Abstract
Bats actively adjust the acoustic features of their sonar calls to control echo information specific to a given task and environment. A previous study investigated how bats adapted their echolocation behavior when tracking a moving target in the presence of a stationary distracter at different distances and angular offsets. The use of only one distracter, however, left open the possibility that a bat could reduce the interference of the distracter by turning its head. Here, bats tracked a moving target in the presence of one or two symmetrically placed distracters to investigate adaptive echolocation behavior in a situation where vocalizing off-axis would result in increased interference from distracter echoes. Both bats reduced bandwidth and duration but increased sweep rate in more challenging distracter conditions, and surprisingly, made more head turns in the two-distracter condition compared to one, but only when distracters were placed at large angular offsets. However, for most variables examined, subjects showed distinct strategies to reduce clutter interference, either by (1) changing spectral or temporal features of their calls, or (2) producing large numbers of sonar sound groups and consistent head-turning behavior. The results suggest that individual bats can use different strategies for target tracking in cluttered environments.
I. INTRODUCTION
Insectivorous bats show great diversity in echolocation call design (Obrist, 1995; Russo and Jones, 2002; Obrist et al., 2004) and actively adjust the timing, spectro-temporal structure, and amplitude of their calls to suit the task at hand. As bats approach targets and obstacles, they reduce the duration and pulse interval of their calls to increase localization accuracy, to minimize ambiguity in pulse-echo assignment, and to obtain information at a faster rate (Griffin, 1958; Kalko and Schnitzler, 1993). Frequency modulated (FM) calls or call components produce echoes that return detailed information about target location and physical characteristics via variation in frequency content (Simmons et al., 1975; Simmons and Stein, 1980).
Bats can adjust the frequency content of calls to avoid signal jamming by conspecifics (Gillam et al., 2007; Bates et al., 2008; Chiu et al., 2009) or to resolve pulse-echo assignment ambiguities (Hiryu et al., 2010). Additionally, bats can change the power spectrum of their calls by apportioning more energy to certain frequencies or harmonics (Hartley and Suthers, 1989; Jakobsen and Surlykke, 2010). This ability to dynamically modify call parameters allows bats to orient in complex environments, which may contain conspecifics, obstacles, and extraneous sounds (Obrist, 1995; Moss and Surlykke, 2010; Jakobsen et al., 2013) from other bats (Ulanovsky et al., 2004) and/or insects (Fullard et al., 1994; Corcoran et al., 2009).
In addition to adjusting its sonar calls, a bat may employ behavioral strategies, such as head turning, to improve detection or localization of targets. Turning the head directly influences the directional aim of the sonar transmission (Ghose and Moss, 2003) and, consequently, echo information carried to the auditory receiver (Aytekin et al., 2004). The width of a bat's sonar beam varies with sound frequency and mouth gape (Jakobsen and Surlykke, 2010; Jakobsen et al., 2013; Kounitsky et al., 2015). Centering the sonar beam on a target may assist the bat in defocusing non-target objects that are off-axis (Bates et al., 2011; Simmons, 2014). Bats also alternate the direction of their sonar beam, which enables them to simultaneously track objects of interest while planning their flight trajectories through obstacles (Surlykke et al., 2009) or towards the next target (Fujioka et al., 2014).
In a cluttered environment, echoes from non-target objects can mask the target through echo-echo overlap, depending on the number and direction of competing echo sources or maskers (Langendijk et al., 2001; Warnecke et al., 2014) or their angular offset (Sümer et al., 2009). Regions in which echoes from non-target objects interfere with target detection have been experimentally determined to be wider at greater distances (Simmons et al., 1988). Bats that use FM calls respond to clutter by producing groups of echolocation calls, referred to as sonar sound groups (SSGs), which consist of two or more pulses close together, flanked by longer intervals (Moss et al., 2006). The bat's alternation between short and long pulse intervals likely facilitates echo assignment (Moss and Surlykke, 2001; Hiryu et al., 2010; Melcón et al., 2011) and may also allow bats to multitask, inspecting close objects while monitoring the greater environment for trajectory planning (Petrites et al., 2009). SSG production is higher when bats attack a moving vs stationary target (Hulgard and Ratcliffe, 2016) or when a target moves erratically instead of predictably (Kothari et al., 2014).
While most bats echolocate for spatial orientation in the environment, those that hunt moving prey must be especially adept at processing echoes quickly in order to inform rapid motor decisions to capture erratically moving targets. The big brown bat (Eptesicus fuscus) is an aerial insectivore that hunts in both open areas and edge spaces where it encounters clutter from foliage, making it a model for studying adaptive adjustments to echolocation calls in different environmental contexts. It can detect and localize target echoes in clutter (Simmons et al., 1989; Aytekin et al., 2010) and discriminate between objects using shape (Griffin et al., 1965; Simmons and Chen, 1989) and surface texture (Falk et al., 2011). In clutter, Eptesicus apportions more power to higher harmonics relative to the fundamental (Sümer et al., 2009; Aytekin et al., 2010), which may allow better separation of target and clutter echoes.
In a previous experiment, Aytekin et al. (2010) examined how the big brown bat adapts its echolocation signals from a resting position in a target tracking task in the presence of a single “distracter” object (a metal pole positioned vertically to one side of the target motion axis). Because the distracter was placed on only one side of the target motion path, it was unclear whether the bats reduced masking echoes from the distracter by moving their head or ears off-axis.
The present study reports on echolocation behavior in big brown bats tracking a target in the presence of one or two distracters. Specifically, we predicted that the bats would reduce call duration and increase bandwidth, sweep rate, and peak frequency when the distracters were close and the angular offset was small. Due to the increased interference created by the addition of a second distracter, we also predicted adjustments in call intervals, with higher SSG production in the two-distracter condition, as well as when the distracters were placed closer to the bat or at small angular offsets from the target. Because turning the head off-axis from the target in the presence of two symmetrically placed distracters would increase clutter interference, we predicted that head turns would be more prevalent in the one-distracter condition.
II. METHODS
A. Animals
Big brown bats (Eptesicus fuscus) were wild-caught in Maryland under a permit from the Department of Natural Resources. Bats were fed mealworms (Tenebrio molitor) only during training and experimental sessions for appetitive motivation, with supplemental feeding provided on non-training days or if daily weight monitoring indicated weight loss beyond minor fluctuations (>5% average weight). Training was initiated with four bats; however, two of these animals became ill or died in the course of the experiment, and complete data sets were obtained for only two animals, Bat 45 and Bat 49. All housing and experiments were conducted with the approval of the University of Maryland Institutional Animal Care and Use Committee.
B. Experimental setup
The experimental setup followed Aytekin et al. (2010). A cable, running along a four-pulley system, was attached to a motorized forcer that slid along a rail to change the position of the target, which the bat tracked from a resting position. An optical sensor (USDIGITAL, EM1-0-200, US Digital, Vancouver, WA) and linear transmissive strip (USDIGITAL, LIN-200-0.5-N) were used to record target distance as the forcer moved. To muffle the sound of the motor, a wooden casement lined with acoustic foam was placed around the rail. The forcer's motion was controlled and recorded by a computer using custom software written in Matlab-2007b. Two microphones used to record echolocation calls were placed at a distance of 2.8 m from the bat, behind, and on either side of, the pulley apparatus.
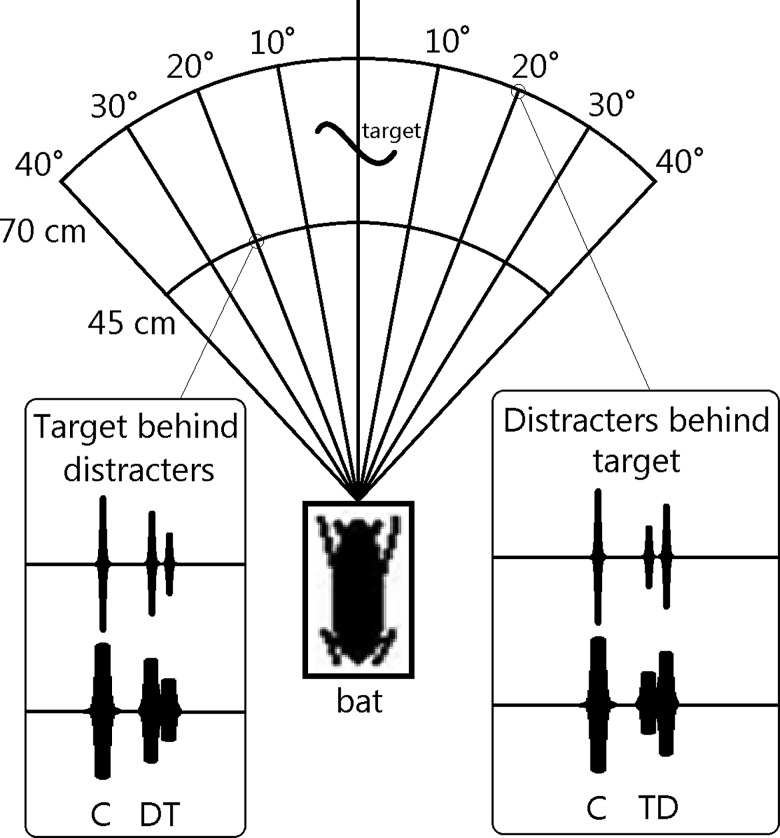
The target was attached to the cable with fishing line and stabilized with an extra loop tethered on both sides of the line to prevent excessive swinging at the beginning and end of programmed movements. For distracter conditions, either one or two distracters were placed at different distances (45 and 70 cm) and angular offsets (10°, 20°, 30°, and 40°) from the platform (Fig. 1). Distracter positions were changed between but not within test days. Each distracter object consisted of a 1.27 cm wide metal rod attached to a baseboard by a flange. Distracters were always placed at the same location on either side of the target motion axis, resulting in a symmetrical arrangement. In the baseline condition, no distracter objects were present. Two sets of baseline data were obtained, one before each clutter experiment. They will be referred to as “baseline 1” and “baseline 2,” respectively.
