Abstract
Studies in mice suggest that rapamycin has a negative impact on glucose homeostasis by inducing insulin resistance. However, results have been inconsistent and difficult to assess because the strains, methods of treatment, and analysis vary among studies. Using a consistent protocol, we surveyed nine inbred strains of mice for the effect of rapamycin on various aspects of glucose metabolism. Across all strains, rapamycin significantly delayed glucose clearance after challenge. However, rapamycin showed no main effect on systemic insulin sensitivity. Analysis of individual strains shows that rapamycin induced higher glucose values at 15 minutes post-challenge in 7/9 strains. However, only three strains show rapamycin-induced reduction in glucose clearance from 15 to 120 minutes. Although pancreatic insulin content was reduced by rapamycin in seven strains, none showed reduced serum insulin values. Although one strain showed no effects of rapamycin on glucose metabolism (129), another showed increased systemic insulin sensitivity (B6). We suggest that rapamycin likely inhibits insulin production and secretion in most strains while having strain-specific effects on glucose clearance without altering systemic insulin sensitivity. This strain survey indicates that genetic differences greatly influence the metabolic response to rapamycin.
Keywords: Insulin sensitivity, Glucose tolerance, Pharmacogenetics
Rapamycin increases life span in mice and several other organisms (1–8), presumably via inhibition of mTORC (mechanistic target of rapamycin complex). mTORC activation is associated with the response to nutrients and cellular turnover and is involved in the regulation of insulin and glucose homeostasis (9,10). Rapamycin can reduce glucose-stimulated insulin secretion as well as islet proliferation (11). Because rapamycin can alter glucose homeostasis, researchers have examined glucose tolerance, insulin sensitivity, and/or weight gain and adiposity in rapamycin-treated mice. The studies principally used C57BL6 mice; but NONcNZO10/LtJ (NcZ10), BKS.Cg-Dock7m +/+ Leprdb/J (BKS-db/db), TALLYHO/NgJ, and KK strains, as well as heterogeneous stocks, have also been tested (9,10,12–22). In general, glucose clearance is delayed in normoglycemic mice, but weight gain/adiposity, insulin sensitivity, and serum insulin values can be unaffected, reduced, or increased. Diabetic mouse models, already glucose intolerant, do not show exacerbation of glucose intolerance and can actually show increased insulin sensitivity with rapamycin treatment (rapa-treatment) (14,19). Such differences in results among these studies are difficult to analyze because of the variation among experimental designs, including mode of rapamycin administration (intraperitoneal injections or encapsulated in diet), fat content of diet, length of treatment, and methods for the analysis of glucose metabolism. The goal of our current study was to determine whether a genetic basis exists for disparate responses to rapamycin by analyzing, across a broad range of well-defined inbred strains, the effects of rapamycin treatment under standardized conditions and analytical procedures. We tested male mice as they are more likely to show perturbations in glucose metabolism, allowing us to increase the number of strains assessed.
Methods
Mice and Test Protocols
Twelve male mice of each of the following strains were obtained from the Jackson Laboratory at 7–10 weeks of age: 129S1/SvImJ (129), A/J, BALB/cJ (BALB), C3H/HeJ (C3H), C57BL/6J (B6), C57BL/6NJ (B6N), C57BLKS/J (BKS), DBA/2J (DBA2), and NON/ShiLtJ (NON). Three additional cohorts of B6 (36 additional mice) and one additional cohort of B6N (12 additional mice) were used to focus on a specific comparison of the B6 and B6N strains (because of the well-established genetic basis for the difference in glucose metabolism between those related strains (23)), to extend the study to longitudinal analysis of B6 mice, and for quality control purposes (to assess testing procedures, drug treatment, and other experimental conditions). The mice were weighed, split into equal groups, and put onto diet (5LA0, 11% fat, Purina) +/– encapsulated rapamycin (14 PPM, rapamycin from LC Labs, Woburn, MA, and microencapsulated by Southwest Research Institute, San Antonio, TX), which replicates the alimentary mode of administration used for humans. All mice were given acidified water ad lib and housed in double pen boxes (three mice per side) with pine shaving bedding. Mice were maintained on a 12 hour light/dark cycle beginning at 6:00 am. The mouse room was maintained at approximately 25°C and 40%–50% humidity. The health status of the room can be viewed as follows: Go to (https://www.jax.org/jax-mice-and-services/customer-support/customer-service/animal-health/health-status-reports); under the subheading “JAX Faculty Strains,” select “D1.” After 