Abstract
Background
The purpose of this study was to investigate whether Orai1 plays a role in the metastasis of osteosarcoma.
Material/Methods
The expression of Orai1 was silenced by small interfering RNAs against Orai1 (Orai1 siRNA) in osteosarcoma MG-63 cells. Various experiments were carried out to detect the changes in migration, invasion, and adhesion ability of these osteosarcoma cells. Furthermore, the activity of Rac1, Wave2, and Ras was detected using Western blot analysis. Moreover, the Rac1 and Ras inhibitors were used to confirm whether the Ras-Rac1-WAVE2 signaling pathway was involved in osteosarcoma metastasis promoted by Orai1.
Results
We found that the migration, invasion, and adhesion ability of MG-63 cells were significantly reduced after silencing Orai1 expression (p<0.05). Moreover, the activity of the Rac1-WAVE2 signaling pathway was significantly inhibited after silencing of Orai1 expression (p<0.05). After the Rac1 inhibitor was added, Orai1 siRNA could not further inhibit migration, invasion, and adhesion of the osteosarcoma cells. Further experiments showed that Ras activity was significantly inhibited after silencing Orai1 expression (p<0.05). Moreover, Orai1 siRNA did not further inhibit the activity of the Rac1-WAVE2 signaling pathway nor did it further inhibit the migration, invasion, and adhesion ability of osteosarcoma cells following the addition of Ras inhibitors.
Conclusions
Orai1 activates the Ras-Rac1-WAVE2 signaling pathway to promote metastasis of osteosarcoma. Abnormal expression or function of Orai1 may be an important cause of osteosarcoma metastasis.
MeSH Keywords: Genes, ras; Neoplasm Metastasis; Osteosarcoma; rac1 GTP-Binding Protein
Background
Osteosarcoma is a highly malignant bone cancer that mainly occurs in children and adolescents. Currently, the survival rate of patients with metastatic osteosarcoma is very low, with an overall 5-year survival rate of just 11–20% [1–4]. Therefore, it is necessary to find an effective method to inhibit the metastasis of osteosarcoma. Metastasis is an important marker for tumor progression to the final stage. Although metastasis accounts for about 90% of cancer-related mortality, the molecular mechanism of tumor metastasis is still poorly understood. Migration, invasion, and adhesion of tumor cells are crucial steps in the process of tumor metastasis [5–7]. Clarifying the regulatory mechanism of cell migration, invasion, and adhesion, and taking comparable therapeutic measures to inhibit metastasis could potentially be new ideas for the treatment of osteosarcoma.
Calcium ions are multifunctional, second messengers that regulate many physiological and pathological processes in cells. Calcium ions regulate intracellular enzyme activity and protein–protein interactions through calmodulin or other calcium-binding proteins [8,9]. The cell membrane is a semi-permeable membrane; calcium ions cannot freely diffuse along the concentration gradient, and rely on calcium ion carriers (proteins) for transport through the cell membrane. The main means of calcium ion influx into the cytoplasmic space is store-operated calcium entry (SOCE). Recently, a calcium channel Orai1 protein, located on the cell membrane, was found to play an important role in regulating the SOCE in mammalian cells. When calcium storage in the endoplasmic reticulum is exhausted, a stromal interaction molecule (STIM) protein approaches the cell membrane and activates the Orai protein, allowing rapid and transient calcium ion influx into the cytoplasm, which is known as SOCE [10,11]. Recent studies have shown that SOCE plays an important role in the progression of various cancers. Some studies have shown that blocking SOCE can inhibit the migration, invasion, and cell movement of cancer cells [12–14]. The Orai1 protein is a multiple transmembrane protein. As an important part of SOCE, Orai1 is overexpressed in a variety of tumor cells. Studies have shown that promotion of Orai1 expression increases tumor progression and tumor cell metastasis [15–17]. However, the involvement of Orai1 in the regulation of osteosarcoma metastasis has not been reported to date. The purpose of this study was to explore whether Orai1 is involved in regulating the metastasis of osteosarcoma, in order to provide new avenues for the treatment of osteosarcoma metastasis.
Material and Methods
Western blot analysis
The total protein was quantified after cell lysis. The same quantity of cell lysis samples was taken for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for 90 min, and then transferred to a PVDF membrane for 45 min. The membrane was incubated with the designated Orai1 and another corresponding antibody at room temperature for 90 min. Thereafter, the corresponding horseradish peroxidase (HRP)-labeled secondary antibody was added. Thereafter, an appropriate amount of chemiluminescence solution was added to the PVDF film, which was then placed in the film box. After the appropriate exposure time, the membranes were photographed and quantified.
Real-time PCR analysis
Total RNA was reverse-transcribed into cDNA (Invitrogen, Carlsbad, CA, USA). The PCR reaction system contained the following: 2 μL of template RNA solution, 1 μL Oligo dT primer solution, 2 μL dNTP mixture solution, 1 μL Ace antiretroviral enzyme solution, 1 μL RNase inhibitor solution, 4 μL RT buffer solution, and 10 μL RNase-free water in a microcentrifuge tube. The reaction conditions were as follows: the first step was 37°C for 30 min and the second step was 84°C for 30 s. The reaction products were preserved at 4°C. Thereafter, real-time quantitative PCR was performed. Reaction conditions were as follows: 12.5 μL RealMasterMix solution, 2 μL template cDNA solution, 1 μL forward primer solution, 1 μL reverse primer solution, and 8.5 μL RNase-free water. Reaction conditions were as follows: the first step was 94°C for 5 min; the second step was 94°C for 60 s, 57°C for 30 s, and 72°C for 30 s, for a total of 30 cycles; the third step was 72°C for 5 min; finally, the reaction ended at 4°C.
