Abstract
Background
This study aimed to provide an update on the occurrence of early urological complications in living-donor and deceased-donor kidney transplantation (KTX).
Material/Methods
Data on all kidney transplant recipients in the Netherlands between January 2005 and December 2015 were retrieved from the prospectively collected Dutch National Organ Transplant Registry Database (NOTR). We assessed the incidence of major urological complications (MUCs) within 3 months after KTX, defined as urinary leakage and ureteral obstruction. Outcomes of living donor and deceased donor kidney transplants were compared. We performed regression analysis to identify predictive factors of urological complications and studied the influence of early urological complications on graft and patient survival. We performed an additional sub-study to explore the influence of preservation of the peri-ureteric connective tissue in living-donor KTX on the occurrence of urological complications.
Results
Among 3329 kidney transplant recipients, urological complications occurred in 208 patients (6.2%) within 3 months after surgery. There were no significant differences in complication rates between recipients from living donors and deceased donors. Multiple regression analysis showed that older donor age and previous cardiac events of the recipient were predictors for the development of urological complications. Graft and patient survival were not affected by early MUCs. The additional sub-study showed that preservation of peri-ureteric tissue within living-donor KTX was not independently associated with urological complications.
Conclusions
Many living- and deceased-donor KTX recipients have early urological complications. MUCs did not affect long-term graft or patient survival.
MeSH Keywords: Anastomotic Leak, Kidney Transplantation, Postoperative Complications, Ureteral Obstruction
Background
Kidney transplantation (KTX) has become the criterion standard in the treatment of patients with end-stage renal disease (ESRD) [1]. KTX is associated with lower mortality and improved quality of life compared with long-term dialysis treatment [2]. However, major urological complications (MUCs), defined as urinary leakage and ureteral stenosis, are still common and can lead to increased morbidity, prolonged hospital stay, and need for a second surgical procedure [3,4]. Most MUCs occur within 3 months after transplantation [1]. The literature on the incidence of MUCs following KTX is limited to numerous, relatively small, single-center studies, mainly performed in recipients from deceased donors. Reported incidences range from 1% to 15% [3,5–10]. Due to various definitions of urological complications, an accurate estimation of the incidence of MUCs following KTX remains difficult. A few studies [4,7,11,12] have compared the incidence of MUCs between living-donor versus deceased-donor KTX, but found no significant differences in complication rates.
MUCs are mainly located at the distal portion of the ureter, most commonly at the site of the ureterovesical anastomosis [1]. Two well-known causes of MUCs are ischemia and technical errors [4,5,13]. During retrieval, the normal blood supply of the ureter is disrupted by dissection of segmental artery branches. Consequently, the renal artery and its branches that traverse in the peri-ureteral tissue are the only blood supply of the distal ureter. For this reason, it is highly recommended to avoid extensive dissection in the “golden triangle” (the area between the ureter, kidney, and renal artery) to prevent ischemia and subsequent urological complications [4,5,12,13]. In living-donor KTX, it can be more difficult to preserve the peri-ureteric connective tissue compared to deceased-donor KTX. However, deceased-donor KTX is accompanied with longer cold ischemia times, which is also associated with ureteral obstruction. Other described risk factors for MUCs following KTX include donor age, male sex of the recipient, African American recipients, delayed graft function, CMV infections, and renal artery multiplicity or arterial reconstructions [10–14].
The incidence of MUCs following KTX has decreased in recent decades with improvement of immunosuppression regimens and refinements in surgical techniques [4–6]. The Leadbetter-Politano (LP) technique has been mainly replaced by the less technically demanding Lich-Gregoir (LG) technique. Several studies have shown that the LG technique significantly reduces the risk of MUCs compared to the LP technique [15–17]. This can possibly be explained by the second cystotomy during the LP technique, which is a potential extra leakage site. Furthermore, there is evidence that routine prophylactic stenting reduces the incidence of MUCs following KTX [1].
The primary aim of this study was to provide an update on the incidence of early MUCs in living-donor and deceased-donor KTX using a recent, large cohort of kidney transplant recipients obtained from the Dutch National Organ Transplant Registry Database (NOTR). Secondary aims of this study were to identify predictive factors for the occurrence of MUCs following KTX and to explore the role of the peri-ureteral tissue preservation in living-donor KTX in a sub-study.
