Abstract
Introduction:
The exact mechanism behind the development of hypospadias is unclear. Research studies on androgen receptor (AR) expression are controversial with results stating all possible outcomes – AR elevated, similar, or reduced when compared to normal.
Aims:
The aim is to study the AR expression and hormone levels in hypospadias patients and compare them with children having normal genitalia.
Methods:
Group 1 (controls) involved patients who underwent circumcision for phimosis while Group 2 involved hypospadias patients who did not receive any preoperative testosterone. Preoperative hormonal assay included luteinizing hormone, follicle-stimulating hormone, and free testosterone levels in all the patients. The foreskin specimen was analyzed for AR expression using immunohistochemistry (anti-AR antibody PathnSitu, clone R441, 1/100 dilution). AR staining was expressed as H score. The H score was calculated by multiplying the intensity of staining and the percentage of stained cells showing cytoplasmic positivity at high power (×40).
Results:
There were 27 patients in Group 1 while 16 in Group 2 (distal 10; proximal 6).There was no significant difference in the age distribution. The mean H score was significantly higher (189.5) in hypospadias patients compared to controls (97.5) and was significantly higher in proximal (220) compared to distal (159) hypospadias. There was no significant difference in hormone levels between groups.
Conclusion:
AR expression was significantly elevated in hypospadias patients. It was higher in proximal compared to distal hypospadias, probably due to end-organ overexpression. Further larger trials are likely to through light into this controversial subject.
Keywords: Androgen receptors, hypospadias, pathology
INTRODUCTION
The exact mechanism behind the development of hypospadias is unclear, despite it being a common congenital anomaly in boys.[1,2,3] Several reports have shown an increase in the incidence of hypospadias over the last 30 years. It is thought that hypospadias is caused by genetic susceptibility in combination with adverse environmental exposure, which disrupts the balance of androgens and estrogens required for normal sexual differentiation of the external genitalia.[4,5,6,7,8]
Androgens are essential for the formation of the male urogenital system. Hence, any defect in the androgen synthesis leading to hypoandrogenism (androgen deficiency) or an androgen receptor (AR) defect may play a causative role in the development of hypospadias.[9] Research studies about the AR expression in hypospadias patients are highly controversial with the results stating all the possible outcomes – AR levels elevated, reduced, or no change when compared to AR levels in normal children.[9,10,11,12,13,14,15,16,17,18,19,20] ARs also show varied expression in the different types of hypospadias. In this study, we have analyzed AR expression and hormone levels in hypospadias patients and compared them with children having normal genitalia.
METHODS
This was a prospective study commenced after taking permission from the institutional ethics committee. Informed consent was obtained from the patients, and complete confidentiality was maintained with regard to the clinical details. Group 1 (controls) involved children with normal genitalia (who underwent circumcision for phimosis), while Group 2 involved hypospadias patients, with bilateral descended testis, who did not receive any preoperative testosterone. Preoperative hormonal assay included luteinizing hormone (LH), follicle-stimulating hormone (FSH), and free testosterone (fTST) levels in all the patients.
The foreskin collected during surgery was fixed in formalin, paraffin embedded, and sectioned. The foreskin specimens were analyzed for AR expression using immunohistochemistry (anti-AR antibody PathnSitu, clone R441, 1/100 dilution). AR staining was expressed as H score which was calculated by multiplying the intensity of staining and the percentage of stained cells showing cytoplasmic positivity [Figure 1] at high power (×40). A total of 100 cells were counted at high power, and the intensity of AR expression was scored 0, 1, 2, and 3 based on the intensity of staining (brown). The total H score was calculated by multiplying the cell score and the number of cells, with a maximum score 300 if all 100 cells scored 3.
Figure 1.
