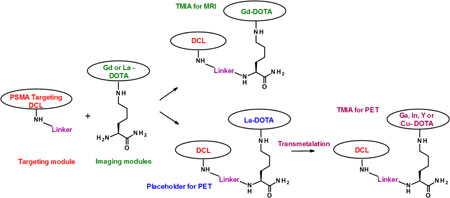
Abstract
A practical, convergent synthesis of prostate-specific membrane antigen (PSMA) targeted imaging agents for MRI, PET, and SPECT of prostate cancer has been developed. In this approach, metals chelated to DOTA were placed on the side chains of lysine early in the synthesis to form imaging modules. These are coupled to targeting modules, in this case consisting of the PSMA-binding urea DCL, bonded to an activated linker. The modular approach to targeted molecular imaging agents (TMIAs) offers distinct advantages. By chelating the MRI contrast metal Gd early, it doubles as a protecting group for DOTA. Standard coupling and deprotection steps may be utilized to assemble the modules into peptides, and the need for tri-t-butyl protection of DOTA requiring removal by strong acid is averted. This enables mild conjugation of the imaging module to a wide variety of targeting agents in the final step. It was further discovered that two labile metals, La3+ or Ce3+, can be used as placeholders in DOTA during the synthesis, then transmetalated in mild acid by Cu2+, Ga3+, In3+, and Y3+, metals used in PET/SPECT. This enables the efficient synthesis of non-radioactive analogs of targeted molecular imaging agents that may be transported or stored until needed. A simple and mild two-step transmetalation, involving de-metalation in dilute acid, followed by rapid chelation of the radioactive metal, may be conveniently performed later at the clinic to provide the TMIAs for PET or SPECT.
Keywords: prostate cancer (PCa), prostate-specific membrane antigen (PSMA), targeted molecular imaging agent (TMIA), magnetic resonance imaging (MRI), positron-emission tomography (PET)
Graphical Abstract
The clinical limitations of non-targeted molecular imaging agents for prostate cancer (PCa) has led to an exponential increase of investigational targeted imaging agents for magnetic resonance imaging (MRI), positron emission tomography (PET), and single-photon emission computed tomography (SPECT) in both preclinical and clinical research over the past five years.[1,2] In particular, the use of targeted PET agents was found to be remarkably effective in the detection, staging, and active surveillance of PCa.[3–8]
Targeted agents for MRI, as well as dual modal agents for SPECT/Optical Imaging, have also been shown to be effective in pre-clinical imaging of active PCa.[9–13] Recently, a study using simultaneous PET/MRI has reported remarkably high accuracy (80–97.5%) in the detection of multiple stages of PCa, underscoring the need for reliable synthetic methods for targeted probes for both PET and MRI of PCa.[14]
The success of targeted molecular imaging agents (TMIAs) for PCa has largely been made possible by the discovery of prostate-specific membrane antigen (PSMA) overexpression in PCa cells.[15–17] PSMA, also known as glutamate carboxypeptidase II (GCPII), NAALADase, and folate hydrolase FOLH1, is a well-characterized trans-membrane glycoprotein.[6] Its expression is elevated with increasing PCa stage and grade making it an excellent biomarker for staging, metastasis detection, and image-guided interventions.[4,6] PSMA is also expressed on the neovasculature of other types of carcinomas such as breast and thyroid, making it an important biomarker in the larger scope of cancer research as well.[18,19]
Elucidation of the structure of the PSMA binding site using protein crystallography led to the development of small molecule inhibitors which included amide, phosphonate, and urea substrate analogs[6,20,21] Among the most effective of these was a small molecule synthesized via a peptidomimetic urea linkage of lysine to glutamic acid in place of the naturally-occurring peptide amide substrate. This urea, designated by the names N-[N-[(S)-1,3-dicarboxypropyl]-carbamoyl]-(S)-L-lysine, DCL, and Glu-Urea-Lys, has emerged as the most effective PSMA inhibitor.[20,21]
