Abstract
The age-related loss of skeletal muscle (sarcopenia) is a major health concern as it is associated with physical disability, metabolic impairments, and increased mortality. The coexistence of sarcopenia with obesity, termed ‘sarcopenic obesity’, contributes to skeletal muscle insulin resistance and the development of type 2 diabetes, a disease prevalent with advancing age. Despite this knowledge, the mechanisms contributing to sarcopenic obesity remain poorly understood, preventing the development of targeted therapeutics. This article will discuss the clinical and physiological consequences of sarcopenic obesity and propose myostatin as a potential candidate contributing to this condition. A special emphasis will be placed on examining the role of myostatin signaling in impairing both skeletal muscle growth and insulin signaling. In addition, the role of myostatin in regulating muscle-to fat cross talk, further exacerbating metabolic dysfunction in the elderly, will be highlighted. Lastly, we discuss how this knowledge has implications for the design of myostatin-inhibitor clinical trials.
Keywords: Aging, muscle wasting, insulin resistance, sarcopenia, atrophy
Introduction
The confluence of the obesity epidemic with the age-related loss of muscle mass (sarcopenia) has resulted in the concept of ‘sarcopenic obesity’ (i.e., the copresence of sarcopenia and obesity) (1, 2). While the prevalence of sarcopenic obesity varies tremendously depending on the operational definition used (2), it is an emerging public health crisis. As Roubenoff stated, “The ‘fat frail’ have the worst of both worlds as they age— increased weakness due to sarcopenia and a need to carry greater weight due to obesity” (3). Sarcopenia reduces the mass of available insulin-responsive skeletal muscle, which promotes insulin resistance, obesity, and the metabolic syndrome (4). It has been suggested that there is a biological connection between insulin resistance, obesity and sarcopenia (3), although the mechanisms that might explain a common etiology are not yet fully understood (5).
One potential contributor to both the metabolic and anabolic defects in sarcopenic obesity is myostatin. Myostatin (also known as growth differentiation factor 8) is a member of the TGF-β superfamily with well-known inhibitory effects on skeletal muscle growth and development. For instance, the landmark work on myostatin by McPherron and colleagues demonstrated that myostatin null animals are 2–3 times larger than wild-type animals and show a large and widespread increase in skeletal muscle mass due to both muscle cell hyperplasia and hypertrophy, which indicates that myostatin functions as a negative regulator of muscle growth (6). However, in addition to its effects on muscle mass, myostatin deficiency has more recently been shown to have beneficial effects on metabolism, adiposity and insulin sensitivity. In this article we will examine the role of myostatin signaling in impairing both skeletal muscle growth and insulin signaling. In addition, the role of myostatin in regulating muscle-to-fat cross talk, further exacerbating metabolic dysfunction in the elderly, will be highlighted.
We believe that a deeper understanding of the vicious cycle that myostain signaling plays in muscle growth and insulin signaling has implications for clinical trials. The field of skeletal muscle pharmacology is at a critical point, and myostatin remains the most popular drug target for attenuating atrophy (7). Anti-myostatin agents have been in clinical trials for more than a decade with the various drug programs most commonly targeting cancer cachexia, muscular dystrophy, inclusion-body myositis, and sarcopenia (7). Many of these trials have reported modest, if not disappointing effects, especially in comparison to pre-clinical data. However, to our knowledge, only one trial is considering targeting sarcopenic obesity as the indication (8). This article will summarize the multiple physiological effects of myostatin, and we hope, make the case for future trials to include outcome measures to not only examine the effects on skeletal muscle mass and physical function, but to also thoroughly examine the potential metabolic effects. We will also provide evidence that sarcopenic obese patients may receive the greatest health benefits from myostatin-inhibition and recommend that future studies examine this specific population, as well as, other conditions where muscle wasting and impaired energy metabolism co-exist.