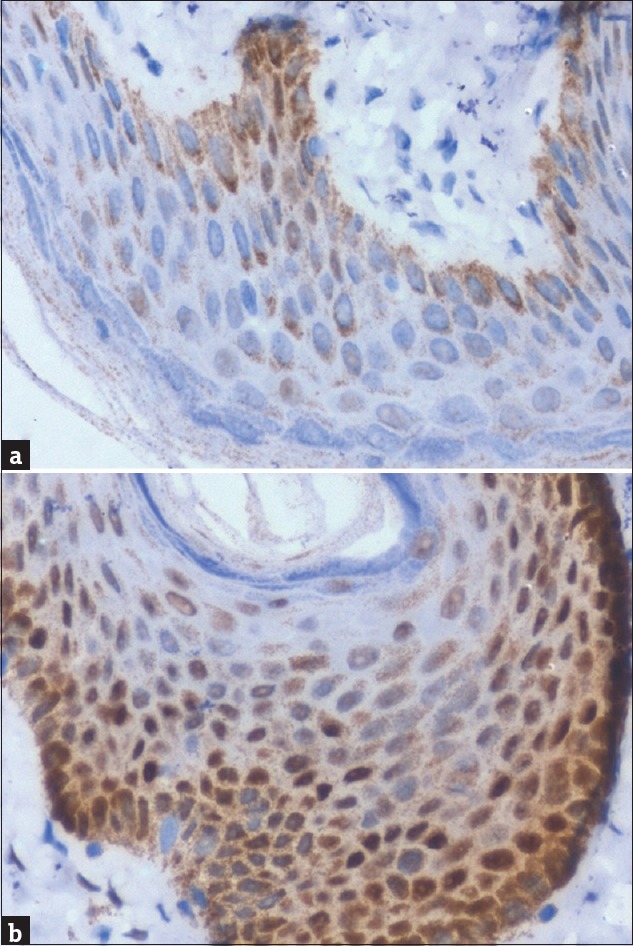
Androgen receptor expression: (a) control–normal foreskin; (b) hypospadias patient. There is increased expression of androgen receptor in hypospadias patient. H score was 80 in control (a) while 175 in that of hypospadias patient (b)
The mean H score was compared between hypospadias patients and controls using Student's t-test. A similar comparison was made between distal and proximal hypospadias patients. The difference was considered statistically significant if P < 0.05.
RESULTS
There were 27 patients in Group 1 and 16 in Group 2. In Group 2, six patients had proximal hypospadias while ten had distal hypospadias. The mean age (range) of the boys in Group 1 was 23 months (15–48) at the time of surgery, while 27 months (12–34) in Group 2. There was no significant difference between the groups. The mean serum FSH level was 0.9 IU/I in Group 1 compared to 0.8 IU/I in Group 2. The mean serum LH level was 0.85 IU/I in Group 1 and 1.1 IU/I in Group 2. The mean fTST level was 6.4 ng/dL in Group 1 and 6.9 ng/dL in Group 2. There was no significant difference in the hormone levels between the groups.
Figure 2 represents the mean H score between the groups. It was significantly higher (P = 0.001) in hypospadias patients (189.5) compared to controls (97.5). Figure 3 represents the mean (standard deviation) H score between proximal and distal hypospadias. It was significantly higher (P = 0.01) in proximal (220) compared to distal (159) hypospadias.
Figure 2.
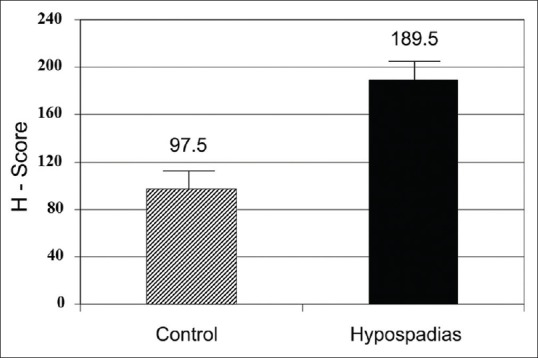
Striped bar represents controls and black bar hypospadias patients. Y-axis represents mean H score. Mean H score (androgen receptor expression) was significantly higher in hypospadias patients
Figure 3.
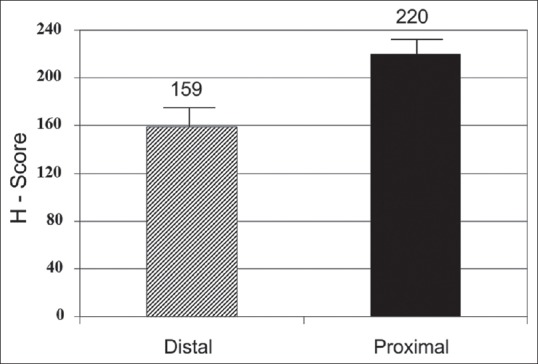
Striped bar represents distal and black bar proximal hypospadias patients. Y-axis represents mean H score. Mean H score (androgen receptor expression) was significantly higher in proximal hypospadias
DISCUSSION
Hypospadias is one of the most common congenital malformations occurring in boys. Hypospadias is considered a multifactorial disorder since both genetic and environmental factors are involved.[1,2] Maternal exposure to chemical pollutants or high concentrations of endocrine disruptors in selected occupations/geographic areas may be additional risk factors for hypospadias. Although in most cases the etiology is unknown, hypospadias has been associated with aberrant androgen signaling during development.[3,4] Interferences in the androgen metabolism, for example, 5α-reductase deficiency, or defects of the AR and its genes are possible etiological factors for hypospadias in a small proportion of patients.[5,6]
Androgen signaling through the AR is critical for normal penile development. Diminished androgen signaling results in a spectrum of incompletely virilized external genitalia: complete androgen insensitivity results in female external genitalia while partial insensitivity results in ambiguous genitalia of varying degrees. AR plays a crucial role in male sex differentiation by mediating the biological effects of androgens. The AR gene resides on chromosome Xq11-12.[5,6,7,8,21]
Pichler et al.[9] and Baskin[10] reported quantitative measurement of differences in the AR in the discarded foreskin collected at the time of surgical repair in boys with hypospadias and compared it with that of a cohort undergoing circumcision. We performed a similar study in Indian children, and our findings are similar to these studies. ARs are overexpressed in hypospadias boys compared to controls, and this overexpression was higher in those with severe hypospadias.