Cell migration experiment
The cells were cultured until about 90% adherence was observed. A straight line was scraped at the bottom of the plate with the tip of a pipette, rinsed 4 times, and subsequently observed and photographed. Thereafter, medium containing 0.5% FBS was added to the culture plate. Incubation plates were placed in a humidified atmosphere at 37°C containing 5% CO2. After 24 h, the culture plate was removed and washed, and then observed and photographed. The results were statistically analyzed.
Cell invasion experiment
Transwell cells with an 8-mm aperture were inserted into 24-orifice plates. Medium (600 mL) containing 0.5% FBS was added to the lower chamber of each Transwell chamber, and 0.5% bovine serum albumin (BSA) was added to the other well as a negative control. The upper portion of the Transwell chamber was coated with matrix gel (5 mg/mL). Before adding the cell suspension, the dry matrix layer was wet again for 2 h with serum-free medium. The preincubated cells were suspended in 0.1 mL RPMI 1640 medium. The preincubated cells were then added to the upper chamber. Incubation plates were placed in a humidified atmosphere at 37°C containing 5% CO2. After 24 h, the remaining cells in the upper chamber were wiped gently with a swab. The cells were stained with crystal violet and counted under a microscope. The results were statistically analyzed.
Cell adhesion experiment
A matrix gel (BD, NY) was coated on a 96-well culture plate and incubated overnight at 4°C. Phosphate-buffered saline (PBS) containing 2% FBS was sealed. The cells were immersed in medium and incubated at 37°C with 5% CO2 for 30–60 min. After removal of the non-adherent cells, crystal violet was added. After 10 min, the 5% SDS was added. Finally, the plate was read at 540 nm. The results were statistically analyzed.
Statistical analysis
The SPSS 16.0 statistical software was used for statistical analysis. The results are expressed as mean±SD (χ±s). The t test or one-way ANOVA was used to evaluate the statistical significance among the groups. A p-value <0.05 was considered statistically significant.
Results
The expression of Orai1 was silenced by small interfering RNAs against Orai1 in MG-63 cells
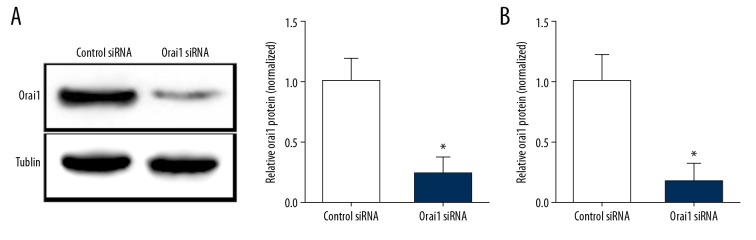
Recent studies have shown that SOCE plays an important role in the progression of various cancers [12–14]. Some studies have shown that blocking SOCE can inhibit the migration, invasion, and movement of cancer cells. The Orai1 protein is a multiple transmembrane protein. As an important part of the SOCE, Orai1 is overexpressed in a variety of tumor cells. Studies have shown that promotion of Orai1 expression increases tumor progression and tumor cell metastasis [15–17]. However, it has not been reported that Orai1 is involved in the regulation of osteosarcoma metastasis. To investigate whether Orai1 is involved in regulating the metastasis of osteosarcoma, we transfected Orai1 siRNA into the osteosarcoma cell line MG-63 to silence the expression of Orai1. Control siRNA was transfected as a negative control. Thereafter, we used Western blot analysis and real-time PCR to detect Orai1 expression and transcription after transfection. Western blot results showed that the protein expression of Orai1 was significantly reduced after transfection with Orai1 siRNA (Figure 1A, p<0.05). Similarly, real-time PCR results showed that the mRNA transcription levels of Orai1 were significantly reduced after transfection with Orai1 siRNA (Figure 1B, p<0.05). These results suggest that we can successfully silence Orai1 expression in MG-63 osteosarcoma cells with Orai1 siRNA.
Figure 1.
The expression of Orai1 was silenced by small interfering RNAs against Orai1 in MG-63 cells. (A) The Orai1 protein levels was examined by Western blot analysis in MG-63 cells silenced by small interfering RNAs against Orai1. (B) The Orai1 mRNA levels was examined by Real-time PCR in MG-63 cells silenced by small interfering RNAs against Orai1.
