Abstract
Endometriosis (EMS) is the most common gynecological disease in women of reproductive age, and it is associated with chronic pelvic pain, dyspareunia and infertility. As a consequence of genetic, immune and environmental factors, endometriotic lesions have high cyclooxygenase (COX)-2 and COX-2-derived prostaglandin E2 (PGE2) biosynthesis compared with the normal endometrium. The transcription of the PTGS2 gene for COX-2 is associated with multiple intracellular signals, which converge to cause the activation of mitogen-activated protein kinases (MAPKs). COX-2 expression can be regulated by several factors, such as estrogen, hypoxia, proinflammatory cytokines, environmental pollutants, metabolites and metabolic enzymes, and platelets. High concentrations of COX-2 lead to high cell proliferation, a low level of apoptosis, high invasion, angiogenesis, EMS-related pain and infertility. COX-2-derived PGE2 performs a crucial function in EMS development by binding to EP2 and EP4 receptors. These basic findings have contributed to COX-2-targeted treatment in EMS, including COX-2 inhibitors, hormone drugs and glycyrrhizin. In this review, we summarize the most recent basic research in detail and provide a short summary of COX-2-targeted treatment.
Keywords: COX-2, PGE2, endometriosis, pain, estrogen
Introduction
Endometriosis (EMS) is a chronic gynecological disease that can usually be seen in women of reproductive age, and is characterized by the presence, transfer and invasion of functional endometrial tissue outside of the uterine cavity 1. Some hypotheses, such as retrograde menstrual reflux 2, ectopic presence of endometrial stem cells (ESCs) 3 and defects in the immune system 4, have been proposed to explain the migration, implantation and survival of the ectopic endometrial tissue and stroma. The incidence rate of EMS is 5-15% of all women of reproductive age and 20-50% of all infertile women 5-7, and the quality of life for endometriosis patients is significantly reduced, due to the increase in symptoms including chronic pelvic pain, dyspareunia, and infertility in comparison with women without EMS 8. The economic impact of EMS is compounded by the latency in the diagnosis of EMS, especially in young women who delay seeking treatment. The diagnosis of EMS is typically delayed by 8-10 years, because of the common misdiagnoses of EMS-induced pelvic pain as menstrual-related abdominal pain 9. EMS can be confirmed by direct visualization using laparoscopy and biopsy. In the past few years, the field of diagnostic biomarkers for EMS has gained increasing attention 10. When considering the theory of retrograde menstrual reflux, a puzzle emerges in that only around one tenth of women develop EMS, whereas retrograde menstruation is observed in most women, suggesting that other factors may also trigger the formation of endometriotic lesions, such as hormones, inflammatory factors, growth factors, angiogenic factors and cancer-related molecules 11.
The cyclooxygenase-2 (COX-2) / prostaglandin E2 (PGE2) pathway is closely related to EMS. There has been a general realization that EMS is a chronic pelvic inflammatory state, characterized by rising numbers of activated peritoneal immune cells, such as macrophages, and pro-inflammatory factors 12-14. COX-2 is thought to play a significant role in the origin and development of EMS 15. In endometrial and endometriotic tissues of women with EMS, elevated expression of COX-2 has also been described 16. COX-2 which is a rate-limiting enzyme in the PGE2 compound 17, is overexpressed in endometriotic tissues and contributes to increased concentrations of PGE2 in EMS patients, which have also been found in the peritoneal fluid (PF), as well as leukotrienes. COX-2/PGE2 signaling biologically activate oxygenated fatty acids, eicosanoids, and has been shown to be involved in various inflammatory pathological process 18. In EMS, they appear to play an important role in disease-associated pain 19, 20, essentially being the target of non-steroidal anti-inflammatory drugs (NSAIDs) 16. These inflammatory mediators, particularly COX-2/PGE2, may also be directly implicated in the pathogenesis of EMS 16, including the regulation of ectopic implantation and the growth of the endometrium, angiogenesis and immunosuppression 21. PGE2 is a major regulator of the immune response and can exert two opposing functions, exerting inflammatory or anti-inflammatory effects 22. Therefore, this paper systematically reviews the elements affecting the level and role of, and targeted drugs for COX-2 in EMS.
COX-2
The enzyme COX was first demonstrated to exist in 1976 and cloned in 1988 23. COX has three isoforms: COX-1, COX-2 and COX-3 24-26. Among these, the COX-1 and COX-2 isoforms are often studied, due to the fact that they are associated with physiological as well as pathological processes. In the gastrointestinal and cardiovascular system, COX-1, a constitutively expressed house-keeping isozyme, is responsible for the basal production of essential PGs 27 that mediate homoeostatic functions. COX-3 is encoded by the COX-1 gene with reserve intron 1 in its mRNA. COX-3 is only expressed in some specific parts of the cerebral cortex and heart, and its exact functions are still unclear 28. The COX-2 isozyme, by contrast, is synthesized at very low levels under normal conditions and can be induced to become over-expressed under pathological conditions. The PTGS2, the gene for COX-2, is located on human chromosome 8 29. The promotor of the immediate-early gene PTGS2 contains a TATA box and binding sites for several transcription factors, including nuclear factor-κB (NF-κB), the cyclic AMP response element binding protein (CRE), and the nuclear factor for interleukin-6 expression (NF-IL-6) 30, 31. COX-2 expression is associated with multiple transcriptional pathways. There is accumulating evidence for the critical involvement of COX-2 in various pathological processes that include inflammation 32, 33, cancer 34-36, neurodegenerative diseases 37, 38 and multidrug resistance 39.
The expression of COX-2 is rapidly upregulated in response to diverse pro-inflammatory and pathogenic stimuli. All signals converge upon the activation of mitogen-activated protein kinases (MAPKs) that regulate COX-2 expression at both the transcriptional and post-transcriptional levels 40. Lipopolysaccharide (LPS) signaling, the most pro-inflammatory mediators, induces the expression of COX-2 in the periphery. Specifically, LPS and other Toll-like receptor (TLR) ligands bind to MyD88-associated receptors and activate MEK/ERK pathway to induce the transcription factor activator protein 1 (AP1). LPS also can induce gene PTGS2 transcription by activating the TRAF6/NIK/Tpl2/IKK/NF-κB pathway 41, 42. Nitric oxide (NO) affects the transcription of PTGS2 in direct and indirect ways; directly, by increasing its catalytic activity, and indirectly, by triggering several signaling cascades that affect the gene transcription. NO and reactive oxygen species (ROS) increase PTGS2 expression 43 via β-catenin/TCF pathway-mediated activation of polyoma enhancer activator 3 (PEA3) 44. Furthermore, several cytokines, including NO, several pro-inflammatory cytokines (e.g. IL-1, IFN-γ) and hypoxia inducible factor-1α (HIF-1α) can induce COX-2 expression through the cAMP/PKA/CREB and JNK/Jun/ATF2 signaling cascades 45-48. Growth factors can induce COX-2 expression in both normal and cancer cells, including insulin-like growth factor (IGF), transforming growth factor-α (TGF-α) and epidermal growth factor (EGF). Notably, this regulatory effect of IGF is mediated by PI3K and Src/extracellular signal-regulated kinase (ERK), while the effects of TGF and EGF are achieved through p38MAPK, ERK1/2 and PI3K 49. There are negative regulators for COX-2 expression. For example, glycogen synthase kinase 3 (GSK3) suppresses COX-2 expression through inhibition of the β-catenin/transcription factor-4 (TCF4) and PKCδ/ERK1/2 signaling pathways 50.
COX-2 expression in EMS
COX-2 is mainly expressed in the endometrial glandular epithelium in healthy women and varies during the menstrual cycle. The expression of COX-2 is at its lowest in the early proliferative phase and gradually increased thereafter, and it maintains a high level throughout the secretory phase 51. In women with EMS, the expression of COX-2 in the endometrial glandular epithelium, endometrial stroma 4 and PF was higher than that in the control group 52, and it also varies throughout the menstrual cycle 53. Cho et al. 54 demonstrated that in EMS patients, the expression of COX-2 was elevated significantly in the eutopic endometrium during the proliferative phase and in ovarian endometriotic tissue during the secretory phase compared with the control groups. In addition, ectopic lesions highly express COX-2 in endometriosis patients with chronic stress 54. Notably, mRNA expression of PTGS2 in the endometrium and ovarian lesions significantly correlates with serum CA-125 and the diameter of endometriomas 54. In recent research, Mei et al. [55]found that the number of COX-2+CD16- NK cells with impaired cytotoxic activity in the abdominal cavity fluid of patients with EMS was markedly higher than that of the control group.
Genetic variation in PTGS2 (COX-2) and the risk of EMS
Gene polymorphisms in PTGS2 are associated with a high risk of many diseases, such as EMS 56, cancer 57, and acute pancreatitis 58. The cloning, sequencing and expression of human PTGS2 cDNA have been previously described 59. There are 51 CpG sites in the promoter region of the COX-2 gene from -590 to +186. Three main transcription factors predominantly regulate COX-2 expression, including NF-κB, NF-IL6, and CRE 60, 61. Moreover, in many cancers, aberrant methylation of promoter CpG island of the COX-2 has been regarded as an alternative mechanism of its abnormal expression and contributes to carcinogenesis 62, 63. Genes associated with endometriosis have abnormal DNA methylation. Wang et al [64]and Zidan et al 56 reported that DNA hypomethylation of the NF-IL6 site within the promoter of the PTGS2 gene was highly correlated with the pathological process of EMS, suggesting that EMS may be an epigenetic disease. Wang et al. 64 found that PTGS2 genotypic frequencies of G to A at the -1195 locus in the promoter region in EMS were significantly different from those in normal women. Moreover, the allele frequency in EMS was markedly higher than that in normal women. The risk of EMS for those carrying two A alleles was 2.19 and 2.41 times greater than that for the to non-A genotype. In addition, Wang et al. 65 demonstrated that on the promoter region of the PTGS2 gene, the -1195 A/G may increase the risk of pain occurrence in women with EMS. The presence of the ancestral allele, -765G/C, of the PTGS2 gene is associated with an increased risk of pathological progression in moderate/severe EMS which is related to fertility, and the expression of COX-2 in the eutopic endometrium of women with EMS has shown a tendency to increase when compared to the control group 66, 67. In a Korean study, the -765C allele was a protective agent against the development of the disease 68.
Regulation of COX-2 expression
Over the years, many epidemiological, pharmacological and laboratory studies have demonstrated that various factors are involved in the regulation of COX-2 expression in EMS (Table 1, Figure 1).
Table 1.
The factors that regulates COX-2 expression in EMS
| Classification | Regulatory factor | Function | Reference |
|---|---|---|---|
| Estrogen | hastens COX-2 expression by activated by NF-κB | Maia et al.2012 | |
| Proinflammatory Cytokine | IL-1β | stimulates the phosphorylation of ERK, p38 and JNK and results in high level of COX-2 | Tamura et al.2002 Huang et al.1998 |
| NGF | increases PTGS2/COX-2 mRNA and protein levels by binding to TrkA | Wang et al.2009 Peng et al.2018 |
|
| Hypoxia | mediates DUSP2 down-regulation, activates ERKs and MAPK, and ultimately results in the hypersensitivity of COX-2 | Wu et al.2005 Wu et al.2011 Teague et al. 2010 Lin et al.2012 Pan et al.2007 Hsiao et al. 2015 |
|
| Environmental pollutants | PCBs | plays a role in the development of endometriosis | Porpora et al. 2013 |
| HCB | activates of cytosolic AhR complex (AhR-dioxin-c-Src), triggers PTGS2 transcription | Smith et.al. 1993 Deger et al.2007 Chiappini et al. 2016 |
|
| Metabolites and metabolic enzymes | omega-3 PUFA | inhibits the activation of NF-κB and decreases the production of pro-inflammatory cytokines to reduces COX-2 expression | Tomio et al. 2013 Attaman et al.2014 |
| IDO | up-regulates COX-2 expression via the activation of JNK signaling pathway | Mei et al.2013 Mei et al. 2012 |
|
| LXA4 | inhibits COX-2 expression | Kumar et al. 2014 | |
| Platelets | increases IL-1β level and increases COX-2 expression | Ding et al. 2015 | |
| Others | COUP-TFII | binds to PTGS2 promoter to inhibit its transcription and IL-1β-induced COX-2 up-regulation | Li et al. 2013 Li et al. 2013 |
Figure 1.