FIG. 1.
Overhead view of experimental setup showing distracter distances and angular offsets (not to scale). Inset diagrams show a call (C), distracter echo (D), and target echo (T) when the target is behind or in front of the distracters at two example distracter positions. When calls are short (top), no overlap of echoes occurs, whereas distracter and target echoes do overlap when the call is long (bottom).
C. Animal training
Bats were trained to sit on a platform in an anechoic room illuminated with low-level, long wavelength light. They were conditioned to associate the initiation of a trial with a sound produced by a clicker. After the presentation of a click, the motor-driven pulley system was used to deliver the target to the bat. During training, the target's initial distance from the platform and its delivery speed were gradually increased until it reached 170 cm and 1.27 m/s, respectively. The two-distracter data presented here were gathered with no additional training beyond that reported in Aytekin et al. (2010). As in the one-distracter experiment, probe trials consisting of a change in the normal pattern of target delivery were interspersed randomly each day of data collection to check for and maintain active engagement in the task.
Data from the one-distracter experiment were obtained in the fall of 2009, after which the bats were given an 8 week break due to metabolic and behavioral changes related to hibernation. We ceased testing during this period to avoid collecting data that could potentially be affected by physiological state, and also to avoid stressing the animals. Testing resumed with the two-distracter experiment in February 2010. A total of 48 768 calls from 936 trials from the one- and two-distracter experiments were examined (Supplemental Table I1), although only one call per trial was used in statistical tests on acoustic parameters (see Sec. II F).
D. Audio recordings and sonar call analysis
Each bat's echolocation calls were recorded with two microphones (Ultrasound Advice), amplified (Ultrasound Advice, SM3), bandpass filtered from 10 to 100 kHz (Krone-Hite 3550), and converted from analog to digital (National Instruments, PCI-6071E). Identification, measurement, and analysis of calls were performed using custom programs written in Matlab, versions 2007b–2015a (see Aytekin et al., 2010 for details). Trials in which the bats did not appear to be engaged in the task (e.g., trials in which the pulse interval pattern did not decrease monotonically, or in which the bat emitted only a few echolocation calls) were excluded from analyses. Additionally, because the bats would sometimes anticipate the arrival of the target by jumping or lunging forward, causing their close-range echolocation calls to differ from the typical pre-capture pattern, we excluded calls that occurred in the final 15 cm of the target's approach to the bat.
For the analysis of temporal variables, calls that were extreme outliers (duration >5 ms or pulse interval >150 ms) were excluded from analysis. Temporal measurements were manually checked and corrected if necessary. Peak frequencies for the fundamental and first harmonic were measured from power spectra of each component. Calls that had durations of less than 1.33 ms were excluded from spectral analyses, because the signal-to-noise ratio of these signals was low and frequency measurements were less reliable. While call intensity was likely adjusted by the bats as part of their strategy to ameliorate echo clutter, we do not report on absolute or relative intensity levels, because microphones were not calibrated, and sensitivity settings changed day-to-day, along with changes in distracter positions.
We characterized SSGs as call clusters with surrounding pulse intervals at least 20% longer than those within the SSG. If three or more calls occurred in a group, an additional criterion of stable pulse intervals (±5%) was applied (see Moss and Surlykke, 2001; Moss et al., 2006). We counted the number of SSGs (doublets, triplets, and quadruplets) in each trial for all conditions.
E. Head turns
To measure head turns as the bats tracked the approaching target, we compared the relative amplitude of echolocation signals picked up by the two microphones positioned to the left and right of the bat. First, the relative amplitude ratio (RAR) was calculated as the ratio between the raw amplitudes of channels 1 and 2, corresponding to right and left floor microphones, respectively. We then subtracted each call's RAR from the close mean ratio for that trial, which was calculated as the average RAR from calls in that trial occurring in the last 5 cm (at target distances of 15–20 cm), when the influence of the distracter(s) was predicted to be minimal and the bat was expected to be vocalizing straight ahead. Calls that were overloaded on both floor microphones were excluded from analysis.
Head turns were counted when consecutive relative amplitude ratio difference (RARD) values in the reduced data set changed from negative to positive or positive to negative, indicating a switch in head direction across the target motion axis. While we do not have video to validate this method, we set a conservative threshold RARD value of ±0.3 to ensure that small deviations of head direction across the target approach axis would not be counted, although this may have resulted in under-counting of head turns. Calls with RARD values below this threshold were eliminated, as were calls with RARD values exceeding ±1, which were not considered reliable.
F. Statistical analysis
To assess the relative importance of the number and location of distracters on bat echolocation behavior we measured eight response variables: two behavioral counts (number of SSGs and number of head turns) from each trial and six acoustic parameters (call duration, pulse interval, bandwidth, and sweep rate of the fundamental, fundamental peak frequency, first harmonic peak frequency) for a single call from each trial when the target distance was at 70 ± 2.5 cm. We adjusted duration and pulse interval measurements by subtracting means for each bat in the absence of any distracters to control for slight differences in baseline values across the one- and two-distracter experiments. SSG and head turn counts were similarly adjusted by subtracting mean baseline values from each trial.
All response variables were then fit to general linear models (GLMs) through a backward stepwise procedure using least squares. Distracter distance (45 or 70 cm), distracter number (one or two), and bat identity (#45 or #49) were categorized as nominal effects while angular offset, which was measured in degrees, was classified as a continuous covariate. We used the minimum Bayesian Information Criterion to select the best model among all possible models. All statistical analyses were performed in JMP 12.1.0.
III. RESULTS
A. Call duration and pulse interval
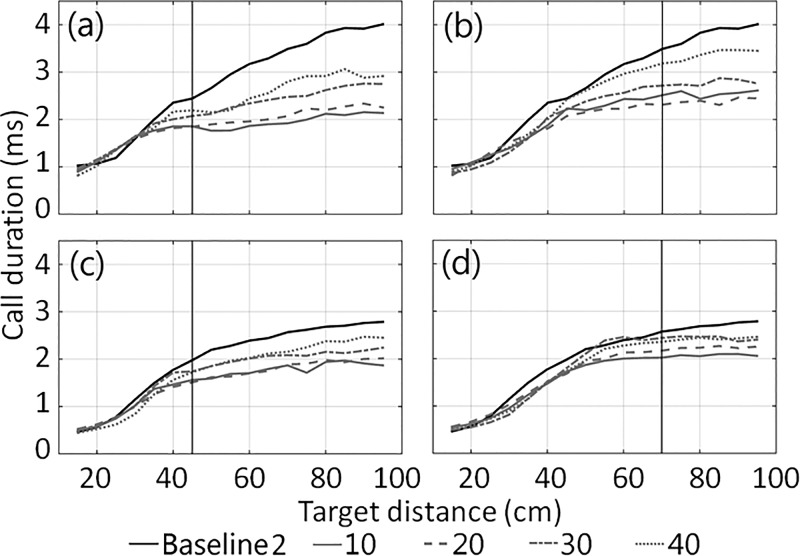
Both bats decreased call duration as the target approached in all conditions (Fig. 2). At target distances beyond 20–30 cm both bats produced shorter duration calls when the distracters were present, when they were placed at the closer distance of 45 cm, and when they were placed at smaller angular offsets (Fig. 2). Once the target had passed the distracters and was within 20–30 cm of the platform, all duration vs target distance profiles for the two-distracter condition converged on baseline levels. For the 45 cm distracter distance, convergence of call duration profiles occurred when the target was at the distracter distance or just after it passed the distracters, while for the 70 cm distracter distance, call duration did not converge to baseline levels until the target was in front of the distracters (∼25 cm in front of the bat). Both bats also tended to use shorter pulse intervals beyond target distances of 40–45 cm when the distracters were present (Supplemental Fig. 11).
FIG. 2.
