Abstract
Antibodies against Rh (CEce) and Kidd (Jka and Jkb) system antigens are mostly implicated in delayed hemolytic transfusion reactions (DHTR), which is a potentially life-threatening complication observed in patients receiving chronic transfusions. Here, we are describing a case of Chido/Roger antibody which presented to our laboratory as DHTR. The clinical presentation and laboratory findings including the immunohematological workups with regard to the reaction are discussed, with a special emphasis on the benefit of identifying such an antibody and obtaining blood unit for transfusion supports the patient with respect to providing a compatible unit.
Keywords: Chido/roger antibody, delayed hemolytic transfusion reaction, red blood cell transfusion
Introduction
Delayed hemolytic transfusion reaction (DHTR) is defined as accelerated destruction of transfused red cells that begins only when sufficient antibody has been produced as a result of an immune response induced by the transfusion.[1] The clinical suspicion arises only 3–10 days following the transfusion when clinical symptoms associated with hemolysis are observed or detected serologically.[1,2] It is mostly seen with patients who have been alloimmunized to red blood cell (RBC) antigens by previous transfusions or pregnancies, but with time the titer of the antibody has been lowered below detectable levels, resulting in not being detected during the pretransfusion testing.[2] The most common antibodies implicated for the same include Rh (CEce) and Kidd (Jka and Jkb) system antigens. However, numerous other specificities have been described.[2,3] Here, we are describing a case of Chido/Roger (Ch/Rg) antibody at our laboratory diagnosed as DHTR.
Case Report
A reference for drop-in hemoglobin (Hb) within 14 days following the last transfusion was received in our laboratory. She was a 41-year-old female, a known case of nephrotic syndrome for 7 years and biopsy-proven membranoproliferative glomerulonephritis. She had received immunosuppression therapy in the form of glucocorticoids, cyclophosphamide, cyclosporine inhibitors, and mycophenolate mofetil over the past 7 years. The patient was started on maintenance hemodialysis in December 2015 in view of the progressive renal dysfunction. All immunosuppressive medications were tapered off. In view of suboptimal Hb levels and in spite of iron and erythropoietin therapy, she was receiving occasional blood transfusions.
During the current admission in August 2016, she was admitted with exacerbation of breathlessness and cough. She was noted to have a history of vesicular lesions over the right thigh and fever for 10 days. On general examination, her vitals were stable. She had pallor and bilateral pedal edema. Her laboratory parameters showed Hb – 5.9 g/dL, RBC count – 2.40 × 106/μL, hematocrit – 20.7%, mean corpuscular volume – 86.3 fL, mean corpuscular hemoglobin – 26.3 pg, mean corpuscular hemoglobin content – 30.4 g%, white blood cell count – 7.22 × 103/μL, and platelet count – 340 × 103/μL. In view of symptomatic anemia, 2 units of packs of red blood cell (PRBC) transfusion were planned, and crossmatch compatible PRBC was issued for the patient. The immediate, during, and posttransfusion periods were uneventful. However, over the next 2 weeks of hospital stay, there was a drop in Hb from 9.0 g/dL (posttransfusion value) to 3.9 g/dL. The peripheral smear showed normocytic normochromic RBCs with spherocytes. The other biochemical parameters showed a total bilirubin of 0.7 g/dL and lactate dehydrogenase of 821 U/L. The other causes for hemolysis were ruled out. Hence, a consultation was requested from the department of transfusion medicine to rule out DHTR.
