Abstract
Background:
Colorado tick fever (CTF) is an acute systemic febrile illness caused by the CTF virus (CTFV). The last national summary of CTF cases in the United States included cases reported through 2001. This study summarizes national surveillance data for CTF from 2002 through 2012 and examines trends in the epidemiology and testing of identified CTF cases.
Methods:
Because CTF is not nationally notifiable, we identified CTF cases through solicited reports from state health departments and diagnostic laboratory records. For all cases, we collected data on age, sex, county of residence, travel history, symptom onset date, laboratory testing, and clinical outcome. Poisson regression was used to examine trends over time in case counts, and simple linear regression and logistic regression were used to examine trends in case characteristics.
Results:
From 2002 through 2012, 75 CTF cases were identified with a median of five cases per year (range 3–14). Forty-seven (63%) cases occurred in males and 49 (65%) occurred in people aged ≥ 40 years. The majority (80%) of cases had onset of illness during May through July. Cases occurred in residents of 14 states but the infections were acquired in six western states. Wyoming had the highest annual incidence of CTF among residents (3.4 cases per million population), followed by Montana (1.5 per million), and Utah (0.5 per million). Over the 11 years, there was an increase in the proportion of cases diagnosed by RT-PCR testing and in the proportion of cases among travelers to another state.
Conclusions:
CTF cases continue to occur annually among residents and visitors to the western United States. Public health prevention messages about decreasing tick exposure should be targeted to residents and travelers who will spend time outdoors in an endemic region during the spring and summer months.
Keywords: Colorado tick fever virus, Coltivirus, Tick-borne diseases, Public health surveillance
Introduction
Colorado tick fever (CTF) is an acute systemic febrile illness caused by the CTF virus (CTFV), a double-stranded RNA virus in the Coltivirus genus and Reoviridae family. The virus is transmitted to humans primarily through the bite of an infected Rocky Mountain wood tick, Dermacentor andersoni. D. andersoni is found in the western United States and Canada at elevations of 4000–10,000 feet, typically in grassy areas near sage or other brush (James et al. 2006, Eisen et al. 2008, Romero and Simonsen 2008). Up to 90% of patients diagnosed with CTF recall a specific tick exposure preceding their illness onset (Goodpasture et al. 1978, Brackney et al. 2010).
The incubation period for CTF is usually 3–4 days (range, 1–14 days). Symptoms can include fever, headache, myalgia, and fatigue and can persist for several weeks in some patients (Goodpasture et al. 1978). Leukopenia is also commonly noted. CTFV infection occasionally results in more severe clinical manifestations, including meningitis, encephalitis, and bleeding disorders. Although fatalities are rare, death has been reported in three children who developed bleeding disorders following CTFV infections (Spruance and Bailey 1973, Centers for Disease Control and Prevention 1972, Goodpasture et al. 1978).
In 2012, CTFV testing was performed at a limited number of facilities in the United States, including the Centers for Disease Control and Prevention (CDC), the Montana Public Health Laboratory, and Focus Diagnostics. Prior to 2012, testing had been available at several additional state health departments (e.g., Utah). The diagnosis of CTF can be made by virus isolation, RT-PCR, or antibody testing (Lambert et al. 2007, Basile 2010).
Although CTF is not a nationally notifiable condition, as of 2012 it was reportable in six states (Arizona, Montana, New Mexico, Oregon, Utah, and Wyoming). Of the additional six states where CTF has historically been identified, it was not reportable in four states (California, Colorado, Idaho, and Nevada). Two states (South Dakota and Washington) had arboviral infections as reportable conditions, but CTF was not specifically listed.
The last national summary of CTF cases in the United States included cases reported between 1987 and 2001 (Marfin and Campbell 2005). This study summarizes national surveillance data for CTF from 2002 through 2012 and examines trends in the epidemiology and testing of identified CTF cases.
