Abstract
RA is a chronic, autoimmune-mediated inflammatory pathology. Long non-coding RNAs (lncRNAs) are a novel group of non-coding RNAs with a length of >200 nucleotides. There are reports emerging that suggest that lncRNAs participate in establishing and sustaining autoimmune diseases, including RA. In this review article, we highlight the functions of lncRNAs in different cell types in RA. Our review indicates that lncRNAs affect various cellular components and are novel candidates that could constitute promising targets for the diagnosis and treatment of RA.
Keywords: RA, lncRNA, FLS, T cells, PBMCs
Rheumatology key messages
LncRNAs are involved in RA via regulating different cells, including fibroblast-like synoviocytes, T cells and peripheral blood mononuclear cells.
LncRNAs are promising hotspots for the diagnosis and treatment of RA.
To identify individual lncRNAs and their specific roles in RA is essential for future study.
Introduction
More than 90% of human genomic DNA can be transcribed into RNA, but only 2% of this RNA represents mRNA that can be translated into protein post-transcriptionally. The remaining 98% of the genome that do not encode protein are referred to as non-coding RNAs [1]. Long non-coding RNAs (lncRNAs) are an accumulation of transcripts with a length >200 nucleotides [2]. Until recently, lncRNAs have not been the focus of intensive research, since lncRNA was considered to be a by-product of RNA polymerase II transcription. This ‘noise’ product produced by gene transcription was supposed to have no biological function associated with it [3]. Nevertheless, recent studies have confirmed that lncRNAs play a role in many basic biological processes, including nucleation, splicing, RNA degradation and translation [4]. Furthermore, lncRNAs can contribute to gene regulation through epigenetic modifications such as methylation, ubiquitination and chromatin remodelling [5]. More importantly, numerous studies have shown that lncRNAs play a key role in the occurrence of the pathological progression of autoimmune diseases, including RA.
RA is a relatively common chronic inflammatory autoimmune disorder that is characteristic of systemic inflammation, peripheral joint destruction and erosion of bone [6]. Moreover, it seriously affects the health of patients, limiting quality of life and leading to severe disabilities. Currently the specific underlying pathological mechanisms that cause RA are still unclear. It is generally believed that several factors, including recurrent infection, cold stimulation, fatigue and environmental factors, play a significant role in the aetiology of RA. Recently, accumulating studies have demonstrated that lncRNAs could play a prominent role in a multitude of biological processes that participate in the pathogenesis of RA [7]. Here we summarize the functions of lncRNAs in several essential cellular compartments that have been identified to be involved in the pathogenesis of RA, including fibroblast-like synoviocytes (FLS), T lymphocytes (T cells) and peripheral blood mononuclear cells (PBMCs).
lncRNAs in different cell types of RA
lncRNAs in FLS of RA
FLS, which are also referred to as synovial fibroblasts or type B synoviocytes, are mesenchymal cells of the synovial joints that have major regulatory functions in joint health. During RA pathology, this cellular compartment can abundantly proliferate and invade cartilage and bone [8], participating in the pathogenesis of RA, and it has been identified as a promising target for RA treatment [9].
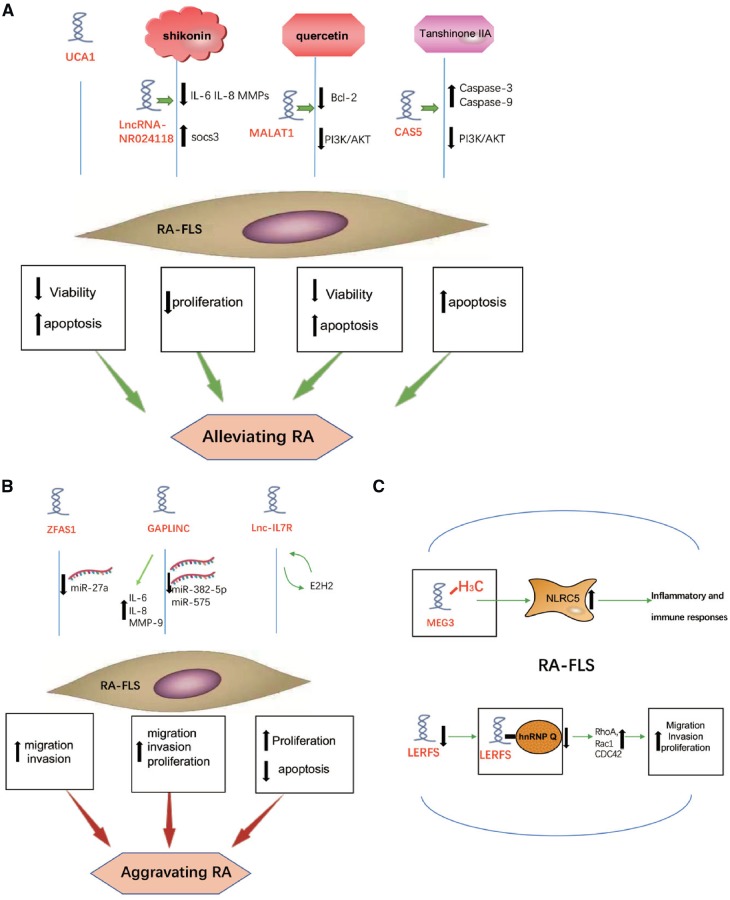
Maternally expressed gene 3 (MEG3) is a long non-coding RNA gene locus that acts as tumour suppressor. A loss of this RNA has been defined as an important marker of various human cancers [10–12]. Interestingly, it has been described in synovial tissues and cells of adjuvant arthritis rat models that nucleotide oligomerization domain-like receptor 5 (NLRC5), a member of the NOD-like receptor family that participates in inflammatory and immune responses [13], is increased dramatically while the expression of MEG3 is markedly decreased [14]. Furthermore, in adjuvant arthritis rat models the MEG3 promoter was methylated in FLS. The methylation inhibitor 5-aza-2-deoxycytidine suppressed MEG3 promoter methylation significantly and simultaneously downregulated NLRC5 expression. Interference with the expression of MEG3 may result in decreased levels of NLRC5 and inflammatory cytokines. Therefore strong expression of MEG3 and downregulation of NLRC5 could reduce the migrative and invasive abilities of RA FLS and it provides a new perspective that MEG3 may potentially alleviate RA symptoms by targeting NLRC5.
