SUMMARY
Allopregnanolone (5α-pregnan-3α-ol-20-one) and its synthetic 3β-methyl analog ganaxolone are positive allosteric modulators of synaptic and extrasynaptic GABAA receptors that exhibit antiseizure activity in diverse animal seizure models, including models of status epilepticus (SE). The two neuroactive steroids are being investigated as treatments for SE, including as a treatment for SE induced by chemical threat agents. Intramuscular injection is the preferred route of administration in the pre-hospital treatment of SE. The objective of this study was to assess the efficacy of intramuscular allopregnanolone and ganaxolone in the treatment of SE induced by the chemical threat agent tetramethylenedisulfotetramine (TETS). The test agents were administered 40 min after the onset of SE when mice are refractory to treatment. Allopregnanolone and ganaxolone (each at 3 mg/kg) terminated SE in, respectively, 92% and 75% of animals and prevented mortality in 85% and 50% of animals; the mean times to termination of behavioral seizures were, respectively, 172 ± 16 s and 447 ± 52 s. In a separate series of experiments, mice were dosed with the neuroactive steroids by intramuscular injection and plasma and brain levels were sampled at various time points following injection to estimate pharmacokinetic parameters. Plasma Cmax values for allopregnanolone and ganaxolone were 645 ng/ml and 550 ng/ml, respectively. Brain exposures of both steroids were approximately 3-fold the plasma exposure. Two compartment pharmacokinetic analysis revealed that the central compartment Vd, CL, t½ (terminal half-life) and F (intramuscular bioavailability) values for allopregnanolone and ganaxolone were, respectively 4.95 L/kg, 12.88 L/kg/hr, 16 min, 97% and 5.07 L/kg, 8.35 L/kg/hr, 25 min, 95%. Allopregnanolone and ganaxolone are effective in the treatment of TETS-induced SE when administered by the intramuscular route. Allopregnanolone is more rapidly acting and modestly more effective, possibly because it has greater potency on GABAA receptors.
Keywords: Refractory status epilepticus, Seizure, Neuroactive steroid, Tetramethylenedisulfotetramine, GABAA receptor, Pharmacokinetics
Introduction
Status epilepticus (SE) is a life-threatening neurological emergency resulting from either the failure of seizure termination or from mechanisms that lead to abnormally prolonged seizures.1 Benzodiazepines, administered by a parenteral route, are the standard-of-care initial treatment for SE, but fail to effectively terminate behavioral and electrographic seizure activity in SE in about one-third of cases.1,2 Benzodiazepines act as positive allosteric modulators of synaptic GABAA receptors. During persistent seizures there are changes in the availability of synaptic GABAA receptors that lead to resistance to benzodiazepines.3–7 Neuroactive steroids, such as allopregnanolone and ganaxolone, have been proposed as alternatives to benzodiazepines in the treatment of SE.8 Like benzodiazepines, such neuroactive steroids are potent positive allosteric modulators of synaptic GABAA receptors. However, in contrast to benzodiazepines, which enhance GABAA receptor responses to GABA but do not activate the receptors in the absence of GABA, the neuroactive steroids can directly activate GABAA receptors.9 Importantly, in addition to actions on synaptic GABAA receptors, neuroactive steroids also act on extrasynaptic GABAA receptors.10 Extrasynaptic GABAA receptors, which are insensitive to benzodiazepines, maintain availability during prolonged seizures11 and are a potential target for the treatment of benzodiazepine-refractory SE. In support of this concept, studies in animal models have indicated that allopregnanolone is effective in the treatment of benzodiazepine-refractory SE.8 Moreover, allopregnanolone has been tried on an emergency basis as a treatment for refractory SE in humans and clinical trials are ongoing.12,13