Myostatin, age and skeletal muscle
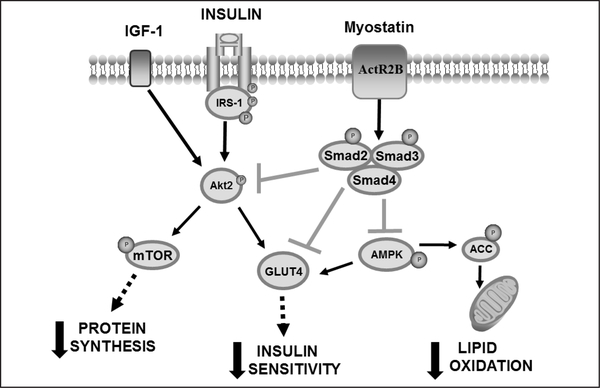
Myostatin is highly expressed in skeletal muscle (6), with lower levels expressed in adipose (9) and cardiac tissue (10). Originally synthesized in its precursor form (52kDa), myostatin is subsequently modified to the mature myostatin form (26kDa) through a series of proteolysis steps. Myostatin inhibits skeletal muscle growth by binding to the myostatin receptor, activin type IIB receptor (ActR2B), leading to phosphorylation of Smad2 and Smad3, which then form a heterodimer with Smad4 to translocate to the nucleus to regulate gene transcription. In addition to the activation of genes involved in the degradation of protein (11), myostatin-induced activation of Smad2 and Smad3 is believed to inhibit the IGF-1/Akt/ mammalian target of rapamycin (mTOR) pathway (11, 12), a critical pathway responsible for stimulating protein synthesis (Figure 1). Current research supports this Smad-mediated pathway as the pathway through which myostatin acts to suppress protein synthesis and potentially other metabolic effects.
Figure 1.
Proposed model of myostatin-induced skeletal muscle wasting and insulin resistance
Myostatin binds to it’s receptor (ActR2B), phosphorylating the Smad2/3 pathway. Myostatin signaling is responsible for inhibiting Akt phosphorylation which reduces skeletal muscle protein synthesis (increasing sarcopenia) and inhibits skeletal muscle insulin sensitivity. Myostatin is also capable of inhibiting AMPK activity which will reduce GLUT4 translocation to the plasma membrane (reducing glucose uptake and insulin sensitivity) and reduce ACC phosphorylation, reducing lipid oxidation. Lastly, myostatin signaling can reduce GLUT4 protein expression which would reduce the availability of glucose transporters and contribute to skeletal muscle insulin resistance.
A number of studies have demonstrated a link between increased myostatin levels, age (13, 14), declining muscle mass (13, 14), and decreased muscular strength (15, 16). An early cross-sectional study determined that myostatin levels increased with both age and declining muscle mass (13). In support of these findings, it was later reported that the odds of males having sarcopenia increased 11% with each 1 ng/ml increase in serum myostatin (17). Since the majority of myostatin is synthesized in skeletal muscle, it is not surprising that elevated levels of myostatin have also been observed in elderly skeletal muscle. Myostatin gene expression was reported to be ~100% higher in elderly men (mean age of 70) compared to young men (mean age of 20), which corresponded with a 40% reduction in type 2 (fast twitch) muscle fiber cross sectional area (14). Myostatin has also been associated with declining muscular strength in aging adults (15, 16). For example, the risk for middle- to older-aged adults to have low grip strength was ~7.5-fold higher if they had elevated myostatin levels (15) and skeletal muscle myostatin mRNA was reported higher in elderly men with weaker muscular strength (16). In contrast, others have not observed a relationship between plasma myostatin and sarcopenia in women (18) or men (19). Part of this discrepancy may be due to difficulties measuring myostatin since it has a close homology with other TGF-β members, making antibody specificity an issue. Another potential issue is subject inclusion criteria. Since many studies exclude overweight and obese individuals, a true depiction of the aging population is not always present in studies. In an attempt to obtain a more accurate measurement of myostatin in a representative adult population, Bergen et al. (20) developed a liquid chromatography with tandem mass spectrometry (LC-MS/MS) assay to measure concentrations of myostatin. This study found that circulating concentrations of mature and propeptide myostatin were higher in older women with elevated body fat, compared to younger women with a lower body fat (20), suggesting that increased body fat could be necessary for elevated myostatin levels to be present in the elderly. It is these individuals that myostatin may contribute to the adverse effects of sarcopenia and insulin resistance.
Myostatin is associated with obesity and insulin resistance
Increased body fat (21) and insulin resistance (22) are commonly associated with aging. Similarly, elevated myostatin levels have been associated with both obesity (23) and insulin resistance (23–26). For example, extremely obese (body mass index, BMI=49 kg/m2) individuals have been reported to have a 35% increase in plasma mature myostatin and a 23% increase in skeletal muscle precursor myostatin, compared to lean controls (BMI=26 kg/m2), with BMI strongly correlated with muscle myostatin (23).