The lncRNA lowly expressed in rheumatoid fibroblast-like synoviocytes (LERFS) has a lower expression in synovial tissues and cells of RA patients than in healthy controls. Furthermore, LERFS suppress the ability of FLS to migrate, invade and proliferate by interacting with heterogeneous nuclear ribonucleoprotein Q (hnRNP Q) [15]. LERFS–hnRNP Q complex binds to the mRNA of RhoA, Rac1 and CDC42, a small GTPase protein that could regulate of FLS motility and proliferation, to target their mRNA, inhibiting its stability and translation, and to negatively regulate its protein expression in healthy controls. In contrast, the decrease in LERFS levels leads to a decrease of LERFS–hnRNP Q complex in RA FLS, which decreases the integration between hnRNP Q and target mRNA, strengthening its stability and translation and upregulating its protein expression. In conclusion, lncRNA LERFS negatively regulate FLS aggression and proliferation, with the downregulated expression of LERFS leading to synovial proliferation and joint destruction of RA.
Previous reports have demonstrated that Tanshinone IIA (Tan IIA) is used to promote apoptosis of RA FLS, thereby hindering the progression of RA [16]. Expression of lncRNA growth arrest-specific 5 (GAS5) is downregulated in RA FLS, however, the GAS5 level is upregulated after treatment with Tan IIA. Apoptosis is significantly repressed after knocking down GAS5 by small interfering RNA (siRNA) transfection [17]. Meanwhile, the knockdown of Gas5 downregulates caspase-3 and caspase-9 levels and activates the phosphoinositide 3-kinase (PI3K)/AKT signalling pathway. Therefore these results show that the pro-apoptotic effect of Tan IIA on RA FLS is achieved by upregulating lncRNA GAS5.
Urothelial carcinoma associated 1 (UCA1) is a novel lncRNA that resides in the chromosome 19p13.12, with three exons and two introns [18]. A series of reports have demonstrated that UCA1 plays a key role in the pathologic processes of diverse disorders. Yan et al. [19] analysed the effect of the activity of lncRNAs on FLS and the pathologic processes of RA. They found that UCA1 shows an enhanced expression in normal FLS compared with that in RA FLS. Down-regulated UCA1 in normal FLS increases cell viability, while upregulated UCA1 in RA FLS reduces cell viability. Furthermore, they also showed that UCA1 induces the apoptosis of FLS by regulating caspase-3 and Wnt6. These results demonstrate that UCA1 could affect the pathological progression of RA by promoting FLS apoptosis.
The association of lncRNA-NR024118 with the inhibition of shikonin on inflammatory response in FLS has been demonstrated [20]. An injury of soft tissue and bone in shikonin-treated arthritis mice can be detected. Shikonin dramatically induces lncRNA-NR024118 and suppressor of cytokine signalling (SOCS3) expression and suppresses secretion and expression of the pro-inflammatory mediators IL-8, IL-6 and various MMPs. In addition, shikonin inhibits the proliferation of FLS and enhances acetylation of histone H3 at the promoter of lncRNA-NR024118 in a dose- and time-dependent manner in RA FLS. Upregulation and siRNA silencing of lncRNA-NR024118 has been shown to enhance and reduce, respectively, the expression of SOCS3. These results suggest that the inhibitory effects of shikonin on the inflammatory response in RA FLS is mediated via affecting lncRNA-NR024118.
The lncRNA ZFAS1 expression level in synovial tissue has been compared between healthy controls and RA patients [21]. The expression of ZFAS1 is enhanced in RA FLS compared with FLS from healthy donors. ZFAS1 siRNA interference markedly suppresses the migratory and invasive phenotype of RA FLS while ZFAS1 overexpression boosts the migration and invasive phenotype of FLS. Importantly, the upregulation of microRNA 27a (miR-27a) almost abolishes the invasive phenotype of RA FLS. However, in a pull-down experiment it was shown that miR-27a allows ZFAS1 to exert its function. These findings indicate that lncRNA ZFAS1 promotes the migration and invasion of FLS via suppression of miR-27a in RA.
Mo et al. [22] recently demonstrated that lncRNA GAPLINC (gastric adenocarcinoma associated, positive CD44 regulator, long intergenic non-coding RNA) affects the functional role of RA FLS. Moreover, GAPLINC is expressed in RA FLS, compared with healthy FLS isolated from trauma patients. In order to verify whether the increased GAPLINC can exert its function in RA FLS, the RA FLS proliferation has been validated by a Cell Counting Kit-8 (CCK-8) assay using an siRNA-mediated GAPLINC knockdown in RA FLS. The CCK-8 results show that GAPLINC knockdown decreases RA FLS proliferation. Moreover, the migratory and invasive abilities of RA FLS are suppressed by GAPLINC silencing. Inflammatory cytokines or proteinases such as IL-6, IL-8 and MMP-9, which are expressed in FLS and intensify the inflammatory response, are also suppressed in GAPLINC knockdown RA FLS. Further studies have illustrated that GAPLINC downregulation leads to an enhanced expression of two anti-inflammatory miRNAs, miR-382-5p and miR-575. These results indicate that GAPLINC could enhance RA FLS tumour-like features, including proliferation, migration and invasion, by downregulating miR-382-5p and miR-575 [23–25].
Quercetin is a ubiquitous dietary antioxidant that has been proven to be helpful in the treatment of arthritis [26, 27]. Pan et al. [28] analysed the mechanisms of quercetin promoting the apoptosis of RA FLS. RA FLS viability is considerably reduced in the wake of elevated concentrations of quercetin, and FLS viability is time-dependently decreased after treatment with the same concentration of quercetin. A PCR array has been used to screen and mark the lncRNAs, and it has been shown that lncRNA MALAT1 (Metastasis Associated Lung Adenocarcinoma Transcript 1) was notably upregulated. Furthermore, knockdown of MALAT1 significantly suppresses the apoptosis of the RA FLS and the protein expression of caspase-3, caspase-9 and Bax. In contrast, Bcl-2 expression is enhanced and the PI3K/AKT pathway is activated in FLS following treatment with siRNA targeting MALAT1. Thus the upregulation of lncRNA MALAT1 is a requirement for the quercetin-induced apoptosis of FLS in RA.
Long non-coding interleukin-7 receptor (lnc‐IL7R) has been authenticated recently. This RNA is considerably upregulated by stimulation with lipopolysaccharide (LPS) and is able to impair LPS‐induced inflammatory responses in the human monocyte line THP1 and human umbilical vein endothelial cells (HUVECs) [29]. Researchers showed that lnc‐IL7R overexpression enhances FLS proliferation [30]. Further work illustrated that lnc‐IL7R can drive cell cycle progression and suppress cell apoptosis, thus enhancing FLS proliferation. Importantly, the function of lnc‐IL7R on proliferation, cell cycle and apoptosis is reversed by knocking down lnc‐IL7R. It was hypothesized and later confirmed that lnc‐IL7R forms a close contact with enhancer of zeste homologue 2 (EZH2), which is a central constituent of polycomb repressive complex 2 (PRC2) to modulate gene expression by affecting histone modification, and lnc‐IL7R serves an essential role in participating in suppression induced by PRC2, which incorporates cyclin‐dependent kinase inhibitor 1A (p21) and cyclin‐dependent kinase inhibitor 2A (p16). EZH2 knockdown almost abolishes the ability of FLS to proliferate, stalls the cell cycle and boosts cell apoptosis. These results demonstrate that lnc‐IL7R could enhance FLS growth by interacting with EZH2.