Materials and Methods
Patient's ethylenediaminetetraacetic acid and the plain sample were received for various immunohematological workups. The ABO (both forward and reverse) and Rh grouping were performed by conventional tube technique (CTT). The direct antiglobulin test (DAT) and indirect antiglobulin test (IAT) were performed initially by CTT and later repeated using column agglutination techniques. For the IAT, in-house prepared O-pooled cells were used and checked in immediate spin, at 37°C and antihuman globulin (AHG) phase. The commercially available antibody screening cells and antibody identification panel cells (Ortho Reagent Red Cells Surgiscreen and Resolve Panel A, NJ, USA) were used for the identification of the antibody along with autocontrol in all the three phases. Titers of immunoglobulin G (IgG) antibody were performed using O–pooled cells in the conventional method, that is, 2 drops of serum and 1 drop of 3%–4% cell suspension were incubated at 37°C for 15 min. The titration was also repeated by increasing the serum drops to 6–8 with 1 drop of 3%–4% cell suspension and increasing the incubation time to 60 min. The plasma neutralization or inhibition test was performed using pooled 2 drops of AB RhD positive plasma and 1 drop of patient's serum, which were incubated at room temperature for 15 min with appropriate controls. Subsequently, indicator cells, that is, C4-coated cells were added and incubated at 37°C for 1 h, followed by IAT using AHG to confirm the complete neutralization of antibodies. The C4-coated cells were obtained by treating the O-pooled donor cells with low ionic strength of 10% sucrose solution.[4,5] The antibody screening was repeated with the neutralized patient's serum. The antibody screening panel was repeated with the ficin-treated cells. For the purpose of transfusion, AHG phase crossmatch was performed using ABO compatible PRBCs.
Results
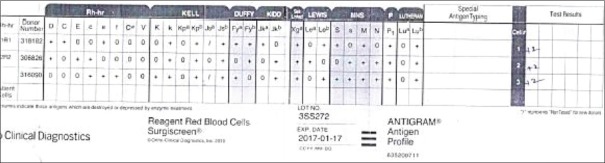
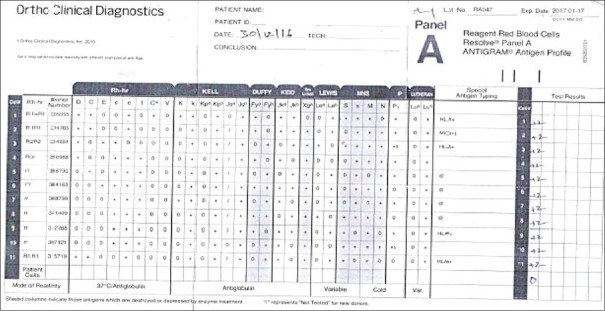
Patient's blood group was typed as “B Rh (D) Positive” with no discrepancy in the forward and reverse typing. The DAT and autocontrol were negative. The IAT with pooled cells gave a negative reaction at IS and 37°C, whereas CTT gave 1+ reaction with AHG. The antibody screening performed with the 3-cell panel gave a consistently 2+ pan reaction [Figure 1]. Similar patterns of the reaction were noted with 11-cell panel also [Figure 2]. The AHG phase crossmatch was 2+ incompatible both for the multiple donor bags and blood relative. The antibody titers were noted to be 1:32. The antibody titers were persistently 1:32 in spite of modification in the technique. The antibody screening using the neutralized serum and ficin-treated red cells gave a negative reaction. Hence, the antibody concluded is belonging to high–titer, low-avidity (HTLA) antibody group, most probably Ch/Rg antibody, and the whole workup is summarized in Table 1.
Figure 1.
Three-cell panel
Figure 2.
Elven-cell panel
Table 1.
Immunohematology procedures performed on the patient
| Test | Result |
|---|---|
| Blood group | B Rh (D) positive (no discrepancy) |
| DAT | Negative |
| IAT | Positive (2+) |
| Autocontrol | Negative |
| 3-cell panel | 2 + (pan reaction) - [Figure 1] |
| 11-cell panel | 2 + (pan reaction) - [Figure 2] |
| AHG phase crossmatch | 2+incompatible |
| Antibody titers | 1:32 |
| Modified antibody titers | 1:32 |
| Antibody screening with plasma neutralized serum | Negative |
| Antibody screening with ficin-treated cells | Negative |
Concluding antibody - Anti-Chido/Roger. DAT=Direct antiglobulin test, IAT=Indirect antiglobulin test, AHG=Antihuman globulin
In view of the low Hb, the patient had received saline phase ABO compatible 4 units of PRBCs over the past 3 months. All the transfusion episodes were uneventful.