Silencing Orai1 expression inhibited the migration, invasion, and adhesion of osteosarcoma cells
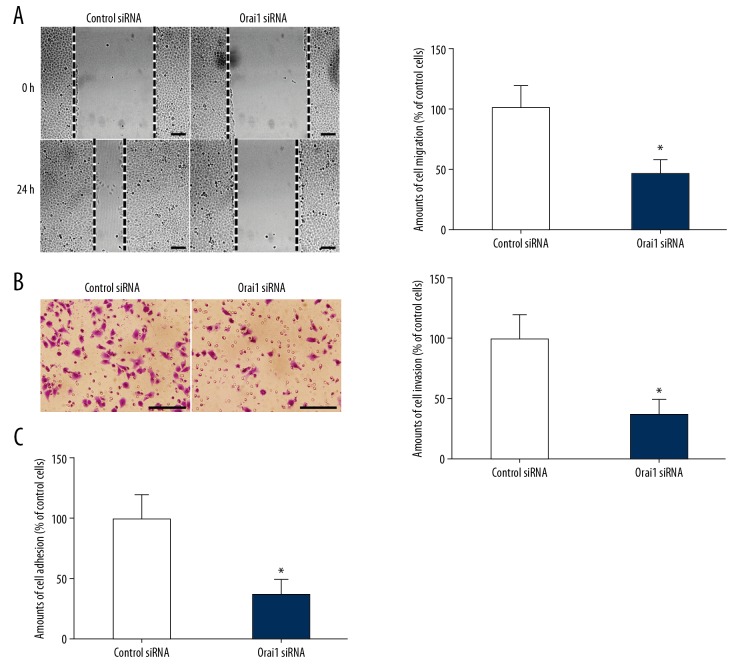
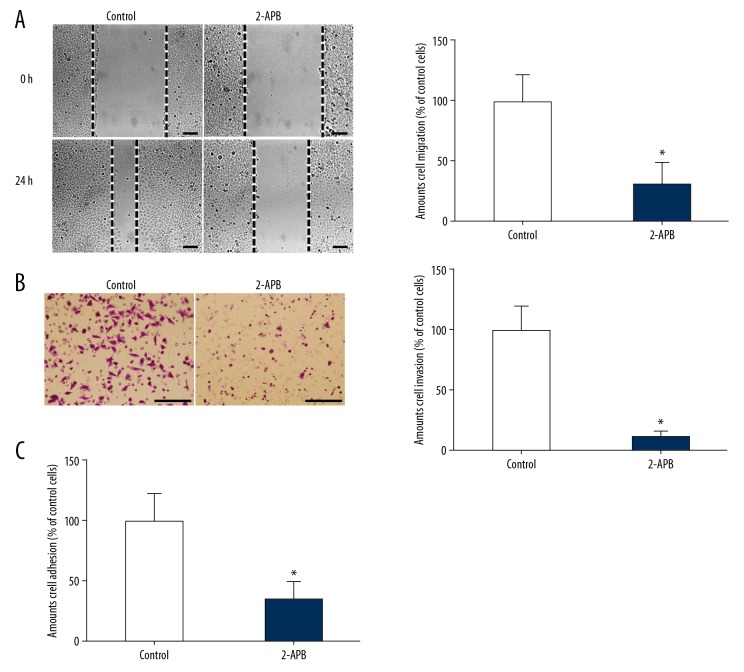
Metastasis is an important marker of tumor progression to the final stage. Although metastasis accounts for about 90% of cancer-related mortality, the molecular mechanism of tumor metastasis is still poorly understood [1–4]. Migration, invasion, and adhesion of tumor cells are important steps in the process of tumor metastasis [5–7]. In order to investigate whether Orai1 can be involved in regulating the metastasis of osteosarcoma cells, we used Orai1 siRNAs to successfully silence the expression of Orai1 in MG-63 osteosarcoma cells. The migration, invasion, and adhesion abilities of osteosarcoma cells were detected through cell migration, invasion, and adhesion experiments. We found that the amount of cell migration evidently decreased after silencing Orai1 expression in MG-63 cells (p<0.05, Figure 2A). In addition, we found that cell invasion ability was significantly decreased after silencing Orai1 expression in MG-63 cells (p<0.05, Figure 2B). Furthermore, we found that the extent of cell adhesion evidently decreased after silencing Orai1 expression in MG-63 cells (p<0.05, Figure 2C). To further confirm that these effects thought to be caused by silencing Orai1 were not actually caused by off-target effects, we used 2-APB, which blocks store-operated Ca2+ entry. Then, the invasion and adhesion abilities of osteosarcoma cells were detected. We found that the amount of cell migration evidently decreased in MG-63 cells treated with 2-APB (p<0.05, Figure 3A). In addition, we found that cell invasion ability was significantly decreased in MG-63 cells treated with 2-APB (p<0.05, Figure 3B). Furthermore, we found that the extent of cell adhesion evidently decreased in MG-63 cells treated with 2-APB (p<0.05, Figure 3C). The results confirmed that the effects thought to be caused by silencing Orai1 were not actually caused by off-target effects. The results confirmed that the expression of Orai1 can promote the migration, invasion, and adhesion of osteosarcoma cells, leading to metastasis of osteosarcoma.
Figure 2.
Silencing Orai1 expression inhibited the migration, invasion, and adhesion in osteosarcoma cells. (A) The amount of cell migration was detected in MG-63 cells silenced by small interfering RNAs against Orai1. (B) The amount of cell invasion was detected in MG-63 cells which were silenced by small interfering RNAs against Orai1. (C) The amounts of cell adhesion were detected in MG-63 cells silenced by small interfering RNAs against Orai1. Scale=100 μm.
Figure 3.
The addition of 2-APB inhibited the migration, invasion, and adhesion in osteosarcoma cells. (A) The amount of cell migration was detected in MG-63 cells treated with 2-APB. (B) The amount of cell invasion was detected in MG-63 cells treated with 2-APB. (C) The amounts of cell adhesion were detected in MG-63 cells which were treated with 2-APB. Scale=100 μm.