Allopregnanolone, an endogenous metabolite of the female sex steroid progesterone, is poorly absorbed when administered by the oral route.14 Ganaxolone, its 3β-methyl synthetic analog, has modestly improved oral bioavailability and oral formulations have been shown in some preliminary clinical trials to be effective in the treatment of epilepsy.15–17 In studies with recombinant synaptic (α1β2γ2 and α1β3γ2) and extrasynaptic (α4β3δ) GABAA receptors isoforms, allopregnanolone and ganaxolone have similar efficacy for potentiation of GABA responses and for direct activation of the receptors but allopregnanolone may be modestly more potent.14,18 Both allopregnanolone and ganaxolone exert anticonvulsant activity when administered parenterally in various animal seizure models, including models of SE.19–24
In the present study we sought to assess the potential of allopregnanolone and ganaxolone for the treatment of refractory SE when administered intramuscularly. Intramuscular injection is the preferred route of administration for a pre-hospital SE treatment.25 Among the uses of such a treatment is as an antidote following exposure to a SE-inducing chemical threat agents. With this latter application in mind, here we used a model of SE in which seizures are induced by tetramethylenedisulfotetramine (TETS), a highly toxic convulsant poison that induces seizures by noncompetitive block of GABAA receptors,18,26 and is considered a chemical threat agent by the National Institutes of Health.27,28 We assessed the activity of allopregnanolone and ganaxolone in the treatment of TETS-induced SE in a mouse model.29 The steroids were administered at a delayed time (40 min after first myoclonic body twitch) when mice are known to be refractory to standard-of-care benzodiazepine treatment.8 In addition, we compared the pharmacokinetic properties of the two steroids following intramuscular administration to assess their potential utility when administered by this route.
Materials and Methods
Animals
Male NIH Swiss mice (22–35 g) served as subjects. The animals were allowed to acclimatize for at least 5 days before use. All procedures used in these studies were conducted in accordance with animal care and use policies of the University of California, Davis Institutional Animal Care and Use Committee and in strict compliance with the Guide for the Care and Use of Laboratory Animals of the National Research Council (National Academy Press, Washington, DC; http://www.nap.edu/readingroom/books/labrats/).
Test substances and drug administration
Tetramethylenedisulfotetramine (TETS), synthesized by a modification of the procedure of Esser et al.30, was a generous gift of Dr. Bruce Hammock. Riluzole (CAS 1744-22-5) was purchased from Oakwood Products (West Columbia, SC) and was used following recrystallization. Allopregnanolone was synthesized under contract with SAFC Pharma (Madison, WI). Ganaxolone was generously provided by Marinus Pharmaceuticals (Radnor, PA).
TETS was initially dissolved in 100% DMSO at a concentration of 1 mg/ml and then further diluted in 0.9% sterile saline at a concentration of 0.2 mg/10 ml. Riluzole was diluted in 0.9% saline (5 mg/ml) with 10% sulfobutyl ethers β-cyclodextrin sodium salts (Captisol®, Ligand Pharmaceuticals, La Jolla, CA) and further diluted in 0.9% saline to a concentration of 10 mg/ 10 ml in 0.9% saline. For testing in the SE model, allopregnanolone was dissolved in 24% Captisol in 0.9% saline at a concentration of 6 mg/ml. Ganaxolone was less soluble so the concentration used was 1.5 mg/ml in 24% Captisol in 0.9% saline. The volume used for intraperitoneal injection of TETS and riluzole was 10 ml/kg. The volumes used for intramuscular allopregnanolone and ganaxolone were 0.5 ml/kg and 2 ml/kg, respectively. For the pharmacokinetic study, allopregnanolone was administered intramuscularly as a 3 mg/ml solution in 12% Captisol in 0.9% saline, using a volume of 1 ml/kg.
TETS-induced status epilepticus model
Mice were pretreated with a single dose of riluzole (10 mg/kg, i.p.) followed 10 min later by a lethal dose of TETS (0.2 mg/kg, i.p.). Allopregnanolone or ganaxolone (3 mg/kg, i.m.) were injected at 40 min after the first myoclonic body twitch. Latency to cessation of SE was defined as the interval between the time of treatment administration and visually assessed termination of continuous seizure activity. Mice were observed for 1 h to determine seizure termination and scored for lethality up to 72 h after dosing. In this study, we did not assess electrographic seizure activity.
