diet.
bMice fed either a high-fat or low-fat diet.
cRapatar is a polymeric formulation of rapamycin fed at 0.75 mg/kg in water.
Effect of DR and Rapamycin on Various Metabolic Pathways
Miller et al. (18) studied the effect of 5 months of DR (60% AL) or rapamycin (14 ppm) on circulating levels of several endocrine factors in UM-HET3 mice (10). It is well established that DR reduces circulating levels of IGF-1 (75,76). Miller et al. (18) also observed that DR reduced plasma levels of IGF-1 significantly but rapamycin did not. In addition, Miller et al. (18) observed that DR but not rapamycin, increased circulating levels of thyroid hormone T4 and reduced circulating levels of leptin. They also studied the effect of rapamycin or DR on circulating levels of FGF-21, a hormone produced by the liver in response to prolonged fast (77). Zhang et al. (78) reported that transgenic mice overexpressing FGF-21 live longer. Miller et al. (18) reported that DR resulted in a dramatic decrease in plasma FGF-21 levels, while rapamycin either had no effect (males) or significantly increased (females) plasma levels of FGF-21. In contrast, Kuhla et al. (79) reported that DR (60% AL) increased plasma levels of FGF-21.
Two groups have compared the effect of DR and rapamycin on liver metabolism. Fok et al. (70) evaluated the effect of 6 months of DR (60% AL) or rapamycin (14 ppm) on the liver metabolome by measuring the levels of over 1,000 metabolites in the livers of male C57BL/6 mice. DR significantly altered the levels of 173 metabolites; however, rapamycin had no significant effect on any of the metabolites studied. Most of the metabolite pathways that were significantly changed by DR were related to regulation of energy status, for example, amino acid, carbohydrate, lipid, and energy (which included the Krebs cycle and oxidative phosphorylation). Interestingly, when mice were treated with both DR and rapamycin, a significant change was observed in an additional 92 metabolites. Thus, a combination of DR and rapamycin had a greater effect on the liver metabolome than DR alone. In a second report, Yu et al. (80) studied the effect of 6 months of DR or rapamycin in same mice on various metabolic pathways in liver. Both DR and rapamycin inhibited lipogenesis and activated lipolysis in liver and increased serum levels of free fatty acids. However, only DR activated β-oxidation, leading to the increased production of ketone bodies by the liver. In contrast, Fang et al. (64) reported that 5 months of rapamycin treatment (4mg/kg i.p.) significantly increased total ketone body in plasma of mice with a heterozygous genetic background.
Recently, Choi et al. (81) studied the effect of DR and rapamycin on the metabolome of yeast. They found that DR had a greater effect on the yeast metabolome than rapamycin, for example, out of 113 metabolites identified, DR significantly altered the levels of ~35% of the metabolites and rapamycin ~10% of the metabolites. Less than 20% of the metabolites that changed were the same for the yeast treated with DR or rapamycin. Choi et al. (81) also found that DR, but not rapamycin, up-regulated genes for β-oxidation in yeast.
In summary, the current studies comparing the effect of DR or rapamycin on various metabolic pathways show that these two manipulations have quite different effects on most of the pathways currently studied. This is particularly striking in the studies in which the metabolome of yeast or mouse was measured.
Effect of DR and Rapamycin on Gene Expression and DNA Methylation
The first report comparing the effect of DR and rapamycin on the expression of specific mRNA transcripts came from Fok et al. (43) in which 2-month-old male C57BL/6 were treated with DR (60% AL) or rapamycin (14 ppm) for 6 months. They reported that DR and rapamycin had significantly different effects on the levels of transcripts for cyclin D1, p16, p21, and all the Sirt genes except Sirt5. Subsequently, Miller et al. (18) measured the expression of 52 hepatic genes involved in xenobiotic metabolism in the livers of 4-month-old male and female UM-HET3 mice treated with DR (60% AL) or rapamycin (14 ppm) for 8 months. They found that the change in pattern of expression of the genes involved in xenobiotic metabolism were “quite distinct” in the DR and rapamycin mice.