Collectively, activated FLS is a key feature of RA. FLS proliferate excessively and play an important role in inflammatory responses, autoimmunity and joint destruction in RA [6]. A number of lncRNAs affect many aspects of FLS (Fig. 1), including viability, apoptosis, proliferation, migration and invasion, to aggravate or alleviate RA via various mechanisms.
Fig. 1.
The role of lncRNAs in RA FLS
(A) UCA1 affects the pathogenesis of RA by reducing viability and inducing apoptosis of FLS. LncRNA-NR024118 is involved in the inhibition of shikonin on inflammation in FLS. MALAT1 is important to quercetin-induced apoptosis of FLS in RA. GAS5 increases after the Tan IIA treatment in RA FLS and GAS5 upregulates caspase-3 and caspase-9 levels and silences the PI3K/AKT pathway. (B) ZFAS1 promotes the migration and invasion of FLS by inhibiting miR-27a to aggravate RA. GAPLINC enhances inflammatory cytokine or proteinase expression. GAPLINC promotes RA FLS tumour-like features, including proliferation, migration and invasion by downregulating miR-382-5p and miR-575. Lnc-IL7R enhances RA FLS proliferation and suppresses its apoptosis to aggravate RA by interacting with EZH2. (C) In RA FLS, MEG3 promoter is methylated to decrease MEG3 expression, thus the increasing expression of NLRC5 participating in inflammatory and immune responses. Downregulated LERFS leads to a decrease of LERFS-hnRNP Q complex in RA FLS.
lncRNAs in PBMCs of RA
PBMCs are key constituents in host defence responses and are used to identify new disease vectors/variants and therapeutic responses [31]. Furthermore, PBMCs are easier to obtain when compared with synovial tissues. Therefore specific lncRNAs in PBMCs may be used as a general marker for clinical diagnosis of RA [32].
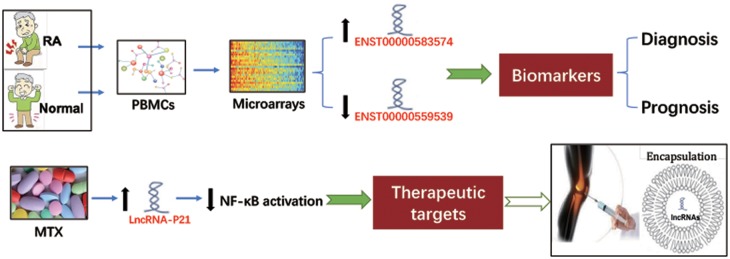
To investigate the expression of lncRNA between RA patients and a normal group, Luo et al. [33] initially used human lncRNA microarrays that included 30 586 lncRNA and 26 109 coding transcripts to profile the patterns of gene expression of PBMCs in RA patients, revealing the potential function of lncRNA in the pathological progression of RA. There were 5045 lncRNA differentially expressed in RA patients (2410 up and 2635 down). The most dramatically upregulated lncRNA was ENST00000583574 (257.7 times) and the most remarkably downregulated was ENST00000559539 (4726.8 times). Most differentially expressed lncRNAs are derived from the intergenic regions (42%), natural antisense (19%) and intronic antisense to the protein-coding locus (15%). Moreover, 135 potential lncRNA–mRNA pairs were verified for 85 abnormally expressed lncRNA and 109 abnormally expressed mRNA. This is the first report to study the relationship between PBMCs and lncRNA in RA.
Another group also utilized microarray technology to analyse the differentially expressed lncRNAs in PBMCs in RA. The bioinformatic pathway and gene ontology analyses found that the lncRNAs could regulate the aberrantly expressed mRNAs, which played a significant role in the pathological progression of RA [34]. For instance, the increased cellular components, including MHC class II protein complex, cell periphery and trans-Golgi network membrane, and the concentrated biological processes, including system development, acute-phase response and cell–cell adhesion, were associated with upregulated mRNAs. The downregulated mRNAs participated in the binding of nucleic acid, a heterocyclic compound. The increased cellular components, including nucleus and intracellular components, and the biological processes, including nucleus and cellular compound metabolic processes, were associated with downregulated mRNAs. Quantitative PCR showed that ENST00000456270 expression was prominently upregulated in PBMCs in RA patients. Moreover, the increasing level of ENST00000456270 was associated with the serum levels of IL-6, TNF-α, and the Simplified Disease Activity Index in RA patients, which demonstrated that ENST00000456270 may serve as a biomarker for assessing and diagnosing RA patients.
Zhang et al. [35] used quantitative RT-PCR to detect the expression of lncRNAs in PBMCs of 65 RA patients and 54 control patients. Meanwhile, three single-nucleotide polymorphisms (SNPs) of lnc0640 and lnc5150 were genotyped. Compared with the healthy controls, the expression of lnc0640 in PBMCs from RA patients was markedly increased, while the expression of lnc5150 was decreased. The expression of lnc0640 and lnc5150 in patients with RA was significantly correlated with CRP and lnc5150 expression was closely related to the ESR. There was a statistical correlation between the TT genotype of rs13039216 in the lnc0640 gene and RA risk reduction. And the risk of variation in the recessive model rs13039216 was reduced. In addition, compared with RF-negative patients, the G allele frequency of the lnc5150 gene rs141561256 polymorphism was notably decreased in RF-positive RA patients. These results show the lnc0640 and lnc5150 participate in RA pathological progression.
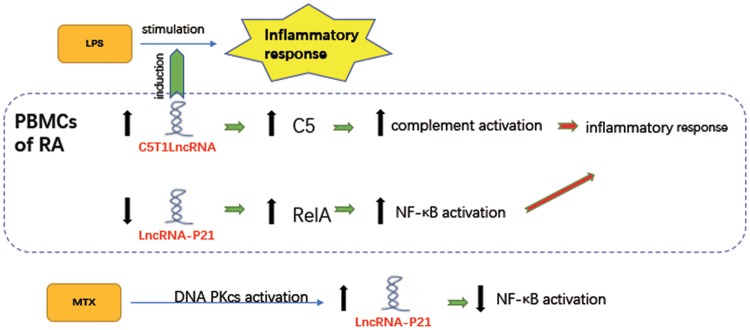
The genomic region of TRAF1-C5 is relatively large and contains several candidate genes associated with validated susceptibility genes of RA that include major histocompatibility complex, class II, DR Beta-1 (HLA-DRB1), lymphoid tyrosine phosphatase (PTPN22) and signal transducer and activator of transcription 4 (STAT4). Messemaker et al. [36] found that the TRAF1-C5 region was related to RA. TRAF1 served as a target in the treatment of RA that could negatively regulate TNF-α signalling, while the C5 expression level increased in the joints of RA patients [37, 38]. The C5T1lncRNA-regulated gene transcription level could be identified in the same genomic region. The expression of C5T1lncRNA was positively associated with C5 mRNA and PBMCs in numerous tissues. C5T1lncRNA induction was determined in LPS-stimulated PBMCs in a dose-dependent manner. Furthermore, the expression of C5T1lncRNA was strongly linked to C5 in PBMCs stimulated by LPS. Knocking down C5T1lncRNA reduced C5 mRNA levels in hepatocytes and in FLS. However, levels of TRAF1 mRNA were not influenced [36]. Therefore C5T1lncRNA, which has been considered as a novel lncRNA, is completely located in the relevant area and is expressed in several cells associated with RA to regulate the transcript levels of C5.