Blood and brain collection and processing for pharmacokinetic analysis
Mice received an intramuscular injection of either allopregnanolone or ganaxolone at a dose of 3 mg/kg. At various times after the injection, animals were sacrificed and trunk blood was collected in chilled heparinized tubes and the whole brain was removed. Mice were not fasted prior to blood and brain collection. The blood was centrifuged at 1500 × g for 10 min to separate the plasma. Brains were snap frozen on dry ice. Plasma and brain specimens were stored at −80 °C until processed for extraction of the analytes. At the time of analysis, the tissues were thawed.
The whole frozen brain from each mouse was placed in a 15 ml Falcon centrifuge tube containing 2 ml of H2O and 3 to 5-5/32-inch stainless steel grinding balls. The tube was shaken in a Geno/Grinder automated cell lyser (SPEX SamplePrep, Metuchen, MJ) at 1500 strokes per min for 1 min. One ml of 10 ng/ml D4-allopregnanolone solution in acetonitrile was then added to the tube. The tube was again shaken in the Geno/Grinder at the same rate for 1 min. The tube was centrifuged at 4000 × g for 30 min.
The resulting total brain homogenate supernatant was loaded onto a Waters Oasis HLB 3 ml (60 mg reversed-phase sorbent) extraction cartridge that had been pre-equilibrated by passage of 3 ml acetonitrile and then 3 ml of H2O. The cartridge was washed once with 3 ml of 40%/60% acetonitrile/H2O, and the analytes were eluted with 3 ml of 100% acetonitrile. When extracting allopregnanolone from plasma, one microliter of 10 μg/ml D4-allopreganolone (internal standard) solution in 100% methanol was added to 200 μl of mouse plasma. The spiked plasma was then loaded onto Waters Oasis HLB 1 ml (30 mg reversed-phase sorbent) extraction cartridge that was also pre-equilibrated by the same method described above. The cartridge was then washed once with 1 ml of 20%/80% acetonitrile/H2O, followed by another wash with 1ml of 40%/60% acetonitrile/H2O. The analytes were also eluted with 3 ml of 100% acetonitrile. The eluents resulting from either brain or plasma extraction were dried by air stream and reconstituted with 200 μl of 50%/50% acetonitrile/H2O. Ten μl of the solution was injected into a Waters Acquity UPLC with a BEH C18 1.7 μm, 2.1 mm × 50 mm column. The following mobile phases were used: A – H2O with 0.1% formic acid, and B – acetonitrile with 0.1% formic acid. Mobile phase A was used for purging and 50%/50% acetonitrile/H2O was used for needle washing between each injection. The elution gradient program was as follows: 0–1 min, 50%B; 2.5 min, 72.5%B; 2.51 min, 95%B; 2.51–4.75 min, 95%B; 4.76–7 min, 50%B. The flow rate was 0.4 ml/min and the column temperature was set at 50 °C. The autosampler temperature was set at 10 °C. The output of the UPLC was fed to a Waters Xevo TQ-S triple quadrupole MS/MS system, which was used to ionize target molecules and monitor the ion m/z fragmentation transitions from 319.20 → 283.30 for allopregnanolone quantification, 323.3 → 287.3 for D4-allopregnanolone quantification and 333.2 → 297.3 for ganaxolone quantification at MRM (multiple reaction monitoring) mode.
Pharmacokinetic parameters calculations
Descriptive and 2-compartment pharmacokinetic parameters were estimated with Phoenix WinNonlin 7.0 (Certara, Princeton, NJ).
Data analysis
Time to cessation of TETS-induced persistent seizures activity is expressed as mean ± S.E.M.; the significance of the difference in the responses of treatment groups with respect to control is based on unpaired t-test. Differences were considered statistically significant when the probability of error was less than 0.05 (P < 0.05).
Results
TETS status epilepticus model
Administration of TETS to mice causes a rapid sequence of immobility, myoclonic body jerks, clonic seizures of the forelimbs and/or hindlimbs, tonic seizures, and death.26 The rapid lethality in mice (often occurring in ~20 min) is inconsistent with reports of prolonged SE in human victims of TETS poisoning.31,32 In mice, death usually occurs rapidly after the onset of tonic extension. We have found that blocking tonic seizures with riluzole prevents lethality. Animals pretreated with riluzole prior to the administration of TETS exhibit clonic seizures and continuous ictal activity that persists for more than 1 h. However, as shown in Figure 2, most vehicle-treated animals eventually expire.