Currently, the PCa TMIAs most widely investigated are 68Ga-labeled DCL probes for PET imaging, with the agent 68Ga-PSMA-11 (also known as 68Ga-DKFZ-PSMA-11) being most prevalent in clinical studies.[3–7,14] While 68Ga-DKFZ-PSMA-11 employs an acyclic metal-chelating group, HBED-CC, the alternative and well-known cyclic chelator, 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA), is at the core of many other reported TMIAs for both PET and MRI of PCa.[17,22–24] DOTA is also the chelating moiety in 177Lu-PSMA-617, an analogous radiotherapeutic agent in current clinical use. for PCa therapy.[25]
The cyclic structure of DOTA allows it to bind strongly to a wide variety of metals. In studies measuring kinetic and thermodynamic stability of chelated lanthanide metals it has been shown that DOTA is superior in complexing gadolinium (Gd3+) with minimal displacement of the metal in blood or other tissues compared to open chain chelating agents such as diethylenetriaminepentaacetic acid (DTPA).[26,27]
The stability of Gd within the cyclic DOTA chelating agents versus the weaker binding acyclic chelating agents such as DPTA agents is particularly advantageous in MRI. In contrast to the relatively safe cyclic complexes such as the DOTA-containing Dotarem®, open chain analogs such as Magnevist® can lead to gadolinium toxicity due to nephrogenic systemic fibrosis (NSF).[28]
When Gd-DOTA complexes are coupled to small molecules or small peptides, they maintain excellent stability, water solubility, and bioavailability.[26] For these reasons, DOTA continues to be widely utilized as the chelator of choice in MRI contrast agents.
Targeted PET imaging agents provide high sensitivity and functional information and may be fused with images from MRI or CT to provide higher spatial resolution. While not as sensitive as PET, MRI is described as exquisite in precisely depicting the internal prostatic anatomy, margins, tumor extent, and surrounding tissues.[23] Additionally, in preoperative reviews, the advantages of high resolution MRI allow surgeons to significantly improve decisions regarding the preservation or resection of neurovascular bundles while performing radical prostatectomies.[24]
Utilizing the targeting agent DCL, a series of small molecules containing Gd-DOTA targeted to PSMA was synthesized and found to be remarkably effective for the MRI of PCa in mouse models.[10] Apart from this study, there have been few reports of contrast agents for MRI which rely on DCL or other PSMA inhibitors as targeting moieties.
As part of our overall research goals, we developed a modular, peptide-based synthesis of TMIAs in which imaging modules, comprised of dyes or chelated metals bonded to the side chain of lysines, may be conjugated to a wide variety of targeting agents.[29,30] Given the prominence of DCL based, PSMA-targeted agents for PET/SPECT and MRI in pre-clinical and clinical research, we felt it was important to explore this method as an alternative means of assembling metal-based PSMA targeted imaging agents, for both modalities. This example would also demonstrate the wider utility of the modular method in molecular imaging research.
In the existing syntheses of metal based targeted agents for PET, SPECT, and MRI, the metal is routinely introduced in the last step.[31–34] This generally requires incorporation of an activated linker form of DOTA near the end of the synthesis or the incorporation of a tri-(t-butyl) ester protection scheme on DOTA, which further requires the use of relatively harsh trifluoroacetic acid (TFA) in the final steps. These conditions may preclude the use of desirable functional groups and may adversely restrict the order of synthesis. If harsh TFA is required in the final steps it could degrade many targeting peptides, proteins, or other delicate small targeting molecules.
In the approach presented here, metals chelated to DOTA are placed on the side chains of amino acids early in the synthesis to form imaging modules. We verified an earlier report that by chelating the metal early, it can double as a protecting group for DOTA, averting the need for the tri-t-butyl protection.[35] The imaging module may thus be incorporated into peptides using standard peptide synthesis conditions, or coupled to linkers and conjugated to targeting agents.