In addition to its role in apoptosis, p53, a tumour suppressor protein, also has anti-inflammatory effects [39]. LincRNA-p21 plays a key role in regulating the responses of cells to p53 [40]. Spurlock et al. [41] attempted to investigate the correlation of p53, lincRNA-p21 and nuclear factor (NF)-κB activity in the context of RA and MTX treatment by analysing blood samples from RA sufferers. They found that RA patients had a lower basal expression level of lincRNA-p21 and a higher expression level of phosphorylated p65 (RelA), the marker of NF-κB activation. Compared with RA sufferers who were treated with low-dose MTX, RA sufferers who did not receive MTX therapy showed lower expression levels of lincRNA-p21 and higher expression levels of RelA. In PBMC lines and primary cell culture, MTX induced expression of lincRNA-p21 by the activation of DNA-dependent protein kinase catalytic subunit and lincRNA-p21 directly inhibited NF-κB activity.
To summarize, lncRNAs regulate the aberrantly expressed mRNAs in PBMCs, which participate in the pathological progression of RA (Fig. 2). Meanwhile, differentially expressed lncRNAs in PBMCs from RA patients could be used as targets for potential diagnosis and treatment of RA.
Fig. 2.
Regulatory roles and expression of lncRNAs in PBMCs of RA
The expression of C5T1lncRNA is positively associated with C5 in PBMCs of RA. Upregulating C5 promotes complement activation to aggravate inflammatory response. Furthermore, C5T1lncRNA induces LPS-stimulated inflammatory response in PBMCs in a dose-dependent manner [36]. RA patients have a lower basal expression level of lincRNA-p21 and a higher expression level of phosphorylated p65 (RelA), the marker of NF-κB activation. MTX induces expression of lincRNA-p21 by the activation of DNA PKcs and lincRNA-p21 directly inhibits NF-κB activity [41].
lncRNAs in T lymphocytes (T cells) of RA
T cells from RA patients show several aberrant functions, for instance, premature aging, self-reactivity and T cell subset disorder [42–44]. Employing soluble cytotoxic T lymphocyte antigen 4 recombinant proteins to inhibit T cell activity could relieve the inflammation of RA sufferers [45].
A multitude of SNPs in the protein tyrosine phosphatase non-receptor type 2 (PTPN2) locus are connected with many autoimmune diseases, including juvenile idiopathic arthritis [46], type 1 diabetes [47] and RA [48]. It has been reported that knockout PTPN2 mice die from grievous inflammatory diseases at 3–5 weeks after birth [49] and PTPN2 was identified as a negative regulator of T cell receptor signalling to prevent autoimmune diseases [50]. PTPN2 could restrain the differentiation of CD8+ T cells after antigen cross-presentation [51]. Houtman et al. [52] investigated the autoimmune disease and link with the PTPN2 locus. The results revealed that autoimmune disease–associated SNPs in the PTPN2 locus are highly linked to differential DNA methylation at four CpG sites downstream of PTPN2 and expression of lncRNA LINC01882 and RP11-973H7.1 occur primarily in T cells in RA patients. To investigate the function of LINC01882 in T cells, LINC01882 was silenced in Jurkat T cells and it could be shown that there were six genes upregulated and six genes downregulated. The most significantly upregulated gene was BZRAP1, whose role in immune cells was not identified. MAP2K4, mediating the response of cells to cytokine signalling and stress, and ZEB1, encoding the transcription factor that inhibits the expression of IL-2 in T cells, were downregulated by LINC01882 knockdown models [53]. Taken together, these results demonstrate that LINC01882 participated in the activation of T cells of RA.
In another study, Lu et al. [54] evaluated whether several abnormally expressed lncRNAs in T cells from RA patients could facilitate the inflammatory response. They identified that the expression of lncRNA LOC100652951 and LOC100506036 was considerably increased in T cells from patients with RA. RA sufferers had a lower expression level of LOC100652951 and female RA patients had a lower expression level of LOC100506036 after the use of biologic agents. Furthermore, the expression level of LOC100506036, rather than LOC100652951, was notably enhanced in Jurkat T cells activated by phorbol 12-myristate 13-acetate and ionomycin. Silencing LOC100506036 decreased the production of IFN-γ and nuclear factor of activated T cells in Jurkat T cells. In addition, knockdown of LOC100506036 repressed sphingomyelin phosphodiesterase 1 (SMPD1), which may further regulate secretion of IL-2 and cytotoxic granules [55–57], T cell apoptosis and differentiation [58, 59]. Together, the expression of lncRNA LOC100652951 and LOC100506036 were considerably increased in T cells from patients with RA, and the latter could regulate numerous genes including SMPD1 and nuclear factor of activated T cells along with promoting RA inflammatory responses.
T cells play a central role in the pathogenesis of RA and promote RA bone injury by directly contacting other pathogenic cells or secreting mediators. The above-mentioned lncRNAs regulate inflammation in RA via activation, apoptosis and differentiation of T cells (Fig. 3). They are likely to manage RA persistently by regulating lncRNAs in T cells in the joints.
Fig. 3.
Regulatory roles and expression of lncRNAs in T cells of RA
BZRAP1, whose role in immune cells is not identified, is remarkably upregulated after knocking down LINC01882. MAP2K4, mediating the response of cells to cytokine signalling and stress, and ZEB1, coding the transcription factor that inhibits the expression of IL-2 in T cells, are downregulated by LINC01882 knockdown [53]. The expression of LINC01882, lncRNA LOC100652951 and LOC100506036 were remarkably increased in T cells from RA patients, LOC100506036 regulates IFN-γ, nuclear factor and SMPD1 to activate T cells [55–57].
lncRNAs in other cellular components of RA
Monocytes are the core participants in the coordination of complex immune responses [60]. Yang et al. [61] established the relation between 17 kb lncRNA non-coding transcripts in T cells (NTT) and monocytes. It was found that NTT was expressed in primary monocytes and in macrophages derived from monocytes, and was adjusted by C/EBP, a crucial transcription factor for monocytes. Together, C/EBP/NTT was overexpressed in PBMCs from patients initially diagnosed as RA without any treatment, however, these genes were downregulated in the wake of treatment. Moreover, lncRNA NTT was found to regulate inflammation in monocytes and its activation was closely associated with the differentiation of monocytes and macrophages and was involved in RA pathological progression.