The studies by Fok et al. (43) and Miller et al. (18) led our group to conduct a comprehensive analysis of the liver transcriptome of male C57BL/6 mice maintained on either DR, rapamycin (14 ppm), or a combination of DR and rapamycin for 6 months (70). As shown in Figure 3, principal component analysis of the transcriptome data showed that the DR mice form a separate group from control mice fed AL or mice fed rapamycin. Using a fold change of >15% and a false discovery rate of q < 0.05, Fok et al. (70) identified over 2,500 genes, out of the 25,600 transcripts analyzed, that changed significantly by either DR or rapamycin. Only ~20% of these 2,500 genes were changed similarly by DR and rapamycin, that is, ~80% of the transcripts that changed were unique to either DR or rapamycin treatment. DR had a greater effect on up-regulated genes, and rapamycin had a greater effect on down-regulated genes. Ingenuity Pathway Analysis of the transcriptomic data revealed that about two-thirds of the pathways identified were unique to either DR or rapamycin. Of those pathways altered by both DR and rapamycin, protein ubiquitination, mTOR signaling, mitochondrial function, and the Nrf2 pathways received the highest scores. One of the most interesting outcomes of the study by Fok et al. (70) was the major increase in the number of genes (more than 1,800) that were significantly changed by a combination of DR and rapamycin, suggesting a potential synergistic effect. Rapamycin combined with DR not only increased the number of transcripts that were significantly changed, but also increased the fold change in the expression of many of the transcripts. As would be expected, more pathways were significantly changed by the combination of DR and rapamycin than DR or rapamycin alone. Subsequently, Fok et al. (82) measured the transcriptome of adipose tissue obtained from the same mice and found even a greater difference between mice fed DR and rapamycin. The expression of only six genes changed significantly with rapamycin while over a thousand changed with DR.
Recently, Choi et al. (81) compared the effect of DR and rapamycin on the transcriptome of yeast. Principal component analysis showed that the transcriptome data appeared as distinct clusters. DR had a much greater effect on the transcriptome that rapamycin; DR significantly changed the expression of 2,006 genes compared to 439 genes for rapamycin. Approximately 25% of the genes showed similar changes for both DR and rapamycin. They also found that the changes in the yeast transcriptome were associated with different pathways/processes. Thus, the transcriptome data from yeast is quite comparable to data obtained from the liver and adipose tissue of mice.
Because rapamycin is known to affect translation at the step of initiation (83), comparing the effect of DR and rapamycin on protein synthesis is important. Rabinovitch’s group measured the synthesis and turnover of proteins in the liver of 3- and 25-month-old female C57BL/6 mice treated with DR (60% AL) or rapamycin (14 ppm) for 10 weeks (84). Compared to age-matched controls, DR increased the protein half-lives 35%–60% while rapamycin increased protein half-lives 15%. The effect of rapamycin and DR on protein turnover and abundance differed greatly between canonical pathways with DR most closely recapitulating the young phenotype. They also found that DR reduced polysome loading while rapamycin increased loading. Thus, the study by Karunadharma et al. (84) indicates that DR and rapamycin have different effects at the level of translation.
More recently, investigators have compared the effect of DR and rapamycin on epigenetic regulation, specifically DNA methylation. Using an epigenetic-clock they had developed for mouse liver, Wang et al. (85) reported that both DR (60% AL) and rapamycin (42 ppm) showed a reduction in the clock; however, the decrease was ~30% greater for DR than rapamycin. Cole et al. (86) measured DNA methylation changes across all 42 million CpG sites in the liver genome at the single-nucleotide level in mice fed AL compared to dwarf mice and mice fed either DR (60% AL) or rapamycin (42 ppm). Age-associated hypomethylation was suppressed in both DR and dwarf mice and to a lesser extent in rapamycin-treated mice. Hypermethylation was also suppressed by DR but not rapamycin. Both DR and rapamycin also induced changes in DNA methylation (hypomethylation or hypermethylation) at sites that did not change with age; however, rapamycin showed greater changes in DNA methylation than DR.
Summary
Once it was established that the DR’s effect on lifespan arose because DR retarded aging, questions were raised about how a manipulation as simple as reducing food consumption could trigger such a dramatic effect on the lifespan of an animal. To answer this question, Dave Sharp proposed in 2004 that changes in nutrient sensing through mTOR signaling was responsible for how an organism sensed DR at the molecular level. More importantly, his proposal was experimentally testable. When Harrison et al. (6) demonstrated that the lifespan of mice could be increased by feeding mice rapamycin to reduce mTOR signaling, the research community assumed that rapamycin was mimicking DR and that the effect of DR and rapamycin on lifespan were likely to be occurring primarily through similar pathways/mechanisms. This concept was supported by the fact that DR and rapamycin are two of the most robust manipulations known to retard aging, increasing lifespan across a wide range of evolutionarily diverse species.