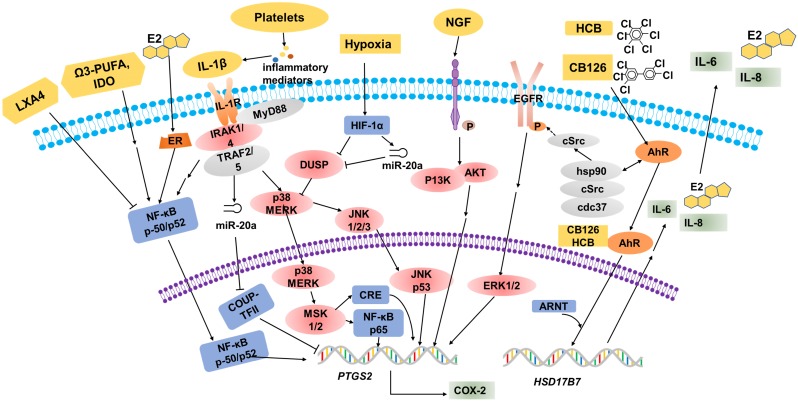
Multiple factors regulate COX-2 expression. Estrogen (E2), omega-3 PUFA and IL-1β promote COX-2 expression through the NF-κB signaling pathway. IL-1β stimulates the phosphorylation of MERK, p38 and JNK, then CRE and NF-κB p65 bind to sites on the COX-2 promoter to increase COX-2 expression. In hypoxic conditions, activated HIF-1α will suppress DUSP2 expression directly, and then result in the hypersensitivity of COX-2 in response to proinflammatory stimuli (e.g. IL-1β). Elevated NGF markedly upregulates the expression of PTGS2/COX-2 via the PI3K/AKT signaling pathway. Environmental pollutants, for example HCB and CB126, are known to act via the AhR. These organic compounds have some biological effects mediated by the activation of the cytosolic AhR complex (AhR-dioxin-c-Src), and regulate PTGS2 transcription indirectly. The combination of organic compounds and AhR induces HSD17B7 expression and results in the upregulation of E2, IL-6 and IL-8, which will further promote COX-2 expression.
Estrogen
Estradiol and progesterone are core hormones regulating the function of endometrial tissue. In the course of different phases of the menstrual cycle, each steroid hormone is estimated to regulate the translation of hundreds of genes successively 15, 69. Ectopic and eutopic endometrial tissues have apparently similar histological changes in response to estradiol and progesterone, and both tissues express immunoreactive estrogen and progesterone receptors (PRs). This locally produced estrogen in both the ectopic and eutopic endometrium is considered to exert a crucial role in the regulation of the immunological mechanisms responsible for controlling the development of EMS 15. Local estrogen production hastens prostaglandin synthesis by stimulating COX-2 activity, thus creating a positive feedback loop of augmented estrogen formation and enhanced inflammation. The synthesis of proinflammatory PGs such as COX-2-derived PGE2, can be activated by NF-κB and increased by estrogen in the endometrium 70. The synthesis of aromatase seems to play a pivotal role in the development of EMS, which is stimulated by PGs and other inflammatory mediators in endometrial cells but not in aromatase-negative endometrial cells 71. Thus, a large amount of local estrogen production will further enhance PG synthesis by activating COX-2 expression.
Proinflammatory Cytokines
It has been reported that ectopic ESCs are hypersensitive to the stimulating effect of cytokines, such as interleukin-1β (IL-1β), in terms of overexpression of COX-2 46. IL-1β can accelerate the synthesis of COX-2 at the mRNA, protein, and enzyme activity levels in a model system of EMS. Notably, IL-1β can activate MAPK-dependent signaling by binding to the CRE site at -571/-564 of the COX-2 promoter to increase IL-1β-induced COX-2 expression 46. COX-2 gene induction by IL-1β involves the ERK1/2 and NF-κB signaling pathway, because IL-1β stimulates the phosphorylation of ERK, p38 and JNK 72-74. Nerve growth factor (NGF), a core endocrine regulator for the growth of neurons, plays crucial roles in the regulation of neuronal survival and maturation 75. In inflamed tissues in numerous diseases, overexpressed NGF regulates immune responses; directly or indirectly: directly, by influencing innate and adaptive immune responses, and indirectly inducing the release of immune-active neuropeptides and neurotransmitters76. NGF is believed to contribute to pathological pain associated with various medical conditions, such as cancer and rheumatoid arthritis (RA) 77. Elevated NGF levels markedly increase the expression of PTGS-2/COX-2 at the mRNA and protein levels as well as PGE2 secretion in women with EMS. This association may be regulated by enhanced nerve bundle density and by COX-2/PGE2 stimulation via the high-affinity Trk receptor 78-80.
Hypoxia
Hypoxia, which plays a key role in immunity and inflammation under both physiological and pathological conditions, arises when cellular oxygen demand exceeds supply 81. Hypoxia triggers a profound change in gene transcription, and hypoxia-inducible factor (HIF) is a master regulator 82. HIF-1α is one of the major transcriptionally active isoforms of HIF that have been described 83. Dual-specificity phosphatase-2 (DUSP2) which is a nuclear phosphatase that can specifically dephosphorylate p38 MAPK and ERK 84, is markedly downregulated in stromal cells of ectopic endometriotic tissues, leading to prolonged activation of p38 MAPK and ERK and increased COX-2 expression 85. HIF-1α suppresses DUSP2 expression directly, leads to sustained activation of p38 MAPK and ERK, and ultimately contributes to aberrant COX-2 synthesis in ectopic endometriotic stromal cells 86. The ERK and p38 MAPK signaling pathways have been reported to play important roles in the modulation of PGE2 synthesis in ectopic endometrial cells, and abnormal phosphorylation of ERK and/or p38 MAPK may lead to over-expression of COX-2 in ectopic lesions 45, 87. Down-regulation of hypoxia-mediated DUSP2 leads to more activated ERKs and p38 MAPK, and ultimately results in the hypersensitivity of COX-2 in response to proinflammatory stimuli. In addition, microRNAs (miRNAs) are related to tissue repair, hypoxia, inflammation, cell proliferation, extracellular matrix remodeling, apoptosis and angiogenesis in EMS 88. It has been demonstrated that the expression of miR-20a induced by hypoxia is relatively high in ectopic endometrial tissues compared to that in eutopic endometrial tissues 86, 89. Interestingly, DUSP2 is a target of miR-20a. A previous study suggested that hypoxia-induced miR-20a expression leads to downregulation of DUSP2 expression, and results in the overexpression of downstream ERK-regulated genes, such as angiogenic, and mitogenic factors, and COX-2 87. Taken together, these data strongly support the hypothesis that hypoxia is a vital factor that potentiates PTGS2 gene sensitivity in ESCs 90.
Environmental pollutants
During the last few years, increasing evidence has emerged in support of the relationship between exposure to chemicals with endocrine disruption potential and hormone-related gynecological diseases shows steadily increased 91. Environmental organochlorine pollutants, particularly polychlorinated biphenyls (PCBs) and dioxins, are thought to be involved in the development of EMS 94. Dioxin-like 92, 93 rather than non-dioxin-like PCB congeners 94 tend to be responsible for the pathological risk of EMS, according to current epidemiological evidences. Huang et al. 95 found that CB126 (a dioxin-like PCB) enhances estradiol (E2) biosynthesis and promotes the secretion of both IL-6 and IL-8. CB126 is known to act via the aromatic hydrocarbon receptor (AhR). Using DMF to inhibit this receptor can abolish the effects induced by CB126 96. The gene expression of HSD17B7, rather than aromatase (CYP19A) or HSD17B1, is up-regulated after exposure to CB126. For local E2 production in endometriotic lesions, CYP19A was previously thought to be significant 97, 98. The expression of HSD17B7 can be enhanced by LPS and IL-1β which can be observed in ESCs. Thus, the development of EMS can be promoted by the interaction between the endocrine and immune systems and CB126 may provoke this process through stimulation of both E2 synthesis and the inflammatory response. This may support the idea that PCB-induced EMS is related to COX-2. Another type of organochlorinated pollutant, hexachlorobenzene (HCB), is a “dioxin-like” organic compound that binds to AhR 99, accumulating in lipid tissue and inducing the synthesis of xenobiotic metabolic enzymes. These organic compounds have some biological effects which are mediated by the activation of the cytosolic AhR complex (AhR-dioxin-c-Src), triggering membrane actions where c-Src activates growth factor receptors, and nuclear actions where AhR regulates gene transcription including for COX-2 100, 101. Chiappini et al 102 found that exposure to HCB enhanced COX-2, PGE2 and EP4 expression, and c-Src kinase activation in T-HESC, thereby contributing to EMS development through both hormonal regulation and immune function.
Metabolites and metabolic enzymes
In vivo and in vitro studies have demonstrated that omega-3 polyunsaturated fatty acids (omega-3 PUFAs) have potential antiapoptotic, anti-inflammatory, antiangiogenic, and antiproliferative effects 103. Omega-3 PUFAs block the activation of NF-κB, cut down the production of pro-inflammatory cytokines such as IL-6, TNF-α and IL-1, and reduce COX-2 expression to protect against the development of EMS 104, 105. In particular, the 12/15-LOX-pathway products of eicosatetraenoic acid (EPA) may be critical mediators in suppressing EMS104. In inflammatory bowel disease (IBD), PUFAs of the n-3 series have reported to exert an inhibitory action on PTGS2 gene expression in vivo using a genetically-modified mouse 106; they compete with arachidonic acid (AA) for binding to the COX-2 catalytic site and finally obstructed prostaglandin formation 107. Indoleamine 2,3-dioxygenase (IDO) has the capacity of tryptophan consumption and the generation of proapoptotic metabolites, thus it was confirmed to be an immune modulator 108 and to be highly expressed in EMS-derived eutopic and ectopic ESCs; it also upregulates COX-2 expression by means of the activation of the JNK signaling pathway 109, 110, along with the enhancement of cell survival, proliferation and invasion. In the canonical JNK pathway, activated JNK can lead to phosphorylation of the transcriptional activation domain of c-Jun; this phosphorylated domain constitutes AP-1, a kind of transcription factors which is acted on the human IDO gene promoter region 111, with c-Fos 112. Subsequently, G protein-coupled receptors regulate MAPK signaling pathways that result in specific response gene expression, including the genes associated with cell proliferation, apoptosis and invasion 113. Lipoxins are endogenous eicosanoids, generally produced via a transcellular biosynthetic pathway, the functions of which exhibit both pro-resolving and anti-inflammatory properties 114. In vivo studies, Lipoxin A4 (LXA4) mediates anti-inflammatory activities through multiple receptors115, and the best characterized lipoxin A4 receptors is (ALX)/formyl peptide receptor 2 (FPR2). LXA4 treatment significantly attenuated COX-2 and PGE2 levels in both endometriotic lesions and peritoneal fluid cells, which might be the result of downregulating CYP19a1 expression or via direct transcriptional repression 116.
Platelets
Inflammation and coagulation are intricately entwined: inflammation stimulates the coagulation cascade and coagulation modulates the inflammatory response in many ways 117, 118. Platelets are aggregated in endometriotic lesions, concomitantly with the elevated levels of VEGF and microvessel density. A co-culture system of endometriotic stromal cells and platelets led to enhanced cellular proliferation, and increased COX-2 expression. Analysis of the underlying mechanisms demonstrated that platelet granules contain a variety of inflammatory mediators, such as, IL-1β, which induce the expression of COX-2 in a dose-dependent manner in both normal ESCs and ectopic ESCs 119.