Fig. 2.
Comparison of the effects of allopregnanolone and ganaxolone, each at a dose of 3 mg/kg, on survival in the mouse TETS status epilepticus model. Bars indicate percent of animals alive at 1 h, 24 h and 72 h after injection of TETS at a dose of 0.2 mg/kg, i.p. Number of animals in each group is shown within the bars. Chi-square analysis rejected the null hypothesis of no difference in alive and dead frequency at 72 h values among the 3 groups with P < 0.001. P values by 2 × 2 contingency analysis using two-tailed Fisher’s exact test for vehicle versus allopregnanolone and ganaxolone comparisons are <0.001 and 0.029, respectively; P value for comparison between allopregnanolone and ganaxolone is 0.15.
Comparison of allopregnanolone and ganaxolone for termination of TETS-induced status epilepticus
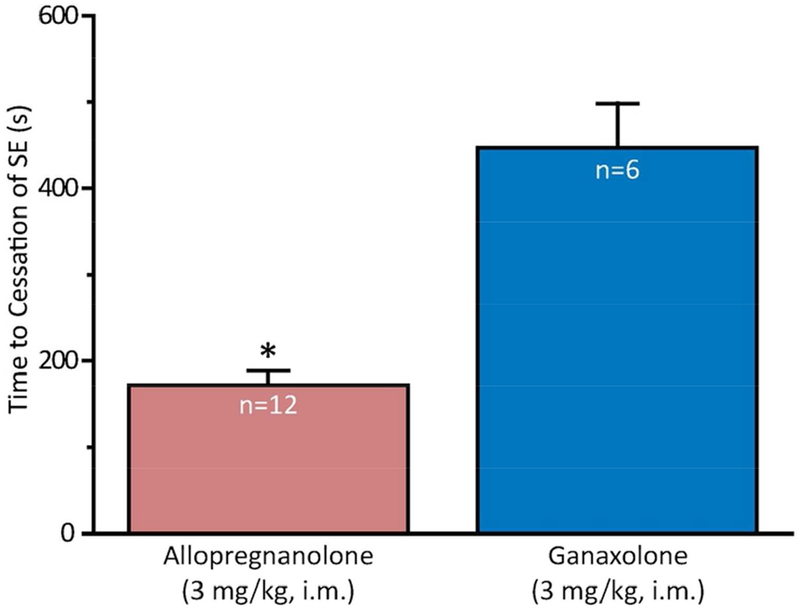
SE that has persisted for 40 min in the riluzole-TETS model is relatively resistant to termination by benzodiazepines (unpublished). Intramuscular administration of allopregnanolone or ganaxolone, each at a dose of 3 mg/kg administered 40 min after seizure onset, terminated behavioral clonic seizure activity in, respectively, 12 of 13 (92%) animals and 6 of 8 (75%) animals. Among the animals in which the treatment terminated behavioral seizures, allopregnanolone was more rapid than ganaxolone (Fig. 1).
Fig. 1.
Comparison of intramuscular allopregnanolone and ganaxolone, each at a dose of 3 mg/kg, for time to termination of behavioral seizures in mouse TETS status epilepticus model. The treatment was administered 40 min after the first myoclonic twitch. Allopregnanolone terminates SE more rapidly than ganaxolone (P < 0.001). Number of animals in each group is shown within the bars.
Long-term survival after treatment with allopregnanolone and ganaxolone in the TETS status epilepticus model
Animals experiencing TETS-induced SE were monitored for 72 h after TETS exposure. Twenty of 22 vehicle treated animal expired. In contrast, 11 of 13 (85%) allopregnanolone treated mice survived and 4 of 8 (50%) ganaxolone treated mice survived (Fig. 2).