Material and Methods
Data on all recipients of kidney transplants in the Netherlands between January 2005 and December 2015 were retrieved from the prospectively collected Dutch National Organ Transplant Registry Database (NOTR). Patients were included if the occurrence of urological complications within 3 months after KTX was registered, including urinary leakage and ureteral obstruction (requiring ureteral stenting, placement of a nephrostomy tube, ureteral dilatation, or surgical reconstructions). Other data retrieved from the database were recipient characteristics (i.e., gender, age, body mass index (BMI), history of diabetes, vascular event or cardiac event, dialysis, and previous transplantations), donor characteristics (i.e., type of donor, gender, age, BMI, and comorbidity such as diabetes and hypertension), surgical parameters (i.e., cold ischemia time and side of transplanted kidney), and postoperative data on graft function, graft survival, and patient survival. We did not perform data imputation for missing variables.
The main endpoint was the incidence of MUCs (Clavien-Dindo grade 3 or higher) within 3 months after KTX, defined as urinary leakage or ureteral obstruction requiring placement of a nephrostomy tube, double-J catheter, endodilatation, or redo surgery to improve the drainage of the KTX. In addition, we assessed the incidence of MUCs after 1 year and compared the complication rates between kidney transplants from living donors and deceased donors.
To identify independent predictors of early MUCs following KTX, we performed binary logistic regression analysis. We included recipient characteristics, donor characteristics, and surgical parameters in the regression model as single predictors; variables with a significance level of P<0.1 were included in the multiple predictor regression model. Outcomes were reported as odds ratio and the corresponding 95% confidence intervals.
The influence of MUCs on graft and patient survival was estimated using the Kaplan-Meier method, and groups were compared with log-rank tests. We performed separate analyses for recipients of living-donor and deceased-donor kidneys.
Sub-study
We explored the influence of the peri-ureteral connective tissue on MUCs in living-donor KTX by a small matched case-control sub-study. Video recordings of laparoscopic donor nephrectomy procedures at the Radboudumc were available in patients who underwent living-donor KTX within the last 5 years. We screened the medical database of KTX recipients at the Radboudumc between 2014 and 2017 to identify recipients with postoperative MUCs. The accompanying donors were retrieved from the medical records. A matched control group was composed of living donors, based on sex, age, BMI, side of donor nephrectomy, and year of surgery, of whom the recipient did not develop an early urological complication. An independent research physician compiled videos of the ureteral dissection during the laparoscopic donor nephrectomy (obtained from the laparoscopic video records). Three blinded surgeons, experienced in laparoscopic donor nephrectomies, observed the videos in randomised order and assessed the amount of peri-ureteral connective tissue of both the proximal and the distal ureter on a 3-point Likert scale (1=bold; 2=some surrounding connective tissue; 3=a large amount of surrounding connective tissue). Mean scores were compared between both groups to assess whether the amount of peri-ureteric connective tissue in living donors is associated with the risk of ureteral leakage following KTX. We assessed the relative inter-observer agreement by using the Kendall coefficient of concordance (Kendall’s W). The Kendall’s W is higher when the observer disagreements are in close proximity along the ordinal scale, and the Kendall’s W is lower when the disagreements are divergent.
Statistical analyses were performed using SPSS® IBM Statistics 22. Categorical data were presented as absolute numbers of patients, and percentages and continuous data were presented as mean±standard deviation. Patient demographics of recipients and donors were analyzed using the chi-square test (for categorical variables) or the independent-sample t test (for continuous variables). For all analyses, statistical significance was defined as P<0.05.
The study was performed in accordance with the Helsinki Declaration of 1975 and with the ethics standards of the local ethics committee, the Central Committee on Research involving Human Subjects, Arnhem-Nijmegen, the Netherlands.
Results
Patient characteristics
A total of 8976 kidney transplant recipients were registered between 2005 and 2015 in the NOTR database. However, in 63% of these patients, no registration of MUCs was performed. Due to these missing data on the primary outcome, 3329 recipients could be included in this study. There were no significant differences in age, sex, or BMI between the included and excluded patients. Our patient cohort included 1829 recipients from living donors and 1500 recipients from deceased donors. The characteristics of the included patients are presented in Table 1.