Orai1 promotes the metastasis of osteosarcoma cells by activating the Rac1-WAVE2 signaling pathway
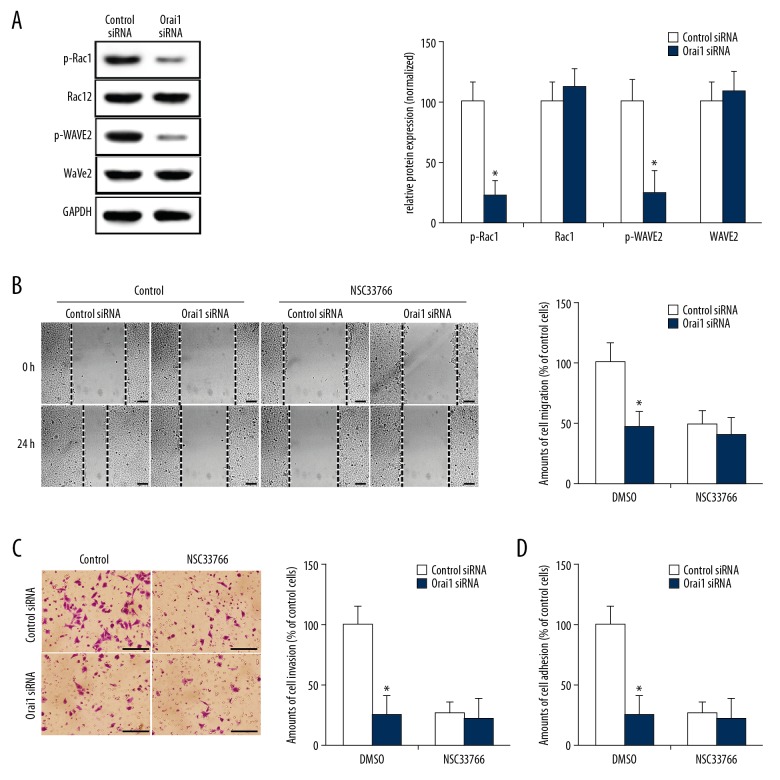
Rac1 is an important member of the Rho family and is a key factor regulating the assembly of actin in cells, regulating the formation of filamentous pseudopodia, lamellar pseudopodia, and stress fibers [18,19]. WAVE2 is an important effector molecule downstream of Rac1. Rac1 induces the formation and secretion of matrix metalloproteinase by activating WAVE2 [20]. Studies have found that the Rac1-WAVE2 signaling pathway plays an important role in the process of tumor metastasis [21,22]. To identify whether Orai1 participates in regulation of the Rac1-WAVE2 signaling pathway in osteosarcoma cells, we successfully silenced Orai1 expression in MG-63 osteosarcoma cells with Orai1-targeted siRNA, and then detected the expression and activity of Rac1 and WAVE2 in the cells by Western blot analysis. We found that after silencing Orai1 expression using siRNA, p-Rac1 and p-WAVE2 (the activated forms of Rac1 and WAVE2) were significantly reduced compared to their levels in the control group (Figure 4A, p<0.05). These results confirmed that silencing Orai1 expression in osteosarcoma cells significantly inhibits the activity of Rac1-WAVE2 signaling pathways, suggesting that Orai1 promotes the metastasis of osteosarcoma cells by activating the Rac1-WAVE2 signaling pathway. To further confirm the hypothesis, NSC33788 (a Rac1 function-blocking antibody) was used. We found that Orai1 siRNA significantly reduced the extent of cell migration, invasion, and adhesion in the control group (p<0.05, Figure 4B–4D). However, the addition of NSC33788 reduced the extent of cell migration, invasion, and adhesion in the control siRNA-transfected groups to levels comparable to those of Orai1 siRNA-transfected groups. The Orai1 siRNA did not further reduce the cell migration, invasion, and adhesion after blocking Rac1 (p>0.05, Figure 4B–4D). These results indicated that the Rac1-WAVE2 signaling pathway was required for Orai1-promoted cell migration, invasion, and adhesion in osteosarcoma cells.
Figure 4.
Orai1 promotes the metastasis of osteosarcoma cells by activating the Rac1-WAVE2 signaling pathway. (A) The expression levels of phosphorylated Rac1 (p-Rac1), Rac1, phosphorylated WAVE2 (p-WAVE2) and WAVE2 were detected in MG-63 cells which were silenced by small interfering RNAs against Orai1. (B) After incubation with NSC33788, the amount of cell migration was detected in MG-63 cells that were silenced by small interfering RNAs against Orai1. (C) After incubated with NSC33788, the amount of cell invasion was detected in MG-63 cells which were silenced by small interfering RNAs against Orai1. (D) After incubation with NSC33788, the amount of cell adhesion was detected in MG-63 cells that were silenced by small interfering RNAs against Orai1. Scale=100 μm.