2 weeks of treatment, an insulin tolerance test (ITT) was performed. Food was removed from the mice at 7:00 am. At about 10:00–10:30 am, mice were weighed and bled (1–3 µL) from the tail tip, glucose was measured (OneTouch Ultra, Lifescan), and mice were injected i.p. with 1.0 U/kg insulin (Humulin, Eli Lilly) in phosphate-buffered saline. Glucose was measured additionally from the tail tip at 15, 30, and 60 minutes. After 3 weeks of treatment, a glucose tolerance test (GTT) was performed. At about 5:00 pm on the day before the test, the mice were moved to clean cages and food was removed for fasting overnight. At about 10:00–10:30 am the following morning, the mice were weighed, bled for glucose measurement, and injected with 1 g/kg glucose from a 10% glucose solution in phosphate-buffered saline. Glucose was measured additionally at 15, 30, 60, and 120 minutes. The mice were bled from the retro-orbital sinus for the 0 and 15 minute time points and from the tail tip for the remaining time points. For each ITT and GTT, two or three age-matched B6 males on 4% fat irradiated diet (5LG6, Purina) were run as positive and quality controls. Food consumption was measured after 4–5 weeks on diets for 6–7 days by weighing the grain in the hopper before and after the allotted time, with wastage (food in the bedding) also measured and accounted for. Mice were sacrificed after 6 weeks on the diets. The mice were weighed and bled for sera from the retro-orbital sinus before sacrifice by cervical dislocation. Pancreas and a portion of the liver were frozen in liquid nitrogen and stored at –80°C. Epididymal, retroperitoneal, and inguinal fat pads were weighed, frozen in liquid nitrogen, and stored at –80°C. Pancreatic insulin content (PIC) was determined by extracting the insulin from the weighed pancreas by homogenization in acid/ethanol (1.5% HCl, 70% EtOH) and insulin concentration determined by ELISA (Meso Scale Discovery). Aliquots of acid/ethanol samples were neutralized in an equal volume of 1 M Tris (pH 7.5) before further dilution (50-fold) in “Diluent 100” provided in the ELISA kit. One cohort of B6 mice was extended on diets for an additional 17 weeks (23 weeks total), with a second ITT and GTT performed when the mice had been on diets for 20 and 21 weeks, respectively. Sera from sacrifice were measured for glucose, triglycerides, total cholesterol, and non–esterified free fatty acids using the UniCel DxC 600 Synchron clinical system (Beckman Coulter, Inc., Brea, CA), and for insulin by ELISA (Meso Scale Discovery).
Statistical Analysis
Analysis of variance (JMP, V14.2; SAS Institute, Inc., Cary, NC) was used for within-strain comparisons for the effect of treatment. GTT and ITT data were analyzed using R, v3.5.1 and the MESS, v0.5.5 package. Area under the curve (AUC) values were calculated using the “auc” function, provided in MESS, using linear interpolation.
Results
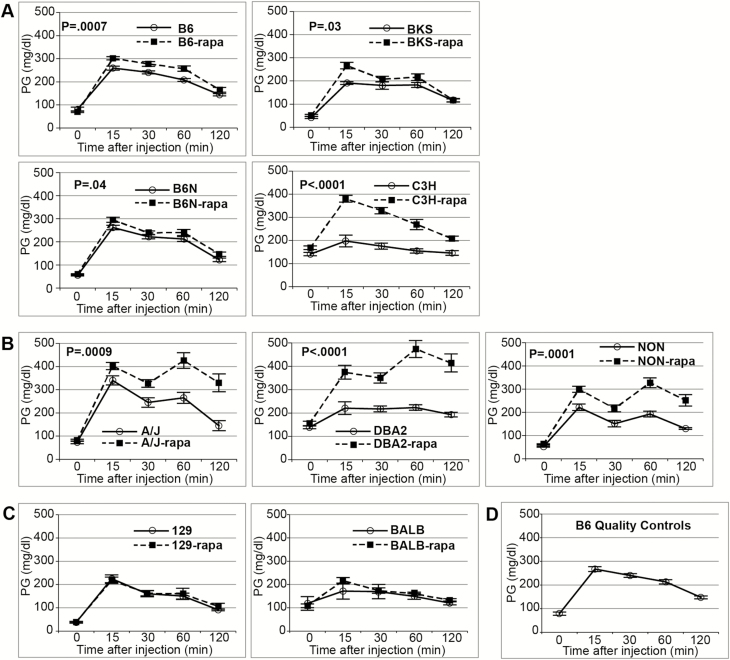
We analyzed effects of rapamycin treatment on phenotypes related to glucose metabolism in nine strains of inbred mice widely used in biological research. Encapsulated rapamycin was administered through the diet (rapa-treatment), which replicates the alimentary mode of administration used for humans. We observed a range of responses to rapamycin in the strains tested, from no differences in 129 to many alterations in DBA2 (Table 1). Across all strains, rapamycin showed a significant main effect reducing glucose clearance in the GTT (calculated by AUC). Although there was no difference among the strains in overnight-fasted plasma glucose value (the zero time point of the GTT), rapa-treated mice show a significantly higher glucose value at 15 minutes post-challenge, which remained significantly higher at 120 minutes. However, there was no effect of rapamycin on the mean decrease in plasma glucose from 15 to 120 minutes (Table 2). Analysis of the individual strains explains this result. Rapamycin increased the glucose value at 15 minutes post-challenge in seven strains, whereas it had no effect on the 15 minutes glucose value in two strains (129 and BALB). The seven affected strains showed significantly increased AUC indicating delayed glucose clearance. However, mean decrease from 15 to 120 minutes is inconsistent across the nine strains. Four strains showed no difference (129, BALB, B6, and B6N), three showed reduced clearance (A/J, DBA2, and NON), and two showed increased clearance (BKS, and C3H; Figure 1; Supplementary Table 1). Thus, although rapa-treatment inhibited the first phase response to glucose challenge in 7/9 strains, only 3 of these showed an inability to clear glucose over the second phase response. Five strains showed significantly elevated morning-fasted plasma glucose levels with rapa-treatment, although only two (DBA2 and NON) cross the diabetes threshold of 250 mg/dL (Table 1 and Supplementary Table 3).