Pharmacokinetic studies comparing equivalent doses of allopregnanolone and ganaxolone
Plasma and brain levels of allopregnanolone and ganaxolone were determined in naïve mice at intervals following intramuscular dosing. As shown in Figure 3, comparable plasma levels were achieved rapidly with both steroids (Cmax, 645 ng/ml for allopregnanolone and 550 ng/ml for ganaxolone; Table 1). Brain levels rose more slowly and peaked at 10 min in both cases. However, the peak brain concentration and brain exposure (AUC) of ganaxolone was greater than of allopregnanolone, which is likely explained by the modestly greater hydrophobicity of ganaxolone than allopregnanolone (logP values 5.423 and 5.042, respectively; calculated using ACD/Labs software V11.02). For both steroids, the brain exposure was approximately 3-fold the plasma exposure. Both steroids had high bioavailability indicating that they were nearly completely absorbed with intramuscular injection.
Fig. 3.
Plasma and brain levels of allopregnanolone and ganaxolone after intramuscular administration in mice, in both cases at a dose of 3 mg/kg. Each data point represents the mean ± S.E.M. of values from 6 to 8 mice. Dosing occurred at 0 time; blood samples were collected from 0.5 to 120 min for allopregnolone and 2 to 120 min for ganaxolone. The graph on the left shows the early data points on an expanded scale.
Table 1.
Descriptive pharmacokinetic parameters
| Analyte | Tissue | Tmax (s) | Cmax (ng/ml; ng/g) | t½ (min) | AUC 0→last (min•ng/ml; min•ng/g) | AUC 0→∞ (min•ng/ml; min•ng/g) | AUC brain/AUC plasma 0→last | AUC brain/AUC plasma 0→ ∞ | F |
|---|---|---|---|---|---|---|---|---|---|
| Allopregnanolone | Plasma | 60 | 645 | 16 | 12486 | 13538 | 2.94 | 2.97 | 0.97 |
| Brain | 600 | 845 | 36743 | 40213 | |||||
| Ganaxolone | Plasma | 120* | 550 | 25 | 17285 | 20502 | 2.93 | 3.02 | 0.95 |
| Brain | 600 | 1239 | 50647 | 61952 |
Allopregnanolone and ganaxolone were administered to mice at a dose of 3 mg/kg, i.m. Animals were sacrificed at various time points after injection and blood and brain was collected for analysis. Descriptive pharmacokinetic parameters are Cmax, maximal plasma or brain concentration, where plasma concentration is in ng/ml and brain concentration is in ng/g; Tmax, time to reach Cmax; t½, terminal half-life; AUC 0→last, area under the plasma concentration time curve to the last time point (7200 second) sampled; AUC 0→∞, area under the plasma concentration time curve extrapolated to infinity; F, bioavailability with intramuscular injection determined as the [(plasma AUC 0→∞) × CL1] ÷ 3 mg/kg, where CL1 is from Table 2.
First blood collection was at 120 s; Cmax value corresponds with this initial collection.
Two-compartment pharmacokinetic analysis indicated similar volumes of distribution for allopregnanolone and ganaxolone but the clearance of allopregnanolone from the central compartment was 54% greater than the clearance of ganaxolone (Table 2). This corresponds with a greater terminal half-life for ganaxolone, indicating that the synthetic analog has greater metabolic stability. Nevertheless, both allopregnanolone and ganaxolone have a sufficient duration of action to be effective in the TETS SE model.
Table 2.
Two-compartment pharmacokinetic analysis parameters
| Analyte | V1 (L/kg) | V2 (L/kg) | CL1 (L/kg/hr) | CL2 (L/kg/hr) |
|---|---|---|---|---|
| Allopregnanolone | 4.95 | 6.03 | 12.88 | 5.98 |
| Ganaxolone | 5.07 | 5.64 | 8.35 | 8.92 |
Parameters determined by two-compartment analysis with WinNonlin 7.0. V1, central volume of distribution; V2, peripheral volume of distribution; CL1, central compartment clearance; CL2, peripheral compartment clearance.