Initially, studies with yeast and C. elegans supported the concept that DR and mutations in TOR signaling increased lifespan through similar pathways; however, it became clear as more studies were published that DR and rapamycin had different effects on a number of pathways and processes in mice as described above. For example, while it is well documented that both DR and rapamycin increase the lifespan of various strains of mice, the effect of rapamycin on lifespan is greater in female than male mice, while DR increases the lifespan of male and female mice similarly in most strains of mice. In addition, the lifespans of many genetically modified mouse models are affected differently by DR and rapamycin. One of the most pronounced differences in DR and rapamycin is their effect on insulin sensitivity; DR improves insulin sensitivity while rapamycin tends to make mice glucose intolerant and insulin resistance (43,59). Using an unbiased approach to study the transcriptome and metabolome as well as the synthesis of proteins, investigators have shown that most of the changes in transcripts, metabolites, or newly synthesized proteins in liver were unique to each manipulation (70,80–82,84). Thus, while DR and rapamycin affect some of the transcripts/proteins/metabolites similarly, the overwhelming majority of the changes are unique to either DR or rapamycin. Because similar effects were observed in tissues from mice as well as yeast, these data provide strong evidence that DR and rapamycin are affecting tissues/cells differently.
Based on the current data, we believe that it is likely that the effect of DR and rapamycin lifespan/aging in mice occurs primarily through different pathways even though DR and rapamycin have similar effects on some pathways (eg, mTOR signaling and autophagy). This leads us to predict that a combination of DR and rapamycin will have a greater effect on lifespan/aging than either DR or rapamycin alone. Unfortunately, there are very few studies that have evaluated the effect of combining DR and rapamycin. Bjedov et al. (16) reported that feeding rapamycin to Drosophila on a DR-diet resulted in a greater increase in lifespan. Studies from our group on the transcriptome and metabolome show that a combination of rapamycin and DR significantly increased the number of transcripts or metabolites that were changed compared to the number altered by either DR or rapamycin alone (70). It should be noted that the combination of DR and rapamycin also resulted in a larger change in the expression of many of the transcripts that DR alone. In addition, it is possible that combining DR with rapamycin might negate negative effects of rapamycin, for example, insulin resistance (Figure 2). For example, combining metformin with rapamycin improved glucose tolerance (73) and appeared to increase the lifespan of the mice more than rapamycin alone (72). Therefore, it would be important in the future to determine whether combining DR and rapamycin results in an increase in lifespan over that compared to mice fed DR alone. If the combination of DR and rapamycin results in a greater increase in lifespan than DR alone, it would be strong evidence that the effect of DR and rapamycin increase lifespan occur through different pathways/mechanisms. In addition, this result would demonstrate that combining manipulations that increase lifespan would lead to the development of an intervention that would be more effective at retarding aging.
Funding
The efforts of authors were supported by National Institute of Aging (NIA) grants R01AG045693 (A.U. and A.R.), KO1AG 056655-01A1 (A.U.), R01 AG 050797 (A.S.), the Nathan Shock Centers of Oklahoma (P30 AG050911) and San Antonio (P30 AG013319), the Oklahoma Center for Advancement of Science and Technology HR17-098 (A.U.), the Oklahoma Center for Adult Stem Cell Research (A.R.), the American Federation of Aging Research 17132 (A.U.), and a Senior Career Research Award (A.R.) from the Department of Veterans Affairs.
Conflict of Interest
None reported.
Acknowledgments
We also thank Dr. David Sharp for reviewing the manuscript and informing us of how he came to propose that rapamycin would increase the lifespan of mice.