Materials and Methods
We defined a confirmed CTF case as a patient with CTFV isolated in viral culture, CTFV RNA detected by RT-PCR, or a four-fold or more rise in CTFV-specific quantitative immunoglobulin G (IgG) or neutralizing antibody titers between acute- and convalescent-phase specimens. We defined a probable case as a patient with anti-CTFV IgM antibodies in serum. We identified cases through soliciting reports from state health department and laboratory testing performed at the CDC Arbovirus Diagnostic Laboratory from 2002 through 2012. State health departments of the 12 states where CTF has been identified historically were contacted actively to solicit additional cases not reported previously.
For all confirmed and probable cases, we collected data on age, sex, county of residence, travel history, symptom onset date, laboratory testing, and clinical outcome. Data were analyzed using SAS statistical software (v. 9.2, SAS Institute, Cary, NC); county of residence was mapped using ArcMap, (version 10, ESRI, Redlands, CA). Categorical variables were described as counts and proportions, and continuous variables were described by median and range. Poisson regression was used to examine trends over time in case counts, and simple linear regression and logistic regression were used to examine trends in case characteristics. State- and county-specific incidence rates were calculated using 2010 US Census Bureau data.
Results
A total of 95 possible CTF cases were identified from 2002 through 2012. Of these, 75 (79%) were classified as confirmed (n = 65) or probable (n = 10) cases. Of the 20 possible cases that did not meet the confirmed or probable case definition, 11 had anti-CTFV IgG or neutralizing antibodies in a single serum sample, seven had no available laboratory test results, and two had no laboratory testing performed. The remainder of this report will focus on the confirmed and probable CTF cases.
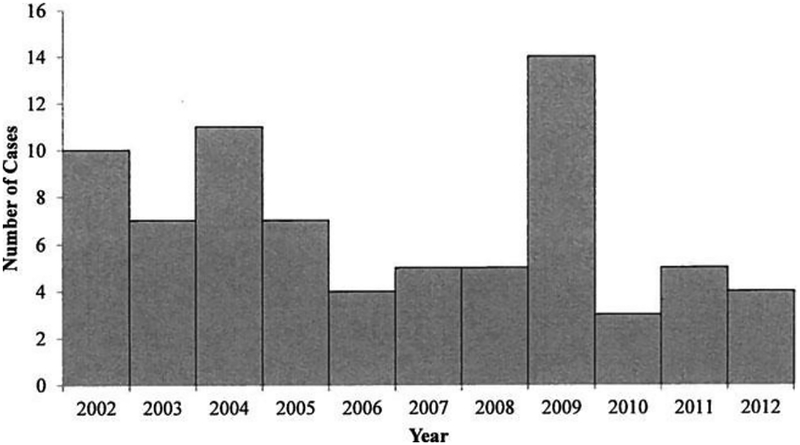
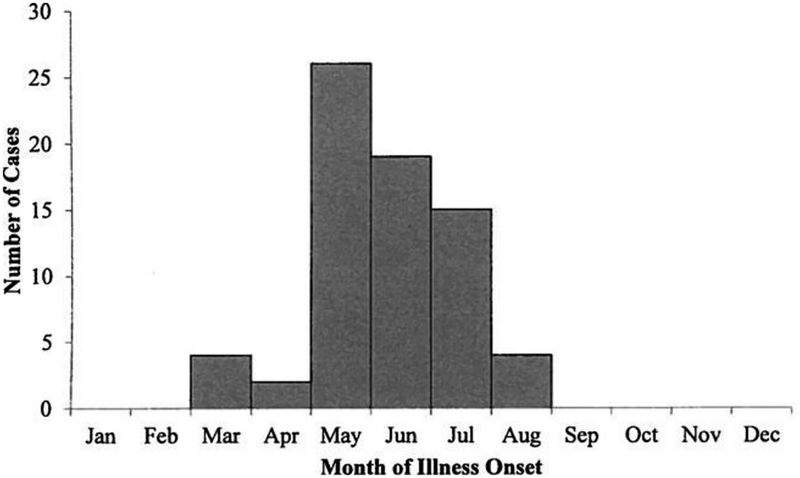
From 2002 through 2012, a median of five CTF cases (range, 3–14) were identified per year (Fig. 1). Forty-seven (63%) case-patients were male (Table 1). Median age was 46 years (range, 2–89) and 49 (65%) cases occurred among people aged ≥ 40 years. Month of illness onset ranged from March through August, with 60 (80%) case-patients having their illness onset during May—July (Fig. 2). Of the 42 case-patients with hospitalization status reported, 13 (31%) were hospitalized during the course of their illness. There were no significant trends over the 11-year time period in the number of cases reported per year, the sex or age distribution of cases, hospitalization rates, or seasonality.