As a member of the lncRNAs, lncRNA-AF085935 was related to HCV in the serum of patients with RA. Compared with healthy donors, serum IL-10, IL-17 and lncRNA-AF085935 in were significantly increased in patients with RA associated with HCV. The serum levels of IL-10 and HCV RNA were remarkably increased after corticosteroid treatment, while the serum levels of IL-17 were notably decreased, with negligible levels of lncRNA-AF085935 observed. Therefore lncRNA-AF085935 plays a role in detecting early RA associated with HCV and provides a novel strategy in the treatment of RA associated with HCV [62].
LncRNA growth arrest-specific 5 (GAS5), a glucocorticoid receptor inhibitor in the manner of its RNA ‘glucocorticoid response element’, was accumulated in the wake of starvation-induced cellular growth arrest [63]. In the whole blood of patients with multiple sclerosis and clinically isolated syndrome with the earliest clinical manifestations of multiple sclerosis, GAS5 levels were significantly increased [64]. In addition, the levels of GAS5 in CD4 T cells and whole blood were slightly affected by viral infection. The abundance of GAS5 in whole blood was decreased in sufferers with bacterial sepsis [65]. GAS5 levels were not sensitive to fasting in immune organs, including the spleen and thymus, in contrast to the metabolic organs such as the liver, fat and skeletal muscles [66]. Together, GAS5 regulating glucocorticoid receptor transcriptional activity through its decoy RNA ‘glucocorticoid response element’ plays a role in the immunological function and pathological/pathophysiological regulation of autoimmunity, inflammation and infectious diseases.
Remarkably upregulated expression of lncRNA HOX transcript antisense RNA (HOTAIR) was detected in PBMCs and serum exosomes of patients with RA, which could accelerate the migration of macrophages. In addition, Song et al. [67] confirmed upregulated HOTAIR suppressed MMP-2 and MMP-13 expression in osteoclasts and RA synoviocytes, and its expression dramatically decreased in LPS-treated RA chondrocytes. The cell proliferation and inflammatory factors IL-17 and IL-23 were remarkably repressed in HOTAIR-overexpressed chondrocytes [68]. Another study showed that the overexpression of miR-138 partly reversed the effects on cell proliferation and inflammation by upregulating HOTAIR in LPS-induced chondrocytes [69]. In addition, the activation of NF-κB treated by LPS was notably repressed by the overexpression of HOTAIR, which is a significant actor in the activation of NF-κB in macrophages [70]. In conclusion, HOTAIR alleviates RA by inhibiting the miR-138 and NF-κB pathway.
More recently, Shaker et al. [71] found that the mRNA expression levels of HOTAIR and lncRNA-Cox2, related to the activation or suppression of immunomodulator gene expression, were markedly upregulated in serum from RA patients compared with healthy subjects. Meanwhile, HOTAIR and lncRNA-Cox2 could distinguish between RA and healthy control groups using the receiver operating characteristics curve. Thus HOTAIR and lncRNA-Cox2 in serum are able to serve as non-invasive biomarkers for diagnosing RA.
The expression of H19 RNA is at a basal level in all tissues except skeletal muscle after birth. However, its expression reoccurs in diverse tumours, so H19 RNA may be a tumour biomarker [72]. H19 expression in synovial tissue from patients with RA was upregulated compared with normal/joint trauma controls. Positive signals of antisense probe for H19 were observed in the stroma, diffuse infiltrates and lining layer regions of RA synovium by in situ hybridization. What’s more, quantitative RT-PCR was used to confirm the expression of H19 in both isolated macrophages and synovial fibroblasts. Meanwhile, it was encouraging to find that the expression of H19 in RA synovial fibroblasts was dramatically upregulated in the context of starvation, either in the presence or absence of stimulation with IL-1β, TNF-α or platelet-derived growth factor BB (PDGF-BB). Moreover, the expression of H19 was downregulated or upregulated by the inhibitors of MAP kinase ERK-1/2 and the phosphatidylinositol 3-kinase after stimulation of IL-1β or PDGF-BB, respectively [73]. Thus H19 RNA may be a biomarker for embryonal dedifferentiation of adult synovial tissue and inflammatory response in RA. The function and related mechanism of lncRNAs in RA is quite versatile (Table 1). If we can comprehend explicitly the mechanism of action of lncRNA in RA, it will provide new ideas for the diagnosis and treatment of RA and other autoimmune-related diseases.
Table 1.
LncRNA expression traits in other cellular components of RA
| LncRNA | Expression | Sample | Pathway | Functional role | Related gene | Reference |
|---|---|---|---|---|---|---|
| lncRNA NTT | Up | THP-1 cells (human monocytic leukaemia cell line) | C/EBP regulating | Regulating inflammation in monocytes | C/EBP | [61] |
| lncRNA-AF085935 | Up | Serum of RA patients associated with HCV | IL-10, IL-17 expression increasing | Detecting early RA associated with HCV | HCV RNA | [62] |
| GAS5 | Down | CD4 T cells and B cells obtained from RA and SLE patients | Regulated GR transcriptional activity through its decoy RNA ‘GRE’ | Regulating effect on autoimmunity, inflammation and infectious diseases. | GRE | [63] |
| HOTAIR | Up | Serum exosome and blood mononuclear cells in RA patients | Repressing the activation of MMP-13 and MMP-2; inhibiting miR-138 and NF-κB | Alleviating RA | MMP-13 MMP-2 | [67, 69, 70] |
| H19 | Up | Synovial tissue in RA and OA patients; isolated synovial fibroblasts and macrophages | None | Be a tumour markerRelating to TIMP-2 in synovial fibroblasts | PI3k ERK-1/2 | [73] |
| HOTAIR and lncRNA-Cox2 | Up | Serum from RA patients | None | Discriminating RA patients from healthy folks | IL-6 and MMP-9 | [71] |
GR: glucocorticoid receptor; GRE: glucocorticoid response element.
lncRNAs as targets for diagnosis and treatment of RA
In recent years, studies of lncRNAs in RA have focussed on functional changes and related molecular mechanisms of lncRNAs in various dysregulated cells of RA patients. The ultimate objective is to seek the potential application of lncRNAs in clinical diagnosis and therapy of RA in the future.