Table 1.
Recipient and donor characteristics and surgical parameters.
| Variable | All kidney transplantations n=3329 |
Living donor kidney transplantations n=1829 (54.9%) |
Deceased donor kidney transplantations n=1500 (45.1%) |
P-value |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Gender (Male) | 2002 (60.1%) | 1100 (60.1) | 902 (60.1) | 1.000 |
| Age (y) | 50.7±15.2 | 47.9±15.3 | 54.1±14.4 | <0.001 |
| BMI (kgm−2) | 25.5±4.5 | 25.2±4.5 | 25.8±4.5 | <0.001 |
| Dialysis (yes) | 2174 (65.3%) | 729 (39.9%) | 1445 (96.3%) | <0.001 |
| Diabetes mellitus (yes) | 477 (14.3%) | 222 (12.3%) | 255 (17.3%) | <0.001 |
| Previous kidney transplantation (yes) | 286 (8.6%) | 143 (7.8%) | 143 (9.5%) | 0.080 |
| Donor characteristics | ||||
| Gender (male) | 1651 (49.6%) | 855 (46.7%) | 796 (53.1%) | <0.001 |
| Age (y) | 52.1±13.1 | 52.8±11.7 | 51.3±14.6 | 0.001 |
| BMI (kgm−2) | 25.6±4.0 | 25.9±3.6 | 25.3±4.4 | <0.001 |
| Surgical parameters | ||||
| Side of transplanted kidney (left) | 2134 (64.1%) | 1421 (77.7%) | 713 (47.5%) | <0.001 |
| Cold ischaemia time (h) | 8.4±7.3 | 2.6±0.8 | 15.2±5.5 | <0.001 |
| Recipient outcomes | ||||
| DGF (yes) | 535 (16.1%) | 42 (2.3%) | 493 (32.9%) | <0.001 |
| Graft failure (yes) | 276 (8.3%) | 74 (4.0%) | 202 (13.5%) | <0.001 |
| Deceased recipients | 408 (12.3%) | 121 (6.6%) | 287 (19.1%) | <0.001 |
BMI – body mass index; DGF – delayed graft function; h – hours; yrs – years. Continuous variables are presented as mean±SD, categorical variables are presented as n (%).
Incidence of urological complications
Overall, among 3329 kidney transplant recipients, MUCs occurred in 208 patients (6.2%) within 3 months after surgery. After 1 year, this number increased to 236 (7.8%), as shown in Table 2. Urinary leakage mainly occurred within the first 3 months, including 83 patients (2.5%). The number of patients with ureteral obstruction was 142 (4.3%) after 3 months and increased to 174 (5.8%) after 1 year. There were no significant differences in the number of MUCs between recipients from living donors or deceased donors.
Table 2.
Urological complications.
| All patients | Living donor KTX | Deceased donor KTX | P-value | |
|---|---|---|---|---|
| Follow-up: 3 months | n=3329 | n=1829 | n=1500 | |
| Urological complications | 208 (6.2%) | 117 (6.4%) | 91 (6.1%) | 0.70 |
| Urinary leakage | 83 (2.5%) | 45 (2.5%) | 38 (2.5%) | 0.89 |
| Ureteral obstruction | 142 (4.3%) | 79 (4.3%) | 63 (4.2%) | 0.87 |
| Follow-up: 1 year | n=3023 | n=1695 | n=1328 | |
| Urological complications | 236 (7.8%) | 132 (7.8%) | 104 (7.8%) | 0.97 |
| Urinary leakage | 84 (2.8%) | 46 (2.7%) | 38 (2.9%) | 0.81 |
| Ureteral obstruction | 174 (5.8%) | 97 (5.7%) | 77 (5.8%) | 0.93 |
KTX – kidney transplantation. Variables are presented as n (%).
Predictive factors of urological complications
We performed binary logistic regression to identify predictive factors for the occurrence of MUCs within 3 months after transplantation. In the regression analysis, recipient age, recipient BMI, recipients with diabetes or cardiac events, donor age, and donor sex were significantly associated with the occurrence of MUCs and were therefore analyzed in a multiple regression model. Donor age and previous cardiac events within the recipient were identified as predictors for the development of early MUCs following KTX (Table 3).