With the ability to conjugate the targeting group in the last step under mild conditions, and with no further treatment of strong acids or bases, a variety of targeting systems including sensitive small molecules, peptides, and proteins and antibodies may be coupled to a given modular imaging system in the final steps of the synthesis.
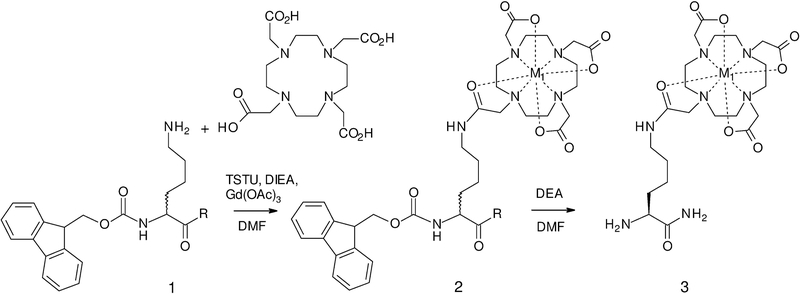
The optimized, one-step synthesis of the imaging modules containing chelating metals is shown in Scheme 1. In this approach, the starting Fmoc-Lys-NH2 (1a) was prepared by amidation of the commercially available Fmoc-Lys(Boc)-OH, followed by removal of the Boc group. The acid form, Fmoc-dLys-OH (1b), available commercially, is useful for creating imaging modules that may brought into in peptides involving standard coupling and Fmoc deprotection steps. The use of dLys stems from related work in which a second imaging module is added to form dual modal agents and is used to impart an increase in proteolytic stability.[29]
Scheme 1.
Synthesis of imaging modules: 1a: R = NH2, 1b: R = OH; 2a: R = NH2, M1 = Gd (for MRI); 1b, 2b: R = OH, M1 = Gd; 2c: R = NH2, M1 = La (placeholder for PET); 3a: M1 = Gd, 3b: M = La (placeholder for PET)
This method can be applied to the synthesis of gadolinium modules for MRI directly (M1 = Gd, Scheme 1), with no further transmetalation necessary. To facilitate the synthesis of radiolabeled agents for PET and SPECT, we made use of literature reports that two metals, lanthanum and cerium (M1 = La3+ or Ce3+), are significantly more labile than Gd, and determined that these chelates are stable to conditions of standard peptide syntheses.[36,37] The two metals can thus be used as placeholders, then transmetalated by Cu2+, Ga3+, In3+, and Y3+, chosen as non-radioactive models of the clinically relevant PET species.
In this synthesis of TMIAs for PSMA, we utilized imaging modules containing La3+ as a placeholder (2c and 3b in Scheme 3) as it is displaced more rapidly than Ce3+, but we showed earlier that Ce3+ may be used in cases where a more stable placeholder may be preferable.
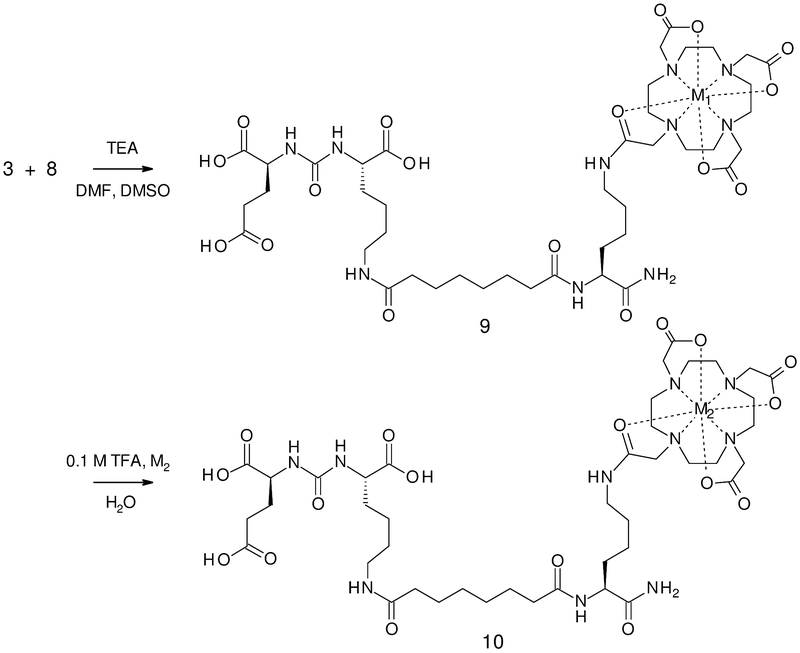
Scheme 3.