The mechanism of this overexpression may be through the genetic pathway ZEB1, which is known to be estrogen responsive, and may be an important mediator of estrogen-driven aberrant androgen signaling in hypospadias.[11,12] It is still unclear whether the overexpression of AR is a cause of hypospadias or an effect of exposure to estrogens like compounds during fetal life. There are several other studies with conflicting reports on AR expression.[13,14,15,16,17,18,19,20] Although none of our patients reported any medical/chemical exposure to estrogenic compounds, we could not rule out maternal exposure to estrogens like compounds common in pesticide-contaminated fruits/vegetables. There was no significant difference in serum-free testosterone, LH, and FSH levels between the groups in our study, indicating the absence of any hormonal dysfunction.
Agras et al.[22] evaluated the influence of exogenous estrogen. Their quantitative results indicated that developing females expressed higher levels of AR than males throughout ontogeny. AR expression in the genital tubercle increased in response to in utero estrogen exposure with estrogen-treated females having the highest AR expression, followed by estrogen-treated males with hypospadias. These findings could be the result of relatively lower levels of androgens in female genital tubercles, indicating that the organism is trying to compensate for lower levels of androgens by increasing AR expression. The organism reacted by upregulating AR expression so that it could respond better to any androgen that might have still been present.
The pitfalls of this study are small numbers. Further larger trials are likely to throw more light into the etiopathogenesis of these complex malformations. We have analyzed only those patients who did not undergo preoperative testosterone treatment. Further studies could address whether testosterone supplementation could up/downregulate AR expression. Furthermore, further studies could address whether prior knowledge of AR status could determine testosterone resistance, noted in some patients with severe hypospadias.
CONCLUSION
The results of our study show that AR expression was significantly elevated in hypospadias patients. It was higher in proximal compared to distal hypospadias, probably due to end-organ overexpression.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Paulozzi LJ. International trends in rates of hypospadias and cryptorchidism. Environ Health Perspect. 1999;107:297–302. doi: 10.1289/ehp.99107297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lund L, Engebjerg MC, Pedersen L, Ehrenstein V, Nørgaard M, Sørensen HT, et al. Prevalence of hypospadias in Danish boys: A longitudinal study, 1977-2005. Eur Urol. 2009;55:1022–6. doi: 10.1016/j.eururo.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Schnack TH, Zdravkovic S, Myrup C, Westergaard T, Christensen K, Wohlfahrt J, et al. Familial aggregation of hypospadias: A cohort study. Am J Epidemiol. 2008;167:251–6. doi: 10.1093/aje/kwm317. [DOI] [PubMed] [Google Scholar]
- 4.Rocheleau CM, Romitti PA, Dennis LK. Pesticides and hypospadias: A meta-analysis. J Pediatr Urol. 2009;5:17–24. doi: 10.1016/j.jpurol.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalfa N, Philibert P, Sultan C. Is hypospadias a genetic, endocrine or environmental disease, or still an unexplained malformation? Int J Androl. 2009;32:187–97. doi: 10.1111/j.1365-2605.2008.00899.x. [DOI] [PubMed] [Google Scholar]
- 6.Vilela ML, Willingham E, Buckley J, Liu BC, Agras K, Shiroyanagi Y, et al. Endocrine disruptors and hypospadias: Role of genistein and the fungicide vinclozolin. Urology. 2007;70:618–21. doi: 10.1016/j.urology.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Kim KS, Torres CR, Jr, Yucel S, Raimondo K, Cunha GR, Baskin LS, et al. Induction of hypospadias in a murine model by maternal exposure to synthetic estrogens. Environ Res. 2004;94:267–75. doi: 10.1016/S0013-9351(03)00085-9. [DOI] [PubMed] [Google Scholar]