Average call duration plotted against target distance for Bat 45 [(a) and (b)] and Bat 49 [(c) and (d)] in the two-distracter condition when the distracters were located at 45 cm [(a) and (c)] and 70 cm [(b) and (d)]. Averages were calculated across trials within 5 cm bins. Baseline 2 is reproduced across both distracter distances for comparison. Distracter distance is shown as a vertical black line.
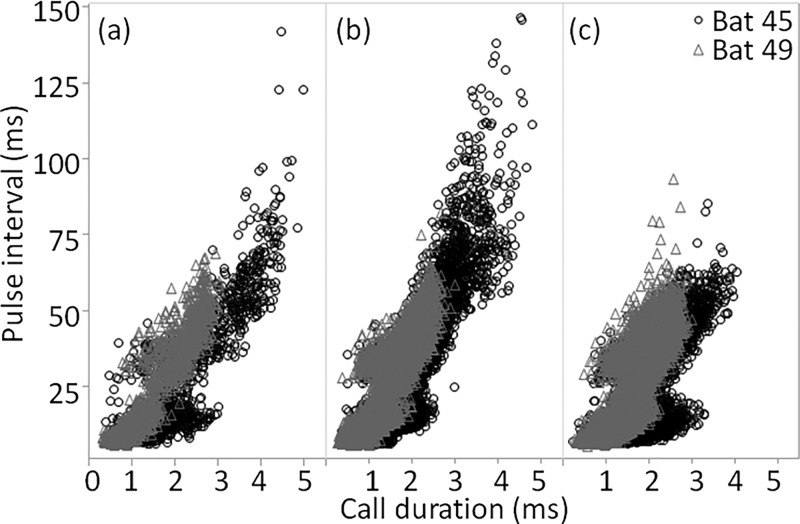
The two bats differed in the range of call durations and pulse intervals they used in the baseline condition, with Bat 45 using shorter duration calls than Bat 49, from 4 ms at a target distance of 100 cm down to 1 ms at 15 cm, compared to 2.8 ms down to 0.5 ms for Bat 49 (Fig. 2). Bat 45 also used shorter pulse intervals than Bat 49, ranging from 60 ms at 100 cm down to 10 ms at 15 cm, compared to 50 ms down to 7 ms for Bat 49 (Supplemental Fig. 11). Scatter plots of call duration against previous pulse interval show that Bat 49 had less variability in its calls than Bat 45 regardless of the number of distracters presented, and differences in pulse interval were smaller than differences in call duration between the bats in the two-distracter condition (Fig. 3).
FIG. 3.
Pulse interval plotted against duration for all calls except outliers (duration >5 ms or pulse interval >150 ms) recorded in the baseline (a), one-distracter (b), and two-distracter (c) conditions.
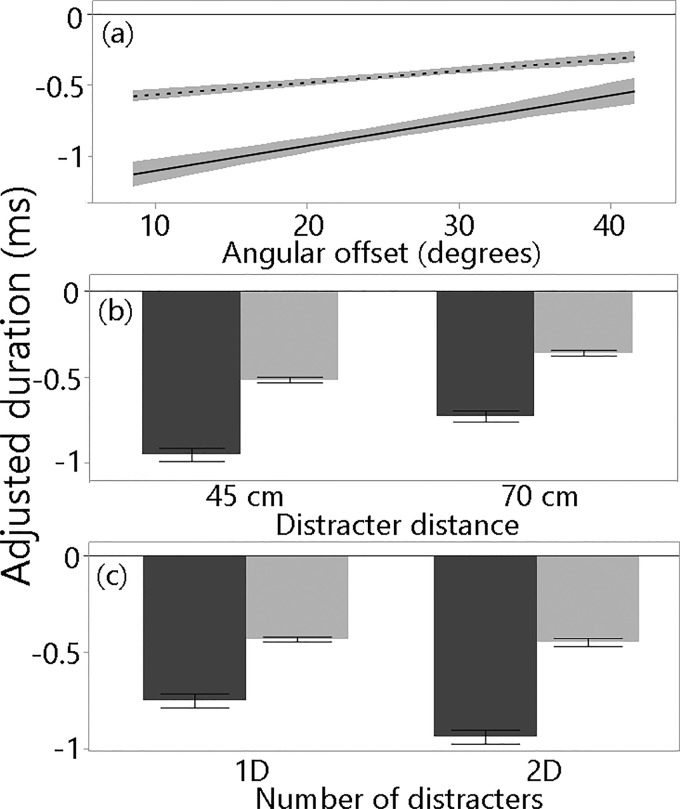
The analysis of baseline-adjusted durations for calls produced when the target was near 70 cm revealed that both bats used shorter calls when distracters were present (Fig. 4). The GLM explained nearly half of the variance in call duration, and included every experimental factor examined [angular offset, distracter distance, and number of distracters (Table I)]. The largest source of variation in call duration was bat identity, with Bat 45 reducing its call duration more across distracter conditions than Bat 49 (Fig. 4). Angular offset was the second largest source of variation, with both bats using shorter durations as angular offset decreased, but the bat identity by angular offset interaction was significant, reflecting the steeper slope of adjustment exhibited by Bat 45 [Fig. 4(a)]. Both bats reduced their call durations more when the distracter distance was 45 cm as compared to 70 cm [Fig. 4(b)]. The effect of the number of distracters also significantly differed between the bats: Bat 49 used similar call durations regardless of the number of distracters present, while Bat 45 used shorter calls when two distracters were presented [Fig. 4(c)].
FIG. 4.
(a) Lines of fit (with confidence intervals) through adjusted duration for one call per trial made when the target distance was 70 cm (±2.5 cm), relative to baseline, across angular offsets. (b) Means and standard errors of adjusted duration for one call per trial made when the target distance was 70 cm (±2.5 cm) from the platform when the distracter distance for was 45 cm or 70 cm, and (c) in the one- and two-distracter conditions.
TABLE I.
Best GLMs for five parameters of echolocation calls emitted when the target distance was near 70 cm, adjusted by baseline means. Interaction effects are denoted by an asterisk between factors. F values are given for all factors included in each model, with significance level indicated by asterisks (* = p ≤ 0.01, ** = p ≤ 0.001, *** = p ≤ 0.0001). Factors not included in the final model for a given parameter are denoted with a dash (—), and overall model statistics are given at the bottom.
| Source | Call duration | Pulse interval | Peak frequency (fundamental) | Peak frequency (first harmonic) | Bandwidth | Sweep rate |
|---|---|---|---|---|---|---|
| Bat | 314.3*** | — | 1535.0*** | 169.0*** | 13.5** | 116.3*** |
| Angular offset | 161.1*** | 10.2* | 59.0*** | 24.5*** | 73.4*** | 192.5*** |
| Distracter distance | 66.8*** | 18.2*** | — | 14.8*** | 3.2 | 124.4*** |
| Number of distracters | 31.2*** | — | 213.5*** | 115.3*** | 51.1*** | 3.4 |
| Bat*Angular offset | 19.2*** | — | 19.3*** | 0 | 17.5*** | 61.7*** |
| Bat*Distracter distance | — | — | — | 5.8 | 18.1*** | 3.9 |
| Bat*Number of distracters | 11.1** | — | 32.7*** | 20.7*** | — | 26.6*** |
| Angular offset*Distracter distance | — | — | — | 4.8 | 0.0 | 17.9*** |
| Angular offset*Number of distracters | 15.0*** | — | — | — | 23.0*** | 11.2** |
| Distracter distance*Number of distracters | 9.9* | — | — | 7.9* | 34.1*** | — |
| Bat*Angular offset*Distracter distance | — | — | — | 24.7*** | 16.5*** | 12.5** |
| Bat*Angular offset*Number of distracters | — | — | — | — | — | — |
| Bat*Distracter distance*Number of distracters | — | — | — | — | — | — |
| Angular offset*Distracter distance*Number of distracters | — | — | — | — | — | — |
| Model n | 664 | 664 | 879 | 879 | 879 | 879 |
| Model R2 | 0.47 | 0.04 | 0.67 | 0.29 | 0.22 | 0.38 |
| Model F | 73.6*** | 13.7*** | 356.1*** | 37.8*** | 25.3*** | 53.8*** |
In contrast to call duration, pulse interval changed very little across experimental conditions (Supplemental Fig. 11). Notably, the GLM for pulse intervals of calls made near a target distance of 70 cm included just two factors, did not include bat identity, and explained only 4% of the variation in pulse interval (Table I). Both bats used shorter pulse intervals when angular offset was small [Supplemental Fig. 2(a)1] and produced shorter pulse intervals when the distracter was at 45 cm, but the difference between distracter distances was more pronounced for Bat 45 [Supplemental Fig. 2(b)1].