FIG. 1.
Number of confirmed and probable Colorado tick fever cases by year, 2002–2012.
Table 1.
Characteristics of Confirmedand Probable CTF Cases: United States, 2002–2012
| Characteristic | CTF cases (n = 75) No. (%) |
|---|---|
| Male | 47 (63) |
| Age (years) | |
| 0–19 | 11 (15) |
| 20–39 | 13 (17) |
| 40–59 | 30 (40) |
| ≥ 60 | 19 (25) |
| Unknown | 2 (3) |
| State of residence | |
| Wyominga | 21 (28) |
| Montanaa | 16 (21) |
| Utaha | 14 (19) |
| Oregona | 7 (9) |
| Colorado | 7 (9) |
| Idaho | 2 (3) |
| Otherb | 8 (11) |
Colorado tick fever (CTF) was specifically listed as reportable disease condition during the study period.
One case each was identified among residents of Arizona,a California, Iowa, Missouri, Nebraska, New Mexico,a Pennsylvania, and Washington. Each of these cases was associated with travel to a state listed in the table.
FIG. 2.
Number of confirmed and probable Colorado tick fever (CTF) cases by month of illness onset. Month of illness onset was known for 70 (93%) of 75 confirmed and probable CTF cases.
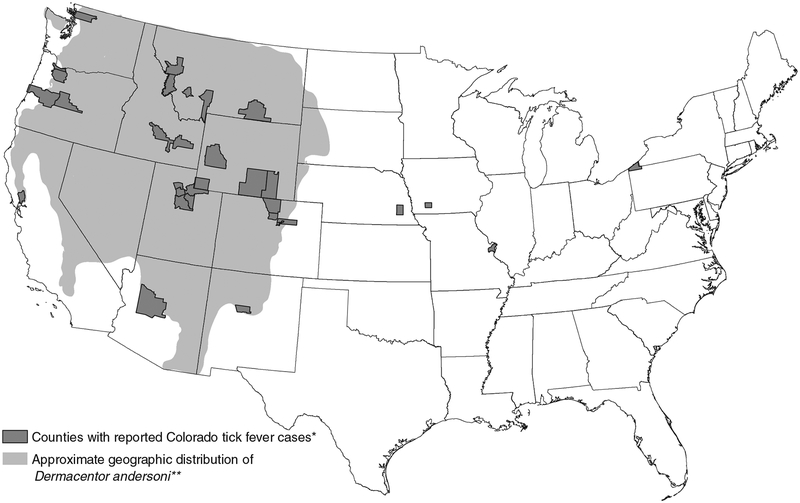
From 2002 through 2012, the average annual incidence of reported cases was 0.02 per million population (range, 0.01–0.05). Case-patients were residents of 14 states (Fig. 3). Wyoming had the most cases (n = 21) identified among their residents, which might have been partially due to enhanced diagnosis and surveillance conducted in Sublette County during 2009. Five additional states had more than one case identified among their residents: Montana (16 cases), Utah(14), Oregon (7), Colorado (7), and Idaho (2) (Table 1). Wyoming had the highest annual incidence of reported CTF among residents (3.4 cases per million population), followed by Montana (1.5 per million) and Utah (0.5 per million). All other states had a reported annual incidence ≤ 0.2 cases per million population. The six states where CTF is a notifiable condition accounted for 60 (80%) of all reported CTF cases. Sublette County, Wyoming, had the highest number of reported cases (n = 16) and average annual incidence among residents (142 cases per million population) (Table 2). Six (38%) of the cases from Sublette County were reported in 2009 during their enhanced surveillance efforts; three additional CTF cases were identified that year among travelers to Sublette County. No other county reported more than six cases total during the 11 years.