Table 1.
Summary of Effects of Rapamycin Treatment on Glucose Metabolism Phenotypes in Nine Inbred Strains Grouped by Prevalence of Effects
| Strains | Decreased insulin sensitivity | Decreased pancreatic insulin content | Delayed glucose clearance | Elevated PG | Glucose intolerance | Change in sera insulin | Reduced weight gain | Change in fat pad weights |
|---|---|---|---|---|---|---|---|---|
| 129 | No | No | No | No | No | No | No | No |
| BALB | No | Yes* | No | No | No | Yes↑ | No | Yes↑* |
| B6N | No | Yes | Yes | No | No | No | No | No |
| BKS | No | No | Yes | No | No | No | No | No |
| C3H | No | Yes | Yes | Yes | No | No | na*** | No |
| B6 | No** | Yes | Yes | Yes | No | No | Yes | No |
| NON | No | Yes | Yes | Yes | Yes | No | No | No |
| A/J | No | Yes* | Yes | Yes | Yes | No | No | Yes↑ |
| DBA2 | No | Yes | Yes | Yes | Yes | No | Yes | Yes↓ |
| Totals | 0/9 | 7/9 | 7/9 | 5/9 | 3/9 | 1/9 | 2/8 | 3/9 |
*Suggestive difference, p = .06–.09;
**B6 shows increased insulin sensitivity;
***Although all other strains consumed equal amounts of rapa-treated or untreated diets, C3H shows reduced food consumption in rapa-treated mice that is likely the primary cause of reduced weight gain in this strain.
Table 2.
Main Effects of Rapamycin on All Mice Tested
| Term glucose (mg/dL) | Term insulin (ng/mL) | GTT | ITT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (mg/dL) | 15 (mg/dL) | 120 (mg/dL) | AUC (×1,000) | 0–15 (mg/dL) | 15–120 (mg/dL) | 0 (mg/dL) | 60 (mg/dL) | AUC raw (×1,000) | AUC % (×1,000) | |||
| Strain | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 |
| Treat | <.0001 | n.s. | n.s. | <.0001 | <.0001 | <.0001 | <.0001 | n.s. | =.0003 | n.s. | =.02 | n.s. |
| Treat × strain | =.0003 | n.s. | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | <.0001 | n.s. | =.06 | =.07 | n.s. |
| UNT | 158 ± 8 | 1.5 ± .3 | 90 ± 7 | 236 ± 9 | 137 ± 12 | 21.8 ± 1.2 | 147 ± 10 | 99 ± 10 | 130 ± 4 | 107 ± 5 | 6.7 ± 0.2 | 5.2 ± 0.1 |
| RAPA | 209 ± 7 | 1.4 ± .3 | 91 ± 6 | 306 ± 10 | 210 ± 12 | 30.1 ± 1.2 | 215 ± 9 | 96 ± 9 | 145 ± 4 | 108 ± 5 | 7.2 ± 0.2 | 5.0 ± 0.1 |
Note: Values are mean ± SEM. Rapamycin has no effect on overnight-fasted glucose (GTT-0) but does increase morning-fasted glucose (ITT-0). Rapamycin increases the glucose value at the 15-minute time point of the GTT but has no effect on the amount of glucose cleared from 15 to 120 minutes. Area under the curve (AUC) for ITT is calculated using both the absolute PG values as well as using the percentage of the baseline value. Lack of significance for AUC % indicates the significant effect seen for the raw AUC is driven by the significant elevation in the ITT-0 time point value. n = 49–50 (untreated), n = 54 (RAPA-treated). Cohorts 2–4 for B6 and cohort 2 for B6N not included to keep contribution of each strain balanced. AUC = area under curve; GTT = glucose tolerance test; ITT = insulin tolerance test; RAPA = rapamycin; UNT = untreated.
Figure 1.
Glucose tolerance test (GTT) values are mean ± SEM. (A) Strains with rapamycin-induced delayed glucose clearance. Delayed glucose clearance is due principally to higher than normal glucose values at 15 minutes after challenge, after which clearance proceeds at normal (B6, B6N) or increased (BKS, C3H) rate by 120 minutes after challenge (Supplementary Table 1). (B) Strains with rapamycin-induced glucose intolerance. Elevated glucose at 15 minutes is followed by reduced clearance rate from 15 to 120 minutes (see Supplementary Table 5). (C) Strains that show no effect of rapamycin on glucose clearance. (D) Aggregate result for age-matched B6 on 4% fat diet run with each GTT for quality control. p Values determined by analysis of variance of area under the curve. All mice were 11–14 weeks old and had been on the treatment or control diet for 3 weeks when tested. N for each strain (untreated, rapa-treated): 129 (6, 6); A/J (5, 6); BALB (4, 6); B6 (24, 24); B6N (10, 12); BKS (6, 6); C3H (6, 6); DBA2 (6, 6); NON (6, 6); B6 quality controls (34).