Orai1 promotes the metastasis of osteosarcoma cells by activating the Ras-Rac1-WAVE2 signaling pathway
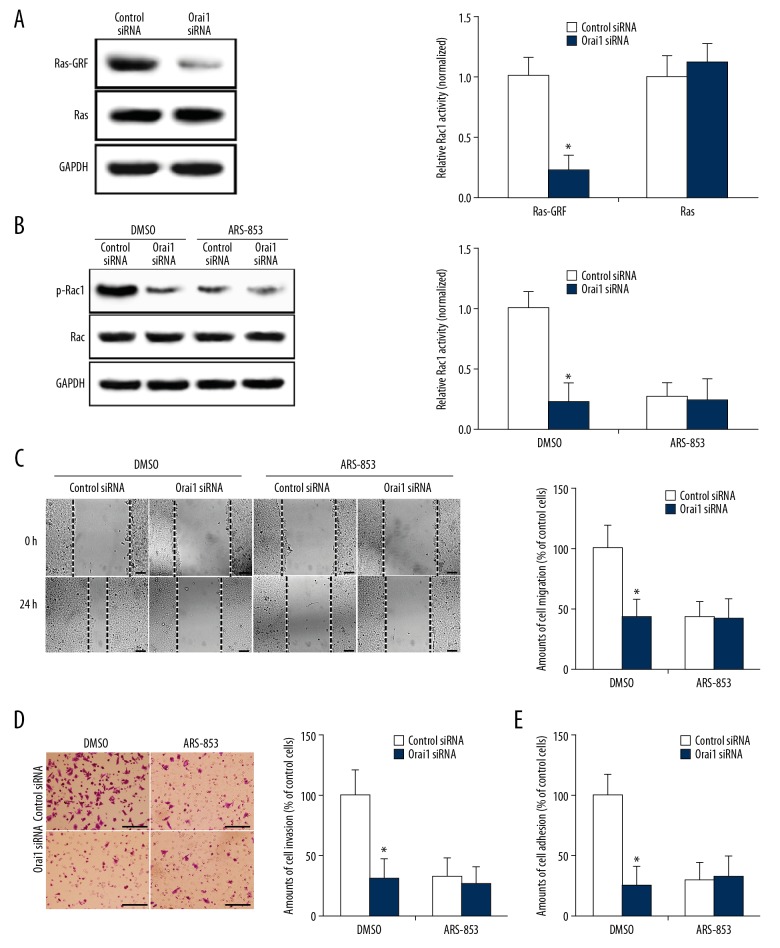
The mechanism by which Orai1 activates Rac1 is unclear. Studies have shown that activated Ras can stimulate Rac1 methylation and induce Rac1 activation [23]. The Ras-specific guanine-nucleotide exchange factor (Ras-GRF) is an activator of Ras and reflects Ras activity. As a second messenger, calcium ions can directly bind to the Ras-GRF and significantly increase Ras activity [24]. To further clarify the mechanism by which Orai1 promotes the metastasis of osteosarcoma cells, we silenced the expression of Orai1 and detected the changes of Ras and Ras-GRF in osteosarcoma cells. The results showed that there was no obvious abnormality in the expression of Ras after silencing Orai1 expression. However, Ras-GRF was significantly inhibited (p<0.05, Figure 5A). The results suggest that Orai1 expression can promote the activation of Ras in osteosarcoma cells. Therefore, it is speculated that Orai1 can activate the Rac1 signaling pathway by activating Ras. To further confirm this hypothesis, ARS-853 (a Ras function-blocking antibody) was used. We found that Orai1 siRNA significantly reduced Rac1 activity (p<0.05, Figure 5B). However, the addition of ARS-853 reduced the amount of Rac1 activity in the control siRNA-transfected groups to levels comparable to that of Orai1 siRNA-transfected groups. Orai1 siRNA did not further reduce Rac1 activity after blocking Ras (p>0.05, Figure 5B). The result indicated that Ras activity is needed in the Orai1-promoted Rac1-WAVE2 signaling pathway in osteosarcoma cells. Furthermore, we found that Orai1 siRNA significantly reduced the extent of cell migration, invasion, and adhesion in the control group (p<0.05, Figure 5C–5E). However, the addition of ARS-853 reduced the extent of cell migration, invasion, and adhesion in control siRNA-transfected groups to levels comparable to those of Orai1 siRNA-transfected groups. Orai1 siRNA did not further reduce the cell migration, invasion, and adhesion after blocking Ras (p>0.05, Figure 5C–5E). These results indicated that Ras activity is required for Orai1 to promote cell migration, invasion, and adhesion of osteosarcoma cells. These results suggest that Orai1 promotes the metastasis of osteosarcoma cells by activating the Ras-Rac1-WAVE2 signaling pathway.
Figure 5.
Orai1 promotes the metastasis of osteosarcoma cells by activating the Ras-Rac1-WAVE2 signaling pathway. (A) The expression levels of Ras-GRF and Ras were detected in MG-63 cells silenced by small interfering RNAs against Orai1. (B) After being incubated with ARS-853, the expression levels of phosphorylated Rac1 (p-Rac1) and Rac1 were detected in MG-63 cells silenced by small interfering RNAs against Orai1. (C) After being incubated with ARS-853, the amounts of cell migration were detected in MG-63 cells silenced by small interfering RNAs against Orai1. (D) After incubation with ARS-853, the amounts of cell invasion were detected in MG-63 cells silenced by small interfering RNAs against Orai1. (E) After incubation with ARS-853, the amounts of cell adhesion were detected in MG-63 cells silenced by small interfering RNAs against Orai1. Scale=100 μm.
Discussion
Osteosarcoma is a highly malignant bone tumor, mainly occurring in children and adolescents. The treatment of osteosarcoma has improved significantly over the past 30 years and multi-drug chemotherapy has improved the 5-year survival rate of patients with osteosarcoma to 60~78%; however, the survival rate of patients with osteosarcoma remains low, with an overall 5-year survival rate of just 11–20% [1–4]. Therefore, it is necessary to find a new method to effectively inhibit the metastasis of osteosarcoma. Metastasis is an important marker of tumor progression to the final stage. Tumor cell metastasis includes invasion into surrounding tissues, infiltration into circulation, migration, and survival in distant organs. Although metastasis accounts for about 90% of cancer-related mortality, the molecular mechanism of tumor metastasis is still poorly understood [5–7]. Migration, invasion, and adhesion of tumor cells run through the whole process of tumor metastasis. Clarifying the regulatory mechanism of cell migration, invasion, and adhesion, and taking corresponding therapeutic measures to inhibit metastasis, can become new concepts and methods for treatment of osteosarcoma.