- 8.Klip H, Verloop J, van Gool JD, Koster ME, Burger CW, van Leeuwen FE, et al. Hypospadias in sons of women exposed to diethylstilbestrol in utero: A cohort study. Lancet. 2002;359:1102–7. doi: 10.1016/S0140-6736(02)08152-7. [DOI] [PubMed] [Google Scholar]
- 9.Pichler R, Djedovic G, Klocker H, Heidegger I, Strasak A, Loidl W, et al. Quantitative measurement of the androgen receptor in prepuces of boys with and without hypospadias. BJU Int. 2013;112:265–70. doi: 10.1111/j.1464-410X.2012.11731.x. [DOI] [PubMed] [Google Scholar]
- 10.Baskin L. Quantitative measurement of the androgen receptor in prepuces of boys with and without hypospadias. BJU Int. 2013;112:159–60. doi: 10.1111/j.1464-410X.2012.11783.x. [DOI] [PubMed] [Google Scholar]
- 11.Qiao L, Tasian GE, Zhang H, Cao M, Ferretti M, Cunha GR, et al. Androgen receptor is overexpressed in boys with severe hypospadias, and ZEB1 regulates androgen receptor expression in human foreskin cells. Pediatr Res. 2012;71:393–8. doi: 10.1038/pr.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiao L, Tasian GE, Zhang H, Cunha GR, Baskin L. ZEB1 is estrogen responsive in vitro in human foreskin cells and is over expressed in penile skin in patients with severe hypospadias. J Urol. 2011;185:1888–93. doi: 10.1016/j.juro.2010.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Liu BC, Lin GT, Lin CS, Lue TF, Willingham E, et al. Up-regulation of estrogen responsive genes in hypospadias: Microarray analysis. J Urol. 2007;177:1939–46. doi: 10.1016/j.juro.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Liu B, Lin G, Willingham E, Ning H, Lin CS, Lue TF, et al. Estradiol upregulates activating transcription factor 3, a candidate gene in the etiology of hypospadias. Pediatr Dev Pathol. 2007;10:446–54. doi: 10.2350/06-04-0079.1. [DOI] [PubMed] [Google Scholar]
- 15.Feyaerts A, Forest MG, Morel Y, Mure PY, Morel-Journel N, Mallet D, et al. Endocrine screening in 32 consecutive patients with hypospadias. J Urol. 2002;168:720–5. [PubMed] [Google Scholar]
- 16.Hughes IA, Deeb A. Androgen resistance. Best Pract Res Clin Endocrinol Metab. 2006;20:577–98. doi: 10.1016/j.beem.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Holmes NM, Miller WL, Baskin LS. Lack of defects in androgen production in children with hypospadias. J Clin Endocrinol Metab. 2004;89:2811–6. doi: 10.1210/jc.2003-032098. [DOI] [PubMed] [Google Scholar]
- 18.Celayir S, Eliçevik M, Tireli G, Dervisoǧlu S, Sander S. Expression of estrogen and androgen receptors in children with hypospadias: Preliminary report. Arch Androl. 2007;53:83–5. doi: 10.1080/01485010601166862. [DOI] [PubMed] [Google Scholar]
- 19.Zhao W, Yin J, Yang Z, Xie J, Zhang Y, Xu W, et al. Meta-analysis of androgen insensitivity in preoperative hormone therapy in hypospadias. Urology. 2015;85:1166–72. doi: 10.1016/j.urology.2015.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Terakawa T, Shima H, Yabumoto H, Koyama K, Ikoma F. Androgen receptor levels in patients with isolated hypospadias. Acta Endocrinol (Copenh) 1990;123:24–9. doi: 10.1530/acta.0.1230024. [DOI] [PubMed] [Google Scholar]
- 21.Aaronson IA, Cakmak MA, Key LL. Defects of the testosterone biosynthetic pathway in boys with hypospadias. J Urol. 1997;157:1884–8. [PubMed] [Google Scholar]
- 22.Agras K, Willingham E, Liu B, Baskin LS. Ontogeny of androgen receptor and disruption of its mRNA expression by exogenous estrogens during morphogenesis of the genital tubercle. J Urol. 2006;176:1883–8. doi: 10.1016/S0022-5347(06)00613-6. [DOI] [PubMed] [Google Scholar]