Coupling of imaging module (3) with targeting module (8) to form the TMIAs: 9a: M1 = Gd, and TMIAs for PET: 9b: M2 = La (placeholder for PET). 10a: M2 = Ga, 10b: M2 = Cu, 10c: M2 = In, and 10d: M2 = Y
To optimize the transmetalation step for the TMIAs, we modified methods by Sherry and others[36,37], used for measuring kinetics of acid-promoted displacement of La3+ and Ce3+ from their DOTA chelates using the modules, with the goal of providing the mildest conditions possible while completely removing the placeholder. While the transmetalation can be carried out with the metal present, the long reaction times are required which are not conducive for radiolabels. After verifying that the rate limiting step in mild acid was the de-metalation, we solved this by developing a two-step method of stirring the placeholder module or TMIA in dilute acid (0.2 M TFA) first, for 16–24 hours to de-metallate, followed by adding the PET metal which chelates to the DOTA rapidly.
To confirm complete removal of the placeholder metal, each step and all final products were monitored to the detection limit of the La3+ species by LC-MS. Using this method we found that Cu2+ and Y3+ chelation was complete, but the La3+ competed with Ga3+ and In3+ for re-metalation under the above conditions after neutralization. To ensure complete transmetalation in those cases, a method was devised to adhere the de-metallated TMIA in mild acid solution to a C-18 SPE cartridge or HPLC column, washing away the La3+, then treating the column with a 5mM solution of Ga3+ or In3+, and then eluting the pure product with an acetonitrile - water gradient.
To synthesize the targeting group DCL, two main methods are reported for preparation, with various modifications in the majority of subsequent studies.[20,21] These methods paved the way for a number of clinical trials including PSMA- targeted SPECT/CT and positron emission tomography (PET) using 18F labelled PSMA urea inhibitor and a targeted agent for endoradiotherapy of PCa.[22,34,38–40]
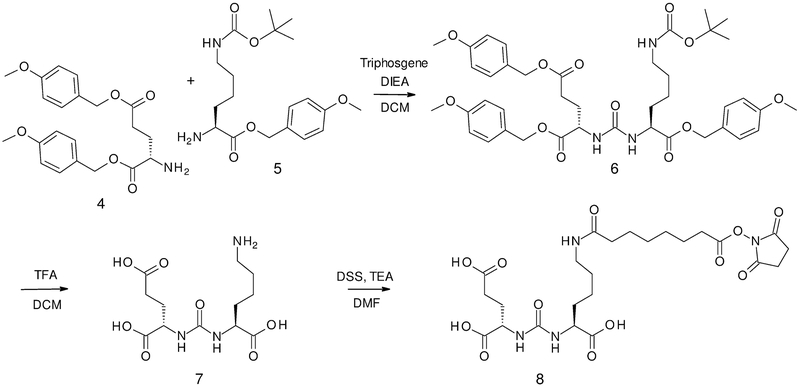
Our route to DCL is a modification of the synthesis reported by the Pomper group. It begins with the orthogonally protected glutamic acid, Boc-Glu(PMB)-OPMB, available from Boc-Glu-OH, with selective deprotection in acetonitrile to yield the deprotected amine, H-Glu(PMB)-OPMB (4) in high yield. The triply differentially protected lysine, Fmoc-Lys(Boc)-OPMB prepared from the readily available Fmoc-Lys(Boc)-OH, provided the deprotected amine, H-Lys(Boc)-OPMB (5), shown in Scheme 2. The urea precursors (4) and (5) were then coupled together as reported to form the protected form of DCL.