Although the understanding of tumor metastasis has made great progress in recent decades, the role of calcium ions in tumor metastasis is still not well understood. Calcium ion is a multifunctional second messenger that regulates many physiological and pathological processes of cells. Calcium ions regulate intracellular enzyme activity and protein–protein interactions through calmodulin or other calcium-binding proteins. The cell membrane is a semi-permeable membrane, and calcium ions cannot freely diffuse along the concentration gradient; rather, they rely on being carried by calcium ions on the cell membrane. The main means of calcium ions influx into cytoplasm space is store-operated calcium entry (SOCE). Recently, a calcium channel Orai protein, which is located on the cell membrane, has been found to play an important role in regulating SOCE in mammalian cells. When the calcium storage in the endoplasmic reticulum is exhausted, STIM protein approaches the cell membrane and activates the Orai protein, allowing rapid and transient calcium ions influx into the cytoplasm, which is SOCE. Recent studies have shown that SOCE plays an important role in the progression of various cancers [8–11]. Some studies have shown that blocking SOCE can inhibit the migration, invasion, and cell movement of cancer cells. Orai1 protein is a transmembrane protein. As an important part SOCE, Orai1 is overexpressed in a variety of tumor cells. Studies have shown that promotion of Orai1 expression increases tumor progression and tumor cell metastasis [12–14]. Many studies have reported that the calcium channel protein Orai1 is overexpressed in a variety of tumor cells and plays a very important role in maintaining malignant phenotypes such as tumor cell adhesion, invasion, and migration [15–17]. Studies have found that in melanoma cells, the Orai1-mediated calcium signaling pathway can promote the degradation of extracellular matrix by melanoma cells, thus promoting the invasion of melanoma and distant organ metastasis. Other studies have found that after knocking out the Orai1 gene in melanoma cells, the activity of the CamkII-Raf-ERK1/2 signaling pathway mediated by the calcium ion signaling pathway was significantly reduced, and the proliferation of melanoma cells was significantly inhibited. Some studies reported that Orail expression was significantly increased in glioma [25–28]. Inhibiting Orai1 in glioma can significantly reduce receptor-dependent calcium ion influx and inhibit Pyke activity, thereby inhibiting glioma cell invasion and metastasis [29,30]. However, it has not been reported whether Orai1 is involved in osteosarcoma metastasis. In the present study, we transfected small interfering RNAs against Orai1 (Orai1 siRNA) into the osteosarcoma cell line MG-63 to silence the expression of Orai1. We found that the migration, invasion, and adhesion of osteosarcoma cells were significantly decreased after silencing Orai1 expression, which reflected the ability of tumor metastasis. The above results confirmed that the expression of Orai1 can promote the migration, invasion, and adhesion of osteosarcoma cells, leading to the metastasis of osteosarcoma.
Tumor metastasis is a complex process involving multiple links and steps, and the ability of tumor cells to adhere, migrate, and invade is closely related to tumor metastasis [1–4]. In the process of tumor cell metastasis, the front end of the cell is extended forward, and adhesive plaque is synthesized to provide anchors for the front part of the cell, thus pulling the cell forward. At the same time, the assembly of the adhesive plaque in the tail of the cell makes the tail retract and complete the metastatic process [5–7]. Rac1 is an important member of the Rho family and a key factor regulating the assembly of actin in cells, regulating the formation of filamentous pseudopodia, lamellar pseudopodia, and stress fibers. It has been reported that tumor cell metastasis first requires cell polarization. The cytoskeletal components are distributed at the cell poles under the action of directional induction signals. Rac1 activation promotes actin aggregation at the cell front, leading to the formation of lamellar and filamentous pseudopodia. The formation of cellular pseudopodia creates a driving force for the cells to move forward, and causes the cells to polarize and elongate, forming a spindle shape [18,19]. WAVE2 is an important effector molecule downstream of Rac1. To further determine whether Orai1 can promote osteosarcoma metastasis by activating the Rac1-WAVE2 signaling pathway, we silenced the expression of Orai1 and detected the changes of Rac1 and WAVE2 in osteosarcoma cells. The results showed that after silencing Orai1 expression, the expression of Rac1 and WAVE2 cells showed no obvious abnormality, while the active forms of Rac1 and WAVE2 were significantly inhibited. These results suggest that Orai1 expression can activate the Rac1-WAVE2 signaling pathway in osteosarcoma cells and promote the metastasis of osteosarcoma. To further confirm this hypothesis, we used NSC33788 (a Rac1 function-blocking antibody). We observed that Orai1 siRNA significantly reduced the cell migration, invasion, and adhesion in the control group. However, the addition of NSC33788 reduced the migration, invasion, and adhesion in the control siRNA-transfected groups to levels comparable to those of Orai1 siRNA-transfected groups. Orai1 siRNA did not further reduce cell migration, invasion, and adhesion after blocking Rac1, indicating that the Rac1-WAVE2 signaling pathway is required for Orai1-promoted cell migration, invasion, and adhesion of osteosarcoma cells.