Others
Chicken ovalbumin upstream promoter-transcription factor II (COUP-TFII, also known as NR2F2) is an orphan nuclear receptor that has a pivotal impact in embryonic implantation and placentation, indicating that it is a key regulator in uterine physiology 120-122. In normal and endometriotic stroma, the expression levels of COUP-TFII mRNA and protein have been dentified to be different, which highlights its potential functions in endometriotic pathogenesis. In normal endometrial tissue, COUP-TFII directly binds to the PTGS2 promoter to inhibit its transcription and diminish IL-1β-induced COX-2 over-expression 123. In endometriotic stroma, cytokines IL-1β, TNF-α and TGF-β1 can repress COUP-TF II expression mediated by miR-302a, then suppress the binding of COUP-TFII to the COX-2 promoter 123. Therefore, the decreased COUP-TFII results in the derepression of COX-2 in ESCs 124. However, the detailed mechanism requires further research.
The role of COX-2 in EMS
Cell proliferation and apoptosis
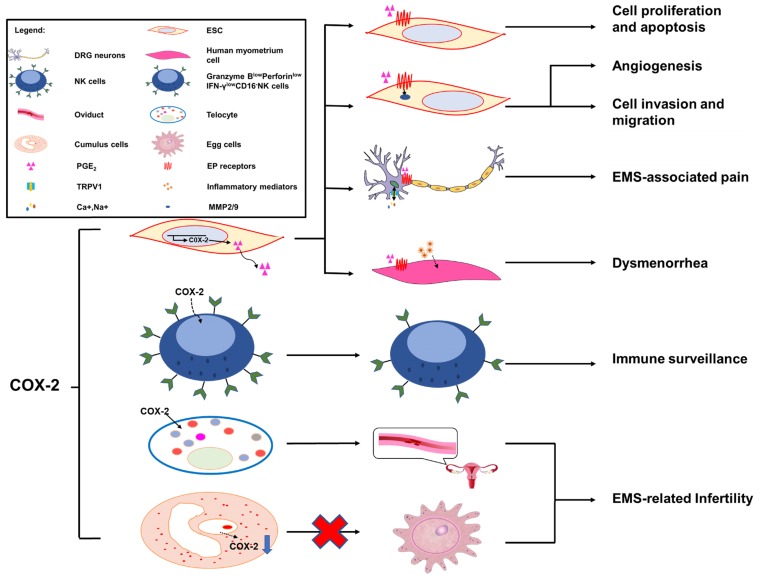
The growth of endometriotic lesions is a process tightly regulated by a delicate balance between proliferation and apoptosis in endometrial cells. This abnormal survival ability has been associated with the concomitant overproduction of antiapoptotic factors and underproduction of proapoptotic factors 125. As shown in Figure 2, COX-2-induced PGE2 is a significant antiapoptotic mediator; it can activate cell survival and antiapoptotic pathways to prevent cells from undergoing programmed cell death or apoptosis. The binding of PGE2 and its receptors, EP2 and EP4, regulates these complex molecular interactions and promotes the survival of human ESCs outside the uterus via multiple trans-activating complex signaling pathways (such as c-Src/β-arrestin 1/EGFR/ERK1/2, c-Src/βarrestin1/TNFαR1, IL-1βR1/IκB/NFκB or Gsα/axin/β-catenin)128. Selective inhibitors of EP2 and EP4 impair ESC survival pathways and facilitate interactions between antiapoptotic proteins (Bcl-2/Bcl-XL) and proapoptotic proteins (Bax/Bad) leading to an augmentation of the release of cytochrome c and activation of the caspase-3/PARP pathways 126. The results indicated that administration of NS-398, a kind of selective COX-2-inhibitor, and siRNA can significantly reduce COX-2 concentration, PGE2 production, and endometriotic epithelial and stromal cell proliferation 127. Laschke et al. 127 showed that in an EMS mouse model, treatment with NS-398 applied to endometrial grafts led to a tendency towards decreased cell proliferation, along with a sustained reduction in proliferating cell nuclear antigen (PCNA) expression; in addition, an increased number of apoptotic cells was observed, as indicated by an upregulation of activated caspase-3. Furthermore, epithelial cell lines stably transfected to overexpress the PTGS2 gene appear to have a higher proliferation rate and to inhibit apoptosis by means of reacting with cyclin D to elongate the G1 phase of the cell cycle 128, 129. Therefore, the administration of selective COX-2 inhibitors to the ectopic and eutopic endometrium may contribute to an inhibition in proliferative potential and a growth rate in apoptosis 130.
Figure 2.
The role of COX-2 in EMS. Overexpression of COX-2 has been demonstrated to be a master regulator in the progression of endometriosis. A high level of COX-2 can promote cell proliferation and suppress cell apoptosis via trans-activating multiple complex signaling pathways, which are triggered by PGE2 and its receptors, EP2 and EP4. In addition, MMP-2/9 activity regulated by PGE2 is be involved in angiogenesis, and ESC migration and invasion, via the intracellular MAPK, AKT and Wnt signaling pathways. COX-2 can induce COX-2+CD16-NK cell (Granzyme BlowPerforinlowIFN-γlowCD16-NK cell) differentiation in the peritoneal fluids of patients with endometriosis, which is beneficial to the immune escape of endometriotic lesions. The COX-2/PGE2/EP2-EP4 signaling decreases the threshold and enhances the excitability of nociceptor sensory fibers through TRPV1 and SCN11A, and contributes to EMS-associated pain. A high level of COX-2/PGE2 and COX-2-induced inflammatory mediators increase uterine tone and contractions and cause pain. TCs are important in maintaining the structural and reproductive functional normality of the oviduct, while overproduced COX-2 may damage the functions of TCs, which will lead to infertility. The low production of COX-2 in cumulus cells is regarded as a possible mechanism of EMS-related infertility.
Cell invasion and migration
PGE2 exerts its biological effects through G protein-coupled receptors and by activating multiple cell signaling pathways. These G protein-coupled receptors are designated according to the four subtypes of the PGE receptor (EP1, EP2, EP3 and EP4) 131. Previous studies have illustrated that EP receptors intracellularly trans-activate the MAPK, AKT and Wnt signaling pathways, resulting in the modulation of cell apoptosis, proliferation, invasion, migration, angiogenesis, pain and immunomodulation 132, 133. Administration of COX-2 inhibitors decreases the survival, migration and invasion of endometriotic cells as a result of decreased production of PGE2 127, 134. Additionally, COX-2-associated migration and invasion are decreased when COX-2 is inhibited in endometriotic cells, and are mediated by matrix metalloproteinase (MMP)-2 and MMP-9 in humans 135. In addition, there is an interesting observation that COX-2 inhibitors produce more detrimental effects on invasion compared with migration in endometriotic cells; however, the underlying molecular mechanisms of these selective effects are unknown 21.
Angiogenesis
In the pathological process of EMS, the development of new blood vessels represents a core factor, because the long-term survival and growth of the exfoliated endometrium requires an effective blood supply; this is a major prerequisite at ectopic lesions. The development of the ectopic endometrium relies on angiogenesis, which is a characterizing factor of EMS 48. MMPs, a group of zinc-dependent proteolytic enzymes, are mainly involved in extracellular matrix degradation to promote cellular invasion, migration and angiogenesis 136, 137. In vitro, some evidence suggests that PGE2 dramatically increases MMP-2 activity as well as tube formation 138. Blocking the expression of COX-2 and/or a phosphorylated protein kinase (AKT) suppresses MMP-2 activity and endothelial tube formation, indicating that the MMP-2 activity modulated by PGE2 is potentially involved in angiogenesis. Moreover, treatment with a chemical inhibitor can specifically inhibit MMP-2 by significantly inhibiting cellular migration, invasion and tube formation. Furthermore, a notable decrease in endometrial lesion numbers was observed after applying inhibitors of MMP-2 and COX-2 to the mouse model of EMS. Collectively, COX-2 can promote angiogenesis indirectly via the involvement of MMP-2 activity during EMS progression 138. In particular, COX-2 inhibitors could exert an anti-angiogenic effect on endometriotic lesions. On one hand, the angiogenic factor vascular endothelial growth factor (VEGF) plays an important role in the pathogenesis of EMS 48, and selective COX-2 inhibitors suppress the expression of VEGF in endometrial grafts initially 127 and in tumor researches 139. On the other hand, in a study on hamsters, firm platelet adhesion to the endothelium of microvessels was increased when treated with a selective inhibitor of COX-2 140.
EMS-associated pain: chronic pelvic pain and dysmenorrhea
COX-2 is inducible and is involved in pain- and inflammation-associated pathological pathways 141. Increased expression levels of COX-2 in central nervous system (CNS) regions within the pain-processing pathway were found at the spinal 142, thalamic and cortical levels 143, and in dorsal root ganglion (DRG) neurons 144. COX-2 expression is viewed as a sensitive and responsive biomarker of centralized inflammatory pain in the CNS 142. In a rat EMS model, sympathetic and sensory C and Aδ fibers innervated endometriosis lesions, which expressed calcitonin gene related peptide (CGRP) and TRPV1 proteins, thereby contributing to the formation of the proinflammatory microenvironment of DRG neurons from L1-S1. Neurons from L1-S1 innervate the pelvis and pelvic organs and increase pelvic floor hyperalgesia 145. Greaves et al 143 found that in an EMS mouse model, the COX-2/PGE2 signaling pathway was overexpressed. PGE2 plays a significant role in the pathophysiology of COX-2-induced EMS 143. PGE2 acts on peripheral nociceptors, lowering the threshold and enhancing the excitability of nociceptor sensory fibers through TRPV1 and Nav1.9 voltage-gated sodium channels (SCN11A) 146, and induces chronic inflammatory pain through EP2 and EP4 147, 148. Localized peripheral inflammation increases the expression of EP4 protein in L5 DRG neurons. Inhibition of EP4 decreases the PGE2-induced sensitization of DRG neurons and the release of the neuropeptides SP and CGRP 147, 148. At the level of the PTSG2 gene, the -1195 A/G on the promoter region of the COX-2 gene may increase the risk of pain occurrence in patients with EMS, possibly by affecting the rate of gene expression, especially in patients with the pain phenotype 66.
Dysmenorrhea, defined as painful cramps in the lower abdomen that occurs with menstruation, is one of three main characteristics of EMS 149. Primary dysmenorrhea is one of two types of dysmenorrhea, caused by an increased or unbalanced level of endometrial prostaglandins, most importantly PGE2, during menstruation 150. COX-2-derived PGE2 increases uterine tone and contractions, and causes pain 151. COX-2 can induce the production of a large number of inflammatory mediators, including PGs 152, and contribute to dysmenorrhea in patients with EMS.
EMS-related infertility
Around 20-50% of the EMS population is estimated to be infertile 153. Telocytes (TCs; previously considered to be interstitial Cajal-like cells, ICLC), a peculiar type of stromal cell, have been identified in many organs, including the endometrium, myometrium and fallopian tube 154, and have been reported to be decreased in women with EMS and tubal ectopic pregnancy 155. Structural and reproductive functional abnormalities of the oviduct are observed as a result of TC damage 156. In oviduct tissues, overproduced COX-2 may be responsible for the TC damage 157. The pathologic niche of EMS is considered to have deleterious effect on oocyte quality. Cumulus cells are indirect biomarkers of this 158-160. In eutopic and ectopic endometrial tissues from women with EMS, the transcription of PTGS2 is upregulated 15, 161, 162. By contrast, the transcript levels of PTGS2 in cumulus cells of infertile women with EMS are decreased 163. Reduced PTGS2/COX-2 expression may lead to an impairment of oocyte quality, which is regarded as a possible mechanism of EMS-related infertility 163.