Scheme 2.
Synthesis of PSMA targeting agent DCL (7) and targeting module DCL-DSS (8).
A key modification in our synthesis of DCL was the complete deprotection of all the protecting groups on the urea using TFA in dichloromethane to yield the fully deprotected DCL (7), shown in Scheme 2. Similar to the use of Cbz for differential protection of the lysine by Maresca, et al[38], this avoided the selective deprotection of Boc in the presence of PMB groups. As applied to our modular method, this also avoided treatment by strong acid which would degrade the Gd3+ and placeholder chelates after their incorporation into the TMIA.
Initially, a two-step procedure was utilized to attach the suberate linker, DSS, onto the metal-chelated imaging module, followed by conjugation of the DCL in its protected or deprotected form in the last step. However, for our approach, a preferred method was devised in which DCL, in its deprotected form (7), was coupled first to the linker DSS to form the targeting module (8) directly, as shown in Scheme 2.
A similar approach to the targeting module, DCL-DSS (8), was previously reported in the syntheses of similar PSMA targeted MRI agents, and dual agents for OMI\SPECT.[10,12,21] In those reports, a two-step procedure couples the tri-PMB or tri-butyl protected DCL to the DSS. In the approach in Scheme 2, targeting module (8) is synthesized in one step from the entirely deprotected DCL (7). We also found that the activated DCL-DSS module could be conveniently purified by reverse phase HPLC under mildly acidic conditions
The modular synthesis of the TMIA for MRI (9a) was then completed in one convergent step by coupling imaging module (3) to targeting module (8) as shown in Scheme 3. This TMIA containing DOTA-chelated Gd3+, is analogous to the single Gd agent described earlier in the report on PSMA targeted contrast agents for MRI.[10]
The non-radioactive, model TMIAs for PET were synthesized by first forming the analog containing the La3+ placeholder metal (9b) by the same convergent step, followed by transmetalation by one of the two step methods described earlier for imaging module (2c). This involved a slow, de-metalation in mild acid, followed by rapid chelation in solution for Cu2+ (10b) and Y3+ (10d) or on a SPE cartridge for Ga3+ (10a) and In3+ (10c) to produce the model TMIAs for PET/SPECT.
As in the transmetalation reactions described for the imaging module (2c), complete removal of the placeholder, and 100 % re-metalation by the four metals was verified to the detection limit by LC-MS.
Relaxivity (r1) values of four compounds including two Fmoc protected modular intermediates (2A and 2B), the final targeted molecular imaging agent (9a), and a sample of Gd-DOTA (Dotarem®) were measured at 40 MHz. The r1 values are presented in Table 1 showing that the Gd containing modules and the TMIA have r1 values comparable to Gd-DOTA (Dotarem®). TMIA (9a) was also measured at 200 MHz to yield a similar r1 of 4.26 mM−1s−1 (see Supporting Information).
Table 1.
Relaxation rates of contrast agents.
| Contrast Agent | r1 (mM−1s−1) 1 T |
|---|---|
| F-Lys(Gd-DOTA)–NH2 (2a) | 4.88 |
| F-dLys(Gd-DOTA)–OH (2b) | 4.45 |
| DCL-DSS-Lys(Gd–DOTA)–NH2 (9a) | 4.66 |
| Gd-DOTA (Dotarem®) | 4.22 |
In summary, a modular method for the synthesis of TMIAs was applied to the synthesis of PSMA-targeted imaging agents for MRI, PET, and SPECT of prostate cancer. In the approach, metals utilized for MRI (Gd3+), or placeholders (La3+, Ce3+) for radioactive metals for PET (Cu2+, Ga3+, In3+, and Y3+) were chelated to DOTA and placed on the side chains of protected lysines early in the synthesis to form imaging modules. In one convergent step, these were coupled directly to a targeting module consisting of the PSMA-binding urea DCL bonded to an activated linker.