Since skeletal muscle is considered the primary target for insulin-mediated glucose uptake (27), research efforts have focused on identifying cellular mechanism(s) in muscle that could contribute to insulin resistance. Of particular interest, a clear relationship between elevated myostatin levels and insulin resistance has been presented in a number of studies (23–26). Skeletal muscle myostatin gene expression has been found to be negatively associated with whole-body insulin sensitivity (r=−0.43, P<0.05) in overweight and obese (BMI range of 26–46 kg/m2), middle- to older-aged individuals (54–77 years) (25). Increased skeletal muscle myostatin mRNA has also been reported in a number of different conditions associated with insulin resistance including dysglycaemia (24), type 2 diabetes (28) and first degree relatives of type 2 diabetics (28). An important finding by Guo et al. (26) was that myostatin knockout mice were more insulin sensitive than wild type mice, even after controlling for increased muscle mass, suggesting myostatin has detrimental effects on insulin sensitivity, independent of decreased muscle mass. This would be especially detrimental for individuals with sarcopenic obesity since they would suffer from the reduced glucose uptake due to decreased muscle mass, but also the independent inhibitory action of myostatin on insulin sensitivity.
In line with these findings, interventions known to increase insulin sensitivity and decrease body fat including exercise training and gastric bypass surgery have demonstrated significant declines in skeletal muscle myostatin (25, 29). Increasing evidence suggests that therapeutic interventions of this nature may be especially effective in older individuals. When 12 weeks of aerobic training was combined with minor diet restrictions to induce weight loss in older individuals (aged 54–77 years), skeletal muscle myostatin mRNA decreased by ~40%, along with an 18% increase in insulin sensitivity and 11% decrease in body fat (25). Unfortunately, potential changes in skeletal muscle strength and function were not reported in this previous study; however, a recent study treating old mice with an anti-myostatin antibody (ATA 842) increased insulin-stimulated whole body glucose metabolism, muscle mass, and grip strength (30).
Taken together these findings suggest that myostatin may provide a cellular mechanism that could promote sarcopenic obesity, including declining muscle mass, increased body fat, and insulin resistance in skeletal muscle, especially in susceptible elderly. The following will provide an overview of these potential mechanisms in both skeletal muscle and adipose tissue.
Myostatin inhibits skeletal muscle Akt phosphorylation
The IGF-1 and insulin signaling pathways responsible for protein synthesis and glucose uptake, respectively, converge on the Akt molecule making it an attractive target to explain myostatin-induced muscle mass loss and insulin resistance. The first evidence of Akt inhibition by myostatin occurred when studying this myokine with respect to inhibiting protein synthesis. IGF-1 typically induces protein synthesis and muscle hypertrophy through the Akt/mTOR/p70S6K pathway. Overexpression of myostatin in C2C12 (mouse) muscle cells demonstrated a decrease in IGF-1 induced Akt phosphorylation and prevented cell hypertrophy (Figure 1) (31). In contrast, myostatin inhibition restored both Akt phosphorylation and hypertrophy (31), providing further support for a link between myostatin-induced inhibition of Akt phosphorylation and the cessation of muscle growth.
A number of studies have since been conducted providing evidence that myostatin also exerts a negative effect on insulin signaling through the inhibition of Akt phosphorylation (Figure 1) (26, 32, 33). Pharmacological injection of myostatin into mice resulted in reduced insulin-stimulated Akt phosphorylation in the gastrocnemius and vastus lateralis, likely contributing to the whole-body insulin resistance also observed (32). In contrast, myostatin-null mice have been reported to exhibit augmented muscle mass, enhanced insulin sensitivity, and elevated levels of insulin-stimulated Akt phosphorylation in the gastrocnemius (26, 33). The inhibitory effects of myostatin on Akt can also be extended to conditions of obesity induced by high fat feeding. Tang et al. (34) reported that mice treated with an anti-myostatin polycolonal antibody had a reversal of diet-induced insulin resistance, in conjunction to an 86% elevation in Akt phosphorylation in the quadriceps muscle compared to mice not treated with the antibody. Collectively, these findings support myostatin-induced inhibition of Akt as a potential mechanism for the muscle wasting and insulin resistance observed with sarcopenic obesity.