Immune surveillance
The transcription of the aromatase gene is favored by epigenetic changes in the endometrium, allowing endometrial cells to survive in ectopic locations by producing enough estrogen to protect them from destruction by activated macrophages 70. Local estrogen production accelerates PG synthesis by stimulating the activation of COX-2, thus creating a positive-feedback sequence of facilitated estrogen formation and enhanced inflammation 70. Therefore, the increased inflammation in EMS may reflect the overexpression of estrogen, which alone activates COX-2 and NF-κB to increase inflammation and PG production. In a recent study, a high level of COX-2+CD16-NK cells was observed in the peritoneal fluid of patients with EMS 55. COX-2 can induce the differentiation of low-cytotoxicity CD16-NK cells (with low levels of Granzyme B, Perforin and IFN-γ), and promote the immune escape of endometriotic lesions. In addition, these COX-2+CD16-NK cells promote the proliferation and restrict the apoptosis of ectopic lesions; however, the mechanism needs further study 23. The population of Foxp3+ regulatory T (Treg) cells is upregulated in the PF of EMS patients, which contributes to the local dysfunctional immune microenvironment in EMS and the immune escape of ectopic endometrial tissue. The estrogen-IDO1-MRC2 axis is involved in regulating the differentiation and function of Treg cells 164. It was reported that Treg cells upregulate the expression of MMP2 and COX-2 and promote the survival, migration and invasion of endometriotic cells 165. In the gastric tumor microenvironment, COX-2 expression is also strongly correlated with Foxp3, a reliable marker of Treg cells 166. Yuan X.Y et al 150 found that Treg cells could express high levels of COX-2, and produced a high level of PGE2. PGE2 binds to EP2 and EP4 and triggers the cAMP Csk inhibitory pathway to suppress T-cell immune responses. Foxp3high Treg cells suppress the proliferation of autologous CD4+CD25- T cells, which can be reversed by COX inhibitors and PGE2 receptor-specific antagonists. These data show that in the development of gastric cancer, tumor-infiltrating Treg cells can induce immune suppression via the COX-2/PGE2 axis 150,167.
Anti-EMS drugs targeting COX-2 (Figure 3)
Figure 3.
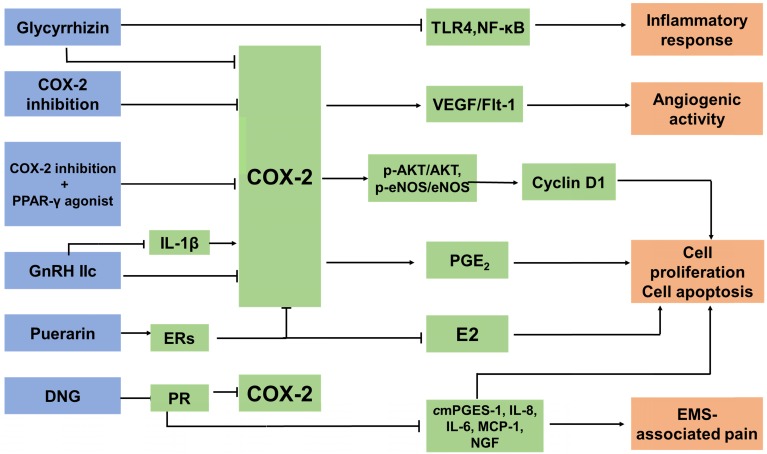
The anti-EMS strategy of targeted COX-2. There are three main types of anti-EMS drugs targeting COX-2: COX-2 inhibitors, hormone drugs and other drugs. They inhibit COX-2 expression in different ways. Treatment with COX‐2 inhibitors significantly decreases microvessel density and macrophage numbers, and is associated with decreased expression of VEGF and Flk-1. Combining the inhibition of COX-2 with peroxisome proliferator-activated receptor (PPAR)-γ agonists suppresses cell proliferation and induces apoptosis by decreasing the expression of p-Akt/Akt and p-eNOS/eNOS. GnRH-II decreases the COX-2 secretion of the ectopic, eutopic and normal ESC in EMS, and it can reverse the IL-1β-induced expression of COX-2 in ESCs. DNG, a selective PR agonist, downregulates the mRNA expression of CYP19A1, COX-2, mPGES-1, IL-8, IL-6, MCP-1, VEGF and NGF, and PGE2 production, as well as suppressing the development of endometriotic lesions and relieving EMS-associated pain. Glycyrrhizin is able to attenuate the expression of COX-2 and dramatically diminishes LPS-induced TLR4 expression and NF-κB activation in MEECs. As a result, it can inhibit the LPS-induced inflammatory response. Puerarin can inhibit the expression of P450arom and COX-2 in the ectopic endometrium, restrict the levels of E2 and PGE2, and block the positive feedback mechanism of E2 synthesis.
COX-2 inhibitors
COX-2 is an essential therapeutic target for anti-inflammatory drugs, which are known as nonsteroidal anti-inflammatory drugs (NSAIDs), including naproxen and diclofenac, as well as newer COX-2 selective inhibitors such as Celebrex (celecoxib; Pfizer). A clinical trial recruited 28 women (age range 23-39 years) who were diagnosed with EMS by laparoscopy. They were treated with a placebo or a COX-2 specific inhibitor. It was found that the administration of NSAIDs was safe and effective in the management of EMS-related pain and might block angiogenesis in endometriotic foci, which was considered to be a long-term effect in that it may help prevent relapses of EMS 168. In a rat model, a new selective COX-2 enzyme inhibitor, dexketoprofen trometamol, remarkably reduced the development of experimentally-induced endometriotic lesions, both macroscopically and microscopically 169, 170. COX-2 induces the production of PGE2 and E2, which are known to increase VEGF expression 171. The binding of VEGF to the Fms-like tyrosine kinase 1 (Flt-1) receptor 170 leads to an upregulation in mitogenesis, migration enhancement, and the release of various proteolytic enzymes. It has been demonstrated that treatment with parecoxib downregulates the expression of VEGF and Flk-1, and reinforces its antiangiogenic activity in rat endometriotic lesions 172. It was reported that patients with EMS showed increased numbers of activated macrophages in the PF 14, 173, which are the primary source of VEGF produced in areas of inflammation 14. Treatment with COX-2 inhibitors significantly decreases microvessel density and macrophage numbers, and is associated with decreased expression of VEGF and Flk-1 172, 174. In mouse model, the group administered with COX-2 inhibitors showed a low concentration of PGE2. Combined use of COX-2 inhibitors and telmisartan may be more effective in the treatment of endometriotic lesions. Combining the inhibition of COX-2 with the peroxisome proliferator-activated receptor (PPAR)-γ agonist telmisartan appears to be a promising strategy in EMS as it suppresses cell proliferation and induces apoptosis. Decreased expression of p-Akt/Akt and downstream p-eNOS/eNOS in parecoxib/telmisartan-treated lesions has also been shown experimentally 175. However, COX-2 inhibitors may damage the gastrointestinal tract, and induce the development of erosions and ulcers, with potential complications of protein loss, stricture formation, bleeding and perforation 176. The side effects of COX-2 inhibitors should be monitored.
Hormone drugs
Type-II gonadotropin releasing hormone (GnRH II), a secondary form of GnRH, is distributed in discrete regions of the central and peripheral nervous systems and in nonneural tissues; GnRH-II functions in the nervous system and, notably, in areas associated with sexual behavior 177. GnRH-II has the effect of promoting apoptosis, especially on the ectopic ESC, as a result of inhibiting the secretion of IL-8 protein and the level of COX-2 mRNA and IL-8 mRNA in endometriotic cells, and in the case of the downregulation of endogenous GnRH-II expression it can lead to the initiation and development of EMS 178. In addition, GnRH-II decreases VEGF secretion in the ectopic, eutopic and normal ESC in EMS in vitro, which contributes to the downregulation of the number of newly-formed blood vessels 177. The IL-1β-induced expression of COX-2 in ESC can be reversed by GnRH-II 179. Dienogest (DNG) is a selective progesterone receptor (PR) agonist. One of the current clinical anti-EMS strategies is oral administration of DNG. However, PR has been reported to appear as two major isoforms, PR-A and PR-B, and they have mostly distinct physiological functions 180. DNG exerts therapeutic efficacy against the pain and progression of EMS regardless of PR expression patterns. It was reported that DNG downregulates the mRNA expression of CYP19A1, COX-2, mPGES-1, IL-8, IL-6, monocyte chemoattractant protein (MCP)-1, VEGF and NGF, and PGE2 production in human endometriotic epithelial cell lines that specifically express either PR-A or PR-B 181, 182.
Other drugs
Glycyrrhizin, a triterpene isolated from the roots and rhizomes of licorice (Glycyrrhiza glabra), has been shown to have anti-inflammatory effects. Wang et al 182 found that glycyrrhizin was able to attenuate the expression of inducible nitric oxide synthase (iNOS) and COX-2 in mouse endometrial epithelial cells (MEECs). Furthermore, glycyrrhizin dramatically diminishes LPS-inducing TLR4 expression and NF-κB activation in MEECs. As a result, it can inhibit the LPS-induced inflammatory response. Glycyrrhizin may be used as a potential agent for the treatment of EMS, partly by targeting COX-2 183. Another traditional Chinese medicine, puerarin, extracted from Radix puerariae, is widely known as a natural conditioner of selective estrogen receptors (ERs) 184. Puerarin can inhibit the expression of P450arom and COX-2 in the ectopic endometrium, restrict the levels of E2 and PGE2, and block the positive feedback mechanism of E2 synthesis. It could be a potential therapeutic agent for the treatment of EMS in clinic 185.
Conclusion and future perspectives
Under the regulation of hormone, hypoxia and so on, the increased COX-2 in the glandular epithelial cells and ESCs of ectopic lesions leads to the high proliferation, low level of apoptosis, high invasion and angiogenesis, and impaired cytotoxic NK cell differentiation, which further promotes the occurrence and development of EMS. By producing PGE2 to induce EMS-related pain, COX-2 in endometriotic cells can further accelerate the development of EMS. Many drugs and COX-2 inhibitors play an important role in the treatment of EMS by targeting COX-2, especially for EMS-related pain. However, further investigation of their actions, apart from analgesic functions, is needed, which will enlarge therapeutic horizon of these drugs in EMS. For example, considering the important role of COX-2 in the survival, invasion, angiogenesis and immune escape of ectopic lesions, COX-2 may be an important indicator for predicting the recurrence of EMS. Prophylactic drugs may become available in high-risk populations. COX-2-targeting treatments may inhibit the growth of the ectopic intima, relieve pain, reduce angiogenesis and remove residual lesions. By analyzing the expression level of COX-2 and the PGE2 concentration in the endometriotic microenvironment, there is potential to provide individualized and precise treatment for preventing the recurrence of EMS.
Acknowledgments
This study was supported by the Major Research Program of National Natural Science Foundation of China (NSFC, No. 31970798, 91542108, 81471513, 31671200 and 81571509), the Shanghai Rising-Star Program 16QA1400800, and the Innovation-oriented Science and Technology Grant from NPFPC Key Laboratory of Reproduction Regulation (CX2017-2), and the Program for Zhuoxue of Fudan University.
Authors' contributions
ZZL performed the literature search, drafted the manuscript and prepared the figure. HLY, SYH, KKC, JM, WJZ, XUQ, XQW, RZ and DJL helped to perform revisions and critically discussed the completed manuscript. MQL designed, supervised and critically reviewed the complete manuscript.