References
- 1. McCay CM, Crowell MF, and Maynard LA. The effect of retarded growth upon the length of life span and upon the ultimate body size. 1935. Nutrition. 1989;5(3):155–171; discussion 172. doi: 10.3410/f.717977201.793470731 [DOI] [PubMed] [Google Scholar]
- 2. Taormina G, Mirisola MG. Calorie restriction in mammals and simple model organisms. Biomed Res Int. 2014;2014:308690. doi: 10.1155/2014/308690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barbet NC, Schneider U, Helliwell SB, Stansfield I, Tuite MF, Hall MN. TOR controls translation initiation and early G1 progression in yeast. Mol Biol Cell. 1996;7:25–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a [DOI] [PubMed] [Google Scholar]
- 5. Sharp ZD, Bartke A. Evidence for down-regulation of phosphoinositide 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR)-dependent translation regulatory signaling pathways in Ames dwarf mice. J Gerontol A Biol Sci Med Sci. 2005;60:293–300. [DOI] [PubMed] [Google Scholar]
- 6. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaeberlein M, Kennedy BK. Ageing: a midlife longevity drug? Nature. 2009;460:331–332. doi: 10.1038/460331a [DOI] [PubMed] [Google Scholar]
- 8. Richardson A, Galvan V, Lin AL, Oddo S. How longevity research can lead to therapies for Alzheimer’s disease: the rapamycin story. Exp Gerontol. 2015;68:51–58. doi: 10.1016/j.exger.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arriola Apelo SI, Lamming DW. Rapamycin: an InhibiTOR of aging emerges from the soil of Easter Island. J Gerontol A Biol Sci Med Sci. 2016;71:841–849. doi: 10.1093/gerona/glw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Richardson A. Rapamycin, anti-aging, and avoiding the fate of Tithonus. J Clin Invest. 2013;123:3204–3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature. 2013;493:338–345. doi: 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson SC, Yanos ME, Bitto A, et al. Dose-dependent effects of mTOR inhibition on weight and mitochondrial disease in mice. Front Genet. 2015;6:247. doi: 10.3389/fgene.2015.00247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kaeberlein M, Powers RW III, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535 [DOI] [PubMed] [Google Scholar]
- 14. Hansen M, Taubert S, Crawford D, Libina N, Lee SJ, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x [DOI] [PubMed] [Google Scholar]
- 15. Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bjedov I, Toivonen JM, Kerr F, et al. Mechanisms of life span extension by rapamycin in the fruit fly Drosophila melanogaster. Cell Metab. 2010;11:35–46. doi: 10.1016/j.cmet.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller RA, Harrison DE, Astle CM, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell. 2014;13:468–477. doi: 10.1111/acel.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang Y, Bokov A, Gelfond J, et al. Rapamycin extends life and health in C57BL/6 mice. J Gerontol A Biol Sci Med Sci. 2014;69(2):119–130. doi: 10.1093/gerona/glt056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fok WC, Chen Y, Bokov A, et al. Mice fed rapamycin have an increase in lifespan associated with major changes in the liver transcriptome. PLoS One. 2014;9:e83988. doi: 10.1371/journal.pone.0083988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bitto A, Ito TK, Pineda VV, et al. Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. Elife. 2016;5:e16351. doi: 10.7554/eLife.16351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. [DOI] [PubMed] [Google Scholar]
- 23. Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci U S A. 2006;103:7901–7905. doi: 10.1073/pnas.0600161103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flurkey K, Astle CM, Harrison DE. Life extension by diet restriction and N-acetyl-L-cysteine in genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2010;65:1275–1284. doi: 10.1093/gerona/glq155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, et al. Effects of sex, strain, and energy intake on hallmarks of aging in mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bhattacharya A, Bokov A, Muller FL, et al. Dietary restriction but not rapamycin extends disease onset and survival of the H46R/H48Q mouse model of ALS. Neurobiol Aging. 2012;33:1829–1832. doi: 10.1016/j.neurobiolaging.2011.06.002 [DOI] [PubMed] [Google Scholar]