Myostatin inhibits skeletal muscle Ampk and lipid oxydation
Genetic deletion of myostatin has been shown to increase skeletal muscle AMP-activated protein kinase (AMPK) expression and activity (33, 35, 36), which could contribute to the improvements in insulin sensitivity and lipid metabolism observed in myostatin deleted mice. AMPK is considered a metabolic switch, becoming activated when the intracellular AMP/ATP ratio becomes elevated, resulting in the upregulation of ATP generating systems, including glucose uptake and fatty acid oxidation (33). AMPK regulates both insulin-dependent and –independent glucose uptake by stimulating the translocation of the glucose transporter, GLUT4, to the plasma membrane; therefore, it is possible that AMPK induced stimulation of GLUT4 during conditions of myostatin deficiency contributes to increased insulin sensitivity. Based on this knowledge, it is conceivable that the upregulation of myostatin in obese, elderly individuals could decrease skeletal muscle AMPK activity which would have a detrimental effect on insulin sensitivity (Figure 1), likely further predisposing these individuals to sarcopenic obesity and type 2 diabetes.
The metabolic benefits of myostatin inhibition appear to be most evident during conditions of high fat feeding. Myostatin null mice resist adipose accumulation and have a preservation of insulin sensitivity during high fat conditions (34). Knowing the metabolic effects attributed to AMPK, it is not unreasonable to suggest the increased activity of this protein could stimulate the preferential shuttling of fat from storage to oxidation in these mice. It is not surprising myostatin deficient mice also have increased phosphorylation of acetyl-CoA carboxylase (ACC) (33), since it is located downstream of AMPK. Phosphorylation of ACC results in the reduction of malonyl-CoA, leading to the inhibition of fatty acid synthesis and the increase in lipid oxidation, further supporting the role of increased lipid oxidation as a mechanism through which myostatin deficient mice appear metabolically protected from conditions of high fat intake.
Age associated skeletal muscle mitochondrial dysfunction is believed to contribute to obesity and declines in insulin sensitivity with aging (37), although the mechanism(s) remain unclear. Increasing evidence suggests that myostatin may have detrimental effects on mitochondrial function. Myostatin null mice have been shown to have increased PGC-1α expression in skeletal muscle (33, 35). Our group (38) and others (39) have documented the role that PGC-1 has in stimulating mitochondrial biogenesis, preferentially shutting lipids towards oxidation versus storage, and increasing GLUT4 expression. The inhibition of myostatin has also been shown to upregulate skeletal muscle oxidation genes (24, 36), including carnitine palmitoyltransferase 1 (CPT-1) (35), the mitochondrial protein responsible for shuttling lipid into the mitochondria for oxidation. Collectively, these findings suggest that elevated myostatin levels in the obese, elderly could inhibit mitochondrial function and AMPK activity, resulting in reduced glucose uptake, and diminished lipid oxidation (Figure 1). These metabolic disturbances will likely contribute to the further progression of saropenic obesity and the onset of insulin resistance in the elderly.
Myostatin inhibits Glut4 expression
The decline in skeletal muscle GLUT4 often parallels the age-associated decline in insulin sensitivity (22). Of interest, myostatin inhibition has been shown to increase skeletal muscle GLUT4 expression in young rodents (34, 36), old mice (40), and double-muscled cattle (myostatin knockout model) (41) compared to controls. Cell culture studies involving myoblasts (muscle cells) isolated from double-muscled cattle, demonstrate increased glucose uptake under basal and insulin-stimulated conditions and increased gene expression for glucose transporters GLUT1 and GLUT4, compared to control cells (41). Increased GLUT4 and GLUT1 protein expression has also been observed in mice given a local injection of myostatin propeptide (decreasing myostatin expression). The role of myostatin in regulating GLUT4 was further strengthened when addition of myostatin to muscle cells resulted in the suppression of GLUT4 and MEF2c mRNA (41). MEF2C, like PGC-1, is a transcription factor responsible for GLUT4 expression; therefore, it is possible that inhibiting myostatin upregulates key transcription factors responsible for the upregulation of GLUT4. Collectively, these findings suggest that myostatin may act to inhibit insulin sensitivity not only by inhibiting the signal responsible for initiating GLUT4 translocation (ie., Akt, AMPK) but also by reducing the number of transporters available for glucose transport (ie., GLUT4) (Figure 1).