Table 3.
Multiple predictor regression analysis for urological complications within 3 months after KTX.
| Parameters | Single predictor analysis [OR (95% CI)] | P-value | Multiple predictor analysis [OR (95% CI)] | P-value |
|---|---|---|---|---|
| Recipient characteristics | ||||
| Age (y) | 1.014 (1.004–1.024) | 0.007 | 1.007 (0.995–1.018) | 0.242 |
| Sex (Male) | 1.242 (0.926–1.666) | 0.148 | – | – |
| BMI (kgm−2) | 1.034 (1.003–1.066) | 0.031 | 1.027 (0.995–1.060) | 0.102 |
| Dialysis (yes) | 1.100 (0.816–1.484) | 0.531 | – | – |
| Diabetes (yes) | 1.371 (0.950–1.978) | 0.092 | 1.181 (0.803–1.736) | 0.404 |
| Vascular event (yes) | 0.692 (0.381–1.258) | 0.228 | – | – |
| Cardiac event (yes) | 1.574 (1.076–2.302) | 0.019 | 1.549 (1.057–2.269) | 0.031 |
| Previous KTX (yes) | 0.880 (0.521–1.488) | 0.633 | – | – |
| Donor characteristics | ||||
| Age (y) | 1.018 (1.006–1.029) | 0.003 | 1.017 (1.005–1.029) | 0.004 |
| Sex (male) | 0.778 (0.587–1.033) | 0.082 | 0.783 (0.587–1.043) | 0.094 |
| BMI (kgm−2) | 1.026 (0.992–1.062) | 0.130 | – | – |
| Type of donor (living) | 1.058 (0.797–1.404) | 0.695 | – | – |
| Surgical parameters | ||||
| Side of transplanted kidney (left) | 0.993 (0.741–1.330) | 0.960 | – | – |
| Cold ischaemia time (h) | 1.000 (0.999–1.000) | 0.264 | – | – |
BMI – body mass index; KTX – kidney transplantation; h – hours; yrs – years. Variables are presented as odds ratio (OR) with the 95% confidence interval (95% CI).
Impact of urological complications on long-term graft and patient survival
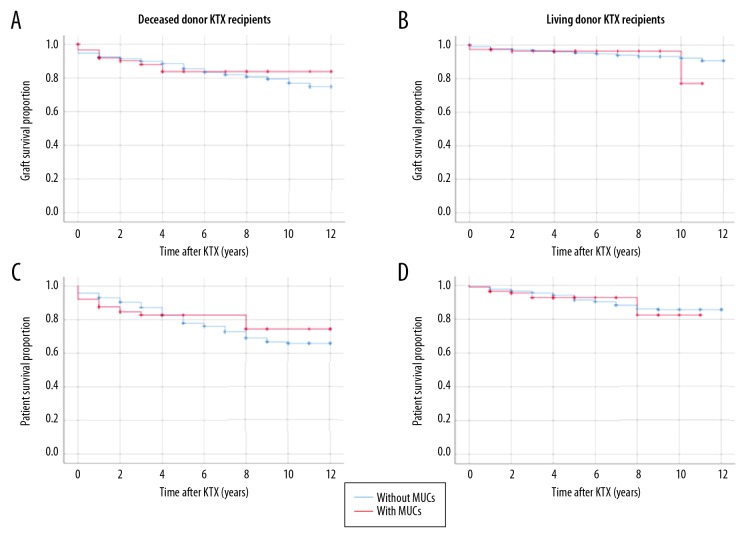
Long-term graft survival and patient survival are depicted in Figure 1. No influence was found of early MUCs within 3 months after KTX on the long-term graft survival in both deceased donor recipients (log-rank test 0.034, p=0.853) and living donor recipients (log-rank test 0.163, p=0.686) or patient survival in both deceased-donor recipients (log-rank test 0.435, p=0.510) and living-donor recipients (log-rank test 0.414, p=0.520), with a median follow-up time of 4.3 years.
Figure 1.