- 27. Livi CB, Hardman RL, Christy BA, et al. Rapamycin extends life span of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging (Albany NY). 2013;5:100–110. doi: 10.18632/aging.100533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sharp ZD, Lee WH, Nikitin AY, et al. Minimal effects of dietary restriction on neuroendocrine carcinogenesis in Rb+/- mice. Carcinogenesis. 2003;24:179–183. [DOI] [PubMed] [Google Scholar]
- 29. Christy B, Demaria M, Campisi J, et al. p53 and rapamycin are additive. Oncotarget. 2015;6:15802–15813. doi: 10.18632/oncotarget.4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hursting SD, Perkins SN, Phang JM. Calorie restriction delays spontaneous tumorigenesis in p53-knockout transgenic mice. Proc Natl Acad Sci U S A. 1994;91:7036–7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harrison DE, Archer JR, Astle CM. Effects of food restriction on aging: separation of food intake and adiposity. Proc Natl Acad Sci U S A. 1984;81:1835–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sataranatarajan K, Ikeno Y, Bokov A, et al. Rapamycin increases mortality in db/db mice, a mouse model of type 2 diabetes. J Gerontol A Biol Sci Med Sci. 2016;71:850–857. doi: 10.1093/gerona/glv170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khapre RV, Patel SA, Kondratova AA, et al. Metabolic clock generates nutrient anticipation rhythms in mTOR signaling. Aging (Albany NY). 2014;6:675–689. doi: 10.18632/aging.100686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel SA, Chaudhari A, Gupta R, Velingkaar N, Kondratov RV. Circadian clocks govern calorie restriction-mediated life span extension through BMAL1- and IGF-1-dependent mechanisms. FASEB J. 2016;30:1634–1642. doi: 10.1096/fj.15-282475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fang Y, Hill CM, Darcy J, et al. Effects of rapamycin on growth hormone receptor knockout mice. Proc Natl Acad Sci U S A. 2018;115:E1495–E1503. doi: 10.1073/pnas.1717065115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Comas M, Toshkov I, Kuropatwinski KK, et al. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging (Albany NY). 2012;4:715–722. doi: 10.18632/aging.100496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Komarova EA, Antoch MP, Novototskaya LR, et al. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging (Albany NY). 2012;4:709–714. doi: 10.18632/aging.100498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core component of the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coschigano KT. Aging-related characteristics of growth hormone receptor/binding protein gene-disrupted mice. Age (Dordr). 2006;28:191–200. doi: 10.1007/s11357-006-9004-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sun LY, Spong A, Swindell WR, et al. Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. Elife. 2013;2:e01098. doi: 10.7554/eLife.01098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park JH, Glass Z, Sayed K, et al. Calorie restriction alleviates the age-related decrease in neural progenitor cell division in the aging brain. Eur J Neurosci. 2013;37:1987–1993. doi: 10.1111/ejn.12249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dogan S, Johannsen AC, Grande JP, Cleary MP. Effects of intermittent and chronic calorie restriction on mammalian target of rapamycin (mTOR) and IGF-I signaling pathways in mammary fat pad tissues and mammary tumors. Nutr Cancer. 2011;63:389–401. doi: 10.1080/01635581.2011.535968 [DOI] [PubMed] [Google Scholar]
- 43. Fok WC, Zhang Y, Salmon AB, et al. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci Med Sci. 2013;68:108–116. doi: 10.1093/gerona/gls127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Markaki M, Tavernarakis N. Metabolic control by target of rapamycin and autophagy during ageing - a mini-review. Gerontology. 2013;59:340–348. doi: 10.1159/000348599 [DOI] [PubMed] [Google Scholar]
- 45. Ma L, Dong W, Wang R, et al. Effect of caloric restriction on the SIRT1/mTOR signaling pathways in senile mice. Brain Res Bull. 2015;116:67–72. doi: 10.1016/j.brainresbull.2015.06.004 [DOI] [PubMed] [Google Scholar]
- 46. Phillips-Farfán BV, Rubio Osornio Mdel C, Custodio Ramírez V, Paz Tres C, Carvajal Aguilera KG. Caloric restriction protects against electrical kindling of the amygdala by inhibiting the mTOR signaling pathway. Front Cell Neurosci. 2015;9:90. doi: 10.3389/fncel.2015.00090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yilmaz OH, Katajisto P, Lamming DW, et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature. 2012;486(7404):490–495. doi: 10.1038/nature11163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Margolis LM, Rivas DA, Berrone M, et al. Prolonged Calorie Restriction Downregulates Skeletal Muscle mTORC1 signaling independent of dietary protein intake and associated microRNA expression. Front Physiol. 2016;7:445. doi: 10.3389/fphys.2016.00445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bartke A, Wright JC, Mattison JA, Ingram DK, Miller RA, Roth GS. Extending the lifespan of long-lived mice. Nature. 2001;414:412. doi: 10.1038/35106646 [DOI] [PubMed] [Google Scholar]