Discussion
Allopregananolone and ganaxolone have similar activity on synaptic and extrasynaptic GABAA receptors.14,18 Both agents have been proposed as treatments for SE, and there is evidence that intravenously administered allopregnanolone may be of benefit in the treatment of refractory SE in humans.12,13 To be optimally useful in the pre-hospital emergency treatment of SE, intramuscular dosing is preferred.25 In the present study, we have shown that intramuscular administration of both neuroactive steroids are effective in stopping SE induced by the chemical threat agent TETS; both agents also improve survival. Although the steroids are able to terminate behavioral seizures, we have found that they do not eliminate electrographic epileptiform activity resulting from TETS exposure (unpublished observations). It remains to be determined whether continued epileptiform discharges lead to irreversible pathology. To the extent that ongoing epileptiform activity contributes to morbidity, additional neuroprotective strategies may be required. With intramuscular administration, high plasma and brain levels of allopregnanolone are present for a relatively brief time. Longer periods of exposure may confer greater neuroprotection; this could be achieved with repeated injection or other strategies that increase the duration of delivery.
Although both allopregnanolone and ganaxolone at a dose of 3 mg/kg terminated TETS-induced SE, there were some differences. Allopregnanolone terminated SE more rapidly and was associated with greater survival. This is despite the fact that the plasma exposure (AUC) for ganaxolone is modestly greater than that of allopregnanolone, likely due to the enhanced metabolic stability of ganaxolone. Brain exposure for both steroids is about 3-fold the plasma exposure. The greater brain exposure than plasma exposure is expected given that both steroids are highly lipid soluble. The modestly greater plasma exposure of ganaxolone translates into a correspondingly greater brain exposure (~40%-50%). Our present observations are consistent with prior studies that have found greater brain concentrations than plasma concentrations following administration of allopregnanolone and ganaxolone in mice, rats and rabbits.14,33 In spite of the greater brain levels of ganaxolone, allopregnanolone more rapidly terminated TETS-induced SE and had a greater beneficial effect on survival. This is likely due to the modestly more potent activity of allopregnanolone on GABAA receptors.14,18 For example, while allopregnanolone and ganaxolone had similar maximal efficacy for activation of α1β3γ2 synaptic GABAA receptors and α4β3δ extrasynaptic GABAA receptors in the absence of GABA as assessed by a potentiometric dye assay, allopregnanolone had EC50 values of 9 nM and 27 nM for α1β3γ2 and α4β3δ, respectively, whereas the corresponding values for ganaxolone were 24 nM and 40 nM.18 Similarly, the EC50 values for potentiation of GABA responses on α1β3γ2 GABAA receptors in the potentiometric dye assay in the presence of GABA were 1.7 and 20 nM for allopregnanolone and ganaxolone, respectively. In an electrophysiological patch clamp assay with α1β3γ2 GABAA receptors in the presence of GABA, the corresponding values were 71.3 nM and 114.8 nM. In all cases, the EC50 values of allopregnanolone were modestly less than those of ganaxolone (between 50% to 1100%), reflecting greater allopregnanolone potency.
An additional difference between allopregnanolone and ganaxolone pharmacokinetics in the brain is that ganaxolone levels peak and fall whereas allopregnanolone levels are relatively flatter during the critical period when seizure termination occurs (5 to 15 min; Figure 3). This is likely the case because of the higher lipophilicity of ganaxolone (log P of 5.3 versus 4.9 for allopregnanolone), which causes ganaxolone to initially concentrate in brain to higher levels after which it redistributes to body fat. Maintaining a flatter distribution in brain is another factor that may account for the superior efficacy of allopregnanolone.
Allopregnanolone has the further advantage over ganaxolone as an intramuscular therapeutic agents in that it can be solubilized at higher concentrations using cyclodextrins. This is major benefit for intramuscular delivery, given that the injection volume is limited. However, alternative ganaxolone parenteral formulations may be available that allow concentrations greater than those that can be achieved with cyclodextrins obviating this negative factor.34
In summary, the present study has found that the neuroactive steroids allopregnanolone and ganaxolone when administered intramuscularly are both effective in terminating behavioral seizures and improving survival in mice exhibiting SE induced by the chemical threat agent TETS. However, at an equal dose of 3 mg/kg, allopregnanolone had a more rapid onset of action and a greater proportion of animals treated with allopregnanolone survived. The greater speed of allopregnanolone is especially desirable in the treatment of SE in which seizures of longer duration may be more difficult to treat and associated with greater morbidity and lethality.
KEY POINTS.