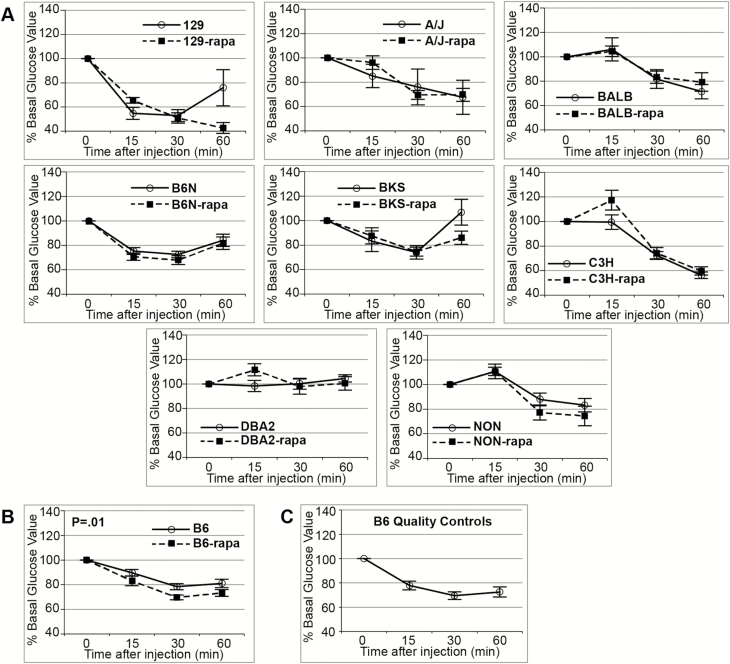
Using the raw PG values of the ITT to calculate AUC appears to indicate a significant main effect of rapamycin to decrease insulin sensitivity. However, when the PG values are converted to % of baseline (the 0 time point of the ITT), we observe no effect of treatment, indicating that the difference from the calculation using unadjusted glucose values is due to the significant rapa-induced elevation of the 0 time point value (Table 2). Analysis of the individual strains shows no effect of rapamycin on systemic insulin sensitivity with the exception of the B6 strain that shows increased insulin sensitivity by the ITT (Figure 2; Supplementary Table 2). Reduced PIC is a common effect of rapa-treatment seen in 7/9 strains (Table 1). PIC values were 52%–82% of untreated controls, depending on the strain. However, no strains showed decreased morning-fasted serum insulin levels with only BALB showing an increase, although still well within the normal range (Supplementary Table 3).
Figure 2.
Insulin tolerance test (ITT) values are mean ± SEM. (A) Strains with no effect of rapamycin on insulin sensitivity. (B) B6 showed increased insulin sensitivity with rapamycin. (C) Aggregate age-matched B6 on 4% fat diet run with each ITT for quality control. Data converted to % of baseline (0 time point of the ITT). p Values determined by analysis of variance of area under the curve. All mice were 10–13 weeks old and had been on the treatment or control diet for 2 weeks when tested. N for each strain (untreated, rapa-treated): 129 (6, 6); A/J (6, 6); BALB (4, 6); B6 (24, 24); B6N (10, 12); BKS (6, 6); C3H (6, 6); DBA2 (6, 6); NON (6, 6); B6 quality controls (33).
Effects of rapa-treatment on body composition varied across the nine strains (Table 1 and Supplementary Tables 3 and 4). Any effects on body composition generally cannot be explained in terms of food intake as it was unaltered in 8/9 strains (Supplementary Table 4). The one exception, C3H, showed decreased food intake (3.0 vs. 4.8 g/day/mouse) in rapa-treated mice, which likely contributed to their diminished weight gain.
Rapa-treatment did not elevate circulating free fatty acids in any strain with A/J the only strain showing significantly reduced values. Circulating triglycerides were increased in BALB and decreased in A/J and DBA/2 rapa-treated mice. Total cholesterol values were unaffected in four strains, significantly decreased in one strain (BKS), and significantly increased in four strains (A/J, B6, B6N, and NON; Supplementary Table 3).