Myostatin and adipose tissue
It has been widely documented that the inhibition of myostatin is associated with reduced adipose tissue (34), suggesting myostatin acts either directly or indirectly on adipose tissue. Adipocytes (fat cells) are generally classified into three different categories: white adipocytes, brown adipocytes, and beige adipocytes. The majority of fat in humans is categorized as white adipose tissue (WAT), composed of large (25–200 μM) white adipocytes that contain a unilocular (single) lipid droplet, few mitochondria, and preferentially store energy as triglycerides (42, 43). Another type of fat, sparsely located in humans, is brown adipose tissue (BAT), consisting of small (15–60 μM) brown adipocytes, having multi-locular (multiple) lipid droplets, high mitochondrial density, and preferentially burn energy through non-shivering thermogenesis, food intake, and hormonal influence (42, 43). Similar to skeletal muscle plasticity, adipose tissue has the ability to adapt to physiological conditions, including exposure to cold, exercise, or certain hormones. It is under these conditions that a third type of fat cell is induced, beige adipocytes (also referred to as brown-like or brite adipocytes) which tend to be located in WAT, but have similar metabolic properties as brown adipocytes, including increased cellular respiration (44).
Whereas adipose tissue has historically been considered detrimental to health, the recent finding that adult humans have depots of BAT (45, 46) and can induce beige adipocytes has redirected the thinking about the metabolic influence of adipose tissue. Both age and obesity are inversely related to BAT activity (45, 46). Further, a reduced brown adipose phenotype (expression of brown adipogenic genes) has been associated with a higher prevalence of insulin resistance (47), and the activation of BAT (by cold) has been demonstrated to enhance glucose uptake and whole-body insulin sensitivity (48). These findings suggest that the loss of BAT or beige adipocytes during aging could predispose the elderly to obesity and type 2 diabetes.
Current research suggests that elevated levels of myostatin could contribute to this accelerated loss of BAT and/or beige adipocytes. WAT from myostatin null mice adopt a BAT-like phenotype with increased expression of genes typically observed in brown/beige adipocytes including UCP1, PPARα and PGC-1α (35, 49). Further, Choi and co-workers (50) reported that myostatin deficient mice had an elevated energy expenditure compared to wild-type controls, even after controlling for their excess lean mass. Since BAT was not measured in this latter study (50), it is unclear if increases in BAT or beige adipocytes contributed to the elevated energy expenditure, but it does provide evidence of increased energy use in tissue, which could contribute to the decreased adipose tissue found in myostatin deficient mice. Despite myostatin clearly having an effect on adipose tissue, these effects appear to occur indirectly through skeletal muscle. Results comparing muscle specific myostatin knock out versus fat specific myostatin knock out indicate the reduction in adipose tissue mass in myostatin deficient mice is the indirect result of metabolic or myokine changes in skeletal muscle (26).
Myostatin inhibition increases the myokine irisin
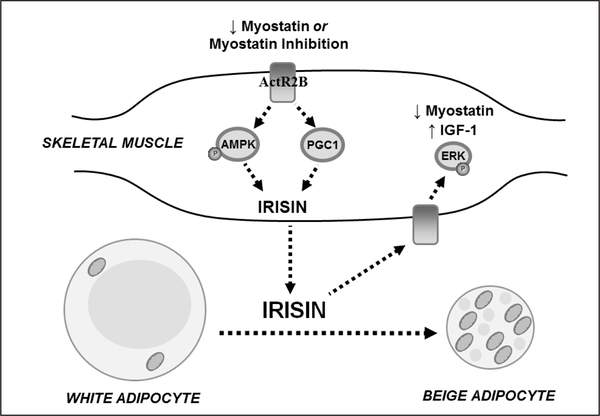
One suggested pathway through which myostatin may act indirectly on adipose tissue is through the AMPK-PGC1α-irisin (Fndc5) pathway (Figure 2). The myokine irisin, increases in skeletal muscle in response to elevations in AMPK phosphorylation and PGC-1 expression (35); therefore, it is not surprising that myostatin deficient mice have increased skeletal muscle irisin expression (35). Irisin levels have been reported to be low in obese individuals and type 2 diabetics (51) and irisin has been shown to induce beige cell markers in adipocytes derived from WAT (35) providing convincing evidence that myostatin induced inhibition of this protein may adversely affect fat metabolism in older, obese adults. Taken together the muscle fat cross-talk via myostatin regulated irisin may be a mechanism through which myostatin inhibition indirectly regulates adipose metabolism.