Kaplan-Meier survival analysis: influence of early urological complications following KTX on graft and patient survival. (A) Graft survival, deceased-donor KTX recipients (log-rank test 0.034, p=0.853); (B) Graft survival, living-donor KTX recipients (log-rank test 0.163, p=0.686); (C) Patient survival, deceased-donor KTX recipients (log-rank test 0.435, p=0.510); (D) Patient survival, living-donor KTX recipients (log-rank test 0.414, p=0.520).
Influence of peri-ureteric connective tissue preservation on MUCs (sub-study)
We identified 12 recipients with postoperative MUCs. In 8 of these patients, urinary leakage was confirmed by a MAG3 renogram, retrograde pyelography, or raised creatinine levels in the drainage fluid. The accompanying donors were retrieved from the medical records. A matched control group was composed of 12 living donors in which the recipient did not develop an early urological complication. As shown in Table 4, no significant differences were found in the amount of peri-ureteric connective tissue between patients with MUCs and patients without MUCs. Furthermore, there were significant differences between the scores of the observers. The Kendall’s W for inter-observer agreement was 0.679 (p=0.002) for the average ureteral score.
Table 4.
Assessment of preservation of peri-ureteric connective tissue.
| Patients without urinary leakage [mean±SD] | Patients with urinary leakage [mean±SD] | P-value | ||
|---|---|---|---|---|
| Ureteral score [1–3] (mean of all observers) | ||||
| Proximal | 2.3±0.3 | 2.5±0.5 | 0.107 | |
| Distal | 1.7±0.5 | 1.7±0.5 | 1.000 | |
| Average | 2.0±0.3 | 2.1±0.4 | 0.318 | |
| Observer 1 [mean±SD] | Observer 2 [mean±SD] | Observer 3 [mean±SD] | P-value | |
| Ureteral score [1–3] | ||||
| Proximal | 2.1±0.6 | 2.5±0.5 | 2.8±0.5 | <0.001 |
| Distal | 1.5±0.4 | 1.6±0.6 | 1.9±0.7 | 0.059 |
| Average | 1.8±0.5 | 2.0±0.5 | 2.3±0.4 | <0.001 |
Ureteral score: 1=bold; 2=some surrounding connective tissue; 3=a lot of surrounding connective tissue. Variables are presented as mean±SD.
Discussion
Within our cohort of 3329 kidney transplant recipients in the Netherlands between January 2005 and December 2015, 208 patients (6.2%) developed MUCs within 3 months after surgery, which is comparable with reported numbers (6.0–8.7%) in other recent, smaller studies (1000–1500 patients) [7,18,19]. Our patient cohort consisted of 83 patients (39.9%) with urinary leakage and 142 patients (68.3%) with ureteral obstruction, and all required an endoscopic, radiological, or surgical intervention. Fifty-five percent of our included patients received a living-donor kidney graft. We found no influence of donor type (living versus deceased donor) on the incidence of MUCs in kidney transplantation, despite shorter cold ischemia times and shorter periods of dialysis in recipients of living-kidney donors. This finding is in line with earlier smaller studies [4,7,11,12]. For this reason, we combined recipients of deceased donors and living donors in our regression analysis, which revealed that older donor age and previous cardiac events in the recipient are predictors for development of urological complications. These 2 factors might be associated with impaired quality of ureteral vascularization, which can result in ischemia and subsequent MUCs. It is generally recognized that living-donor KTX is superior to deceased-donor KTX in terms of graft survival and patient survival [20]; therefore, we performed separate survival analyses for recipients of deceased and living donors. In both groups, the occurrence of MUCs did not affect graft or patient survival, which might be explained by the early recognition and treatment of MUCs. Permanent renal injury can mostly be prevented by ureteral stenting or placement of a nephrostomy tube. However, the accompanying morbidity should not be underestimated.
In contradiction to many previous studies stressing the importance of preservation of the peri-ureteric connective tissue to prevent ischemia of the distal ureter [4,5,12,13], our sub-study did not reveal a direct association between peri-ureteric tissue preservation and postoperative MUCs in living-donor KTX. Likewise, Ooms and colleagues hypothesized that a shorter length of the ureter would be accompanied by better vascularization, but found no influence of ureteral length on MUCs [21]. In summary, visual judgement of the amount of peri-ureteric tissue preservation appears to be a subjective and unreliable way to assess vascularization of the distal ureter. Intraoperative indocyanine green (ICG) fluorescence has been proven to be a simple and safe technique to assess the vascularization of the ureter during robotic radical cystectomy, and minimized the number of postoperative uretero-enteric strictures [22]. The use of ICG fluorescence to assess distal ureter vascularization and the ureterovesical anastomosis during KTX could possibly prevent distal ureter ischemia, and consequently decrease the incidence of MUCs.