Longitudinal B6 Study
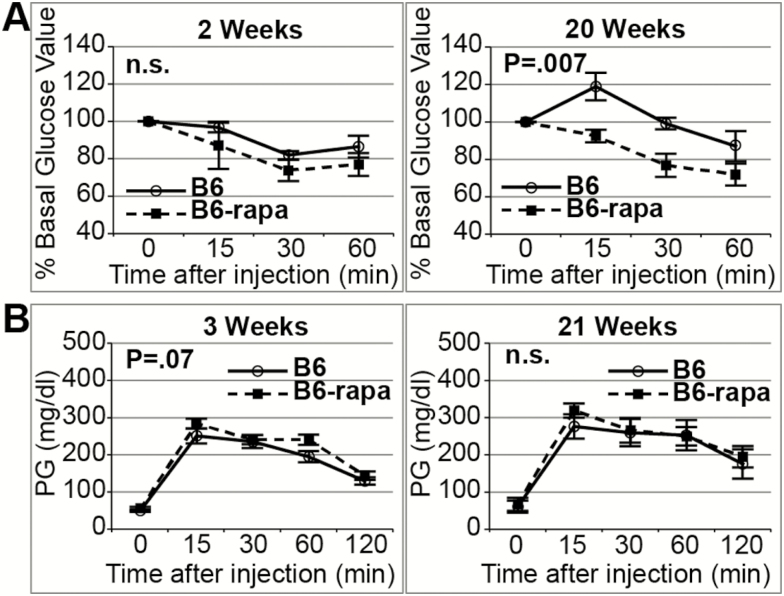
Rapa-treatment was continued past the first 6 weeks for one cohort of B6 mice. An ITT was performed at 20 weeks of treatment and a GTT at 21 weeks, followed by sacrifice at 23 weeks. Rapa-treatment for 20 weeks sustained the increased insulin sensitivity observed at 2 weeks (Figure 3A; p = 0.005 for treatment, no effect of duration of treatment, two-way analysis of variance). In addition, the moderately delayed glucose clearance produced after 3 weeks of rapa-treatment was not exacerbated by 21 weeks of treatment (Figure 3B). In addition, although 6 weeks of rapa-treatment showed significantly higher morning-fasted plasma glucose levels, by 23 weeks there was no difference. The diminished PIC value observed at 6 weeks of rapa-treatment remained the same after 23 weeks of treatment. Body weight gain was diminished at both 6 and 23 weeks of rapa-treatment. In fact, although untreated mice gained an additional approximately 3.4 g from 6 to 23 weeks of the study, the rapa-treated mice lost approximately 0.5 g. Although the effect of rapamycin on adipose tissue weights was not evident at 6 weeks of treatment, after 23 weeks, epididymal and inguinal fat pads showed significant reductions. A number of phenotypes were unaffected by rapa-treatment in B6 at both 6 and 23 weeks of treatment: serum insulin, serum triglycerides, serum total cholesterol, and serum fatty acids (Supplementary Table 5).
Figure 3.
Effect of treatment duration on B6 mice. Values are mean ± SEM. (A) Insulin tolerance test (ITT) at 2 and 20 weeks of treatment in a subgroup of B6 mice (n = 5–6 per treatment) drawn from the mice that were tested after 2 weeks of treatment. The increased insulin sensitivity we observed at 2 weeks of rapa-treatment in B6 mice was sustained after 20 weeks of treatment (main effect of treatment, p = 0.005; main effect of time, n.s.; interaction of treatment with time, n.s., two-way analysis of variance (ANOVA) for area under the curve [AUC] %). (B) Glucose tolerance test (GTT) in the same subgroup of B6 mice, tested after 3 weeks and 21 weeks of treatment. The rapamycin-induced delayed glucose clearance we observed at 3 weeks of treatment was indicated as a trend in this smaller sample size (nominal effect for the treatment at 3 weeks, p = 0.07); this trend was not altered with treatment duration (no interaction of treatment with time) even as the AUC increased moderately with time (15%, p = 0.05, two-way ANOVA for AUC).
Discussion
The nine inbred strains tested show a range of effects of rapamycin on glucose metabolism from none in 129 to many effects in DBA2, demonstrating considerable genetic influence on the metabolic response to rapamycin. B6, the strain most commonly used in studies of rapamycin effects on glucose metabolism in normoglycemic mice (9,13,17,18,22), lies in the middle of the range of effects.
Surprisingly, none of the strains exhibited rapamycin-induced insulin resistance as measured by the insulin tolerance test, despite the rapamycin-induced elevations of circulating glucose and delayed glucose clearance in a majority of the strains. Similarly, in a panel of five mouse models of type 2 diabetes (T2D) and in a study of genetically heterogeneous normoglycemic UM-HET3 mice, we observed that rapamycin treatment did not elevate insulin resistance (10,20); in fact, in three of the five T2D mouse models, rapamycin increased systemic insulin sensitivity, as it did for B6 mice in this study. Our test procedures do effectively identify insulin resistance in mice, as evidenced by the untreated control groups in the strain panel of T2D mouse models (20) and by DBA/2 mice in this study.
Rapamycin-induced delayed glucose clearance was the most common result, with 7/9 strains showing the phenotype. Our results point to a need to consider the different phases of the GTT when inferring mechanisms by which rapamycin alters glucose tolerance. Analysis of glucose tolerance can be partitioned into two segments: the first phase is defined by the initial insulin response to challenge from 0 to 15 minutes post-injection when a relatively large amount of insulin is released to the circulation in a burst; the second phase includes the secondary insulin response, hepatic gluconeogenesis, as well as uptake of glucose in multiple individual tissues, from 15 to 120 minutes post-injection. Our study demonstrates that rapa-treatment generally elevates glucose in the first phase but interacts with genotype to differentially affect glucose clearance in the second phase. In the seven strains with rapamycin-induced alteration of glucose clearance, all showed increased 15-minute glucose values. However, during the second phase, rapa-treatment increased glucose clearance rate in BKS and C3H mice and had no effect on glucose clearance rate in B6 and B6N mice. Thus, all four of these strains clear glucose effectively during the second phase despite the reduced response during the first phase, suggesting that the effect of rapamycin on glucose tolerance was not driven by a generalized effect on insulin resistance in these strains, particularly given the lack of reduced insulin sensitivity in the ITT.