B. Peak call frequencies
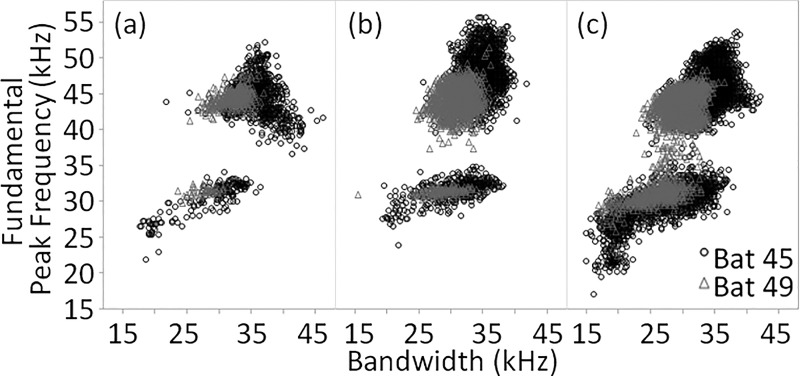
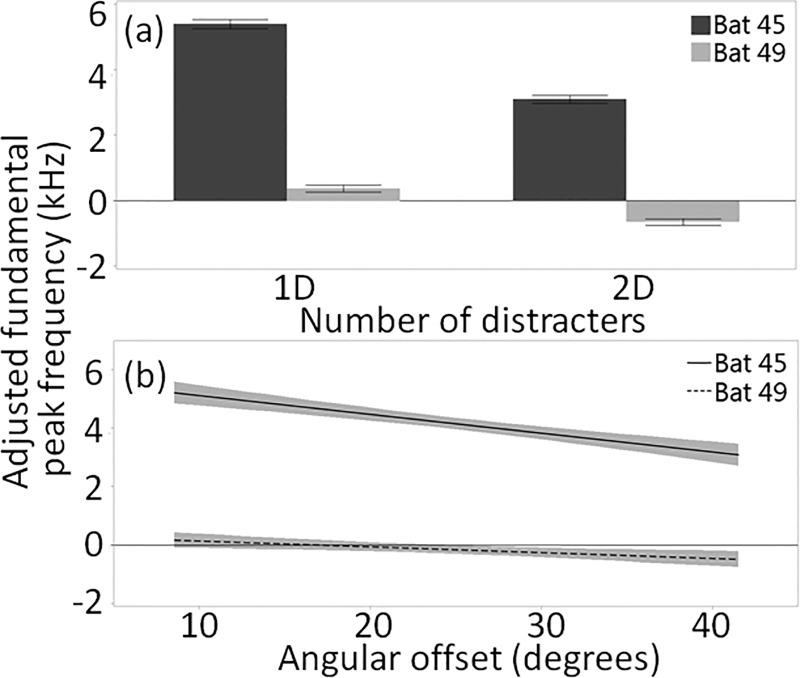
Bat identity explained the most variation in fundamental peak frequency and contributed heavily to the model's explanatory power (R2 = 0.67, Table I). Bat 45 exhibited more variability in fundamental peak frequency than Bat 49 in response to clutter (Fig. 5). Bat 45 also used higher fundamental peak frequencies in distracter conditions relative to baseline while Bat 49 made only slight changes to fundamental peak frequency in distracter conditions relative to baseline [Fig. 6(a)] and across angular offsets [Fig. 6(b); Supplemental Fig. 3(a)1]. These differences are reflected in the significance of two interaction effects (bat identity by number of distracters, and bat identity by angular offset) in the GLM for fundamental peak frequency.
FIG. 5.
Fundamental peak frequency plotted against bandwidth for all calls for which frequency could be reliably estimated (duration <1.33 ms) recorded in the baseline (a), one-distracter (b), and two-distracter (c) conditions.
FIG. 6.
(a) Means and standard errors of adjusted fundamental peak frequency for one call per trial made when the target distance was 70 cm (±2.5 cm) by the number of distracters. (b) Lines of fit (with confidence intervals) to adjusted fundamental peak frequency of one call per trial made when the target distance was 70 cm (±2.5 cm), relative to baseline, across angular offsets.
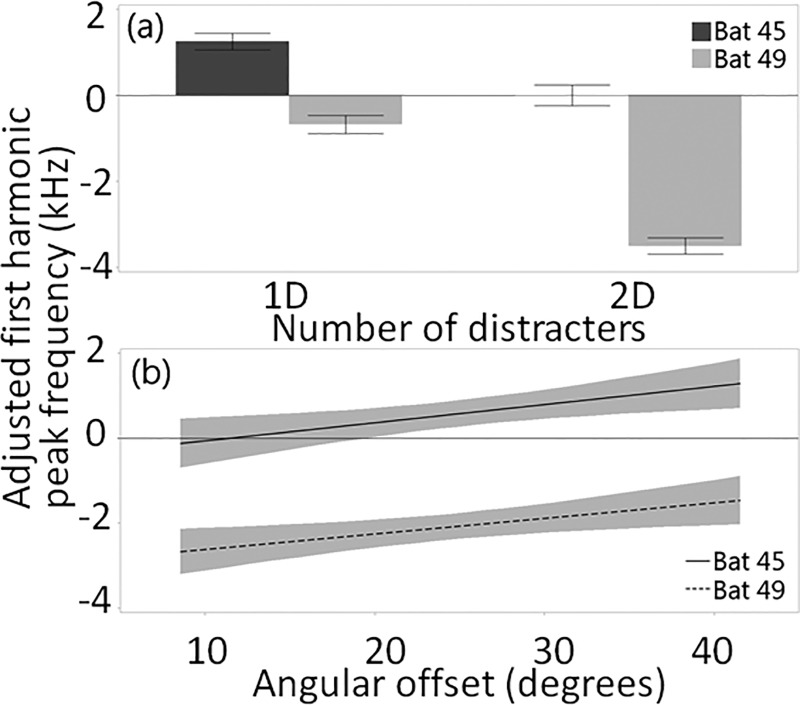
As with peak frequency of the fundamental, bat identity explained the most variation in first harmonic peak frequency, followed by the number of distracters. But by contrast, Bat 49 lowered its first harmonic peak frequency in distracter conditions, especially when two distracters were present, while Bat 45 did not change its first harmonic frequency relative to baseline in the two-distracter condition [Supplemental Fig. 3(b)1], although it did slightly increase it in the one-distracter condition [Fig. 7(a)]. Accordingly, the bat identity by the number of distracters interaction effect was significant in the model (Table I). Bat 49 used lower first harmonic peak frequencies at small angular offsets, and Bat 45 showed little difference from baseline unless angular offset was large [Fig. 7(b)].
FIG. 7.
(a) Means and standard errors of adjusted first harmonic peak for one call per trial made when the target distance was 70 cm (±2.5 cm) by the number of distracters. (b) Lines of fit (with confidence intervals) to adjusted first harmonic peak frequency of one call per trial made when the target distance was 70 cm (±2.5 cm), relative to baseline, across angular offsets.
C. Bandwidth and sweep rate
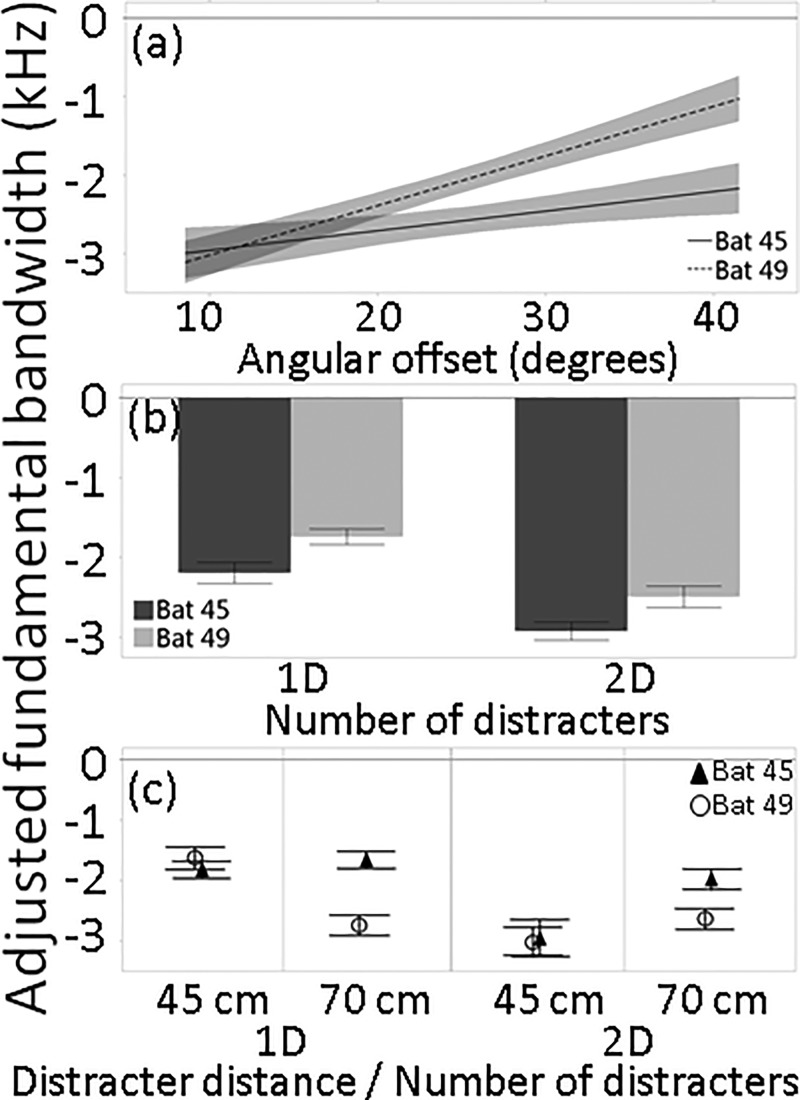
Both bats lowered bandwidth when distracters were present, particularly at low angular offsets [Fig. 8(a)] and when two distracters were present [Fig. 8(b)]. These were the two largest sources of variation in the GLM (R2 = 0.22, Table I). As with peak frequency, Bat 45 exhibited more variability in bandwidth than Bat 49 in response to clutter (Fig. 5). Changes to bandwidth were generally larger for Bat 45, and while differences between bats were clear at the 70 cm distracter distance, they virtually disappeared when the distracter distance was 45 cm [Fig. 8(c)]. Accordingly, the number of distracters by distracter distance interaction effect was the third largest source of variation in bandwidth (Table I).