Limitations of the present study are its retrospective design and the amount of missing data. Due to missing data on our primary outcome, we could include only 37.1% of the registered 8976 kidney transplant recipients. Nevertheless, due to the multicentre design of the present study, the number of patients in our study cohort exceeded the number included in earlier published studies. Distinguishing between urinary leakage and ureteral obstruction can be quite challenging for 3 reasons: 1) possible variations in definition between centers, 2) the association between ureteral obstruction and early urinary leakage, and 3) a comparable first-line treatment (i.e., drainage). Due to these difficulties, divergent incidences of urinary leakage and ureteral obstruction are reported; therefore, we decided to combine these 2 MUCs in our analyses. Due to missing data on donor characteristics (medical history) and the kidney graft (vascular anatomy), we could not include these parameters into the regression analysis. Moreover, we intended to include surgical parameters (e.g., ureteral stenting, stent design, duration of stenting, uretero-vesical anastomosis technique and suture type, and vascular reconstructions) into the regression analysis; unfortunately, these variables were not registered in the database. However, in recent decades, routine prophylactic ureteral stenting and an extravesical ureterovesical anastomosis have become standard practice in Dutch transplantation centers. Although none of the observers in our sub-study found an association between peri-ureteric tissue preservation and the occurrence MUCs, there were significant differences between the mean scores of the different observers, and the Kendall’s W analysis revealed moderate inter-observer agreement. This emphasizes the fact that visual judgement of the amount of peri-ureteric tissue preservation is rather subjective.
Conclusions
Many KTX recipients experience early MUCs, despite recent refinements in surgical techniques. Although MUCs did not affect graft survival or patient survival in our study, they are associated with increased morbidity. Prospective studies are needed to develop strategies to reduce the number of MUCs following KTX.
Acknowledgements
We thank all our colleagues in the various Dutch transplant centers for submitting their data to the Dutch Organ Transplant Registry, and we also thank Cynthia Konijn of the Dutch Transplant Foundation (Nederlandse Transplantatie Stichting) for assistance with data management.
Footnotes
Source of support: Departmental sources
Author disclosure statement
Dr. Michiel C. Warlé reports grants from Merck Sharp and Dohme, outside the submitted work. The other authors have no competing financial interests.
References
- 1.Wilson CH, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD004925.pub3. Cd004925. [DOI] [PubMed] [Google Scholar]
- 2.Tonelli M, Wiebe N, Knoll G, et al. Systematic review: kidney transplantation compared with dialysis in clinically relevant outcomes. Am J Transplant. 2011;11(10):2093–109. doi: 10.1111/j.1600-6143.2011.03686.x. [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Punatar CB, Jadhav KK, et al. Routine double-J stenting for live related donor kidney transplant recipients: It doesn’t serve the purpose, but does it serve a better purpose? Investig Clin Urol. 2018;59(6):410–15. doi: 10.4111/icu.2018.59.6.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Streeter EH, Little DM, Cranston DW, Morris PJ. The urological complications of renal transplantation: A series of 1535 patients. BJU Int. 2002;90(7):627–34. doi: 10.1046/j.1464-410x.2002.03004.x. [DOI] [PubMed] [Google Scholar]
- 5.Buttigieg J, Agius-Anastasi A, Sharma A, Halawa A. Early urological complications after kidney transplantation: An overview. World J Transplant. 2018;8(5):142–49. doi: 10.5500/wjt.v8.i5.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalgic A, Boyvat F, Karakayali H, et al. Urologic complications in 1523 renal transplantations: The Baskent University experience. Transplant Proc. 2006;38(2):543–47. doi: 10.1016/j.transproceed.2005.12.116. [DOI] [PubMed] [Google Scholar]