True glucose intolerance with rapamycin treatment is seen in A/J, DBA2, and NON mice. In these strains, not only is the 15-minute glucose value higher in rapa-treated than in untreated mice, but glucose clearance rate also was reduced during the second phase (Supplementary Table 1). DBA2 is an inherently insulin resistant strain; DBA2 mice expressed the insulin resistant phenotypes of elevated serum insulin levels as well as lack of insulin sensitivity by ITT, although neither ITT nor serum insulin values were altered by rapa-treatment. Glucose intolerance was drastically exacerbated by rapa-treatment despite the lack of effect on the underlying insulin sensitivity phenotype, thus suggesting that the involvement of diminished glucose-stimulated insulin secretion in DBA2 mice, that may result from the significant reduction in PIC (52% of untreated, p < .0001). NON is known to have reduced insulin secretion capacity, possibly due to a defect in the β-cells of the islets resulting in moderate glucose intolerance (24). Rapamycin reduces PIC in NON males (to 70% of untreated, p = 0.007), likely further inhibiting insulin secretion potential, and thus driving a more pronounced glucose intolerance. It is quite surprising that A/J mice show rapamycin-induced glucose intolerance and elevated glucose, despite having no effect on normal insulin sensitivity, because the strain is considered resistant to the development of glucose intolerance and diabetes (https://www.jax.org/strain/000646). PIC values in A/J are modestly reduced by rapamycin treatment (to 69% of untreated, p = 0.09), but the level remains equivalent to or higher than levels in untreated mice of most other strains. Serum insulin values in A/J mice, while unaltered by rapamycin treatment, are quite low (0.35 ng/mL) compared to other normoglycemic strains. In all three of these strains, there may also be rapamycin-induced reduction in tissue-specific glucose uptake, which would extend elevated glucose levels in the blood.
Only two strains (DBA2 and NON) in this panel show rapa-treated mice with plasma glucose levels above the diabetes threshold of 250 mg/dL, suggesting that these are uncommon effects of rapamycin treatment in normoglycemic mice. Given that both of these strains can be considered prediabetic, these effects of rapamycin may be linked to their underlying diabetic susceptibilities. It is interesting to contrast these effects with those in our previous report that showed, under the same protocol, that rapamycin had no effect on glucose intolerance in fully diabetic models while also increasing insulin sensitivity in inherently insulin resistant strains (20). Interestingly, exacerbation of hyperglycemia in diabetic models has been seen in the NcZ10 (19) and TALLYHO/NgJ (20) strains, but not in BKS-db/db or KK strains (12,14,16,20,21), furthering the idea that rapamycin interacts with the genetic and physiological context of the individual to shape outcomes for glucose metabolism.
A review of previous reports indicates that rapamycin can inhibit insulin production and secretion as well as pancreatic islet proliferation (11). Indeed, reduced pancreatic insulin content was seen here in 7/9 strains. In these strains, the decrease in PIC ranges from 18% to 48% below values in untreated cohorts. Although decreases in PIC were not reflected in reduced circulating insulin values, they might reflect decreased potential for glucose-stimulated insulin secretion, a well-documented effect of rapamycin (11). Given that rapamycin increases life span in heterogeneous mice, it is not likely that rapamycin would reduce stored insulin to zero over time, as that would induce a type 1 diabetes phenotype and lead to premature death. In our study, B6 mice treated with rapamycin for 23 weeks had similar PIC values to B6 mice treated for 6 weeks. Therefore, the likely long-term scenario for normoglycemic strains is for chronic rapamycin-induced inhibition of insulin production without destruction of the islets.
Reduced weight gain in 2/8 strains, in the absence of reduced food consumption, suggests that rapamycin may have a beneficial side effect for some patients. Although only one of these two strains showed reduced fat pad weights, this could be a function of the length of treatment. For example, although B6 mice show no effect on fat pad weights after 6 weeks of treatment, despite reduced weight gain, the same fat pads show significant reductions after 23 weeks of treatment along with significantly reduced weight gain. Eight of nine strains showed equivalent food consumption between treatment groups, although analysis is underpowered (two pens per treatment for most strains) such that significance within strain could not be calculated. The one exception was C3H, which showed reduced consumption in the rapamycin-treated cohort. Because this prevented an unequivocal evaluation of a direct effect of rapamycin on body weight, the C3H strain was removed from consideration of weight reduction effects of rapamycin in Table 1. Differential food consumption between strains could contribute to differential effects of rapamycin. Although 7/9 strains consumed between 2.7 and 4.0 g/day/mouse, 129 consumed less (2.2 g/day/mouse in rapa-treated, body weight = 24.4 g) and NON consumed more (7.2 g/day/mouse in rapa-treated, body weight = 34.7 g), although it is important to note that 129 was the lightest strain and NON the heaviest in the survey. The 129 strain also showed no effects of rapamycin whereas NON showed induction of hyperglycemia above the diabetes threshold. Calculating food intake across all strains showed a significant main effect of strain, but no effect of treatment (Supplementary Table 4). We did not determine blood concentration of rapamycin, so we cannot rule out potential differences in rapamycin dosage and metabolism among the strains.