Figure 2.
Proposed model of myostatin inhibition leading to beneficial muscle growth and adipocyte beige phenotype
Inhibition or reduced myostatin increases PGC-1 levels and the activity of AMPK, resulting in the increased expression of the myokine, irisin. Irisin is released into circulation inducing a beige adipocyte phenotype and increasing skeletal muscle IGF-1 expression and decreasing myotatin expression via the extracellular signal–regulated kinase (ERK) pathway to promote muscle growth.
Of particular interest, low irisin has been suggested as a potential marker for sarcopenia (52, 53), in part based on findings that circulating irisin levels are lower in sarcopenic individuals compared to normal controls, and serum irisin is positively associated with grip strength (52). Although the specific mechanism through which low irisin may be involved in muscle wasting remains unclear, irisin treatment of muscle cells has been shown to increase IGF-1 and decrease myostatin expression, respectively (54), suggesting a potential role in stimulating skeletal muscle protein synthesis (Figure 2). Collectively, these findings suggest that the myostatin induced inhibition of irisin can contribute to increased fat mass and decreased muscle mass, which is especially detrimental for the elderly since it further predisposes them to sarcopenic obesity.
Summary comments & implications for clinical trials
For more than two decades myostatin has been recognized as a master negative regulator of skeletal muscle mass. More recent data indicates that myostatin expression also exerts effects on glucose and fat metabolism, which in the case of sarcopenic obesity, could create a vicious cycle as described in the preceding sections. For instance, if increased levels of myostatin result in low levels of muscle tissue mass while concomitantly inhibiting insulin signaling, muscle mitochondrial biogenesis, lipid oxidation, and energy expenditure, this myokine (myostatin) could conceptually be a key contributor to sarcopenic obesity. As such, we propose myostatin be considered, and further investigated, as a potential candidate contributing to sarcopenic obesity.
Moreover, we propose that the multiple physiological effects of myostatin be considered when designing mysostatin-inhibitor clinical trials. There are a number of completed and ongoing clinical trials examining the effects of compounds designed to inhibit the myostatin pathway (7), and the vast majority of these do not obtain measures related to energy metabolism. Accordingly, we recommend that future trials include outcome measures to not only examine the effects on skeletal muscle mass and physical function, but to thoroughly examine the potential metabolic effects. Further, we recommend due thought be given to examining the effectiveness of myostatin-inhibition in sarcopenic obese patients as well as other conditions where muscle wasting and impaired energy metabolism co-exist.
Acknowledgments:
This work was supported by grants from the NIH’s National Institute of Diabetes and Digestive and Kidney Diseases (R15DK102115 to LAC) and the National Institute on Aging (R01AG044424 to BCC).
Footnotes
Conflict of interest: None declared by authors.