Diverse bioinformatic and high-throughput technologies have been used to investigate the role of lncRNAs in RA, such as the above-mentioned microarray analysis and gene ontology terminology. Luo et al. [33] used human lncRNAs microarrays that included 30 586 lncRNAs and 26 109 coding transcripts to evaluate the differential expression of lncRNAs in PBMCs of RA patients and healthy controls. There were 5045 (2410 up and 2635 down) differentially expressed lncRNAs in PBMCs of RA patients. The largest upregluated one was ENST00000583574 (257.7 times) and the largest downregulated one was ENST00000559539 (4726.8 times). Gene ontology analysis also predicted that a large number of differentially expressed lncRNAs are closely associated with inflammation and immune response [33], suggesting that these abnormally expressed lncRNAs in PBMCs may be used to indicate the progress or prognosis of RA with high sensitivity and specificity [74]. In addition, Spurlock et al. [41] found that MTX can induce lincRNA-p21 by activating DNA PKcs. The specific response of lincRNA-p21 to MTX reminded us that it is possible to use the encapsulation technique to deliver lncRNA to specific tissues, such as a joint cavity, for RA treatment. A previous study also showed that nanofibres encapsulating siRNA-treated RA mice have achieved promising results [75]. It is known that lncRNAs show a relatively low conservation between species, various structures and complex molecular regulatory mechanisms. Currently it is difficult to find strong evidence of lncRNAs to replace or replenish conventional clinical biomarkers or therapy in RA. However, it is believed that lncRNAs will have wider use in RA with a deeper understanding of the specific role of individual lncRNAs in various essential cells of RA (Fig. 4).
Fig. 4.
Two examples of lncRNAs as targets for diagnosis and treatment of RA
The differential expression of lncRNAs in PBMCs could be used as potential diagnostic or prognostic biomarkers for RA. LncRNAs may also serve as potential therapeutic targets and an encapsulation technique can be used to deliver lncRNAs to a specific tissue, such as a joint cavity, to treat RA.
Conclusion and future perspectives
RA is a chronic and common autoimmune disorder and its aetiology is still elusive. There are still knowledge gaps. although the underlying pathogenesis has been extensively described [6]. There have been reports of a significant role for lncRNAs in RA. However, the functional role of lncRNAs in RA is largely unexplored. It is a challenge to identify individual lncRNAs and their specific roles in RA pathological progression. Structure is the basis of function, and function cannot be separated from its specific molecular structure. Due to the relatively long length, lncRNAs possess the characteristics of being large molecules, with high molecular weight and complex spatial structure, with lower sequence conservation of secondary structures [76, 77]. Thus identifying the spatial structure of lncRNAs and their interactions is essential for understanding their function and mechanisms. In addition, in order to carry out much deeper research on lncRNAs in different cellular components in RA, cutting-edge technology should be applied. The application of single-cell sequencing could increase the precision of research on lncRNAs, which helps us to better understand the specific role of lncRNAs at the single cell level [78]. Finally, in spite of a large number of lncRNAs serving as biomarkers for assessing and diagnosing RA patients, researchers need to study more clinical samples and further validate the role of lncRNAs in RA patients.
Funding: This study was supported by the National Natural Science Foundation of China (81673444), Natural Science Foundation of Anhui Province for Young Scholars (1708085QH200) and Grants for Scientific Research of BoShiKeYan from Anhui Medical University (4501041101). The authors would like to acknowledge Prof Heinrich Korner for critical reading of the manuscript. YLF and JT drafted the manuscript. DFH, YWG, WMH and WW revised the manuscript.
Disclosure statement: The authors have declared no conflicts of interest.
References
- 1. Gezer U, Ozgur E, Cetinkaya M, Isin M, Dalay N.. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol Int 2014;38:1076–9. [DOI] [PubMed] [Google Scholar]
- 2. Liu C, Bai B, Skogerbo G. et al. NONCODE: an integrated knowledge database of non-coding RNAs. Nucleic Acids Res 2005;33:D112–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Bakel H, Hughes TR.. Establishing legitimacy and function in the new transcriptome. Brief Funct Genomic Proteomic 2009;8:424–36. [DOI] [PubMed] [Google Scholar]
- 4. Wang KC, Chang HY.. Molecular mechanisms of long noncoding RNAs. Mol Cell 2011;43:904–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu K-S, Li T-P, Ton H, Mao X-D, Chen Y-J.. Advances of long noncoding RNAs-mediated regulation in reproduction. Chin Med J 2018;131:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ogishima H, Tsuboi H, Umeda N. et al. Analysis of subclinical synovitis detected by ultrasonography and low-field magnetic resonance imaging in patients with rheumatoid arthritis. Mod Rheumatol 2014;24:60–8. [DOI] [PubMed] [Google Scholar]
- 7. Jiang H, Ma R, Zou S. et al. Reconstruction and analysis of the lncRNA-miRNA-mRNA network based on competitive endogenous RNA reveal functional lncRNAs in rheumatoid arthritis. Mol Biosyst 2017;13:1182–92. [DOI] [PubMed] [Google Scholar]
- 8. Bottini N, Firestein GS.. Duality of fibroblast-like synoviocytes in RA: passive responders and imprinted aggressors. Nat Rev Rheumatol 2013;9:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mor A, Abramson SB, Pillinger MH.. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol 2005;115:118–28. [DOI] [PubMed] [Google Scholar]
- 10. Wong C-M, Anwar SL, Krech T. et al. Loss of imprinting and allelic switching at the DLK1-MEG3 locus in human hepatocellular carcinoma. PLoS ONE 2012;7:e49462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qin R, Chen Z, Ding Y. et al. Long non-coding RNA MEG3 inhibits the proliferation of cervical carcinoma cells through the induction of cell cycle arrest and apoptosis. Neoplasma 2013;60:486–92. [DOI] [PubMed] [Google Scholar]
- 12. Liu J, Li Q, Zhang KS. et al. Downregulation of the long non-coding RNA Meg3 promotes angiogenesis after ischemic brain injury by activating notch signaling. Mol Neurobiol 2017;54:8179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Benko S, Magalhaes JG, Philpott DJ, Girardin SE.. NLRC5 limits the activation of inflammatory pathways. J Immunol 2010;185:1681–91. [DOI] [PubMed] [Google Scholar]