FIG. 8.
(a) Lines of fit (with confidence intervals) to adjusted fundamental bandwidth of one call per trial made when the target distance was 70 cm (±2.5 cm), relative to baseline, by the number of distracters and across angular offsets. (b) Means and standard errors of adjusted fundamental bandwidth for one call per trial made when the target distance was 70 cm (±2.5 cm) by number of distracters and (c) by distracter distance and number of distracters.
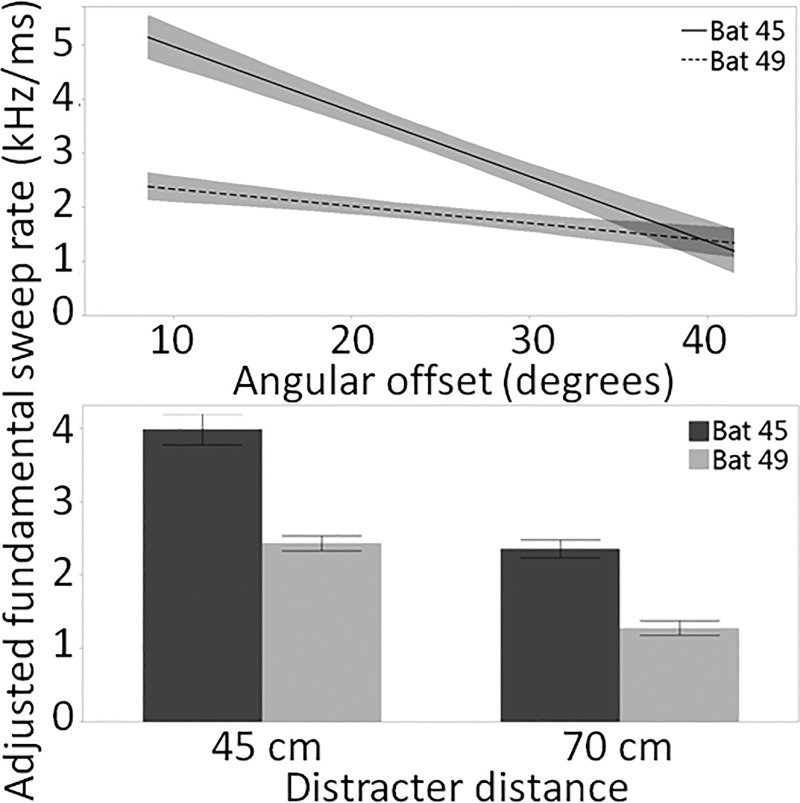
Sweep rate increased when angular offset was low [Fig. 9(a)] and when the distracter distance was 45 cm [Fig. 9(b)]. As with bandwidth, angular offset explained the most variation in the model for sweep rate (R2 = 0.38), followed by distracter distance, bat identity, and the bat identity by angular offset interaction effect. Interestingly, the difference between bats was less pronounced in the two-distracter condition than the one-distracter condition [Supplemental Fig. 4(a)1] and the bats appeared to more drastically increase sweep rate with decreasing angular offset when the distracter distance was 45 cm [Supplemental Fig. 4(b)1], as reflected in the significance of these two-way interactions in the GLM (Table I).
FIG. 9.
(a) Lines of fit (with confidence intervals) to adjusted fundamental sweep rate of one call per trial made when the target distance was 70 cm (±2.5 cm), relative to baseline, across angular offsets. (b) Means and standard errors of adjusted fundamental sweep rate for one call per trial made when the target distance was 70 cm (±2.5 cm) by distracter distance.
D. SSGs
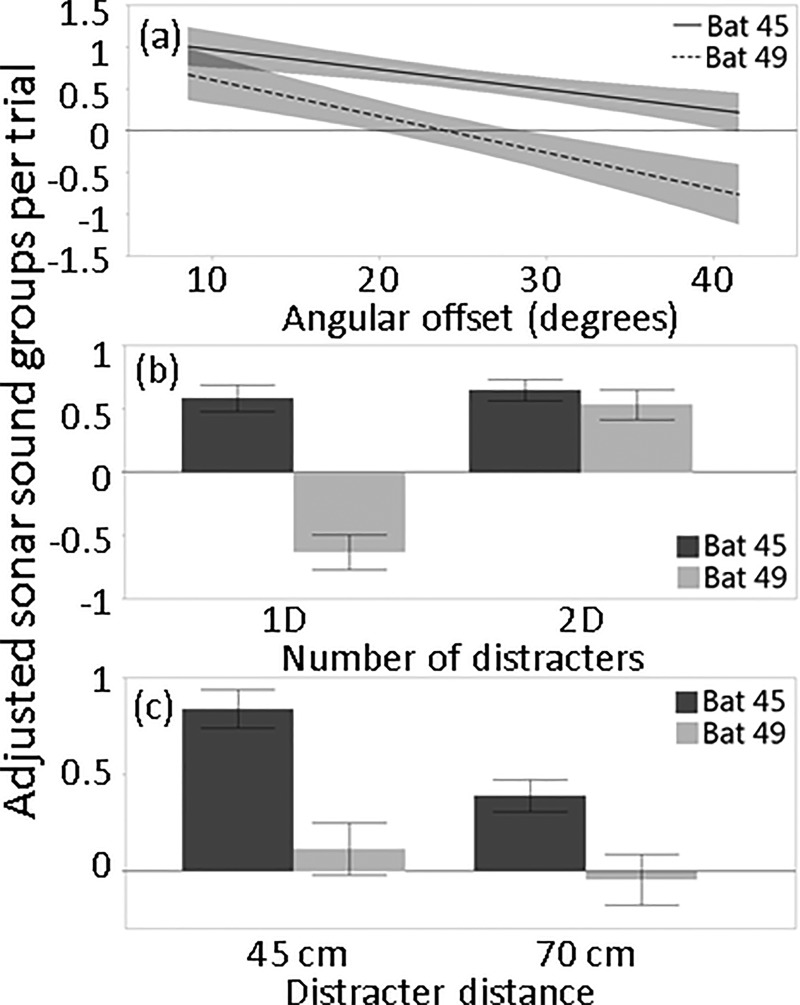
Both bats produced more SSGs with decreasing angular offset [Fig. 10(a)] and when the distracter distance was 45 cm as opposed to 70 cm [Fig. 10(c)]. The number of distracters also influenced adjusted SSGs per trial but this effect differed by bat, with Bat 49 using fewer SSGs in the one-distracter condition and Bat 45 using more SSGs regardless of how many distracters were present [Fig. 10(b)]. This was reflected in the significance of the bat identity by the number of distracters interaction term in the model (Table II). While Bat 45's adjusted mean SSGs per trial were higher than Bat 49's in all conditions, Bat 49 produced more SSGs overall and in every experimental condition compared to Bat 45 (Supplemental Fig. 51). This was due at least in part to Bat 49's abnormally high use of SSGs in baseline 1, which exceeded all conditions except the 10° angular offset [Supplemental Fig. 5(a)1].
FIG. 10.
(a) Lines of fit (with confidence intervals) through number of SSGs per trial across angular offsets, adjusted by mean baseline SSGs per trial. (b) Means and standard errors of adjusted SSGs per trial by the number of distracters present, and (c) distracter distance.
TABLE II.