- 7.Dinckan A, Tekin A, Turkyilmaz S, et al. Early and late urological complications corrected surgically following renal transplantation. Transpl Int. 2007;20(8):702–7. doi: 10.1111/j.1432-2277.2007.00500.x. [DOI] [PubMed] [Google Scholar]
- 8.Krajewski W, Dembowski J, Kolodziej A, et al. Urological complications after renal transplantation – a single centre experience. Cent European J Urol. 2016;69(3):306–11. doi: 10.5173/ceju.2016.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lempinen M, Stenman J, Kyllonen L, Salmela K. Surgical complications following 1670 consecutive adult renal transplantations: A single center study. Scand J Surg. 2015;104(4):254–59. doi: 10.1177/1457496914565419. [DOI] [PubMed] [Google Scholar]
- 10.Slagt IK, Ijzermans JN, Visser LJ, et al. Independent risk factors for urological complications after deceased donor kidney transplantation. PLoS One. 2014;9(3):e91211. doi: 10.1371/journal.pone.0091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Englesbe MJ, Dubay DA, Gillespie BW, et al. Risk factors for urinary complications after renal transplantation. Am J Transplant. 2007;7(6):1536–41. doi: 10.1111/j.1600-6143.2007.01790.x. [DOI] [PubMed] [Google Scholar]
- 12.Rahnemai-Azar AA, Gilchrist BF, Kayler LK. Independent risk factors for early urologic complications after kidney transplantation. Clin Transplant. 2015;29(5):403–8. doi: 10.1111/ctr.12530. [DOI] [PubMed] [Google Scholar]
- 13.Karam G, Maillet F, Parant S, et al. Ureteral necrosis after kidney transplantation: Risk factors and impact on graft and patient survival. Transplantation. 2004;78(5):725–29. doi: 10.1097/01.tp.0000131953.13414.99. [DOI] [PubMed] [Google Scholar]
- 14.Arpali E, Al-Qaoud T, Martinez E, et al. Impact of ureteral stricture and treatment choice on long-term graft survival in kidney transplantation. Am J Transplant. 2018;18(8):1977–85. doi: 10.1111/ajt.14696. [DOI] [PubMed] [Google Scholar]
- 15.Alberts VP, Idu MM, Legemate DA, et al. Ureterovesical anastomotic techniques for kidney transplantation: a systematic review and meta-analysis. Transpl Int. 2014;27(6):593–605. doi: 10.1111/tri.12301. [DOI] [PubMed] [Google Scholar]
- 16.Pleass HC, Clark KR, Rigg KM, et al. Urologic complications after renal transplantation: A prospective randomized trial comparing different techniques of ureteric anastomosis and the use of prophylactic ureteric stents. Transplant Proc. 1995;27(1):1091–92. [PubMed] [Google Scholar]
- 17.Slagt IK, Dor FJ, Tran TC, et al. A randomized controlled trial comparing intravesical to extravesical ureteroneocystostomy in living donor kidney transplantation recipients. Kidney Int. 2014;85(2):471–77. doi: 10.1038/ki.2013.464. [DOI] [PubMed] [Google Scholar]
- 18.Neri F, Tsivian M, Coccolini F, et al. Urological complications after kidney transplantation: Experience of more than 1,000 transplantations. Transplant Proc. 2009;41(4):1224–26. doi: 10.1016/j.transproceed.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 19.Zavos G, Pappas P, Karatzas T, et al. Urological complications: Analysis and management of 1525 consecutive renal transplantations. Transplant Proc. 2008;40(5):1386–90. doi: 10.1016/j.transproceed.2008.03.103. [DOI] [PubMed] [Google Scholar]
- 20.Cecka JM. The UNOS Scientific Renal Transplant Registry – ten years of kidney transplants. Clin Transpl. 1997:1–14. [PubMed] [Google Scholar]
- 21.Ooms LS, Slagt IK, Dor FJ, et al. Ureteral length in live donor kidney transplantation; Does size matter? Transpl Int. 2015;28(11):1326–31. doi: 10.1111/tri.12635. [DOI] [PubMed] [Google Scholar]
- 22.Ahmadi N, Ashrafi AN, Hartman N, et al. Use of indocyanine green to minimise uretero-enteric strictures after robotic radical cystectomy. BJU Int. 2019;124(2):302–7. doi: 10.1111/bju.14733. [DOI] [PubMed] [Google Scholar]