FIG. 3.
Approximate geographic distribution of D. andersoni ticks and counties of residence for confirmed and probable Colorado tick fever (CTF) virus disease cases, United States, 2002–2012. (*) All cases were acquired in states where local transmission of CTF virus has been reported previously. Two additional cases were reported from Colorado with unknown county of residence. (**) Derived from James et al. (2006).
Table 2.
Number of Confirmed and Probable CTF Cases and Average Annual Incidence per Million Population by County of Residence for Counties with More Than One Case:United States, 2002–2012
| CTF cases | ||
|---|---|---|
| County and state of residence | No. | Rate per million |
| Sublette, Wyominga | 16 | 141.9 |
| Missoula, Montana | 6 | 5.0 |
| Salt Lake, Utah | 5 | 0.4 |
| Big Horn, Montana | 4 | 28.3 |
| Wasatch, Utah | 4 | 15.5 |
| Ravalli, Montana | 4 | 9.0 |
| Deschutes, Oregon | 3 | 1.7 |
| Davis, Utah | 3 | 0.9 |
| Carbon, Wyoming | 2 | 11.4 |
| Multnomah, Oregon | 2 | 0.3 |
| Albany, Wyoming | 2 | 5.0 |
| Boulder, Colorado | 2 | 0.6 |
Active surveillance for Colorado tick fever (CTF) occurred in Sublette County, Wyoming, in 2009.
On the basis of known history of travel and exposure, all case-patients were likely infected with CTFV in an endemic area in one of six states (Colorado, Idaho, Montana, Oregon, Utah, or Wyoming). Nine counties in Wyoming, Montana, Utah, and Oregon were the likely exposure location for at least 46 (61%) cases. Eleven (15%) case-patients were likely exposed during travel to another state, including four travelers from states where local transmission of CTFV has not been reported (Iowa, Missouri, Nebraska, Pennsylvania). The proportion of travel-associated cases increased during the study period, with only one (2%) of 44 cases from 2002 through 2007 identified in travelers to other states compared with 10 (32%) of 31 from 2008 through 2012 (p < 0.01).
Diagnostic testing
Diagnostic testing for the 75 cases was performed at the CDC (40 cases), a state health department laboratory (26 cases), and/or a commercial laboratory (11 cases); two cases had samples tested at more than one location. For 72 (96%) cases, the diagnosis was confirmed by using one method, including a four-fold or more rise in IgG or neutralizing antibody titers for 33 (44%) cases, RT-PCR for 21 (28%) cases, IgM antibody testing for 10 (13%) cases, and viral culture for 8 (11%) cases. Diagnosis was made by more than one method for three (4%) cases; specifically, one case was confirmed by a four-fold or more rise in titers and RT-PCR, one by RT-PCR and viral culture, and one by IgM antibody testing and viral culture. The proportion of cases that were laboratory-confirmed by a four-fold or more rise in antibody titers decreased from 77% (27/35) during 2002–2005 to 18% (7/40) during 2006–2012 (p < 0.01). This finding corresponded with the use of CTFV RT-PCR, which became routinely available in 2006, with 58% (23/40) of cases confirmed by RT-PCR during 2006–2012. There was no significant trend in the proportion of cases diagnosed by viral culture or IgM antibody testing during the study period.
All of the 23 serum specimens tested by RT-PCR were collected ≤ 15 days after illness onset and all were positive for CTFV RNA (Table 3). One (7%) of 14 specimens collected < 14 days after illness onset was positive for CTFV neutralizing antibodies compared to 18 (90%) of 20 specimens collected ≥ 14 days after onset of illness (p < 0.01). Similarly, two (20%) of 10 specimens collected within 2 weeks after illness onset were positive for CTFV IgG antibodies versus eight (89%) of nine collected at ≥ 14 days (p < 0.01). Data on the timing of specimens obtained for viral culture and IgM antibody testing were not available.
Table 3.