Best GLMs for per-trial totals of two behavioral parameters, adjusted by baseline means. Interaction effects are denoted by an asterisk between factors. F values are given for all factors included in each model, with significance level indicated by asterisks (* = p ≤ 0.01, ** = p ≤ 0.001, *** = p ≤ 0.0001). Factors not included in the final model for a given parameter are denoted with a dash (—), and overall model statistics are given at the bottom.
| Source | SSGs | Head turns |
|---|---|---|
| Bat | 46.0*** | 4.0 |
| Angular offset | 59.4*** | 6.0 |
| Distracter distance | 8.6* | 20.0*** |
| Number of distracters | 47.2*** | 37.83*** |
| Bat*Angular offset | 9.0* | — |
| Bat*Distracter distance | — | — |
| Bat*Number of distracters | 34.8*** | 19.2*** |
| Angular offset*Distracter distance | — | — |
| Angular offset*Number of distracters | — | 7.9* |
| Distracter distance*Number of distracters | 11.8** | — |
| Bat*Angular offset*Distracter distance | — | — |
| Bat*Angular offset*Number of distracters | — | — |
| Model n | 857 | 857 |
| Model R2 | 0.17 | 0.10 |
| Model F | 26.0*** | 16.3*** |
The bats differed most markedly in their use of doublets (SSGs consisting of two calls), and, as with total SSGs, all three types generally increased at low angular offsets (Supplemental Fig. 61). Overall, it appears that the bats differed in their overall use of SSGs, but both bats changed their production of SSGs similarly with angular offset and distracter distance. However, the effect of number of distracters on SSG production is unclear, and the GLM explained relatively little of the variation in SSGs (R2 = 0.17).
E. Head turns
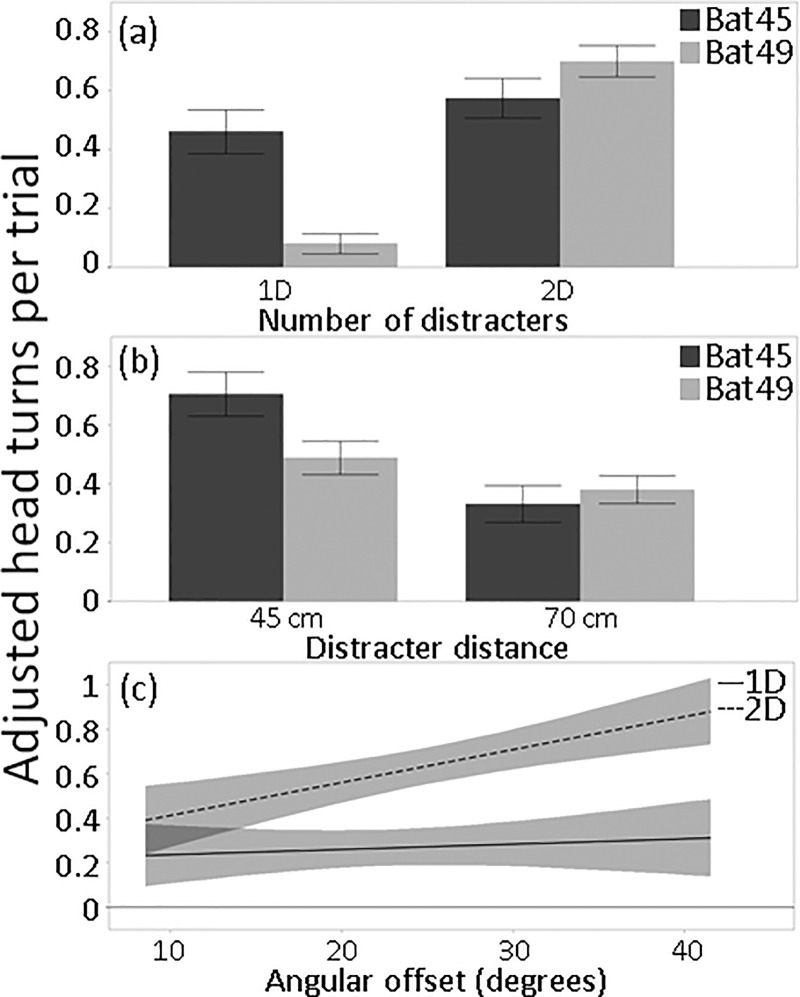
Head turns, as measured by our criteria, were generally infrequent—the highest average adjusted to baseline was fewer than one per trial—and the GLM explained only 10% of the variance in head turns (Table II). Nevertheless, the presence of distracters clearly influenced head movements given that both bats used more head turns in the two-distracter condition than in the one-distracter condition. Bat 49 showed a larger increase in head turns between the one- and two-distracter conditions relative to baseline than Bat 45 [Fig. 11(a)]. It is noteworthy that Bat 45's mean head turns in the one-distracter condition were inflated by an abnormally high number produced at the 20° angular offset [Supplemental Fig. 7(a)1]. The GLM accordingly showed that the bat by number of distracters term was significant (Table II). Both bats also turned their heads more frequently when the distracter was at 45 cm [Fig. 11(b)]. The interaction between angular offset and number of distracters was significant, and showed that more head turns occurred when the angular offset was high, but only in the two-distracter condition [Fig. 11(c)]. RARDs plotted against target distance showed that head turns were prominent and consistent in most trials for Bat 49, but less so for Bat 45, and appeared to be initiated just after the target passed the distracter(s) (Supplemental Fig. 81). The number of distracters, distracter distance, and the interaction between the bat and number of distracters accounted for most of the variation in adjusted head turns while the interaction between angular offset and number of distracters was less influential (Table II).
FIG. 11.
Means and standard errors of head turns, adjusted to baseline, by (a) number of distracters present and (b) distracter distance. (c) Lines of fit (with confidence intervals) to adjusted head turns relative to baseline by angular offset and number of distracters.
IV. DISCUSSION
This study investigated how big brown bats adjust their echolocation behavior when tracking a moving target in a cluttered environment, with differing levels of clutter interference created by distracter objects placed at different distances and angular offsets from the bat. Analysis of the temporal and spectral variation in the calls and head movements with a series of GLMs provides compelling evidence that these bats used different strategies for target tracking in clutter.
A. Effect of distracters on call duration and pulse interval
When the target was close (≤30 cm from the platform), both bats produced calls that were very similar in duration between two-distracter and baseline conditions (Fig. 2), illustrating that the distracters no longer influenced call duration, despite the distracters' large reflecting surfaces compared with the target (1.27 cm compared to 0.38 cm). Both bats used shorter calls when acoustic interference from the distracter echoes was greatest, i.e., the distracter distance was close or angular offset was small. Additionally, the bats showed less change in call duration as the target approached when the distracters were at small angular offsets (Fig. 2). These findings are consistent with earlier reports that bats adjust call duration primarily in response to the nearest object (Aytekin et al., 2010). By using shorter call durations, bats reduce the potential for echo-echo overlap. If target and non-target objects are sufficiently close, such that returning echoes overlap in time, the neural representations of the objects may merge, causing clutter interference (Simmons et al., 1989).
Consistent with a previous study (Aytekin et al., 2010), call durations were influenced by the distracters for a period after the target had passed the distracters, and this zone was larger when the distracter was at 70 cm than at 45 cm (Fig. 2). This may reflect range-dependence in the size of clutter interference zones. In a phantom target echo detection task, Simmons et al. (1988) reported that the size of the clutter interference zone in Eptesicus fuscus increases with range, suggesting that the spatial region over which clutter and target echoes interfere scales with distance. These zones are created by forward, simultaneous, and backward masking, as the target is first behind, then near, then in front of, the distracter object(s) (Simmons et al., 1988).
Both bats systematically shortened pulse interval with target distance, regardless of distracter condition, suggesting that they could track the moving target even when it was behind the distracter(s). For all angular offsets, in both the one- and two-distracter conditions, pulse interval changed with target distance until the target was very close to the bat (∼20 cm), suggesting that the distracters influenced the timing of calls, even well after the target had passed the distracter(s).
The bats exhibited consistent differences in call duration and pulse interval (Fig. 3). Bat 45 used shorter duration calls at small angular offsets, at the distracter distance of 45 cm, and when two distracters were present, while Bat 49 changed its call durations relatively little across distracter conditions (Fig. 4). Similarly, Bat 49 changed its pulse intervals very little across angular offsets and between distracter distances, while Bat 45 clearly reduced its pulse intervals in these conditions (Supplemental Fig. 21). That the bats differed in the temporal parameters of their calls under different distracter conditions reveals that adjustments in sonar call duration and pulse interval differ among individual bats.
B. Effect of distracters on peak frequency
We predicted that the bats would increase the peak frequency of echolocation calls when two distracters were present and at small angular offsets, to sharpen the sonar images created by more directional sonar information carried by higher frequencies. The bats showed opposite patterns of adjustment in peak frequency, with one subject changing only the peak frequency of the fundamental, and the other changing only the peak frequency of the first harmonic (Supplemental Fig. 31).
Counter to our prediction, the bat that made its calls more directional by increasing peak frequency did so in the one-distracter condition and not the two-distracter condition [Supplemental Fig. 3(a)1]. Even at the largest angular distracter offsets, neither bat could have avoided ensonifying the distracters entirely by narrowing their sonar beams (Hartley and Suthers, 1989), and it is therefore likely that additional strategies allowed the bat to disambiguate echo streams from objects in a cluttered environment (Bates et al., 2011; Simmons, 2014; Wohlgemuth et al., 2016).