Abbreviations
- AA
Arachidonic acid
- CNS
Central nervous system
- COX-2
Cyclooxygenase-2
- EMS
Endometriosis
- ESCs
Endometrial stem cells
- GnRH II
II-type gonadotropin releasing hormone
- IL-1β
Interleukin-1β
- MAPK
Mitogen-activated protein kinases
- MMP
Matrix metalloproteinase
- PGE2
Prostaglandin E2
- VEGF
Vascular endothelial growth factor
References
- 1.Frackiewicz EJ. Endometriosis: an overview of the disease and its treatment. J Am Pharm Assoc (Wash) 2000;40(5):645–57. doi: 10.1016/s1086-5802(16)31105-6. quiz 99-702. [DOI] [PubMed] [Google Scholar]
- 2.Griffith JS, Liu YG, Tekmal RR, Binkley PA, Holden AE, Schenken RS. Menstrual endometrial cells from women with endometriosis demonstrate increased adherence to peritoneal cells and increased expression of CD44 splice variants. Fertil Steril. 2010;93(6):1745–9. doi: 10.1016/j.fertnstert.2008.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueira PG, Abrao MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2011;1221:10–7. doi: 10.1111/j.1749-6632.2011.05969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goumenou AG, Matalliotakis IM, Tzardi M, Fragouli YG, Mahutte NG, Arici A. Apoptosis and differential expression of apoptosis-related proteins in endometriotic glandular and stromal cells. J Soc Gynecol Investig. 2004;11(5):318–22. doi: 10.1016/j.jsgi.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24(2):235–58. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 6.Pritts EA, Taylor RN. An evidence-based evaluation of endometriosis-associated infertility. Endocrinol Metab Clin North Am. 2003;32(3):653–67. doi: 10.1016/s0889-8529(03)00045-8. [DOI] [PubMed] [Google Scholar]
- 7.Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D'Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92(1):68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 8.Fuldeore MJ, Soliman AM. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol Obstet Invest. 2017;82(5):453–61. doi: 10.1159/000452660. [DOI] [PubMed] [Google Scholar]
- 9.Greene R, Stratton P, Cleary SD, Ballweg ML, Sinaii N. Diagnostic experience among 4,334 women reporting surgically diagnosed endometriosis. Fertil Steril. 2009;91(1):32–9. doi: 10.1016/j.fertnstert.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Ahn SH, Singh V, Tayade C. Biomarkers in endometriosis: challenges and opportunities. Fertil Steril. 2017;107(3):523–32. doi: 10.1016/j.fertnstert.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–99. doi: 10.1016/S0140-6736(04)17403-5. [DOI] [PubMed] [Google Scholar]
- 12.Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123(2):217–26. doi: 10.1530/rep.0.1230217. [DOI] [PubMed] [Google Scholar]
- 13.Lousse JC, Van Langendonckt A, Gonzalez-Ramos R, Defrere S, Renkin E, Donnez J. Increased activation of nuclear factor-kappa B (NF-kappaB) in isolated peritoneal macrophages of patients with endometriosis. Fertil Steril. 2008;90(1):217–20. doi: 10.1016/j.fertnstert.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Ahn SH, Monsanto SP, Miller C, Singh SS, Thomas R, Tayade C. Pathophysiology and Immune Dysfunction in Endometriosis. Biomed Res Int. 2015;2015:795976. doi: 10.1155/2015/795976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E. et al. Role of estrogen receptor-beta in endometriosis. Semin Reprod Med. 2012;30(1):39–45. doi: 10.1055/s-0031-1299596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lousse JC, Defrere S, Colette S, Van Langendonckt A, Donnez J. Expression of eicosanoid biosynthetic and catabolic enzymes in peritoneal endometriosis. Hum Reprod. 2010;25(3):734–41. doi: 10.1093/humrep/dep408. [DOI] [PubMed] [Google Scholar]
- 17.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res. 2004;43(1):3–35. doi: 10.1016/s0163-7827(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 18.Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA. Prostanoids in health and disease. J Lipid Res. 2009;50(Suppl):S423–8. doi: 10.1194/jlr.R800094-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu MH, Shoji Y, Chuang PC, Tsai SJ. Endometriosis: disease pathophysiology and the role of prostaglandins. Expert Rev Mol Med. 2007;9(2):1–20. doi: 10.1017/S146239940700021X. [DOI] [PubMed] [Google Scholar]
- 20.Ray K, Fahrmann J, Mitchell B, Paul D, King H, Crain C. et al. Oxidation-sensitive nociception involved in endometriosis-associated pain. Pain. 2015;156(3):528–39. doi: 10.1097/01.j.pain.0000460321.72396.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banu SK, Lee J, Speights VJ, Starzinski-Powitz A, Arosh JA. Cyclooxygenase-2 regulates survival, migration, and invasion of human endometriotic cells through multiple mechanisms. Endocrinology. 2008;149(3):1180–9. doi: 10.1210/en.2007-1168. [DOI] [PubMed] [Google Scholar]
- 22.Park GY, Christman JW. Involvement of cyclooxygenase-2 and prostaglandins in the molecular pathogenesis of inflammatory lung diseases. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L797–805. doi: 10.1152/ajplung.00513.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 24.Smith WL, Garavito RM, DeWitt DL. Prostaglandin endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271(52):33157–60. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 25.Kurumbail RG, Stevens AM, Gierse JK, McDonald JJ, Stegeman RA, Pak JY. et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature. 1996;384(6610):644–8. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 26.Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. doi: 10.1146/annurev.biochem.69.1.145. [DOI] [PubMed] [Google Scholar]
- 27.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56(3):387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 28.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS. et al. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A. 2002;99(21):13926–31. doi: 10.1073/pnas.162468699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kraemer SA, Meade EA, DeWitt DL. Prostaglandin endoperoxide synthase gene structure: identification of the transcriptional start site and 5'-flanking regulatory sequences. Arch Biochem Biophys. 1992;293(2):391–400. doi: 10.1016/0003-9861(92)90411-o. [DOI] [PubMed] [Google Scholar]
- 30.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302( Pt 3):723–7. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang YJ, Mbonye UR, DeLong CJ, Wada M, Smith WL. Regulation of intracellular cyclooxygenase levels by gene transcription and protein degradation. Prog Lipid Res. 2007;46(2):108–25. doi: 10.1016/j.plipres.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mitchell JA, Warner TD. Cyclo-oxygenase-2: pharmacology, physiology, biochemistry and relevance to NSAID therapy. Br J Pharmacol. 1999;128(6):1121–32. doi: 10.1038/sj.bjp.0702897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benelli R, Vene R, Ferrari N. Prostaglandin-endoperoxide synthase 2 (cyclooxygenase-2), a complex target for colorectal cancer prevention and therapy. Transl Res. 2018;196:42–61. doi: 10.1016/j.trsl.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Gomes RN, Felipe DCS, Colquhoun A. Eicosanoids and cancer. Clinics (Sao Paulo) 2018;73(suppl 1):e530s. doi: 10.6061/clinics/2018/e530s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu T, Lao X, Zheng H. Influencing COX-2 Activity by COX Related Pathways in Inflammation and Cancer. Mini Rev Med Chem. 2016;16(15):1230–43. doi: 10.2174/1389557516666160505115743. [DOI] [PubMed] [Google Scholar]
- 37.Teismann P, Tieu K, Choi DK, Wu DC, Naini A, Hunot S. et al. Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc Natl Acad Sci U S A. 2003;100(9):5473–8. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mulet M, Blasco-Ibanez JM, Crespo C, Nacher J, Varea E. Early increased density of cyclooxygenase-2 (COX-2) immunoreactive neurons in Down syndrome. Folia Neuropathol. 2017;55(2):154–60. doi: 10.5114/fn.2017.68582. [DOI] [PubMed] [Google Scholar]
- 39.Li W, Cao Y, Xu J, Wang Y, Li W, Wang Q. et al. YAP transcriptionally regulates COX-2 expression and GCCSysm-4 (G-4), a dual YAP/COX-2 inhibitor, overcomes drug resistance in colorectal cancer. J Exp Clin Cancer Res. 2017;36(1):144. doi: 10.1186/s13046-017-0612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsatsanis C, Androulidaki A, Venihaki M, Margioris AN. Signalling networks regulating cyclooxygenase-2. Int J Biochem Cell Biol. 2006;38(10):1654–61. doi: 10.1016/j.biocel.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 41.Berthou F, Ceppo F, Dumas K, Massa F, Vergoni B, Alemany S. et al. The Tpl2 Kinase Regulates the COX-2/Prostaglandin E2 Axis in Adipocytes in Inflammatory Conditions. Mol Endocrinol. 2015;29(7):1025–36. doi: 10.1210/me.2015-1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eliopoulos AG, Dumitru CD, Wang CC, Cho J, Tsichlis PN. Induction of COX-2 by LPS in macrophages is regulated by Tpl2-dependent CREB activation signals. EMBO J. 2002;21(18):4831–40. doi: 10.1093/emboj/cdf478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci U S A. 1993;90(15):7240–4. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu Y, Borchert GL, Phang JM. Polyoma enhancer activator 3, an ets transcription factor, mediates the induction of cyclooxygenase-2 by nitric oxide in colorectal cancer cells. J Biol Chem. 2004;279(18):18694–700. doi: 10.1074/jbc.M308136200. [DOI] [PubMed] [Google Scholar]
- 45.Wu MH, Lin SC, Hsiao KY, Tsai SJ. Hypoxia-inhibited dual-specificity phosphatase-2 expression in endometriotic cells regulates cyclooxygenase-2 expression. J Pathol. 2011;225(3):390–400. doi: 10.1002/path.2963. [DOI] [PubMed] [Google Scholar]
- 46.Wu MH, Wang CA, Lin CC, Chen LC, Chang WC, Tsai SJ. Distinct regulation of cyclooxygenase-2 by interleukin-1beta in normal and endometriotic stromal cells. J Clin Endocrinol Metab. 2005;90(1):286–95. doi: 10.1210/jc.2004-1612. [DOI] [PubMed] [Google Scholar]
- 47.Endale M, Kim TH, Kwak YS, Kim NM, Kim SH, Cho JY. et al. Torilin Inhibits Inflammation by Limiting TAK1-Mediated MAP Kinase and NF-kappaB Activation. Mediators Inflamm. 2017;2017:7250968. doi: 10.1155/2017/7250968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407(6801):249–57. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 49.Chun KS, Surh YJ. Signal transduction pathways regulating cyclooxygenase-2 expression: potential molecular targets for chemoprevention. Biochem Pharmacol. 2004;68(6):1089–100. doi: 10.1016/j.bcp.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Zhou Y, Wang X, Evers BM. Glycogen synthase kinase-3 is a negative regulator of extracellular signal-regulated kinase. Oncogene. 2006;25(1):43–50. doi: 10.1038/sj.onc.1209004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maia HJ, Maltez A, Studard E, Zausner B, Athayde C, Coutinho E. Effect of the menstrual cycle and oral contraceptives on cyclooxygenase-2 expression in the endometrium. Gynecol Endocrinol. 2005;21(1):57–61. doi: 10.1080/09513590500099602. [DOI] [PubMed] [Google Scholar]