Diverse forms of status epilepticus, including status epilepticus caused by chemical threat agents, are often refractory to treatment
The neuroactive steroids allopregnanolone and ganaxolone are potential treatments for refractory status epilepticus
Intramuscular injection is the preferred route of administration for the pre-hospital treatment of status epilepticus
Allopregnanolone and ganaxolone are effective when administered by the intramuscular route in a model of chemical threat agent status epilepticus
Acknowledgments
This research was supported by the CounterACT Program, National Institutes of Health Office of the Director, and the National Institute of Neurological Disorders and Stroke under Grant NS079202. Additional support was provided by the Office of the Assistant Secretary of Defense for Health Affairs under Award No. W81XWH-09-1-0746. Opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
Footnotes
Disclosures
D.Z. and M.R. are named as inventors on patent applications claiming use of neuroactive steroids including allopregnanolone and ganaxolone in the treatment of status epilepticus. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- 1.Trinka E, Brigo F, Shorvon S. Recent advances in status epilepticus. Curr Opin Neurol 2016;29:189–198. [DOI] [PubMed] [Google Scholar]
- 2.Mayer SA, Claassen J, Lokin J, et al. Refractory status epilepticus: frequency, risk factors, and impact on outcome. Arch Neurol 2002;59:205–210. [DOI] [PubMed] [Google Scholar]
- 3.Kapur J, Macdonald RL. Rapid seizure-induced reduction of benzodiazepine and Zn2+ sensitivity of hippocampal dentate granule cell GABAA receptors. J Neurosci 1997;17:7532–7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazarati AM, Baldwin RA, Sankar R, et al. Time-dependent decrease in the effectiveness of antiepileptic drugs during the course of self-sustaining status epilepticus. Brain Res 1998;814:179–185. [DOI] [PubMed] [Google Scholar]
- 5.Naylor DE, Liu H, Wasterlain CG. Trafficking of GABAA receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005;25:7724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodkin HP, Joshi S, Mtchedlishvili Z, et al. Subunit-specific trafficking of GABAA receptors during status epilepticus. J Neurosci 2008;28:2527–2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodkin HP, Sun C, Yeh JL, et al. GABAA receptor internalization during seizures. Epilepsia 2007;48(Suppl. 5):109–113. [DOI] [PubMed] [Google Scholar]
- 8.Rogawski MA, Loya CM, Reddy K, et al. Neuroactive steroids for the treatment of status epilepticus. Epilepsia 2013;54 Suppl 6:93–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kokate TG, Svensson BE, Rogawski MA. Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 1994;270:1223–1229. [PubMed] [Google Scholar]
- 10.Reddy DS, Rogawski MA. Neurosteroids — Endogenous regulators of seizure susceptibility and role in the treatment of epilepsy In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet]. 4th edition Bethesda (MD): National Center for Biotechnology Information (US); 2012. Available from http://www.ncbi.nlm.nih.gov/books/NBK98218/ [PubMed] [Google Scholar]
- 11.Joshi S, Kapur J. GABAA Receptor Plasticity During Status Epilepticus In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper’s Basic Mechanisms of the Epilepsies [Internet]. 4th edition Bethesda (MD): National Center for Biotechnology Information (US); 2012. Available from https://www.ncbi.nlm.nih.gov/books/NBK98149/ [PubMed] [Google Scholar]
- 12.Broomall E, Natale JE, Grimason M, et al. Wainwright MS. Pediatric super-refractory status epilepticus treated with allopregnanolone. Ann Neurol 2014;76:911–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaitkevicius H, Husain AM, Rosenthal ES, et al. First-in-man allopregnanolone use in super-refractory status epilepticus. Ann Clin Transl Neurol 2017;4:411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez Botella G, Salituro FG, Harrison BL, et al. Neuroactive steroids. 1. Positive allosteric modulators of the (γ-aminobutyric acid)A receptor: Structure-activity relationships of heterocyclic substitution at C-21. J Med Chem 2015;58:3500–3511. [DOI] [PubMed] [Google Scholar]