C. Effect of distracters on bandwidth and sweep rate
We expected that the bats would produce calls with higher bandwidth and sweep rate as the distracter number and position created more echo clutter, to sharpen the target image and improve localization accuracy. However, the fundamental bandwidth of calls made when the target was near 70 cm was consistently lower in distracter conditions than in baseline. Although adjusted bandwidth decreased at small angular offsets, adjusted sweep rate (calculated as bandwidth divided by call duration) increased, indicating that higher sweep rates were achieved through reductions in call duration.
Interestingly, the second-largest source of variation identified in the GLM for adjusted bandwidth was the number of distracters, which was not significant in the GLM for adjusted sweep rate. Similarly, the second-largest source of variation in adjusted sweep rate was distracter distance, which was not significant in the GLM for adjusted bandwidth. This result lends support to the assertion made by Boonman and Ostwald (2007) that broader bandwidth calls helps bats correctly count the number of echoes (which would change depending on the number of distracters present) while higher sweep rates help with accuracy of distance estimates based on echo delays (which would change depending on distracter distance).
Additionally, while bat identity was the third largest source of variation in adjusted sweep rate, sweep rates of both bats converged as the angular offset became smaller when there were two distracters present or when the distracter distance was 45 cm (Supplemental Fig. 41), suggesting that under challenging conditions there may indeed be an optimal sweep rate which balances resolution of echoes in a cascade with echo delay acuity (Boonman and Ostwald, 2007).
D. Effect of distracters on use of SSGs
We hypothesized that under more challenging conditions (e.g., when two distracters were present, distracter distance was 45 cm, and angular offset was small), the bats would produce more SSGs to improve spatial resolution and counteract ambiguity in echo assignment. The GLM fit to adjusted SSGs generally supports this hypothesis (Table II), although the R2 was low (0.17). Contrary to our prediction, however, both bats used fewer SSGs in the two-distracter condition than the one-distracter condition (Supplemental Fig. 51). The considerable difference between bats in unadjusted SSG totals per trial, and the significance of bat identity in the model, also suggests that individual bats may rely more heavily on other acoustic or behavioral adjustments in response to a challenging task.
E. Effect of distracters on head turns
We hypothesized that bats would employ more head turns in the one-distracter condition when the distracter distance was 45 cm and angular offset was small, and fewer head turns in the two-distracter condition. As predicted, both bats used more head turns when the distracter was closer [Table II; Fig. 11(b)]. Inspection of RARDs by target distance appeared to show more evidence of head turning at the 45 cm distracter distance when two distracters were present (Supplemental Fig. 81). That the head turns seemed to occur more consistently after the target passed the distracters at 45 cm (relative to at 70 cm) may reflect a greater need or ability of the bats to track the target when it passed in front of the distracters at closer range.
Surprisingly, both bats used fewer head turns in the one-distracter condition and more in the two-distracter condition, relative to baseline [Fig. 11(a)]. This effect contrasts with our prediction, as the echoes returning from the distracter toward which the bat turned its head would be strengthened in amplitude and bandwidth, while echoes from the target would be weakened in amplitude and low-pass filtered. However, examination of the other significant interaction term, angular offset by number of distracters, revealed that adjusted head turns were higher at large angular offsets in the two-distracter condition, while in the one-distracter condition they remained the same across angular offsets [Fig. 11(c)].
Differences in head turning between the one- and two-distracter conditions suggests that bats may not need to employ head turns to disambiguate echo sources when clutter is low or confined to one side of the bat, but head turning might help when clutter is high (e.g., on both sides of the bat), as long as the clutter objects are separated from the target by a sufficiently large azimuthal angle. At high angular offsets, the bats may have been able to turn their heads or move their pinna to more accurately represent the location of the distracters using interaural difference cues, while maintaining the distracters sufficiently off-axis to result in low-pass filtered, “defocused” clutter echoes (Bates et al., 2011). Adjusted head turns were low in both one- and two-distracter conditions at small angular offsets [Fig. 11(c)], presumably because head turning would result in directly ensonifying the distracters and increasing the strength of clutter echoes. Alternatively, the head turn counts may be biased toward more exaggerated head turns due to our use of a conservative RARD threshold, and larger head turns might only benefit the bats when the distracters were placed at large angular offsets.
V. CONCLUSIONS
In this experiment, we showed that bats make adjustments to their echolocation calls and head movements in response to clutter, which we created by introducing one or two distracters at two distances and four angular offsets from an approaching target. Although the bats were stationary rather than free-flying, this design allowed us to systematically investigate the effect of clutter distance and angular offset on echolocation behavior. As hypothesized, call durations shortened as clutter interference increased. Pulse interval was not strongly influenced by clutter, indicating that the bats could still track the target even when it was beyond the distracter(s). Consistent with other studies, the bats used higher sweep rates and more SSGs when clutter was increased. Head turns were used more frequently in the two-distracter condition, but mostly at large angular offsets.
Notably, individual bats used different strategies to track a moving target in the presence of distracter objects. One bat primarily changed the spectro-temporal features of its calls, shortening duration, and increasing peak frequency, while the other used more SSGs and exhibited consistent head turning in high-clutter conditions. While limited to two subjects, this study suggests that call duration, peak frequency, SSGs, and head movements can all be dynamically adjusted to ameliorate clutter interference at different ranges and angular offsets, and that individual bats may use different combinations of vocal and behavioral adjustments to track targets in the presence of other objects.
ACKNOWLEDGMENTS
We thank Dr. Edward Smith for his help in the design and construction of the experimental apparatus and Jenny Finder for her help running bats in an earlier version of this experiment. This work was supported by the Center for Comparative and Evolutionary Biology of Hearing P30 DC04664 to R. Dooling. B.M. was supported on training Grant No. T32 DC000046 from the National Institute of Deafness and Communicative Disorders of the National Institutes of Health awarded to A. N. Popper. We also wish to acknowledge grants from the National Institutes for Health, R01MH056366 and R01EB004750, the Human Frontiers Science Program, RGP0040, and the National Science Foundation, Collaborative Research in Computational Neuroscience, IOS1460149 to C.F.M., which supported the research reported in this manuscript.
Portions of this work were presented in “Echolocating bats adapt their sonar calls to separate echoes from obstacles and prey,” at the 37th annual meeting of the Association for Research in Otolaryngology, San Diego, CA, February 2014.
Footnotes
See supplementary material at http://dx.doi.org/10.1121/1.4962496E-JASMAN-140-034609 for information on the number of trials and calls recorded per condition, and additional figures.