Number and Proportion of Samples Positive for Colorado Tick Fever Virus RNA, Neutralizing Antibodies, and IgG Antibodies for Confirmed and Probable CTF Cases by Number of Days the Specimen Was Collected after Onset of Illness
| RNA | Neutralizing antibodies | IgG antibodies | |
|---|---|---|---|
| Days post illness onset | No. positive/no. tested (%) | No. positive/no. tested (%) | No. positive/no. tested (%) |
| 0–6 | 14/14 (100) | 0/12 (0) | 1/8 (13) |
| 7–13 | 8/8 (100) | 1/2 (50) | 1/2 (50) |
| 14–20 | 1/1 (100)a | 2/4 (50) | 5/6 (83) |
| ≥ 21 | 0/0 (0) | 16/16 (100) | 3/3 (100) |
One specimen collected 15 days after onset of illness. IgG, immunoglobulin G; CTF, Colorado tick fever.
Fatal case report
A single fatality was identified. The patient was an adult male > 80 years of age who lived in an urban county of an endemic state and had no recent travel. He had multiple underlying medical conditions (i.e., chronic obstructive pulmonary disease, chronic renal insufficiency, atrial fibrillation, congestive heart failure, and coronary artery disease) and was admitted to the hospital for 10 days in August for fever and diarrhea. During hospitalization, the patient was noted to be leukopenic (1.9 × 103 leukocytes/μL). The patient tested positive for CTFV infection by both viral culture and IgM and IgG antibody testing. He also had an equivocal result for hantavirus IgM antibodies and was positive for hantavirus IgG antibodies. At 6 days after discharge, he was readmitted for continued weakness, abdominal pain, and diarrhea. He was hypoxic and had bilateral pleural effusions. Sixteen days after being readmitted, he died from progressive respiratory failure with disseminated intravascular coagulation and renal failure. Autopsy findings were consistent with acute exacerbation of interstitial pneumonia.
Discussion
From 2002 through 2012, the number of CTF cases reported in the United States remained relatively low, with less than 15 cases per year. We found no trends over the 11-year period in the incidence, demographic characteristics, or geographic distribution of cases. However, there were increases in the number of cases recognized among travelers and in the proportion of cases diagnosed by RT-PCR testing.
From 1987 through 2001, a median of 55 CTF cases per year was reported in the United States (Marfin and Campbell 2005). However, that number declined consistently over the 15-year period, with a median of 80 cases annually from 1987 through 1994 (range, 55–96) and 18 cases per year from 1995 through 2001 (range, 7–54). This report shows that the drop in the number of reported cases has continued, with a median of only five cases per year from 2002 through 2012. This decline is likely due, at least in part, to changes in testing and reporting practices. For example, Colorado historically reported the largest number of CTF cases (Tsai 1991, Marfin and Campbell 2005). However, CTF was not a notifiable condition in Colorado from 1997 through 2013, and during 2002 through 2012, Wyoming, Montana, Utah, and Oregon each reported more CTF cases than Colorado. In 2009, Wyoming instituted active enhanced CTF surveillance in Sublette County by contacting healthcare providers in the county and offering testing for all suspected CTF cases. This resulted in the identification of nine CTF cases, the highest number of cases reported to the CDC from one state or county since 1998 (Geissler et al. 2014). Of these nine cases, six were residents of the county; these represented 29% of all cases reported from Wyoming during the 11-year time period. The remaining three CTF cases were among travelers to the county. These results suggest that CTF is underdiagnosed and underreported, even in states where it is a notifiable condition and among travelers. Therefore, it is likely that CTF is causing more illness and morbidity among persons living in or visiting endemic areas than is currently recognized.
The seasonality of CTF cases in this report was similar to previous reports, with the majority of cases occurring during May through July (Spruance and Bailey 1973, Goodpasture et al. 1978, Brackney et al. 2010). In studies from the 1970s, most CTF cases were identified among males aged 20–39 years (Spruance and Bailey 1973, Goodpasture et al. 1978). By contrast, we found that 64% of reported cases from 2002 through 2012 occurred in males, but 65% of all cases were in adults aged ≥ 40 years. These findings were similar to those during 1995–2003 from Montana, Utah, and Wyoming, where 69% of the cases were male and the highest incidence was in people aged ≥ 50 years (Brackney et al. 2010). Potential explanations for these trends in the demographic characteristics of CTF cases over the last several decades may include changes in care-seeking behavior, testing, or surveillance practices or true differences in who is being exposed due to increases in recreational activities among persons of all ages and sexes.