Discussion
HTLA shows high titer (>64) but low avidity (1+ or less) for the corresponding antigen in the AHG phase of testing.[4] These are high-frequency antigens and antibodies are IgG type, which do not bind complement. However, the antibody is considered clinically insignificant as it is neither implicated in causing hemolytic transfusion reaction nor hemolytic disease of the newborn. These antibodies are mostly noted among patients with multiple transfusion or in transfused multiparous women similar to our patient.[4] The variable reaction strength and inability to obtain a crossmatch compatible bag are the various immunohemtological challenges associated with the antibodies, making them nebulous antibodies. The various antibodies belonging to this group are Ch/Rg, Knops, Cost, and JMH. The other antibodies which are known to interfere with the reactivity of HTLA group were ruled out such as the combination of anti-Jka and anti-K and anti-Vel, anti-Dib, and anti-Cra, by increasing the serum-to-cell ratio and incubation time to 60 min for IAT.[4] The HTLA antibodies show a similar pattern of reaction irrespective of the modification in the testing. A similar pattern was noted in the above case also. However, in our case, we noted at reaction strength of 1+ by CTT and 2+ by column agglutination technology for the HTLA antibodies. The available literature describes the strength of the reaction should be <1+; however, there is no mention regarding the testing methodology. The titer strength was 1:32 in our case, which was <1:64 as per the standard definition for HTLA antibodies. After ruling out all the other possibilities such as Lutheran or Cartwright system, the final conclusion of HTLA antibodies was made in our case.[4]
Chido and Rogers are a part of the C4 component of the complement system. Ch/Rg antibodies are the IgG type. However, they are not considered clinically significant from the red cell transfusion aspect, as they do not cause a reduction in red cell survival, despite being detected as DHTR antibodies in multiply transfused patients.[4,5] There are various methods to confirm the presence of Ch/Rg antibodies such as testing of the subject's red cells with anti-Ch and Rg sera, plasma inhibition studies, enzyme studies, and using C4-coated cells for testing.[5,6] In our case, we used plasma inhibition technique and enzyme studies for the confirmation Ch/Rg antibodies. Plasma of individual's is known to express Ch/Rh antigens, and inhibition studies are effective at inhibiting anti-Ch/Rg antibodies. Anti-Ch/Rg are the only antibodies neutralized or inhibited by this method.[4,5]
As described in the above case scenario, though the patient had the antibodies, ABO compatible PRBCs should survive normally in vivo, and the patient could tolerate four episodes of transfusion without any adverse reactions associated to the antibodies.
Conclusion
The Ch/Rg antibodies are known to be nebulous antibody. However, it is always essential to decipher these antibodies which not only rule outs the clinical significant one but also helps in providing the appropriate transfusion support to such patients, as every unsolved antibody is a nightmare in part of an immunohematologist and the treating physicians.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient(s) has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initial will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mollison P. Antibody-mediated destruction of red cells. Clin Immunol Newsl. 1985;6:700–3. [Google Scholar]
- 2.Shirey RS, King KE, Ness PM. Hemolytic transfusion reactions: Acute and delayed. In: Hillyer CD, Silberstein L, Ness PM, Anderson, Roback JD, editors. Blood Banking and Transfusion Medicine. 2nd ed. United States of America: Churchill Livingstone; 2007. pp. 668–76. [Google Scholar]
- 3.Klein HG, Anstee DJ, editors. Mollison's Blood Transfusion in Clinical Medicine. 12th ed. West Sussex, UK: John Wiley & Sons, Ltd; 2014. Haemolytic transfusion reactions; pp. 458–98. [Google Scholar]
- 4.Harmening D, Dawson-Batcha FE. Miscellaneous blood group systems. In: Harmening D, editor. Modern Blood Banking & Transfusion Practices. 3rd ed. New Delhi: Jaypee; 1998. pp. 188–98. [Google Scholar]
- 5.Daniels G, editor. Chido/Rodgers Blood Group System. 3rd ed. Bristol, UK: Blackwell Publishing; 2013. pp. 400–10. [Google Scholar]
- 6.Harris JP, Tegoli J, Swanson J, Fisher N, Gavin J, Noades J, et al. Anebulous antibody responsible for cross-matching difficulties (Chido) Vox Sang. 1967;12:140–2. doi: 10.1111/j.1423-0410.1967.tb03078.x. [DOI] [PubMed] [Google Scholar]