The mechanism by which Orai1 activates Rac1 is unclear. Studies have shown that activated Ras can stimulate Rac1 methylation and induce Rac1 activation [23]. Ras plays an important role in tumor cell growth, metastasis, and apoptosis; it a guanosine triphosphate (GTP)-binding protein (an information transfer coupling factor of cells). Ras can be activated by complex networks. First, phosphorylated receptors, such as epidermal growth factor receptor (EGFR), bind directly to growth factor receptor binding proteins (Grb2). These receptors can also indirectly bind to phosphorylated proteins (such as Shc and Syp) containing SRC homologous domain 2 (SH2), and then activate Grb2. Next, the SRC homologous region 3 (SH3) domain of Grb2 is bound to target proteins such as mSos1, mSos2, C3G, and dynamin. Ras is activated by coupling tyrosine phosphorylation after C3G binds to the SH3 domain of connexin Crk. Crk can also activate Ras in combination with mSos1. Grb2 binds to the activated receptor and promotes the localization of the guanosine exchange factor (Sos) protein on the membrane adjacent to Ras. In this way, Sos forms a complex with RAS, and RAS is activated after the combination of GTP and GDP [31,32]. Ras binds to GTP and regulates cell growth, metastasis, and apoptosis by interacting with various effector molecules; these molecules include MAPK (mitotic protein kinase), STAT (signal transduction and activation transcription) and PI3K (phosphoinositol 3 kinase) signaling cascades [33,34].
The Ras-specific guanine-nucleotide exchange factor (Ras-GRF) is an activator of Ras and reflects Ras activity. As a second messenger, calcium ions can directly bind to the Ras-GRF and significantly increase Ras activity [24]. To further clarify the mechanism by which Orai1 promotes the metastasis of osteosarcoma cells, we silenced the expression of Orai1 and detected the changes of Ras and Ras-GRF in osteosarcoma cells. The results showed that Orai1 expression can promote the activation of Ras in osteosarcoma cells. Therefore, it is speculated that Orai1 can activate the Rac1 signaling pathway by activating Ras. To further confirm this hypothesis, ARS-853 (a Ras function-blocking antibody) was used. We found that the activity of Ras is required for Orai1-promoted Rac1-WAVE2 signaling pathway and cell migration, invasion, and adhesion of osteosarcoma cells. The above results suggest that Orai1 promotes the metastasis of osteosarcoma cells by activating the Ras-Rac1-WAVE2 signaling pathway.
Conclusions
Our study found that silencing Orai1 expression in osteosarcoma cells decreased migration, invasion, and adhesion abilities and inhibited activity of the Ras-Rac1-WAVE2 signaling pathway in osteosarcoma. These results suggest that Orai1 activates the Ras-Rac1-WAVE2 signaling pathway to promote the metastasis of osteosarcoma. Abnormal expression or function of Orai1 may be an important cause of osteosarcoma metastasis. Therefore, an in-depth study of the role of Orai1 in the metastasis of osteosarcoma has the potential to provide new ideas for the biological treatment of osteosarcoma metastasis.
Acknowledgement
Thanks to associate professor Tang Juan for her guidance and help in this study.
Footnotes
Source of support: This work was supported by the Fundamental Research Funds of Central Universities (xzy012019123), the Project funded by China Postdoctoral Science Foundation (2017T100763 and 2016M600805), and the National Natural Science Foundation of China (81502330 and 81702668)
Conflict of interest
None.
References
- 1.Lin YH, Jewell BE, Gingold J, et al. Osteosarcoma: Molecular pathogenesis and iPSC modeling. Trends Mol Med. 2017;23(8):737–55. doi: 10.1016/j.molmed.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He JP, Hao Y, Wang XL, et al. Review of the molecular pathogenesis of osteosarcoma. Asian Pac J Cancer Prev. 2014;15(15):5967–76. doi: 10.7314/apjcp.2014.15.15.5967. [DOI] [PubMed] [Google Scholar]
- 3.Tsiambas E, Fotiades PP, Sioka C, et al. Novel molecular and metabolic aspects in osteosarcoma. J BUON. 2017;22(6):1595–98. [PubMed] [Google Scholar]
- 4.Mercatelli D, Bortolotti M, Bazzocchi A, et al. Immunoconjugates for osteosarcoma therapy: Preclinical experiences and future perspectives. Biomedicines. 2018;6(1) doi: 10.3390/biomedicines6010019. pii: E19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhandary SK, Kali A. Significance of serum L-fucose glycoprotein as cancer biomarker in head and neck malignancies without distant metastasis. J Clin Diagn Res. 2013;7(12):2818–20. doi: 10.7860/JCDR/2013/6681.3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanz-Moreno V, Gadea G, Ahn J, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135(3):510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 7.Friedl P, Locker J, Sahai E, et al. Classifying collective cancer cell invasion. Nat Cell Biol. 2012;14(8):777–83. doi: 10.1038/ncb2548. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NT, Han W, Cao WM, et al. Store-operated calcium entry mediated by ORAI and STIM. Compr Physiol. 2018;8(3):981–1002. doi: 10.1002/cphy.c170031. [DOI] [PubMed] [Google Scholar]