The case-fatality ratio for CTF is quite low, with rare fatal cases identified only in children (Centers for Disease Control and Prevention 1972, Spruance and Bailey 1973, Goodpasture et al. 1978). To our knowledge, this report contains the first fatality in an adult patient infected with CTFV. However, the elderly patient had significant underlying medical conditions, and his respiratory failure was attributed to interstitial pneumonia. Although CTFV has been associated previously with one case of atypical pneumonitis (Byrd et al. 1997, Goodpasture et al. 1978), it is unclear what role the virus infection played in his death.
Travel to an endemic area is a known risk factor for developing CTF (Drevets 1957, Midoneck et al. 1994). We found that the proportion of case-patients who became infected while traveling to an endemic area in another state increased during 2002–2012. This could be attributed to increased disease awareness and testing in certain locations (e.g., Sublette County in 2009) or increased recreation in the Rocky Mountain region during the study period (Brackney et al. 2010). Given these findings, prevention messages should be targeted to people who are visiting endemic areas and engaging in behaviors that might place them in contact with ticks, like hiking or camping.
Healthcare providers should consider CTF in the differential diagnosis of febrile illness among persons who have spent time in areas of the Rocky Mountain region, where they may have come in contact with D. andersoni. They should obtain samples and order appropriate laboratory tests based on the timing of the specimen collection. The findings of this study and others suggest that molecular testing (e.g., RT-PCR) is most useful on samples obtained during the first 2 weeks after illness onset when antibody testing is often negative (Calisher et al. 1985, Basile 2010). Samples obtained after the second or third week of the illness are more likely to test positive for CTFV IgG or neutralizing antibodies. Due to the prolonged viremia and sequestration of CTFV in red blood cells, healthcare providers should also advise patients with CTF against donating blood for 6 months following their illness onset to prevent cases of transfusion-associated transmission of CTF (Hughes et al. 1974, Centers for Disease Control and Prevention 1975, Philip et al. 1975). Finally, healthcare providers should report patients who test positive for CTF to their state health department, where applicable.
These data are subject to several limitations. The number of CTF cases identified likely underestimates of the true number of CTF cases because patients may not present for medical care and healthcare providers may not consider CTFV infection in the differential diagnosis or obtain appropriate diagnostic testing. In addition, we only identified cases tested at CDC or reported to CDC by state health departments. Because CTF is not reportable in all states where the disease occurs, case finding and reporting are not standardized across jurisdictions and some cases diagnosed by testing at a commercial laboratory could have been missed. We had limited information regarding the patients’ symptoms and could not include a clinically compatible illness as part of the case definition. As a result, we used conservative criteria for laboratory evidence of recent CTFV infection by excluding reported cases with a single elevated IgG or neutralizing antibody titer. However, given the expected low seroprevalence and lack of viruses in the United States that would result in cross-reactive antibodies, these criteria likely led to underestimating the number of acute disease cases. Finally, states and counties of likely exposure were based on reported travel histories and may not always accurately reflect the location at which the case-patient became infected with CTFV.
Conclusions
CTF remains a cause of febrile illness among persons living in and traveling to areas of the Rocky Mountain region where D. andersoni is found. Public health prevention messages (e.g., reducing exposure to ticks through use of personal protective measures such as use of repellents and long pants) should be targeted to persons who are spending time outdoors in an endemic region during March through August, particularly during the peak CTFV transmission season of May through July.
Acknowledgments
We would like to thank Nicole Lindsey, Jennifer Lehman, and the vector-borne disease surveillance coordinators in local and state health departments.
Footnotes
Author Disclosure Statement
No competing financial interests exist.