- 52.Nandakishore R, Yalavarthi PR, Kiran YR, Rajapranathi M. Selective cyclooxygenase inhibitors: current status. Curr Drug Discov Technol. 2014;11(2):127–32. doi: 10.2174/1570163811666140127123717. [DOI] [PubMed] [Google Scholar]
- 53.Ota H, Igarashi S, Sasaki M, Tanaka T. Distribution of cyclooxygenase-2 in eutopic and ectopic endometrium in endometriosis and adenomyosis. Hum Reprod. 2001;16(3):561–6. doi: 10.1093/humrep/16.3.561. [DOI] [PubMed] [Google Scholar]
- 54.Cho S, Park SH, Choi YS, Seo SK, Kim HY, Park KH. et al. Expression of cyclooxygenase-2 in eutopic endometrium and ovarian endometriotic tissue in women with severe endometriosis. Gynecol Obstet Invest. 2010;69(2):93–100. doi: 10.1159/000261017. [DOI] [PubMed] [Google Scholar]
- 55.Mei J, Zhou WJ, Zhu XY, Lu H, Wu K, Yang HL. et al. Suppression of autophagy and HCK signaling promotes PTGS2(high) FCGR3(-) NK cell differentiation triggered by ectopic endometrial stromal cells. Autophagy. 2018;14(8):1376–97. doi: 10.1080/15548627.2018.1476809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zidan HE, Rezk NA, Alnemr AA, Abd EGA. COX-2 gene promoter DNA methylation status in eutopic and ectopic endometrium of Egyptian women with endometriosis. J Reprod Immunol. 2015;112:63–7. doi: 10.1016/j.jri.2015.06.093. [DOI] [PubMed] [Google Scholar]
- 57.Luo MX, Long BB, Li F, Zhang C, Pan MT, Huang YQ. et al. Roles of Cyclooxygenase-2 gene -765G>C (rs20417) and -1195G>A (rs689466) polymorphisms in gastric cancer: A systematic review and meta-analysis. Gene. 2019;685:125–35. doi: 10.1016/j.gene.2018.10.077. [DOI] [PubMed] [Google Scholar]
- 58.Barbeiro DF, Koike MK, Coelho AM, Da SF, Machado MC. Intestinal barrier dysfunction and increased COX-2 gene expression in the gut of elderly rats with acute pancreatitis. Pancreatology. 2016;16(1):52–6. doi: 10.1016/j.pan.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 59.Hla T, Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992;89(16):7384–8. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995;270(42):24965–71. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- 61.Inoue H, Tanabe T. Transcriptional role of the nuclear factor kappa B site in the induction by lipopolysaccharide and suppression by dexamethasone of cyclooxygenase-2 in U937 cells. Biochem Biophys Res Commun. 1998;244(1):143–8. doi: 10.1006/bbrc.1998.8222. [DOI] [PubMed] [Google Scholar]
- 62.Jing F, Yuping W, Yong C, Jie L, Jun L, Xuanbing T. et al. CpG island methylator phenotype of multigene in serum of sporadic breast carcinoma. Tumour Biol. 2010;31(4):321–31. doi: 10.1007/s13277-010-0040-x. [DOI] [PubMed] [Google Scholar]
- 63.Li B, Liu W, Wang L, Li M, Wang J, Huang L. et al. CpG island methylator phenotype associated with tumor recurrence in tumor-node-metastasis stage I hepatocellular carcinoma. Ann Surg Oncol. 2010;17(7):1917–26. doi: 10.1245/s10434-010-0921-7. [DOI] [PubMed] [Google Scholar]
- 64.Wang D, Chen Q, Zhang C, Ren F, Li T. DNA hypomethylation of the COX-2 gene promoter is associated with up-regulation of its mRNA expression in eutopic endometrium of endometriosis. Eur J Med Res. 2012;17:12. doi: 10.1186/2047-783X-17-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Qu Y, Song W. Genetic variation in COX-2 -1195 and the risk of endometriosis and adenomyosis. Clin Exp Obstet Gynecol. 2015;42(2):168–72. [PubMed] [Google Scholar]
- 66.Wang H, Sun L, Jiang M, Liu L, Wang G. -1195 A/G promoter variants of the cyclooxygenase-2 gene increases the risk of pain occurrence in endometriotic women. Clin Exp Obstet Gynecol. 2016;43(2):254–7. [PubMed] [Google Scholar]
- 67.Cavalcanti V, Ponce TG, Mafra FA, Andre GM, Christofolini DM, Barbosa CP. et al. Evaluation of the frequency of G-765C polymorphism in the promoter region of the COX-2 gene and its correlation with the expression of this gene in the endometrium of women with endometriosis. Arch Gynecol Obstet. 2016;293(1):109–15. doi: 10.1007/s00404-015-3808-9. [DOI] [PubMed] [Google Scholar]
- 68.Kim HY, Cho S, Choi YS, Yang HI, Lee KE, Seo SK. et al. Cyclooxygenase-2 ( COX -2) gene-765G/C polymorphism and advanced-stage endometriosis in Korean women. Am J Reprod Immunol. 2012;68(3):238–43. doi: 10.1111/j.1600-0897.2012.01151.x. [DOI] [PubMed] [Google Scholar]
- 69.Kao LC, Tulac S, Lobo S, Imani B, Yang JP, Germeyer A. et al. Global gene profiling in human endometrium during the window of implantation. Endocrinology. 2002;143(6):2119–38. doi: 10.1210/endo.143.6.8885. [DOI] [PubMed] [Google Scholar]
- 70.Maia HJ, Haddad C, Coelho G, Casoy J. Role of inflammation and aromatase expression in the eutopic endometrium and its relationship with the development of endometriosis. Womens Health (Lond) 2012;8(6):647–58. doi: 10.2217/whe.12.52. [DOI] [PubMed] [Google Scholar]
- 71.Maia HJ, Casoy J, Valente FJ. Is aromatase expression in the endometrium the cause of endometriosis and related infertility? Gynecol Endocrinol. 2009;25(4):253–7. doi: 10.1080/09513590802627647. [DOI] [PubMed] [Google Scholar]
- 72.Tamura M, Sebastian S, Yang S, Gurates B, Fang Z, Bulun SE. Interleukin-1beta elevates cyclooxygenase-2 protein level and enzyme activity via increasing its mRNA stability in human endometrial stromal cells: an effect mediated by extracellularly regulated kinases 1 and 2. J Clin Endocrinol Metab. 2002;87(7):3263–73. doi: 10.1210/jcem.87.7.8594. [DOI] [PubMed] [Google Scholar]
- 73.Huang JC, Liu DY, Yadollahi S, Wu KK, Dawood MY. Interleukin-1 beta induces cyclooxygenase-2 gene expression in cultured endometrial stromal cells. J Clin Endocrinol Metab. 1998;83(2):538–41. doi: 10.1210/jcem.83.2.4533. [DOI] [PubMed] [Google Scholar]
- 74.Huang F, Cao J, Liu Q, Zou Y, Li H, Yin T. MAPK/ERK signal pathway involved expression of COX-2 and VEGF by IL-1beta induced in human endometriosis stromal cells in vitro. Int J Clin Exp Pathol. 2013;6(10):2129–36. [PMC free article] [PubMed] [Google Scholar]
- 75.Sofroniew MV, Howe CL, Mobley WC. Nerve growth factor signaling, neuroprotection, and neural repair. Annu Rev Neurosci. 2001;24:1217–81. doi: 10.1146/annurev.neuro.24.1.1217. [DOI] [PubMed] [Google Scholar]
- 76.Minnone G, De Benedetti F, Bracci-Laudiero L. NGF and Its Receptors in the Regulation of Inflammatory Response. Int J Mol Sci; 2017. p. 18. (5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelleher JH, Tewari D, McMahon SB. Neurotrophic factors and their inhibitors in chronic pain treatment. Neurobiol Dis. 2017;97(Pt B):127–38. doi: 10.1016/j.nbd.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 78.Wang G, Tokushige N, Markham R, Fraser IS. Rich innervation of deep infiltrating endometriosis. Hum Reprod. 2009;24(4):827–34. doi: 10.1093/humrep/den464. [DOI] [PubMed] [Google Scholar]
- 79.Kajitani T, Maruyama T, Asada H, Uchida H, Oda H, Uchida S. et al. Possible involvement of nerve growth factor in dysmenorrhea and dyspareunia associated with endometriosis. Endocr J. 2013;60(10):1155–64. doi: 10.1507/endocrj.ej13-0027. [DOI] [PubMed] [Google Scholar]
- 80.Peng B, Zhan H, Alotaibi F, Alkusayer GM, Bedaiwy MA, Yong PJ. Nerve Growth Factor Is Associated With Sexual Pain in Women With Endometriosis. Reprod Sci. 2018;25(4):540–9. doi: 10.1177/1933719117716778. [DOI] [PubMed] [Google Scholar]
- 81.Taylor CT, Colgan SP. Regulation of immunity and inflammation by hypoxia in immunological niches. Nat Rev Immunol. 2017;17(12):774–85. doi: 10.1038/nri.2017.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148(3):399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mole DR, Blancher C, Copley RR, Pollard PJ, Gleadle JM, Ragoussis J. et al. Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem. 2009;284(25):16767–75. doi: 10.1074/jbc.M901790200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Simoens S, Hummelshoj L, D'Hooghe T. Endometriosis: cost estimates and methodological perspective. Hum Reprod Update. 2007;13(4):395–404. doi: 10.1093/humupd/dmm010. [DOI] [PubMed] [Google Scholar]
- 85.Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L. et al. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol. 2010;2010:369549. doi: 10.1155/2010/369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Owens DM, Keyse SM. Differential regulation of MAP kinase signalling by dual-specificity protein phosphatases. Oncogene. 2007;26(22):3203–13. doi: 10.1038/sj.onc.1210412. [DOI] [PubMed] [Google Scholar]
- 87.Lin SC, Wang CC, Wu MH, Yang SH, Li YH, Tsai SJ. Hypoxia-induced microRNA-20a expression increases ERK phosphorylation and angiogenic gene expression in endometriotic stromal cells. J Clin Endocrinol Metab. 2012;97(8):E1515–23. doi: 10.1210/jc.2012-1450. [DOI] [PubMed] [Google Scholar]
- 88.Teague EM, Print CG, Hull ML. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update. 2010;16(2):142–65. doi: 10.1093/humupd/dmp034. [DOI] [PubMed] [Google Scholar]
- 89.Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13(11):797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- 90.Hsiao KY, Lin SC, Wu MH, Tsai SJ. Pathological functions of hypoxia in endometriosis. Front Biosci (Elite Ed) 2015;7:309–21. doi: 10.2741/E736. [DOI] [PubMed] [Google Scholar]
- 91.Smarr MM, Kannan K, Buck LG. Endocrine disrupting chemicals and endometriosis. Fertil Steril. 2016;106(4):959–66. doi: 10.1016/j.fertnstert.2016.06.034. [DOI] [PubMed] [Google Scholar]
- 92.Porpora MG, Resta S, Fuggetta E, Storelli P, Megiorni F, Manganaro L. et al. Role of environmental organochlorinated pollutants in the development of endometriosis. Clin Exp Obstet Gynecol. 2013;40(4):565–7. [PubMed] [Google Scholar]
- 93.Martinez-Zamora MA, Mattioli L, Parera J, Abad E, Coloma JL, van Babel B. et al. Increased levels of dioxin-like substances in adipose tissue in patients with deep infiltrating endometriosis. Hum Reprod. 2015;30(5):1059–68. doi: 10.1093/humrep/dev026. [DOI] [PubMed] [Google Scholar]
- 94.Trabert B, De Roos AJ, Schwartz SM, Peters U, Scholes D, Barr DB. et al. Non-dioxin-like polychlorinated biphenyls and risk of endometriosis. Environ Health Perspect. 2010;118(9):1280–5. doi: 10.1289/ehp.0901444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huang Q, Chen Y, Chen Q, Zhang H, Lin Y, Zhu M. et al. Dioxin-like rather than non-dioxin-like PCBs promote the development of endometriosis through stimulation of endocrine-inflammation interactions. Arch Toxicol. 2017;91(4):1915–24. doi: 10.1007/s00204-016-1854-0. [DOI] [PubMed] [Google Scholar]
- 96.Guo SW, Simsa P, Kyama CM, Mihalyi A, Fulop V, Othman EE. et al. Reassessing the evidence for the link between dioxin and endometriosis: from molecular biology to clinical epidemiology. Mol Hum Reprod. 2009;15(10):609–24. doi: 10.1093/molehr/gap075. [DOI] [PubMed] [Google Scholar]
- 97.Bulun SE. Endometriosis. N Engl J Med. 2009;360(3):268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 98.Colette S, Donnez J. Endometriosis. N Engl J Med. 2009;360(18):1911–2. doi: 10.1056/NEJMc090328. author reply 2. [DOI] [PubMed] [Google Scholar]
- 99.Smith AG, Carthew P, Francis JE, Cabral JR, Manson MM. Enhancement by iron of hepatic neoplasia in rats caused by hexachlorobenzene. Carcinogenesis. 1993;14(7):1381–7. doi: 10.1093/carcin/14.7.1381. [DOI] [PubMed] [Google Scholar]