Although B6 mice have shown both reduced (17) and increased (18) insulin sensitivity in response to rapamycin, it is surprising that the B6 mice in this study show increased insulin sensitivity with rapa-treatment, as the treatment protocol and substrain used is more similar to that of the study of Liu and colleagues (17) than the study of Makki and colleagues (18). In comparison to Liu and colleagues, the mice are the same B6 substrain (JAX), and rapamycin is delivered encapsulated in diet at the same concentration. Insulin injection for the ITT is also at the same concentration, although the source of insulin is not indicated in the study of Liu and colleagues. The fat content of the diet in this study is intermediate, above that of the low fat and below that of the high-fat diets used in the study of Liu and colleagues, although there are likely differences in the protein and carbohydrate sources. Although Liu and colleagues treated mice for 8 weeks before running the ITT, in this study the B6 mice were treated for 2 and 20 weeks with similar increases in insulin sensitivity. It is interesting to note that the study of Liu and colleagues showed increased body weight in high-fat-diet-fed rapa-treated mice, whereas this study showed decreased body weight with rapa-treatment, suggesting that the difference in effect of rapa-treatment on insulin sensitivity may result indirectly from differences between the studies in effects of rapa-treatment on body composition. This difference between the studies in the effect of rapa-treatment on insulin sensitivity, despite considerable similarities between the studies, indicates that subtle differences in factors such as environment, handling, or diet composition, can contribute significantly to rapamycin effects on glucose metabolism in B6 mice. Makki and colleagues had a rather different protocol, using C57BL/6JRj mice, 60 kcal% fat diet, and rapamycin treatment consisting of weekly i.p. injections for 22 weeks, thus their study is not directly comparable to this study.
Rapa-treated, heterogeneous “HET3” mice have been shown to exhibit delayed glucose clearance while remaining insulin sensitive (10). We tested all four of the strains used to make the HET3 stock (BALB, B6, C3H, DBA2); mice of all four strains showed altered glucose clearance with rapa-treatment, with one showing glucose intolerance. None of the four strains showed a negative effect of rapa-treatment on insulin sensitivity, as assessed by ITT. Thus, the results for the HET3 mice are consistent with the aggregate responses of the background strains.
Inclusion of B6N is of interest as it diverged from B6 in 1951. On a semi-defined, 10% fat diet, B6N showed significantly higher insulin values and lower glucose values compared to B6, indicating strain differences in insulin production and secretion capabilities had emerged (23). Here, B6 showed more effects of rapamycin treatment than B6N (Table 1). Although B6 and B6N showed similar increased values for plasma glucose and reduced body weight gain in rapamycin-treated mice, the differences reach significance in B6 but not B6N. It seems that B6 is somewhat more sensitive to rapamycin-induced alterations to glucose metabolism phenotypes than B6N, likely due to reduced insulin secretion capacity in the B6.
It is interesting to note that although rapa-treated mice showed a significant increase in morning-fasted glucose values (zero time point of the ITT and terminal bleed), there was no difference in overnight-fasted glucose values (zero time point of the GTT). This indicates that the effect of rapamycin on circulating glucose dissipates over the longer fasting time frame, or that the overnight fast drives the glucose value so low that any differences become negligible. However, rapamycin continues to affect the response to glucose challenge in 7/9 strains even after the longer fast.
It is likely that the length of rapamycin treatment will differentially affect glucose metabolism. It has been shown in a heterogeneous mouse population that rapamycin treatment decreased insulin sensitivity after 2 weeks, showed no difference after 6 weeks, and increased sensitivity after 20 weeks. GTTs also improved from delayed glucose clearance at 2 weeks to almost normal at 6 and 20 weeks (15). Similarly, in this study, the one cohort of B6 with extended treatment showed delayed glucose clearance after 3 weeks of treatment, but no difference in clearance after 21 weeks as well as elevated plasma glucose levels at 6 weeks of treatment, but not after 23 weeks. Furthermore, rapa-treated B6 mice show significantly reduced body weight gain after 6 weeks of treatment that is amplified by 23 weeks of treatment. Repeated measures multivariate analysis of variance for body weight of the one B6 cohort on extended treatment confirms significance of treatment (p = 0.02; data not shown). Given the results here for B6, and previously in heterogeneous stocks (10,15), future research should include long-term treatment in multiple strains.
This strain survey of the effects of rapamycin on glucose metabolism, with a consistent protocol, shows that these effects are dependent upon the genetic background of the individual. Allelic differences in the many genes of the mTOR pathway could explain part of these phenotypes, but it would almost certainly involve other genes that regulate glucose clearance, insulin production/secretion, and fat deposition. Rapamycin did not affect insulin sensitivity or induce hyperinsulinemia, despite delaying glucose clearance in four strains and inducing or exacerbating glucose intolerance in an additional three of the nine strains tested. Coupled with the evidence for reduced pancreatic insulin content in 7/9 strains, our results suggest that rapamycin most likely has an inhibitory effect on insulin production and secretion, particularly during the first phase of initial response to glucose challenge.