- 50. Masoro EJ, McCarter RJ, Katz MS, McMahan CA. Dietary restriction alters characteristics of glucose fuel use. J Gerontol. 1992;47:B202–B208. [DOI] [PubMed] [Google Scholar]
- 51. McCarter R, Mejia W, Ikeno Y, et al. Plasma glucose and the action of calorie restriction on aging. J Gerontol A Biol Sci Med Sci. 2007;62:1059–1070. [DOI] [PubMed] [Google Scholar]
- 52. Escrivá F, Gavete ML, Fermín Y, et al. Effect of age and moderate food restriction on insulin sensitivity in Wistar rats: role of adiposity. J Endocrinol. 2007;194:131–141. doi: 10.1677/joe.1.07043 [DOI] [PubMed] [Google Scholar]
- 53. Cameron KM, Miwa S, Walker C, von Zglinicki T. Male mice retain a metabolic memory of improved glucose tolerance induced during adult onset, short-term dietary restriction. Longev Healthspan. 2012;1:3. doi: 10.1186/2046-2395-1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Selman C, Hempenstall S. Evidence of a metabolic memory to early-life dietary restriction in male C57BL/6 mice. Longev Healthspan. 2012;1:2. doi: 10.1186/2046-2395-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gresl TA, Colman RJ, Roecker EB, et al. Dietary restriction and glucose regulation in aging rhesus monkeys: a follow-up report at 8.5 yr. Am J Physiol Endocrinol Metab. 2001;281:E757–E765. doi: 10.1152/ajpendo.2001.281.4.E757 [DOI] [PubMed] [Google Scholar]
- 56. Franssila-Kallunki A, Rissanen A, Ekstrand A, Ollus A, Groop L. Weight loss by very-low-calorie diets: effects on substrate oxidation, energy expenditure, and insulin sensitivity in obese subjects. Am J Clin Nutr. 1992;56(1 Suppl):247S–248S. doi: 10.1093/ajcn/56.1.247S [DOI] [PubMed] [Google Scholar]
- 57. Larson-Meyer DE, Heilbronn LK, Redman LM, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, beta-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29:1337–1344. doi: 10.2337/dc05-2565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Y, Diaz V, Fernandez E, et al. Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY). 2014;6(9):742–754. doi: 10.18632/aging.100688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chang GR, Wu YY, Chiu YS, et al. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105:188–198. doi: 10.1111/j.1742-7843.2009.00427.x [DOI] [PubMed] [Google Scholar]
- 61. Yang SB, Lee HY, Young DM, et al. Rapamycin induces glucose intolerance in mice by reducing islet mass, insulin content, and insulin sensitivity. J Mol Med (Berl). 2012;90:575–585. doi: 10.1007/s00109-011-0834-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lamming DW. Inhibition of the mechanistic target of rapamycin (mTOR)-rapamycin and beyond. Cold Spring Harb Perspect Med. 2016;6(5):pii: a025924. doi: 10.1101/cshperspect.a025924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dominici FP, Arostegui Diaz G, Bartke A, Kopchick JJ, Turyn D. Compensatory alterations of insulin signal transduction in liver of growth hormone receptor knockout mice. J Endocrinol. 2000;166:579–590. [DOI] [PubMed] [Google Scholar]
- 64. Fang Y, Westbrook R, Hill C, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lesniewski LA, Seals DR, Walker AE, et al. Dietary rapamycin supplementation reverses age-related vascular dysfunction and oxidative stress, while modulating nutrient-sensing, cell cycle, and senescence pathways. Aging Cell. 2017;16:17–26. doi: 10.1111/acel.12524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lamming DW, Ye L, Astle CM, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12:712–718. doi: 10.1111/acel.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Reifsnyder PC, Flurkey K, Te A, Harrison DE. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY). 2016;8:3120–3130. doi: 10.18632/aging.101117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Samidurai A, Salloum FN, Durrant D, Chernova OB, Kukreja RC, Das A. Chronic treatment with novel nanoformulated micelles of rapamycin, Rapatar, protects diabetic heart against ischaemia/reperfusion injury. Br J Pharmacol. 