- 100.Degner SC, Kemp MQ, Hockings JK, Romagnolo DF. Cyclooxygenase-2 promoter activation by the aromatic hydrocarbon receptor in breast cancer mcf-7 cells: repressive effects of conjugated linoleic acid. Nutr Cancer. 2007;59(2):248–57. doi: 10.1080/01635580701485585. [DOI] [PubMed] [Google Scholar]
- 101.Haarmann-Stemmann T, Bothe H, Abel J. Growth factors, cytokines and their receptors as downstream targets of arylhydrocarbon receptor (AhR) signaling pathways. Biochem Pharmacol. 2009;77(4):508–20. doi: 10.1016/j.bcp.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Chiappini F, Baston JI, Vaccarezza A, Singla JJ, Pontillo C, Miret N. et al. Enhanced cyclooxygenase-2 expression levels and metalloproteinase 2 and 9 activation by Hexachlorobenzene in human endometrial stromal cells. Biochem Pharmacol. 2016;109:91–104. doi: 10.1016/j.bcp.2016.03.024. [DOI] [PubMed] [Google Scholar]
- 103.Akyol A, Simsek M, Ilhan R, Can B, Baspinar M, Akyol H. et al. Efficacies of vitamin D and omega-3 polyunsaturated fatty acids on experimental endometriosis. Taiwan J Obstet Gynecol. 2016;55(6):835–9. doi: 10.1016/j.tjog.2015.06.018. [DOI] [PubMed] [Google Scholar]
- 104.Tomio K, Kawana K, Taguchi A, Isobe Y, Iwamoto R, Yamashita A. et al. Omega-3 polyunsaturated Fatty acids suppress the cystic lesion formation of peritoneal endometriosis in transgenic mouse models. PLoS One. 2013;8(9):e73085. doi: 10.1371/journal.pone.0073085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Attaman JA, Stanic AK, Kim M, Lynch MP, Rueda BR, Styer AK. The anti-inflammatory impact of omega-3 polyunsaturated Fatty acids during the establishment of endometriosis-like lesions. Am J Reprod Immunol. 2014;72(4):392–402. doi: 10.1111/aji.12276. [DOI] [PubMed] [Google Scholar]
- 106.Gravaghi C, La Perle KM, Ogrodwski P, Kang JX, Quimby F, Lipkin M. et al. Cox-2 expression, PGE(2) and cytokines production are inhibited by endogenously synthesized n-3 PUFAs in inflamed colon of fat-1 mice. J Nutr Biochem. 2011;22(4):360–5. doi: 10.1016/j.jnutbio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 107.Malkowski MG, Thuresson ED, Lakkides KM, Rieke CJ, Micielli R, Smith WL. et al. Structure of eicosapentaenoic and linoleic acids in the cyclooxygenase site of prostaglandin endoperoxide H synthase-1. J Biol Chem. 2001;276(40):37547–55. doi: 10.1074/jbc.M105982200. [DOI] [PubMed] [Google Scholar]
- 108.Palafox D, Llorente L, Alberu J, Torres-Machorro A, Camorlinga N, Rodriguez C. et al. The role of indoleamine 2,3 dioxygenase in the induction of immune tolerance in organ transplantation. Transplant Rev (Orlando) 2010;24(3):160–5. doi: 10.1016/j.trre.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Mei J, Li MQ, Ding D, Li DJ, Jin LP, Hu WG. et al. Indoleamine 2,3-dioxygenase-1 (IDO1) enhances survival and invasiveness of endometrial stromal cells via the activation of JNK signaling pathway. Int J Clin Exp Pathol. 2013;6(3):431–44. [PMC free article] [PubMed] [Google Scholar]
- 110.Mei J, Jin LP, Ding D, Li MQ, Li DJ, Zhu XY. Inhibition of IDO1 suppresses cyclooxygenase-2 and matrix metalloproteinase-9 expression and decreases proliferation, adhesion and invasion of endometrial stromal cells. Mol Hum Reprod. 2012;18(10):467–76. doi: 10.1093/molehr/gas021. [DOI] [PubMed] [Google Scholar]
- 111.Fujigaki H, Saito K, Fujigaki S, Takemura M, Sudo K, Ishiguro H. et al. The signal transducer and activator of transcription 1alpha and interferon regulatory factor 1 are not essential for the induction of indoleamine 2,3-dioxygenase by lipopolysaccharide: involvement of p38 mitogen-activated protein kinase and nuclear factor-kappaB pathways, and synergistic effect of several proinflammatory cytokines. J Biochem. 2006;139(4):655–62. doi: 10.1093/jb/mvj072. [DOI] [PubMed] [Google Scholar]
- 112.Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Derijard B. et al. Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J. 1996;15(11):2760–70. [PMC free article] [PubMed] [Google Scholar]
- 113.Gutkind JS. Regulation of mitogen-activated protein kinase signaling networks by G protein-coupled receptors. Sci STKE. 2000;2000(40):re1. doi: 10.1126/stke.2000.40.re1. [DOI] [PubMed] [Google Scholar]
- 114.Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3-4):141–62. doi: 10.1016/j.plefa.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 115.Chiang N, Serhan CN, Dahlen SE, Drazen JM, Hay DW, Rovati GE. et al. The lipoxin receptor ALX: potent ligand-specific and stereoselective actions in vivo. Pharmacol Rev. 2006;58(3):463–87. doi: 10.1124/pr.58.3.4. [DOI] [PubMed] [Google Scholar]
- 116.Kumar R, Clerc AC, Gori I, Russell R, Pellegrini C, Govender L. et al. Lipoxin A(4) prevents the progression of de novo and established endometriosis in a mouse model by attenuating prostaglandin E(2) production and estrogen signaling. PLoS One. 2014;9(2):e89742. doi: 10.1371/journal.pone.0089742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Levi M, van der Poll T. Inflammation and coagulation. Crit Care Med. 2010;38(2 Suppl):S26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 118.Lipinski S, Bremer L, Lammers T, Thieme F, Schreiber S, Rosenstiel P. Coagulation and inflammation. Molecular insights and diagnostic implications. Hamostaseologie. 2011;31(2):94–102. doi: 10.5482/ha-1134. 4. [DOI] [PubMed] [Google Scholar]
- 119.Ding D, Liu X, Duan J, Guo SW. Platelets are an unindicted culprit in the development of endometriosis: clinical and experimental evidence. Hum Reprod. 2015;30(4):812–32. doi: 10.1093/humrep/dev025. [DOI] [PubMed] [Google Scholar]
- 120.Lin FJ, Qin J, Tang K, Tsai SY, Tsai MJ. Coup d'Etat: an orphan takes control. Endocr Rev. 2011;32(3):404–21. doi: 10.1210/er.2010-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP. et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3(6):e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Petit FG, Jamin SP, Kurihara I, Behringer RR, DeMayo FJ, Tsai MJ. et al. Deletion of the orphan nuclear receptor COUP-TFII in uterus leads to placental deficiency. Proc Natl Acad Sci U S A. 2007;104(15):6293–8. doi: 10.1073/pnas.0702039104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin SC, Li YH, Wu MH, Chang YF, Lee DK, Tsai SY. et al. Suppression of COUP-TFII by proinflammatory cytokines contributes to the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2014;99(3):E427–37. doi: 10.1210/jc.2013-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li X, Large MJ, Creighton CJ, Lanz RB, Jeong JW, Young SL. et al. COUP-TFII regulates human endometrial stromal genes involved in inflammation. Mol Endocrinol. 2013;27(12):2041–54. doi: 10.1210/me.2013-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nasu K, Nishida M, Kawano Y, Tsuno A, Abe W, Yuge A. et al. Aberrant expression of apoptosis-related molecules in endometriosis: a possible mechanism underlying the pathogenesis of endometriosis. Reprod Sci. 2011;18(3):206–18. doi: 10.1177/1933719110392059. [DOI] [PubMed] [Google Scholar]
- 126.Banu SK, Lee J, Speights VJ, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 induces apoptosis of human endometriotic cells through suppression of ERK1/2, AKT, NFkappaB, and beta-catenin pathways and activation of intrinsic apoptotic mechanisms. Mol Endocrinol. 2009;23(8):1291–305. doi: 10.1210/me.2009-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Laschke MW, Elitzsch A, Scheuer C, Vollmar B, Menger MD. Selective cyclo-oxygenase-2 inhibition induces regression of autologous endometrial grafts by down-regulation of vascular endothelial growth factor-mediated angiogenesis and stimulation of caspase-3-dependent apoptosis. Fertil Steril. 2007;87(1):163–71. doi: 10.1016/j.fertnstert.2006.05.068. [DOI] [PubMed] [Google Scholar]
- 128.Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995;83(3):493–501. doi: 10.1016/0092-8674(95)90127-2. [DOI] [PubMed] [Google Scholar]
- 129.DuBois RN, Shao J, Tsujii M, Sheng H, Beauchamp RD. G1 delay in cells overexpressing prostaglandin endoperoxide synthase-2. Cancer Res. 1996;56(4):733–7. [PubMed] [Google Scholar]
- 130.Lee J, Banu SK, Subbarao T, Starzinski-Powitz A, Arosh JA. Selective inhibition of prostaglandin E2 receptors EP2 and EP4 inhibits invasion of human immortalized endometriotic epithelial and stromal cells through suppression of metalloproteinases. Mol Cell Endocrinol. 2011;332(1-2):306–13. doi: 10.1016/j.mce.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 131.Narumiya S, FitzGerald GA. Genetic and pharmacological analysis of prostanoid receptor function. J Clin Invest. 2001;108(1):25–30. doi: 10.1172/JCI13455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang D, Dubois RN. Prostaglandins and cancer. Gut. 2006;55(1):115–22. doi: 10.1136/gut.2004.047100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Buchanan FG, Wang D, Bargiacchi F, DuBois RN. Prostaglandin E2 regulates cell migration via the intracellular activation of the epidermal growth factor receptor. J Biol Chem. 2003;278(37):35451–7. doi: 10.1074/jbc.M302474200. [DOI] [PubMed] [Google Scholar]
- 134.Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84(1):16–21. doi: 10.1016/j.fertnstert.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 135.Yao M, Kargman S, Lam EC, Kelly CR, Zheng Y, Luk P. et al. Inhibition of cyclooxygenase-2 by rofecoxib attenuates the growth and metastatic potential of colorectal carcinoma in mice. Cancer Res. 2003;63(3):586–92. [PubMed] [Google Scholar]
- 136.Brinckerhoff CE, Matrisian LM. Matrix metalloproteinases: a tail of a frog that became a prince. Nat Rev Mol Cell Biol. 2002;3(3):207–14. doi: 10.1038/nrm763. [DOI] [PubMed] [Google Scholar]
- 137.Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8(3):221–33. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jana S, Chatterjee K, Ray AK, DasMahapatra P, Swarnakar S. Regulation of Matrix Metalloproteinase-2 Activity by COX-2-PGE2-pAKT Axis Promotes Angiogenesis in Endometriosis. PLoS One. 2016;11(10):e0163540. doi: 10.1371/journal.pone.0163540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Williams CS, Tsujii M, Reese J, Dey SK, DuBois RN. Host cyclooxygenase-2 modulates carcinoma growth. J Clin Invest. 2000;105(11):1589–94. doi: 10.1172/JCI9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Buerkle MA, Lehrer S, Sohn HY, Conzen P, Pohl U, Krotz F. Selective inhibition of cyclooxygenase-2 enhances platelet adhesion in hamster arterioles in vivo. Circulation. 2004;110(14):2053–9. doi: 10.1161/01.CIR.0000143234.51796.A9. [DOI] [PubMed] [Google Scholar]