- 14. Liu YR, Yang L, Xu QQ. et al. Long noncoding RNA MEG3 regulates rheumatoid arthritis by targeting NLRC5. J Cell Physiol 2019;234:14270–84. [DOI] [PubMed] [Google Scholar]
- 15. Zou Y, Xu S, Xiao Y. et al. Long noncoding RNA LERFS negatively regulates rheumatoid synovial aggression and proliferation. J Clin Invest 2018;128:4510–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jie L, Du H, Huang Q. et al. Tanshinone IIA induces apoptosis in fibroblast-like synoviocytes in rheumatoid arthritis via blockade of the cell cycle in the G2/M phase and a mitochondrial pathway. Biol Pharm Bull 2014;37:1366–72. [DOI] [PubMed] [Google Scholar]
- 17. Li G, Liu Y, Meng F. et al. Tanshinone IIA promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by up-regulating lncRNA GAS5. Biosci Rep 2018;38:pii: BSR20180626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu FT, Dong Q, Gao H, Zhu ZM.. The prognostic significance of UCA1 for predicting clinical outcome in patients with digestive system malignancies. Oncotarget 2017;8:40620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan ZF, Zhao XY, Liu W, Liu XP.. UCA1 impacts progress of rheumatoid arthritis by inducing the apoptosis of fibroblast-like synoviocyte. Eur Rev Med Pharmacol Sci 2018;22:914–20. [DOI] [PubMed] [Google Scholar]
- 20. Yang K-y, Chen D-L.. Shikonin inhibits inflammatory response in rheumatoid arthritis synovial fibroblasts via lncRNA-NR024118. Evid Based Complement Alternat Med 2015;2015:631737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ye Y, Gao X, Yang N.. LncRNA ZFAS1 promotes cell migration and invasion of fibroblast-like synoviocytes by suppression of miR-27a in rheumatoid arthritis. Hum Cell 2018;31:14–21. [DOI] [PubMed] [Google Scholar]
- 22. Mo BY, Guo XH, Yang MR. et al. Long non-coding RNA GAPLINC promotes tumor-like biologic behaviors of fibroblast-like synoviocytes as microRNA sponging in rheumatoid arthritis patients. Front Immunol 2018;9:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu L, Zhao X, Zou H. et al. Hypoxia promotes gastric cancer malignancy partly through the HIF-1α dependent transcriptional activation of the long non-coding RNA GAPLINC. Front Physiol 2016;7:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luo Y, Ouyang J, Zhou D. et al. Long noncoding RNA GAPLINC promotes cells migration and invasion in colorectal cancer cell by regulating miR-34a/c-MET signal pathway. Dig Dis Sci 2018;63:890–9. [DOI] [PubMed] [Google Scholar]
- 25. Zheng Z, Zhu D, Zhao F. et al. Upregulated GAPLINC predicts a poor prognosis in bladder cancer patients and promotes tumor proliferation and invasion. Oncol Lett 2018;15:6770–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lamson DW, Brignall MS.. Antioxidants and cancer, part 3: quercetin. Alternat Med Rev 2000;5:196–208. [PubMed] [Google Scholar]
- 27. Natarajan V, Krithica N, Madhan B, Sehgal PK.. Formulation and evaluation of quercetin polycaprolactone microspheres for the treatment of rheumatoid arthritis. J Pharm Sci 2011;100:195–205. [DOI] [PubMed] [Google Scholar]
- 28. Pan F, Zhu L, Lv H, Pei C.. Quercetin promotes the apoptosis of fibroblast-like synoviocytes in rheumatoid arthritis by upregulating lncRNA MALAT1. Int J Mol Med 2016;38:1507–14. [DOI] [PubMed] [Google Scholar]
- 29. Cui H, Xie N, Tan Z. et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. Eur J Immunol 2014;44:2085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye Z, Xu J, Li S. et al. LncIL7R promotes the growth of fibroblastlike synoviocytes through interaction with enhancer of zeste homolog 2 in rheumatoid arthritis. Mol Med Rep 2017;15:1412–8. [DOI] [PubMed] [Google Scholar]
- 31. Toonen EJ, Barrera P, Radstake TR. et al. Gene expression profiling in rheumatoid arthritis: current concepts and future directions. Ann Rheum Dis 2008;67:1663–9. [DOI] [PubMed] [Google Scholar]
- 32. Pauley KM, Satoh M, Chan AL. et al. Upregulated miR-146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res Ther 2008;10:R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Luo Q, Xu C, Li X. et al. Comprehensive analysis of long non-coding RNA and mRNA expression profiles in rheumatoid arthritis. Exp Ther Med 2017;14:5965–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yuan M, Wang S, Yu L. et al. Long noncoding RNA profiling revealed differentially expressed lncRNAs associated with diseaseactivity in PBMCs from patients with rheumatoid arthritis. PLoS One 2017;12:e0186795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang TP, Zhang Q, Wu J. et al. The expression levels of long noncoding RNAs lnc0640 and lnc5150 and its gene single-nucleotide polymorphisms in rheumatoid arthritis patients. J Cell Biochem 2018;119:10095–106. [DOI] [PubMed] [Google Scholar]
- 36. Messemaker TC, Frank-Bertoncelj M, Marques RB. et al. A novel long non-coding RNA in the rheumatoid arthritis risk locus TRAF1-C5 influences C5 mRNA levels. Genes Immun 2016;17:85–92. [DOI] [PubMed] [Google Scholar]
- 37. Kurreeman FA, Padyukov L, Marques RB. et al. A candidate gene approach identifies the TRAF1/C5 region as a risk factor for rheumatoid arthritis. PLoS Med 2007;4:e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Plenge RM, Seielstad M, Padyukov L. et al. TRAF1-C5 as a risk locus for rheumatoid arthritis—a genomewide study. N Engl J Med 2007;357:1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Komarova EA, Krivokrysenko V, Wang K. et al. p53 is a suppressor of inflammatory response in mice. FASEB J 2005;19:1030–2. [DOI] [PubMed] [Google Scholar]
- 40. Hung T, Wang Y, Lin MF. et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat Genet 2011;43:621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spurlock CF 3rd, Tossberg JT, Matlock BK, Olsen NJ, Aune TM.. Methotrexate inhibits NF-κB activity via long intergenic (noncoding) RNA-p21 induction. Arthritis Rheumatol 2014;66:2947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gizinski AM, Fox DA.. T cell subsets and their role in the pathogenesis of rheumatic disease. Curr Opin Rheumatol 2014;26:204–10. [DOI] [PubMed] [Google Scholar]
- 43. Kobezda T, Ghassemi-Nejad S, Mikecz K, Glant TT, Szekanecz Z.. Of mice and men: how animal models advance our understanding of T-cell function in RA. Nat Rev Rheumatol 2014;10:160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Weyand CM, Yang Z, Goronzy JJ.. T-cell aging in rheumatoid arthritis. Curr Opin Rheumatol 2014;26:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kremer JM, Westhovens R, Leon M. et al. Treatment of rheumatoid arthritis by selective inhibition of T-cell activation with fusion protein CTLA4Ig. N Engl J Med 2003;349:1907–15. [DOI] [PubMed] [Google Scholar]