Funding
This work was supported by the National Institutes of Health (AG022308 to D.E.H.) and by support to the Jackson Laboratory Summer Student Program (Barbara H. Sanford Endowed Scholarship Fund to A.T.). The authors report no conflicts of interest.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
Experiments were conducted by A.T. and P.C.R. Manuscript was written by P.C.R. D.E.H. contributed to experimental design and editing of the manuscript. The authors wish to thank Clinton M. Astle for help procuring the diets; Vicki Ingalls, Nelson Durgin, Leonor Robidoux, and Jennifer Davidson for excellent care and maintenance of the mice; Joanne Currer for her help editing the manuscript; Dr. Kevin Flurkey for reviewing the manuscript; Zoë Reifsnyder for graphic design of the figures; Timothy Stearns for statistical analysis using R. All procedures were approved by the Animal Care and Use Committee of the Jackson Laboratory (Animal Use Summary #99084) and comply with guidelines in accordance with the National Institutes of Health.
References
- 1. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201. doi: 10.1093/gerona/glq178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682. doi: 10.1111/j.1474-9726.2012.00832.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kaeberlein M, Powers RW 3rd, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535 [DOI] [PubMed] [Google Scholar]
- 5. Powers RW 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184. doi: 10.1101/gad.1381406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255 [DOI] [PubMed] [Google Scholar]
- 7. Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004;14:885–890. doi: 10.1016/j.cub.2004.03.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Müller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature. 2003;426:620. doi: 10.1038/426620a [DOI] [PubMed] [Google Scholar]
- 9. Lamming DW, Ye L, Katajisto P, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science. 2012;335:1638–1643. doi: 10.1126/science.1215135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lamming DW, Ye L, Astle CM, Baur JA, Sabatini DM, Harrison DE. Young and old genetically heterogeneous HET3 mice on a rapamycin diet are glucose intolerant but insulin sensitive. Aging Cell. 2013;12:712–718. doi: 10.1111/acel.12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Barlow AD, Nicholson ML, Herbert TP. Evidence for rapamycin toxicity in pancreatic β-cells and a review of the underlying molecular mechanisms. Diabetes. 2013;62:2674–2682. doi: 10.2337/db13-0106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang GR, Wu YY, Chiu YS, et al. Long-term administration of rapamycin reduces adiposity, but impairs glucose tolerance in high-fat diet-fed KK/HlJ mice. Basic Clin Pharmacol Toxicol. 2009;105:188–198. doi: 10.1111/j.1742-7843.2009.00427.x [DOI] [PubMed] [Google Scholar]
- 13. Chang GR, Chiu YS, Wu YY, et al. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci. 2009;109:496–503. PMID: 19372632. doi: 10.1254/jphs.08215FP [DOI] [PubMed] [Google Scholar]
- 14. Deepa SS, Walsh ME, Hamilton RT, et al. Rapamycin modulates markers of mitochondrial biogenesis and fatty acid oxidation in the adipose tissue of db/db mice. J Biochem Pharmacol Res. 2013;1:114–123. PMID: 24010023. [PMC free article] [PubMed] [Google Scholar]
- 15. Fang Y, Westbrook R, Hill C, et al. Duration of rapamycin treatment has differential effects on metabolism in mice. Cell Metab. 2013;17:456–462. doi: 10.1016/j.cmet.2013.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Das A, Durrant D, Koka S, Salloum FN, Xi L, Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem. 2014;289:4145–4160. doi: 10.1074/jbc.M113.521062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Y, Diaz V, Fernandez E, et al. Rapamycin-induced metabolic defects are reversible in both lean and obese mice. Aging (Albany NY). 2014;6:742–754. doi: 10.18632/aging.100688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makki K, Taront S, Molendi-Coste O, et al. Beneficial metabolic effects of rapamycin are associated with enhanced regulatory cells in diet-induced obese mice. PLoS One. 2014;9:e92684. doi: 10.1371/journal.pone.0092684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reifsnyder PC, Doty R, Harrison DE. Rapamycin ameliorates nephropathy despite elevating hyperglycemia in a polygenic mouse model of type 2 diabetes, NONcNZO10/LtJ. PLoS One. 2014;9:e114324. doi: 10.1371/journal.pone.0114324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reifsnyder PC, Flurkey K, Te A, Harrison DE. Rapamycin treatment benefits glucose metabolism in mouse models of type 2 diabetes. Aging (Albany NY). 2016;8:3120–3130. doi: 10.18632/aging.101117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Reifsnyder PC, Ryzhov S, Flurkey K, et al. Cardioprotective effects of dietary rapamycin on adult female C57BLKS/J-Leprdb mice. Ann N Y Acad Sci. 2018;1418:106–117. doi: 10.1111/nyas.13557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fok WC, Zhang Y, Salmon AB, et al. Short-term treatment with rapamycin and dietary restriction have overlapping and distinctive effects in young mice. J Gerontol A Biol Sci Med Sci. 2013;68:108–116. doi: 10.1093/gerona/gls127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nicholson A, Reifsnyder PC, Malcolm RD, et al. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity. 2010;18:1902–1905. doi: 10.1038/oby.2009.477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leiter EH, Herberg L. The polygenics of diabesity in mice. Diabetes Rev. 1997;5:131–148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.