- 141.Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J. et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci U S A. 1994;91(6):2046–50. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vardeh D, Wang D, Costigan M, Lazarus M, Saper CB, Woolf CJ. et al. COX2 in CNS neural cells mediates mechanical inflammatory pain hypersensitivity in mice. J Clin Invest. 2009;119(2):287–94. doi: 10.1172/JCI37098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Greaves E, Horne AW, Jerina H, Mikolajczak M, Hilferty L, Mitchell R. et al. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci Rep. 2017;7:44169. doi: 10.1038/srep44169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amaya F, Samad TA, Barrett L, Broom DC, Woolf CJ. Periganglionic inflammation elicits a distally radiating pain hypersensitivity by promoting COX-2 induction in the dorsal root ganglion. Pain. 2009;142(1-2):59–67. doi: 10.1016/j.pain.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101(30):11094–8. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rush AM, Waxman SG. PGE2 increases the tetrodotoxin-resistant Nav1.9 sodium current in mouse DRG neurons via G-proteins. Brain Res. 2004;1023(2):264–71. doi: 10.1016/j.brainres.2004.07.042. [DOI] [PubMed] [Google Scholar]
- 147.Lin CR, Amaya F, Barrett L, Wang H, Takada J, Samad TA. et al. Prostaglandin E2 receptor EP4 contributes to inflammatory pain hypersensitivity. J Pharmacol Exp Ther. 2006;319(3):1096–103. doi: 10.1124/jpet.106.105569. [DOI] [PubMed] [Google Scholar]
- 148.Samad TA, Sapirstein A, Woolf CJ. Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med. 2002;8(8):390–6. doi: 10.1016/s1471-4914(02)02383-3. [DOI] [PubMed] [Google Scholar]
- 149.Koninckx PR, Ussia A, Adamyan L, Keckstein J, Wattiez A. Primary Dysmenorrhea. J Obstet Gynaecol Can. 2017;39(7):578–9. doi: 10.1016/j.jogc.2017.03.093. [DOI] [PubMed] [Google Scholar]
- 150.Yuan XL, Chen L, Li MX, Dong P, Xue J, Wang J. et al. Elevated expression of Foxp3 in tumor-infiltrating Treg cells suppresses T-cell proliferation and contributes to gastric cancer progression in a COX-2-dependent manner. Clin Immunol. 2010;134(3):277–88. doi: 10.1016/j.clim.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 151.Soontornchaiboon W, Joo SS, Kim SM. Anti-inflammatory effects of violaxanthin isolated from microalga Chlorella ellipsoidea in RAW 264.7 macrophages. Biol Pharm Bull. 2012;35(7):1137–44. doi: 10.1248/bpb.b12-00187. [DOI] [PubMed] [Google Scholar]
- 152.Haidari F, Homayouni F, Helli B, Haghighizadeh MH, Farahmandpour F. Effect of chlorella supplementation on systematic symptoms and serum levels of prostaglandins, inflammatory and oxidative markers in women with primary dysmenorrhea. Eur J Obstet Gynecol Reprod Biol. 2018;229:185–9. doi: 10.1016/j.ejogrb.2018.08.578. [DOI] [PubMed] [Google Scholar]
- 153.Holoch KJ, Lessey BA. Endometriosis and infertility. Clin Obstet Gynecol. 2010;53(2):429–38. doi: 10.1097/GRF.0b013e3181db7d71. [DOI] [PubMed] [Google Scholar]
- 154.Cretoiu SM, Cretoiu D, Suciu L, Popescu LM. Interstitial Cajal-like cells of human Fallopian tube express estrogen and progesterone receptors. J Mol Histol. 2009;40(5-6):387–94. doi: 10.1007/s10735-009-9252-z. [DOI] [PubMed] [Google Scholar]
- 155.Yang XJ, Xu JY, Shen ZJ, Zhao J. Immunohistochemical alterations of cajal-like type of tubal interstitial cells in women with endometriosis and tubal ectopic pregnancy. Arch Gynecol Obstet. 2013;288(6):1295–300. doi: 10.1007/s00404-013-2878-9. [DOI] [PubMed] [Google Scholar]
- 156.Yang J, Chi C, Liu Z, Yang G, Shen ZJ, Yang XJ. Ultrastructure damage of oviduct telocytes in rat model of acute salpingitis. J Cell Mol Med. 2015;19(7):1720–8. doi: 10.1111/jcmm.12548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang XJ, Yang J, Liu Z, Yang G, Shen ZJ. Telocytes damage in endometriosis-affected rat oviduct and potential impact on fertility. J Cell Mol Med. 2015;19(2):452–62. doi: 10.1111/jcmm.12427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Barcelos ID, Vieira RC, Ferreira EM, Martins WP, Ferriani RA, Navarro PA. Comparative analysis of the spindle and chromosome configurations of in vitro-matured oocytes from patients with endometriosis and from control subjects: a pilot study. Fertil Steril. 2009;92(5):1749–52. doi: 10.1016/j.fertnstert.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 159.Barcelos ID, Donabella FC, Ribas CP, Meola J, Ferriani RA, de Paz CC. et al. Down-regulation of the CYP19A1 gene in cumulus cells of infertile women with endometriosis. Reprod Biomed Online. 2015;30(5):532–41. doi: 10.1016/j.rbmo.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 160.Donabela FC, Meola J, Padovan CC, de Paz CC, Navarro PA. Higher SOD1 Gene Expression in Cumulus Cells From Infertile Women With Moderate and Severe Endometriosis. Reprod Sci. 2015;22(11):1452–60. doi: 10.1177/1933719115585146. [DOI] [PubMed] [Google Scholar]
- 161.Chen Q, Zhang C, Chen Y, Lou J, Wang D. Identification of endometriosis-related genes by representational difference analysis of cDNA. Aust N Z J Obstet Gynaecol. 2012;52(2):140–5. doi: 10.1111/j.1479-828X.2011.01405.x. [DOI] [PubMed] [Google Scholar]
- 162.Rakhila H, Carli C, Daris M, Lemyre M, Leboeuf M, Akoum A. Identification of multiple and distinct defects in prostaglandin biosynthetic pathways in eutopic and ectopic endometrium of women with endometriosis. Fertil Steril. 2013;100(6):1650–9.e1. doi: 10.1016/j.fertnstert.2013.08.016. -2. [DOI] [PubMed] [Google Scholar]
- 163.Da LC, Da BM, Donabela FC, Paro DPC, Meola J, Navarro PA. PTGS2 down-regulation in cumulus cells of infertile women with endometriosis. Reprod Biomed Online. 2017;35(4):379–86. doi: 10.1016/j.rbmo.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 164.Wei C, Mei J, Tang L, Liu Y, Li D, Li M. et al. 1-Methyl-tryptophan attenuates regulatory T cells differentiation due to the inhibition of estrogen-IDO1-MRC2 axis in endometriosis. Cell Death Dis. 2016;7(12):e2489. doi: 10.1038/cddis.2016.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li MQ, Wang Y, Chang KK, Meng YH, Liu LB, Mei J. et al. CD4+Foxp3+ regulatory T cell differentiation mediated by endometrial stromal cell-derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 2014;5:e1436. doi: 10.1038/cddis.2014.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ziegler SF. FOXP3: of mice and men. Annu Rev Immunol. 2006;24:209–26. doi: 10.1146/annurev.immunol.24.021605.090547. [DOI] [PubMed] [Google Scholar]
- 167.Mahic M, Yaqub S, Johansson CC, Tasken K, Aandahl EM. FOXP3+CD4+CD25+ adaptive regulatory T cells express cyclooxygenase-2 and suppress effector T cells by a prostaglandin E2-dependent mechanism. J Immunol. 2006;177(1):246–54. doi: 10.4049/jimmunol.177.1.246. [DOI] [PubMed] [Google Scholar]
- 168.Cobellis L, Razzi S, De Simone S, Sartini A, Fava A, Danero S. et al. The treatment with a COX-2 specific inhibitor is effective in the management of pain related to endometriosis. Eur J Obstet Gynecol Reprod Biol. 2004;116(1):100–2. doi: 10.1016/j.ejogrb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 169.Nap AW, Griffioen AW, Dunselman GA, Bouma-Ter SJ, Thijssen VL, Evers JL. et al. Antiangiogenesis therapy for endometriosis. J Clin Endocrinol Metab. 2004;89(3):1089–95. doi: 10.1210/jc.2003-031406. [DOI] [PubMed] [Google Scholar]
- 170.Machado DE, Berardo PT, Landgraf RG, Fernandes PD, Palmero C, Alves LM. et al. A selective cyclooxygenase-2 inhibitor suppresses the growth of endometriosis with an antiangiogenic effect in a rat model. Fertil Steril. 2010;93(8):2674–9. doi: 10.1016/j.fertnstert.2009.11.037. [DOI] [PubMed] [Google Scholar]
- 171.Melincovici CS, Bosca AB, Susman S, Marginean M, Mihu C, Istrate M. et al. Vascular endothelial growth factor (VEGF) - key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455–67. [PubMed] [Google Scholar]
- 172.Liang Y, Xie H, Wu J, Liu D, Yao S. Villainous role of estrogen in macrophage-nerve interaction in endometriosis. Reprod Biol Endocrinol. 2018;16(1):122. doi: 10.1186/s12958-018-0441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kilico I, Kokcu A, Kefeli M, Kandemir B. Regression of experimentally induced endometriosis with a new selective cyclooxygenase-2 enzyme inhibitor. Gynecol Obstet Invest. 2014;77(1):35–9. doi: 10.1159/000356686. [DOI] [PubMed] [Google Scholar]
- 174.Nenicu A, Gu Y, Korbel C, Menger MD, Laschke MW. Combination therapy with telmisartan and parecoxib induces regression of endometriotic lesions. Br J Pharmacol. 2017;174(16):2623–35. doi: 10.1111/bph.13874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Millar RP. GnRH II and type II GnRH receptors. Trends Endocrinol Metab. 2003;14(1):35–43. doi: 10.1016/s1043-2760(02)00016-4. [DOI] [PubMed] [Google Scholar]
- 176.Bjarnason I, Scarpignato C, Holmgren E, Olszewski M, Rainsford KD, Lanas A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology. 2018;154(3):500–14. doi: 10.1053/j.gastro.2017.10.049. [DOI] [PubMed] [Google Scholar]
- 177.Morimoto C, Osuga Y, Yano T, Takemura Y, Harada M, Hirata T. et al. GnRH II as a possible cytostatic regulator in the development of endometriosis. Hum Reprod. 2005;20(11):3212–8. doi: 10.1093/humrep/dei192. [DOI] [PubMed] [Google Scholar]
- 178.Huang F, Wang H, Zou Y, Liu Q, Cao J, Yin T. Effect of GnRH-II on the ESC proliferation, apoptosis and VEGF secretion in patients with endometriosis in vitro. Int J Clin Exp Pathol. 2013;6(11):2487–96. [PMC free article] [PubMed] [Google Scholar]
- 179.Ichioka M, Mita S, Shimizu Y, Imada K, Kiyono T, Bono Y. et al. Dienogest, a synthetic progestin, down-regulates expression of CYP19A1 and inflammatory and neuroangiogenesis factors through progesterone receptor isoforms A and B in endometriotic cells. J Steroid Biochem Mol Biol. 2015;147:103–10. doi: 10.1016/j.jsbmb.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 180.Grimm SL, Hartig SM, Edwards DP. Progesterone Receptor Signaling Mechanisms. J Mol Biol. 2016;428(19):3831–49. doi: 10.1016/j.jmb.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 181.Grandi G, Mueller M, Bersinger NA, Cagnacci A, Volpe A, McKinnon B. Does dienogest influence the inflammatory response of endometriotic cells? A systematic review. Inflamm Res. 2016;65(3):183–92. doi: 10.1007/s00011-015-0909-7. [DOI] [PubMed] [Google Scholar]
- 182.Wang XR, Hao HG, Chu L. Glycyrrhizin inhibits LPS-induced inflammatory mediator production in endometrial epithelial cells. Microb Pathog. 2017;109:110–3. doi: 10.1016/j.micpath.2017.05.032. [DOI] [PubMed] [Google Scholar]
- 183.Namavar JB, Farrokhnia F, Tanideh N, Vijayananda KP, Parsanezhad ME, Alaee S. Comparing The Effects of Glycyrrhiza glabra Root Extract, A Cyclooxygenase-2 Inhibitor (Celecoxib) and A Gonadotropin-Releasing Hormone Analog (Diphereline) in A Rat Model of Endometriosis. Int J Fertil Steril. 2019;13(1):45–50. doi: 10.22074/ijfs.2019.5446.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D. et al. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a two-year randomized, double-blind, placebo-controlled study. J Clin Endocrinol Metab. 2007;92(8):3068–75. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- 185.Yu J, Zhao L, Zhang D, Zhai D, Shen W, Bai L. et al. The effects and possible mechanisms of puerarin to treat endometriosis model rats. Evid Based Complement Alternat Med. 2015;2015:269138. doi: 10.1155/2015/269138. [DOI] [PMC free article] [PubMed] [Google Scholar]