References
- 1.Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care 2008;11:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cauley JA. An Overview of Sarcopenic Obesity. Curr Opin Clin Nutr Metab Care 2015;18:499–505. [DOI] [PubMed] [Google Scholar]
- 3.Roubenoff R Sarcopenic obesity: the confluence of two epidemics. Curr Opin Clin Nutr Metab Care 2004;12:887–888. [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988;37:1595–1607. [DOI] [PubMed] [Google Scholar]
- 5.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J Endocrinol 2016;229:R67–81. [DOI] [PubMed] [Google Scholar]
- 6.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 1997;387:83–90. [DOI] [PubMed] [Google Scholar]
- 7.Garber K No longer going to waste. Nat Biotechnol 2016;34:458–461. [DOI] [PubMed] [Google Scholar]
- 8.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2016 Jan 3-. Identifier NCT03021798, An OBServational Clinical Trial (SARA-OBS) in Sarcopenia and Sarcopenic Obesity in Patients Aged 65 Years and Over (SARA-OBS). Available from: https://clinicaltrials.gov/ct2/show/NCT03021798?term=NCT03021798&rank=1
- 9.Kocsis T, Trencsenyi G, Szabo K, et al. Myostatin propeptide mutation of the hypermuscular Compact mice decreases the formation of myostatin and improves insulin sensitivity. Am J Physiol Endocrinol Metab 2017;312:E150–E160. [DOI] [PubMed] [Google Scholar]
- 10.Sharma M, Kambadur R, Matthews KG, et al. Myostatin, a transforming growth factor-beta superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J Cell Physiol 1999;180:19. [DOI] [PubMed] [Google Scholar]
- 11.Goodman CA, McNally RM, Hoffmann FM, Hornberger TA. Smad3 induces atrogin-1, inhibits mTOR and protein synthesis, and promotes muscle atrophy in vivo. Mol Endocrinol 2013;27:1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morissette MR, Cook SA, Buranasombati C, Rosenberg MA, Rosenzweig A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol 2009;297:C1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yarasheski KE, Bhasin S, Sinha-Hikim I, Pak-Loduca J, Gonzalez-Cadavid NF. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J Nutr Health Aging 2002;6:343–348. [PubMed] [Google Scholar]
- 14.Leger B, Derave W, De Bock K, Hespel P, Russell AP. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res 2008;11:163–175B. [DOI] [PubMed] [Google Scholar]
- 15.Han DS, Chen YM, Lin SY, et al. Serum myostatin levels and grip strength in normal subjects and patients on maintenance haemodialysis. Clin Endocrinol (Oxf) 2011;75:857–863. [DOI] [PubMed] [Google Scholar]
- 16.Patel HP, Al-Shanti N, Davies LC, et al. Lean mass, muscle strength and gene expression in community dwelling older men: findings from the Hertfordshire Sarcopenia Study (HSS). Calcif Tissue Int 2014;95:308–316. [DOI] [PubMed] [Google Scholar]
- 17.Tay L, Ding YY, Leung BP, et al. Sex-specific differences in risk factors for sarcopenia amongst community-dwelling older adults. Age (Dordr) 2015;37:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann M, Halper B, Oesen S, et al. Serum concentrations of insulin-like growth factor-1, members of the TGF-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol 2015;64:35–45. [DOI] [PubMed] [Google Scholar]
- 19.Ratkevicius A, Joyson A, Selmer I, et al. Serum concentrations of myostatin and myostatin-interacting proteins do not differ between young and sarcopenic elderly men. J Gerontol A Biol Sci Med Sci 2011;66:620626. [DOI] [PubMed] [Google Scholar]
- 20.Bergen HR 3rd, Farr JN, Vanderboom PM, et al. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet Muscle 2015;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borkan GA, Hults DE, Gerzof SG, Robbins AH, Silbert CK. Age changes in body composition revealed by computed tomography. J Gerontol 1983;38:673–677. [DOI] [PubMed] [Google Scholar]
- 22.Consitt LA, Van Meter J, Newton CA, et al. Impairments in site-specific AS160 phosphorylation and effects of exercise training. Diabetes 2013;62:3437–3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hittel DS, Berggren JR, Shearer J, Boyle K, Houmard JA. Increased secretion and expression of myostatin in skeletal muscle from extremely obese women. Diabetes 2009;58:30–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjorth M, Pourteymour S, Gorgens SW, et al. Myostatin in relation to physical activity and dysglycaemia and its effect on energy metabolism in human skeletal muscle cells. Acta Physiol (Oxf) 2016;217:45–60. [DOI] [PubMed] [Google Scholar]
- 25.Ryan AS, Li G, Blumenthal JB, Ortmeyer HK. Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults. Obesity (Silver Spring) 2013;21:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One 2009;4:e4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeFronzo RA. Glucose intolerance and aging. Diabetes Care 1981;4:493501. [DOI] [PubMed] [Google Scholar]