References
- Basile AJ. Colorado tick fever virus In: Liu D, ed. Molecular Detection of Human Viral Pathogens. Boca Raton, FL: CRC Press, 2010:779–790. [Google Scholar]
- Brackney MM, Marfin AA, Staples JE, Stallones L, et al. Epidemiology of Colorado tick fever in Montana, Utah, and Wyoming, 1995–2003. Vector Borne Zoonotic Dis 2010; 10:381–385. [DOI] [PubMed] [Google Scholar]
- Byrd RP Jr, Vasquez J, Roy TM. Respiratory manifestations of tick-borne diseases in the southeastern United States. Southern Med J 1997; 90:1–4. [DOI] [PubMed] [Google Scholar]
- Calisher CH, Poland JD, Calisher SB, Warmoth LA. Diagnosis of Colorado tick fever virus infection by enzyme immunoassays for immunoglobulin M and G antibodies. J Clin Microbiol 1985; 22:84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Colorado tick fever—Colorado. MMWR Morb Mortal Wkly Rep 1972; 21:374. [Google Scholar]
- Centers for Disease Control and Prevention. Transmission of Colorado tick fever virus by blood transfusion—Montana. MMWR Morb Mortal Wkly Rep 1975; 24:422, 427. [Google Scholar]
- Drevets CC. Colorado tick fever: Observations on eighteen cases and review of the literature. J Kans Med Soc 1957; 58:448–455. [PubMed] [Google Scholar]
- Eisen L, Ibarra-Juarez LA, Eisen RJ, Piesman J. Indicators for elevated risk of human exposure to host-seeking adults of the Rocky Mountain wood tick (Dermacentor andersoni) in Colorado. J Vector Ecol 2008; 33:117–128. [DOI] [PubMed] [Google Scholar]
- Geissler AL, Thorp E, Van Houten C, Lanciotti RS, et al. Infection with Colorado tick fever virus among humans and ticks in a national park and forest, Wyoming, 2010. Vector Borne Zoonot Dis 2014; 14:675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodpasture HC, Poland JD, Francy DB, Bowen GS, et al. Colorado tick fever: Clinical, epidemiological, and laboratory aspects of 228 cases in Colorado in 1973–1974. Ann Intern Med 1978; 88:303–310. [DOI] [PubMed] [Google Scholar]
- Hughes LE, Casper EA, Clifford CM. Persistence of Colorado tick fever virus in red blood cells. Am J Trop Med Hyg 1974; 23:530–532. [DOI] [PubMed] [Google Scholar]
- James AM, Freier JE, Keirans JE, Durden LA, et al. Distribution, seasonality, and hosts of the Rocky Mountain wood tick in the United States. J Med Entomol 2006; 43:17–24. [PubMed] [Google Scholar]
- Lambert AJ, Kosoy O, Velez JO, Russell BJ, et al. Detection of Colorado tick fever viral RNA in acute human serum samples by a quantitative real-time RT-PCR assay. J Virol Methods 2007; 140:43–48. [DOI] [PubMed] [Google Scholar]
- Marfin A, Campbell G. Colorado tick fever and related Coltivirus infections In: Goodman J, ed. Tick-Borne Diseases of Humans. Washington, DC: ASM Press, 2005:143–149. [Google Scholar]
- Midoneck SR, Richard J, Murray HW. Colorado tick fever in a resident of New York City. Arch Fam Med 1994; 3:731–732. [DOI] [PubMed] [Google Scholar]
- Philip RN, Casper EA, Cory J, Whitlock J. The potential for transmission of arboviruses by blood transfusion with particular reference to Colorado tick fever In: Greenwalt TJ, Jamieson GA, eds. Transmissible Disease and Blood Trans-fusion. New York: Grune & Stratton, 1975:175–195. [Google Scholar]
- Romero JR, Simonsen KA. Powassan encephalitis and Colorado tick fever. Infect Dis Clin North Am 2008; 22:545–559. [DOI] [PubMed] [Google Scholar]
- Spruance SL, Bailey A. Colorado tick fever: A review of 115 laboratory confirmed cases. Arch Intern Med 1973; 131:288–293. [PubMed] [Google Scholar]
- Tsai TF. Arboviral infections in the United States. Infect Dis Clin North Am 1991; 5:73–102. [PubMed] [Google Scholar]