References
- 1. Aytekin, M. , Grassi, E. , Sahota, M. , and Moss, C. F. (2004). “ The bat head-related transfer function reveals binaural cues for sound localization in azimuth and elevation,” J. Acoust. Soc. Am. 116, 3594–3605. 10.1121/1.1811412 [DOI] [PubMed] [Google Scholar]
- 2. Aytekin, M. , Mao, B. , and Moss, C. F. (2010). “ Spatial perception and adaptive sonar behavior,” J. Acoust. Soc. Am. 128, 3788–3798. 10.1121/1.3504707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bates, M. E. , Simmons, J. A. , and Zorikov, T. V. (2011). “ Bats use echo harmonic structure to distinguish their targets from background clutter,” Science 333, 627–630. 10.1126/science.1202065 [DOI] [PubMed] [Google Scholar]
- 3. Bates, M. E. , Stamper, S. A. , and Simmons, J. A. (2008). “ Jamming avoidance response of big brown bats in target detection,” J. Exp. Biol. 211, 106–113. 10.1242/jeb.009688 [DOI] [PubMed] [Google Scholar]
- 4. Boonman, A. , and Ostwald, J. (2007). “ A modeling approach to explain pulse design in bats,” Biol. Cybern. 97, 159–172. 10.1007/s00422-007-0164-2 [DOI] [PubMed] [Google Scholar]
- 5. Chiu, C. , Xian, W. , and Moss, C. F. (2009). “ Adaptive echolocation behavior in bats for the analysis of auditory scenes,” J. Exp. Biol. 212, 1392–1404. 10.1242/jeb.027045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corcoran, A. J. , Barber, J. R. , and Conner, W. E. (2009). “ Tiger moth jams bat sonar,” Science 325, 325–327. 10.1126/science.1174096 [DOI] [PubMed] [Google Scholar]
- 7. Falk, B. , Williams, T. , Aytekin, M. , and Moss, C. F. (2011). “ Adaptive behavior for texture discrimination by the free-flying big brown bat, Eptesicus fuscus,” J. Comp. Physiol. A 197, 491–503. 10.1007/s00359-010-0621-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fujioka, E. , Aihara, I. , Watanabe, S. , Sumiya, M. , Hiryu, S. , Simmons, J. A. , Riquimaroux, H. , and Watanabe, Y. (2014). “ Rapid shifts of sonar attention by Pipistrellus abramus during natural hunting for multiple prey,” J. Acoust. Soc. Am. 136, 3389–3400. 10.1121/1.4898428 [DOI] [PubMed] [Google Scholar]
- 9. Fullard, J. H. , Simmons, J. A. , and Saillant, P. A. (1994). “ Jamming bat echolocation: The dogbane tiger moth Cycnia tenera times its clicks to the terminal attack calls of the big brown bat Eptesicus fuscus,” J. Exp. Biol. 194, 285–298. [DOI] [PubMed] [Google Scholar]
- 10. Ghose, K. , and Moss, C. F. (2003). “ The sonar beam pattern of a flying bat as it track tethered insects,” J. Acoust. Soc. Am. 114, 1120–1131. 10.1121/1.1589754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillam, E. H. , Ulanovsky, N. , and McCracken, G. F. (2007). “ Rapid jamming avoidance in biosonar,” Proc. R. Soc. B 274, 651–660. 10.1098/rspb.2006.0047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griffin, D. R. (1958). Listening in the Dark ( Yale University Press, New Haven, CT: ). [Google Scholar]
- 13. Griffin, D. R. , Friend, J. H. , and Webster, F. A. (1965). “ Target discrimination by the echolocation of bats,” J. Exp. Zool. 158, 155–168. 10.1002/jez.1401580204 [DOI] [PubMed] [Google Scholar]
- 14. Hartley, D. J. , and Suthers, R. A. (1989). “ The sound emission pattern of the echolocating bat, Eptesicus fuscus,” J. Acoust. Soc. Am. 85, 1348–1351. 10.1121/1.397466 [DOI] [PubMed] [Google Scholar]
- 15. Hiryu, S. , Bates, M. E. , Simmons, J. A. , and Riquimaroux, H. (2010). “ FM echolocating bats shift frequencies to avoid broadcast-echo ambiguity in clutter,” Proc. Natl. Acad. Sci. U.S.A. 107, 7048–7053. 10.1073/pnas.1000429107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hulgard, K. , and Ratcliffe, J. M. (2016). “ Sonar sound groups and increased terminal buzz duration reflect task complexity in hunting bats,” Sci. Rep. 6, 21500. 10.1038/srep21500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jakobsen, L. , Brinkløv, S. , and Surlykke, A. (2013). “ Intensity and directionality of bat echolocation signals,” Front Physiol. 4, 89. 10.3389/fphys.2013.00089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jakobsen, L. , and Surlykke, A. (2010). “ Vespertilionid bats control the width of their biosonar sound bean dynamically during prey pursuit,” Proc. Natl. Acad. Sci. U.S.A. 107, 13930–13935. 10.1073/pnas.1006630107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalko, E. K. V. , and Schnitzler, H. U. (1993). “ Plasticity in echolocation signals of European Pipistrelle bats in search flight—implications for habitat use and prey detection,” Behav. Ecol. Sociobiol. 33, 415–428. 10.1007/BF00170257 [DOI] [Google Scholar]
- 20. Kothari, N. B. , Wohlgemuth, M. J. , Hulgard, K. , Surlykke, A. , and Moss, C. F. (2014). “ Timing matters: Sonar call groups facilitate target localization in bats,” Front. Physiol. 5, 168. 10.3389/fphys.2014.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kounitsky, P. , Rydell, J. , Amichai, E. , Boonman, A. , Eitan, O. , Weiss, A. J. , and Yovel, Y. (2015). “ Bats adjust their mouth gape to zoom their biosonar field of view,” Proc. Natl. Acad. Sci. U.S.A. 112, 6724–6729. 10.1073/pnas.1422843112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Langendijk, E. H. , Kistler, D. J. , and Wightman, F. L. (2001). “ Sound localization in the presence of one or two distracters,” J. Acoust. Soc. Am. 109, 2123–2134. 10.1121/1.1356025 [DOI] [PubMed] [Google Scholar]
- 23. Melcón, M. L. , Yovel, Y. , Denzinger, A. , and Schnitzler, H. U. (2011). “ How greater mouse-eared bats deal with ambiguous echoic scenes,” J. Comp. Physiol. A. Neuroethol. Sense. Neural Behav. Physiol. 197, 505–514. 10.1007/s00359-010-0563-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moss, C. F. , Bohn, K. , Gilkenson, H. , and Surlykke, A. (2006). “ Active listening for spatial orientation in a complex auditory scene,” PLoS Biol. 4, 615–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moss, C. F. , and Surlykke, A. (2001). “ Auditory scene analysis by echolocation in bats,” J. Acoust. Soc. Am. 10, 2207–2226. 10.1121/1.1398051 [DOI] [PubMed] [Google Scholar]
- 26. Moss, C. F. , and Surlykke, A. (2010). “ Probing the natural scene by echolocation in bats,” Front Behav. Neurosci. 4, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Obrist, M. K. (1995). “ Flexible bat echolocation: The influence of individual, habitat and conspecifics on sonar signal design,” Behav. Ecol. Sociobiol. 36, 207–219. 10.1007/BF00177798 [DOI] [Google Scholar]
- 28. Obrist, M. K. , Boesch, R. , and Fluckinger, P. F. (2004). “ Variability in echolocation call design of 26 Swiss bat species: Consequences, limits and options for automated field identification with a synergetic pattern recognition approach,” Mammalia 68, 307–322. 10.1515/mamm.2004.030 [DOI] [Google Scholar]
- 29. Petrites, A. E. , Eng, O. S. , Mowlds, D. S. , Simmons, J. A. , and DeLong, C. M. (2009). “ Interpulse interval modulation by echolocating big brown bats (Eptesicus fuscus) in different densities of obstacle clutter,” J. Comp. Physiol. A. Neuroethol. Sense. Neural Behav. Physiol. 195, 603–617. 10.1007/s00359-009-0435-6 [DOI] [PubMed] [Google Scholar]
- 30. Russo, D. , and Jones, G. (2002). “ Identification of twenty-two bat species (Mammalia: Chiroptera) from Italy by analysis of time-expanded recordings of echolocation calls,” J. Zool. 258, 91–103. 10.1017/S0952836902001231 [DOI] [Google Scholar]
- 31. Simmons, J. A. (2014). “ Temporal binding of neural responses for focused attention in biosonar,” J. Exp. Biol. 217, 2834–2843. 10.1242/jeb.104380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Simmons, J. A. , and Chen, L. (1989). “ The acoustic basis for target discrimination by FM echolocating bats,” J. Acoust. Soc. Am. 86, 1333–1350. 10.1121/1.398694 [DOI] [PubMed] [Google Scholar]
- 33. Simmons, J. A. , Freedman, E. G. , Stevenson, S. B. , Chen, L. , and Wohlgenant, T. J. (1989). “ Clutter interference and the integration time of echoes in the echolocating bat, Eptesicus fuscus,” J. Acoust. Soc. Am. 86, 1318–1332. 10.1121/1.398693 [DOI] [PubMed] [Google Scholar]
- 34. Simmons, J. A. , Howell, D. J. , and Suga, N. (1975). “ Information content of bat sonar echoes: Recent research on echolocation in bats identifies some of the kinds of information conveyed by echoes of their sonar sounds,” Am. Sci. 63, 204–215. [PubMed] [Google Scholar]
- 35. Simmons, J. A. , Kick, S. A. , Moffat, A. J. M. , Masters, W. M. , and Kon, D. (1988). “ Clutter interference along the target range axis in the echolocating bat, Eptesicus fuscus,” J. Acoust. Soc. Am. 84, 551–559. 10.1121/1.396832 [DOI] [PubMed] [Google Scholar]
- 36. Simmons, J. A. , and Stein, R. A. (1980). “ Acoustic imaging in bat sonar: Echolocation signals and the evolution of echolocation,” J. Comp. Physiol. 135, 61–84. 10.1007/BF00660182 [DOI] [Google Scholar]
- 37. Sümer, S. , Denzinger, A. , and Schnitzler, U. H. (2009). “ Spatial unmasking in the echolocating big brown bat, Eptesicus fuscus,” J. Comp. Physiol. A. 195, 463–472. 10.1007/s00359-009-0424-9 [DOI] [PubMed] [Google Scholar]
- 38. Surlykke, A. , Ghose, K. , and Moss, C. F. (2009). “ Acoustic scanning of natural scenes by echolocation in the big brown bat, Eptsesicus fuscus,” J. Exp. Biol. 212, 1011–1020. 10.1242/jeb.024620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ulanovsky, N. , Fenton, M. B. , Tsoar, A. , and Korine, C. (2004). “ Dynamics of jamming avoidance in echolocating bats,” Proc. R. Acad. B. 271, 1467–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Warnecke, M. , Bates, M. E. , Flores, V. , and Simmons, J. A. (2014). “ Spatial release from simultaneous echo masking in bat sonar,” J. Acoust. Soc. Am. 135, 3077–3085. 10.1121/1.4869483 [DOI] [PubMed] [Google Scholar]
- 41. Wohlgemuth, M. J. , Kothari, N. B. , and Moss, C. F. (2016). “ Action enhances acoustic cues for 3-D target localization by echolocating bats,” PLoS Biol. 14(9), e1002544. 10.1371/journal.pbio.1002544 [DOI] [PMC free article] [PubMed] [Google Scholar]