- 28.Palsgaard J, Brons C, Friedrichsen M, et al. Gene expression in skeletal muscle biopsies from people with type 2 diabetes and relatives: differential regulation of insulin signaling pathways. PLoS One 2009;4:e6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milan G, Dalla Nora E, Pilon C, et al. Changes in muscle myostatin expression in obese subjects after weight loss. J Clin Endocrinol Metab 2004;89:2724–2727. [DOI] [PubMed] [Google Scholar]
- 30.Camporez JP, Petersen MC, Abudukadier A, et al. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc Natl Acad Sci U S A 2016;113:2212–2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morissette MR, Cook SA, Buranasombati C, Rosenberg MA, Rosenzweig A. Myostatin inhibits IGF-I-induced myotube hypertrophy through Akt. Am J Physiol Cell Physiol 2009;297:C1124–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hittel DS, Axelson M, Sarna N, Shearer J, Huffman KM, Kraus WE. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med Sci Sports Exerc 2010;42:2023–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang C, McFarlane C, Lokireddy S, et al. Myostatin-deficient mice exhibit reduced insulin resistance through activating the AMP-activated protein kinase signalling pathway. Diabetologia 2011;54:1491–1501. [DOI] [PubMed] [Google Scholar]
- 34.Tang L, Liu CT, Wang XD, et al. A prepared anti-MSTN polyclonal antibody reverses insulin resistance of diet-induced obese rats via regulation of PI3K/Akt/mTOR&FoxO1 signal pathways. Biotechnol Lett 2014;36:2417–2423. [DOI] [PubMed] [Google Scholar]
- 35.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1alpha-Fndc5 pathway in muscle. FASEB J 2013;27:1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong J, Dong Y, Dong Y, Chen F, Mitch WE, Zhang L. Inhibition of myostatin in mice improves insulin sensitivity via irisin-mediated cross talk between muscle and adipose tissues. Int J Obes (Lond) 2016;40:434442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petersen KF, Befroy D, Dufour S, et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science 2003;300:1140–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Consitt LA, Bell JA, Koves TR, et al. Peroxisome proliferator-activated receptor-gamma coactivator-1alpha overexpression increases lipid oxidation in myocytes from extremely obese individuals. Diabetes 2010;59:1407–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michael LF, Wu Z, Cheatham RB, et al. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc Natl Acad Sci U S A 2001;98:3820–3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pauly M, Chabi B, Favier FB, et al. Combined Strategies for Maintaining Skeletal Muscle Mass and Function in Aging: Myostatin Inactivation and AICAR-Associated Oxidative Metabolism Induction. J Gerontol A Biol Sci Med Sci 2015;70:1077–1087. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi H, Sato K, Yamaguchi T, et al. Myostatin alters glucose transporter-4 (GLUT4) expression in bovine skeletal muscles and myoblasts isolated from double-muscled (DM) and normal-muscled (NM) Japanese shorthorn cattle. Domest Anim Endocrinol 2014;48:62–68. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez A, Becerril S, Ezquerro S, Mendez-Gimenez L, Fruhbeck G. Crosstalk between adipokines and myokines in fat browning. Acta Physiol (Oxf) 2017;219:362–381. [DOI] [PubMed] [Google Scholar]
- 43.Cinti S The adipose organ at a glance. Dis Model Mech 2012;5:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem 2010;285:7153–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoneshiro T, Aita S, Matsushita M, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring) 2011;19:1755–1760. [DOI] [PubMed] [Google Scholar]
- 46.Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 2009;58:1526–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Enerback S, Smith U. Reduced expression of FOXC2 and brown adipogenic genes in human subjects with insulin resistance. Obes Res 2003;11:1182–1191. [DOI] [PubMed] [Google Scholar]
- 48.Chondronikola M, Volpi E, Borsheim E, et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 2014;63:4089–4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang C, McFarlane C, Lokireddy S, et al. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 2012;55:183193. [DOI] [PubMed] [Google Scholar]
- 50.Choi SJ, Yablonka-Reuveni Z, et al. Increased energy expenditure and leptin sensitivity account for low fat mass in myostatin-deficient mice. Am J Physiol Endocrinol Metab 2011;300:E1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab 2013;98:E769–778. [DOI] [PubMed] [Google Scholar]
- 52.Chang JS, Kim TH, Nguyen TT, Park KS, Kim N, Kong ID. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr Gerontol Int 2017. [DOI] [PubMed] [Google Scholar]
- 53.Kalinkovich A, Livshits G. Sarcopenia--The search for emerging biomarkers. Ageing Res Rev 2015;22:58–71. [DOI] [PubMed] [Google Scholar]
- 54.Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond) 2014;38:1538–1544. [DOI] [PubMed] [Google Scholar]