- 46. Hinks A, Cobb J, Marion MC. et al. Dense genotyping of immune-related disease regions identifies 14 new susceptibility loci for juvenile idiopathic arthritis. Nat Genet 2013;45:664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Todd JA, Walker NM, Cooper JD. et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 2007;39:857–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Eyre S, Bowes J, Diogo D. et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet 2012;44:1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Heinonen KM, Nestel FP, Newell EW. et al. T-cell protein tyrosine phosphatase deletion results in progressive systemic inflammatory disease. Blood 2004;103:3457–64. [DOI] [PubMed] [Google Scholar]
- 50. Wiede F, Shields BJ, Chew SH. et al. T cell protein tyrosine phosphatase attenuates T cell signaling to maintain tolerance in mice. J Clin Invest 2011;121:4758–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wiede F, Ziegler A, Zehn D, Tiganis T.. PTPN2 restrains CD8+ T cell responses after antigen cross-presentation for the maintenance of peripheral tolerance in mice. J Autoimmun 2014;53:105–14. [DOI] [PubMed] [Google Scholar]
- 52. Houtman M, Shchetynsky K, Chemin K. et al. T cells are influenced by a long non-coding RNA in the autoimmune associated PTPN2 locus. J Autoimmun 2018;90:28–38. [DOI] [PubMed] [Google Scholar]
- 53. Wang J, Lee S, Teh CE. et al. The transcription repressor, ZEB1, cooperates with CtBP2 and HDAC1 to suppress IL-2 gene activation in T cells. Int Immunol 2009;21:227–35. [DOI] [PubMed] [Google Scholar]
- 54. Lu MC, Yu HC, Yu CL. et al. Increased expression of long noncoding RNAs LOC100652951 and LOC100506036 in T cells from patients with rheumatoid arthritis facilitates the inflammatory responses. Immunol Res 2016;64:576–83. [DOI] [PubMed] [Google Scholar]
- 55. Stoffel B, Bauer P, Nix M, Deres K, Stoffel W.. Ceramide-independent CD28 and TCR signaling but reduced IL-2 secretion in T cells of acid sphingomyelinase-deficient mice. Eur J Immunol 1998;28:874–80. [DOI] [PubMed] [Google Scholar]
- 56. Church LD, Hessler G, Goodall JE. et al. TNFR1-induced sphingomyelinase activation modulates TCR signaling by impairing store-operated Ca2+ influx. J Leukoc Biol 2005;78:266–78. [DOI] [PubMed] [Google Scholar]
- 57. Herz J, Pardo J, Kashkar H. et al. Acid sphingomyelinase is a key regulator of cytotoxic granule secretion by primary T lymphocytes. Nat Immunol 2009;10:761–8. [DOI] [PubMed] [Google Scholar]
- 58. Brenner B, Ferlinz K, Grassme H. et al. Fas/CD95/Apo-I activates the acidic sphingomyelinase via caspases. Cell Death Differ 1998;5:29–37. [DOI] [PubMed] [Google Scholar]
- 59. Bai A, Moss A, Kokkotou E. et al. CD39 and CD161 modulate Th17 responses in Crohn’s disease. J Immunol 2014;193:3366–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shi C, Pamer EG.. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 2011;11:762–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang CA, Li JP, Yen JC. et al. lncRNA NTT/PBOV1 axis promotes monocyte differentiation and is elevated in rheumatoid arthritis. Int J Mol Sci 2018;19:2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sabry D, Elamir A, Mahmoud RH, Abdelaziz AA, Fathy W.. Role of LncRNA-AF085935, IL-10 and IL-17 in rheumatoid arthritis patients with chronic hepatitis C. J Clin Med Res 2017;9:416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schneider C, King RM, Philipson L.. Genes specifically expressed at growth arrest of mammalian cells. Cell 1988;54:787–93. [DOI] [PubMed] [Google Scholar]
- 64. Katz Sand I. Classification, diagnosis, and differential diagnosis of multiple sclerosis. Curr Opin Neurol 2015;28:193–205. [DOI] [PubMed] [Google Scholar]
- 65. Sutherland A, Thomas M, Brandon RA. et al. Development and validation of a novel molecular biomarker diagnostic test for the early detection of sepsis. Crit Care 2011;15:R149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mayama T, Marr AK, Kino T.. Differential expression of glucocorticoid receptor noncoding RNA repressor Gas5 in autoimmune and inflammatory diseases. Horm Metab Res 2016;48:550–7. [DOI] [PubMed] [Google Scholar]
- 67. Song J, Kim D, Han J. et al. PBMC and exosome-derived Hotair is a critical regulator and potent marker for rheumatoid arthritis. Clin Exp Med 2015;15:121–6. [DOI] [PubMed] [Google Scholar]
- 68. Zhang HJ, Wei QF, Wang SJ. et al. LncRNA HOTAIR alleviates rheumatoid arthritis by targeting miR-138 and inactivating NF-κB pathway. Int Immunopharmacol 2017;50:283–90. [DOI] [PubMed] [Google Scholar]
- 69. Seidl CI, Martinez-Sanchez A, Murphy CL.. Derepression of microRNA-138 contributes to loss of the human articular chondrocyte phenotype. Arthritis Rheumatol 2016;68:398–409. [DOI] [PubMed] [Google Scholar]
- 70. Obaid M, Udden SMN, Deb P. et al. LncRNA HOTAIR regulates lipopolysaccharide-induced cytokine expression and inflammatory response in macrophages. Sci Rep 2018;8:15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Shaker OG, Mahmoud RH, Abdelaleem OO. et al. Expression profile of long noncoding RNAs, lnc-Cox2, and HOTAIR in rheumatoid arthritis patients. J Interferon Cytokine Res 2019;39:174–80. [DOI] [PubMed] [Google Scholar]
- 72. Ariel I, de Groot N, Hochberg A.. Imprinted H19 gene expression in embryogenesis and human cancer: the oncofetal connection. Am J Med Genet 2000;91:46–50. [DOI] [PubMed] [Google Scholar]
- 73. Stuhlmüller B, Kunisch E, Franz J. et al. Detection of oncofetal H19 RNA in rheumatoid arthritis synovial tissue. Am J Pathol 2003;163:901–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhang Y, Xu YZ, Sun N. et al. Long noncoding RNA expression profile in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Res Ther 2016;18:227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Scheinman RI, Trivedi R, Vermillion S, Kompella UB.. Functionalized STAT1 siRNA nanoparticles regress rheumatoid arthritis in a mouse model. Nanomedicine (Lond) 2011;6:1669–82. [DOI] [PubMed] [Google Scholar]
- 76. Holbrook SR. RNA structure: the long and the short of it. Curr Opin Struct Biol 2005;15:302–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Derrien T, Johnson R, Bussotti G. et al. The GENCODE v7 catalog of human long noncoding RNAs: analysis of their gene structure, evolution, and expression. Genome Res 2012;22:1775–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Potter SS. Single-cell RNA sequencing for the study of development, physiology and disease. Nat Rev Nephrol 2018;14:479–92. [DOI] [PMC free article] [PubMed] [Google Scholar]