2017;174:4771–4784. doi: 10.1111/bph.14059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. den Hartigh LJ, Goodspeed L, Wang SA, et al. Chronic oral rapamycin decreases adiposity, hepatic triglycerides and insulin resistance in male mice fed a diet high in sucrose and saturated fat. Exp Physiol. 2018;103:1469–1480. doi: 10.1113/EP087207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fok WC, Bokov A, Gelfond J, et al. Combined treatment of rapamycin and dietary restriction has a larger effect on the transcriptome and metabolome of liver. Aging Cell. 2014;13:311–319. doi: 10.1111/acel.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giannarelli R, Aragona M, Coppelli A, Del Prato S. Reducing insulin resistance with metformin: the evidence today. Diabetes Metab. 2003;29(4 Pt 2):6S28–6S35. [DOI] [PubMed] [Google Scholar]
- 72. Strong R, Miller RA, Antebi A, et al. Longer lifespan in male mice treated with a weakly estrogenic agonist, an antioxidant, an α-glucosidase inhibitor or a Nrf2-inducer. Aging Cell. 2016;15:872–884. doi: 10.1111/acel.12496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Weiss RA, Bernardy J. Induction of fat apoptosis by a non-thermal device: mechanism of action of non-invasive high-intensity electromagnetic technology in a porcine model. Lasers Surg Med. 2019;51:47–53. doi: 10.1002/lsm.23039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ye L, Varamini B, Lamming DW, Sabatini DM, Baur JA. Rapamycin has a biphasic effect on insulin sensitivity in C2C12 myotubes due to sequential disruption of mTORC1 and mTORC2. Front Genet. 2012;3:177. doi: 10.3389/fgene.2012.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sonntag WE, Lynch CD, Cefalu WT, et al. Pleiotropic effects of growth hormone and insulin-like growth factor (IGF)-1 on biological aging: inferences from moderate caloric-restricted animals. J Gerontol A Biol Sci Med Sci. 1999;54:B521–B538. [DOI] [PubMed] [Google Scholar]
- 76. Fontana L, Villareal DT, Das SK, et al. ; CALERIE Study Group Effects of 2-year calorie restriction on circulating levels of IGF-1, IGF-binding proteins and cortisol in nonobese men and women: a randomized clinical trial. Aging Cell. 2016;15:22–27. doi: 10.1111/acel.12400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;26:312–324. doi: 10.1101/gad.184788.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Zhang Y, Xie Y, Berglund ED, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife. 2012;1:e00065. doi: 10.7554/eLife.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kuhla A, Hahn S, Butschkau A, Lange S, Wree A, Vollmar B. Lifelong caloric restriction reprograms hepatic fat metabolism in mice. J Gerontol A Biol Sci Med Sci. 2014;69:915–922. doi: 10.1093/gerona/glt160 [DOI] [PubMed] [Google Scholar]
- 80. Yu Z, Wang R, Fok WC, Coles A, Salmon AB, Pérez VI. Rapamycin and dietary restriction induce metabolically distinctive changes in mouse liver. J Gerontol A Biol Sci Med Sci. 2015;70:410–420. doi: 10.1093/gerona/glu053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Choi KM, Hong SJ, van Deursen JM, Kim S, Kim KH, Lee CK. Caloric restriction and rapamycin differentially alter energy metabolism in yeast. J Gerontol A Biol Sci Med Sci. 2017;73:29–38. doi: 10.1093/gerona/glx024 [DOI] [PubMed] [Google Scholar]
- 82. Fok WC, Livi C, Bokov A, et al. Short-term rapamycin treatment in mice has few effects on the transcriptome of white adipose tissue compared to dietary restriction. Mech Ageing Dev. 2014;140:23–29. doi: 10.1016/j.mad.2014.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Beretta L, Gingras AC, Svitkin YV, Hall MN, Sonenberg N. Rapamycin blocks the phosphorylation of 4E-BP1 and inhibits cap-dependent initiation of translation. EMBO J. 1996;15:658–664. [PMC free article] [PubMed] [Google Scholar]
- 84. Karunadharma PP, Basisty N, Dai DF, et al. Subacute calorie restriction and rapamycin discordantly alter mouse liver proteome homeostasis and reverse aging effects. Aging Cell. 2015;14:547–557. doi: 10.1111/acel.12317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang T, Tsui B, Kreisberg JF, et al. Epigenetic aging signatures in mice livers are slowed by dwarfism, calorie restriction and rapamycin treatment. Genome Biol. 2017;18(1):57. doi: 10.1186/s13059-017-1186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cole JJ, Robertson NA, Rather MI, et al. Diverse interventions that extend mouse lifespan suppress shared age-associated epigenetic changes at critical gene regulatory regions. Genome Biol. 2017;18:58. doi: 10.1186/s13059-017-1185-313059-017-1185-3 [DOI] [PMC free article] [PubMed] [Google Scholar]