- 9.Cai XG, Robert M, Nwokonko NA. Pore properties of Orai1 calcium channel dimers and their activation by the Orai1 ER calcium sensor. J Biol Chem. 2018;293(33):12962–74. doi: 10.1074/jbc.RA118.003424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang CL, Chen YJ, Quintanilla CG, et al. EB1 binding restricts Orai1 translocation to ER-PM junctions and regulates store-operated Ca2+ entry. J Cell Biol. 2018;217(6):2047–58. doi: 10.1083/jcb.201711151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chakraborty S, Bipan KD, Chorna T, et al. Mutant IP3 receptors attenuate store-operated Ca2+ entry by destabilizing STIM-Orai interactions in Drosophila neurons. J Cell Sci. 2016;129(20):3903–10. doi: 10.1242/jcs.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun J. Orai1- and Orai1-mediated Ca2+ oscillation orchestrates invadopodium formation and melanoma invasion. J Cell Biol. 2014;207:535–48. doi: 10.1083/jcb.201407082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang JW, Sun JW, Huang MY, et al. Orai1 overexpression promotes colorectal cancer progression, cell motility and COX-2 expression. Oncogene. 2015;34(33):4358–67. doi: 10.1038/onc.2014.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YJ, Sun JW, Huang MY, et al. Orai1-dependent Ca2+ signaling regulates podosome formation to facilitate cancer cell invasion. Sci Rep. 2017;7:11523. doi: 10.1038/s41598-017-11273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Z, Liu X, Feng B, et al. Orai1, a direct target of microRNA-185, promotes tumor metastasis and is associated with poor prognosis in coloreccancer. Oncogene. 2015;34(37):4808–20. doi: 10.1038/onc.2014.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou YB, Gu P, Li J, et al. Suppression of Orai1 inhibits the migration and invasion of human prostate cancer cells and is associated with PI3K/Akt signaling inactivation. Oncol Rep. 2017;38(5):2629–36. doi: 10.3892/or.2017.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang YD, Wang HY, Pan T, et al. Orai1 silencing inhibits the migration and invasion of A549 cells. Mol Med Rep. 2017;16(3):3283–89. doi: 10.3892/mmr.2017.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanz-Moreno V, Gadea G, Ahn J, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135(3):510–23. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 19.Castillo-Lluva S, Tatham MH, Jones RC, et al. SUMOylation of the GTPase Rac1 is required for optimal cell migration. Nat Cell Biol. 2010;12(11):1078–85. doi: 10.1038/ncb2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng Q, Tian YX, Liang JJ. Mangiferin inhibits cell migration and invasion through Rac1/WAVE2 signalling in breast cancer breast. Cytotechnology. 2018;70(2):593–60. doi: 10.1007/s10616-017-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yokotsuka M, Iwaya K, Tsuyoshi E, et al. Overexpression of HER2 signaling to WAVE2-Arp2/3 complex activates MMP-independent migration in breast cancer. Breast Cancer Res Treat. 2011;126(2):311–18. doi: 10.1007/s10549-010-0896-x. [DOI] [PubMed] [Google Scholar]
- 22.Kamai T, Shirataki H, Nakanishi K, et al. Increased Rac1 activity and Pak1 overexpression are associated with lymphovascular invasion and lymph node metastasis of upper urinary tract cancer. BMC Cancer. 2010;10:164. doi: 10.1186/1471-2407-10-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhanot H, Young AM, Overmeyer JH, et al. Induction of nonapoptotic cell death by activated Ras requires inverse regulation of Rac1 and Arf6. Mol Cancer Res. 2010;8:1358–74. doi: 10.1158/1541-7786.MCR-10-0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keiper M, Stope MB, Szatkowski D, et al. Epac- and Ca2+-controlled activation of Ras and extracellular signal-regulated kinases by Gs-coupled receptors. J Biol Chem. 2004;279(45):46497–508. doi: 10.1074/jbc.M403604200. [DOI] [PubMed] [Google Scholar]
- 25.Umemura M, Baljinnyam E, Feske S, et al. Store-operated Ca2+ entry (SOCE) regulates melanoma proliferation and cell migration. PLoS One. 2014;9(2):e89292. doi: 10.1371/journal.pone.0089292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y, Bao X, Zhang Z, et al. FGF2 promotes metastasis of uveal melanoma cells via store-operated calcium entry. Onco Targets Ther. 2017;10:5317–28. doi: 10.2147/OTT.S136677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stanisz H, Vultur A, Herlyn M, et al. The role of Orai-STIM calcium channels in melanocytes and melanoma. J Physiol. 2016;594(11):2825–35. doi: 10.1113/JP271141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hooper R, Zhang X, Webster M, et al. Novel protein kinase C-mediated control of Orai1 function in invasive melanoma. Mol Cell Biol. 2015;35(16):2790–98. doi: 10.1128/MCB.01500-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shi ZX, Rao W, Wang H, et al. Modeled microgravity suppressed invasion and migration of human glioblastoma U87 cells through downregulating store-operated calcium entry. Biochem Biophys Res Commun. 2015;457(3):378–84. doi: 10.1016/j.bbrc.2014.12.120. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, Chen L, Zhao P, et al. Store-operated Ca(2+) entry regulates glioma cell migration and invasion via modulation of Pyk2 phosphorylation. J Exp Clin Cancer Res. 2014;33:98. doi: 10.1186/s13046-014-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vo U, Vajpai N, Flavell L, et al. Monitoring Ras interactions with the nucleotide exchange factor son of sevenless (Sos) using site-specific NMR reporter signals and intrinsic fluorescence. J Biol Chem. 2016;291(4):1703–18. doi: 10.1074/jbc.M115.691238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simanshu DK, Nissley DV, McCormick F, et al. RAS proteins and their regulators in human disease. Cell. 2017;170(1):17–33. doi: 10.1016/j.cell.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nan Xiaolin, Tamgüney Tanja M, Collisson Eric A, et al. Ras-GTP dimers activate the Mitogen-Activated Protein Kinase (MAPK) pathway. Proc Natl Acad Sci USA. 2015;112(26):7996–8001. doi: 10.1073/pnas.1509123112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tamaskovic R, Schwill M, Nagy-Davidescu G, et al. Intermolecular biparatopic trapping of ErbB2 prevents compensatory activation of PI3K/AKT via RAS–p110 crosstalk. Nat Commun. 2016;7:11672. doi: 10.1038/ncomms11672. [DOI] [PMC free article] [PubMed] [Google Scholar]