- 15.Monaghan EP, Navalta LA, Shum L, et al. Initial human experience with ganaxolone, a neuroactive steroid with antiepileptic activity. Epilepsia 1997;38:1026–1031. [DOI] [PubMed] [Google Scholar]
- 16.Nohria V, Giller E. Ganaxolone. Neurotherapeutics 2007;4:102–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bialer M, Johannessen SI, Levy RH, et al. Progress report on new antiepileptic drugs: a summary of the Eleventh Eilat Conference (EILAT XI). Epilepsy Res 2013;103:2–30. [DOI] [PubMed] [Google Scholar]
- 18.Nik AM, Pressly B, Singh V, et al. Rapid throughput analysis of GABAA receptor subtype modulators and blockers using DiSBAC1(3) Membrane Potential Red Dye. Mol Pharmacol 2017;92:88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kokate TG, Cohen AL, Karp E, et al. Neuroactive steroids protect against pilocarpine- and kainic acid-induced limbic seizures and status epilepticus in mice. Neuropharmacology 1996;35:1049–1056. [DOI] [PubMed] [Google Scholar]
- 20.Carter RB, Wood PL, Wieland S, et al. Characterization of the anticonvulsant properties of ganaxolone (CCD 1042; 3α-hydroxy-3β-methyl-5α-pregnan-20-one), a selective, high-affinity, steroid modulator of the γ-aminobutyric acidA receptor. J Pharmacol Exp Ther 1997;280:1284–1295. [PubMed] [Google Scholar]
- 21.Gasior M, Ungard JT, Beekman M, et al. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology 2000;39:1184–1196. [DOI] [PubMed] [Google Scholar]
- 22.Kaminski RM, Livingood MR, Rogawski MA. Allopregnanolone analogs that positively modulate GABA receptors protect against partial seizures induced by 6-Hz electrical stimulation in mice. Epilepsia 2004;45:864–867. [DOI] [PubMed] [Google Scholar]
- 23.Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther 2000;295:1241–1248. [PubMed] [Google Scholar]
- 24.Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res 2010;89:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silbergleit R, Durkalski V, Lowenstein D, et al. Intramuscular versus intravenous therapy for prehospital status epilepticus. N Engl J Med 2012;366:591–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zolkowska D, Banks CN, Dhir A, et al. Characterization of seizures induced by acute and repeated exposure to tetramethylenedisulfotetramine. J Pharmacol Exp Ther 2012;341:435–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whitlow KS, Belson M, Barrueto F, et al. Tetramethylenedisulfotetramine: old agent and new terror. Ann Emerg Med 2005;45:609–613. [DOI] [PubMed] [Google Scholar]
- 28.Jett DA, Yeung DT. The CounterACT Research Network: basic mechanisms and practical applications. Proc Am Thorac Soc 2010;7:254–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pessah IN, Rogawski MA, Tancredi DJ, et al. Models to identify treatments for the acute and persistent effects of seizure-inducing chemical threat agents. Ann N Y Acad Sci 2016;1378:124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esser T, Karu AE, Toia RF, et al. Recognition of tetramethylenedisulfotetramine and related sulfamides by the brain GABA-gated chloride channel and a cyclodiene-sensitive monoclonal antibody. Chem Res Toxicol 1991;4:162–167. [DOI] [PubMed] [Google Scholar]
- 31.Barrueto F Jr, Furdyna PM, Hoffman RS, et al. Status epilepticus from an illegally imported Chinese rodenticide: “tetramine”. J Toxicol Clin Toxicol 2003;41:991–994. [DOI] [PubMed] [Google Scholar]
- 32.Chau CM, Leung AK, Tan IK. Tetramine poisoning. Hong Kong Med J 2005;11:511–514. [PubMed] [Google Scholar]
- 33.Irwin RW, Solinsky CM, Loya CM, et al. Allopregnanolone preclinical acute pharmacokinetic and pharmacodynamic studies to predict tolerability and efficacy for Alzheimer’s disease. PLoS One 2015;10(6):e0128313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai J. Phase 1 study to determine the pharmacokinetics, pharmacodynamics, and safety of IV ganaxolone in healthy adults. 6th London-Innsbruck Colloquium on Status Epilepticus and Acute Seizures (Salzburg, Austria, April 6-8, 2017). Poster P72. [Google Scholar]