This modular method provides PSMA-targeted agents for MRI directly with no further transmetalation or deprotection steps. By using a placeholder place-holder analogs for PET agents, all synthetic steps leading to the targeted imaging agent can be accomplished without the constraints of time and safety aspects of handling radio-chemicals. Moreover, the agent containing a placeholder can be transported and stored until a small portion of it is needed for transmetalation. The very mild acid conditions for transmetalation are amenable to preparation of PET agents in a clinical setting.
Of the clinically relevant applications for prostate cancer, the method should be applicable to the synthesis of related PSMA TMIAs such as the tailor-made 68Ga-labeled Glu-urea-Lys-(Ahx)-HBED-CC, which contains a lipophilic amino acid bonded to a linker between DOTA-based PET imaging moiety and the DCL targeting moiety.35 It could also be utilized to synthesize targeted radioligand therapeutic agents such as the DOTA based 177Lu-PSMA-617. The method of preparation for these examples, currently in clinical use in patients with metastatic castration-resistant prostate cancer, and closely related analogs now in preclinical studies, should be amenable to this convergent, modular method. [25]
In the broader scope, the ability to conjugate a targeting agent in the last steps under mild conditions offers the use of a given modular imaging system for a wide variety of targeting systems. The imaging modules may be utilized in standard peptide coupling reactions and conjugated directly or via linkers to targeting agents in the final steps of synthesis. The mild conditions in the method are particularly amenable to targeting groups that are acid sensitive as it avoids the use of harsh acid in the final steps synthesis. We hope that others find the modular method useful in the synthesis of a wide variety of TMIAs.
Supplementary Material
Acknowledgments
This research was supported by grant NIH - NCI grants: 1-R15CA219915–01 (H.S.) and 1-R15CA192148–01 (H.S.) and by HHS-009–17SF from the S.A.S. Foundation and institutional support from the NIH grant P30CA06156 (K.L.N).
We thank the Louis Stokes Alliances for Minority Participation (LSAMP) at RIT for support for summer research (N.A.), the RIT Fred L. Emerson Foundation for summer funding (K.J.), the Daniel J. Pasto Award for summer funding (D.D.), and the RIT Honors Program for summer funding (D.D.).
We thank Mr. Furong Sun, Director of the Mass Spectrometry Facility, University of Illinois at Urbana-Champaign for the high resolution mass spectra (HRMS).
We thank Dr. J. Spernyak of the Roswell Park Cancer Center (RPCC) Translational Imaging Shared Resource (TISR) for MR imaging support and RPCC support grant P30 CA016056.
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Dulaney CR, Osula DO, Yang ES, Rais-Bahrami S, Prostate Cancer 2016, 2016, 4897515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Muteganya R, Goldman S, Aoun F, Roumeguère T, Albisinni S, Front. Surg 2018, 5, DOI 10.3389/fsurg.2018.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wüstemann T, Haberkorn U, Babich J, Mier W, Med Res Rev 2019, 39, 40–69. [DOI] [PubMed] [Google Scholar]
- [4].Bouchelouche K, Turkbey B, Choyke PL, Semin Nucl Med 2016, 46, 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Czarniecki M, Mena E, Lindenberg L, Cacko M, Harmon S, Radtke JP, Giesel F, Turkbey B, Choyke PL, Transl Androl Urol 2018, 7, 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gourni E, Henriksen G, Molecules 2017, 22, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Schwarzenboeck SM, Rauscher I, Bluemel C, Fendler WP, Rowe SP, Pomper MG, Afshar-Oromieh A, Herrmann K, Eiber M, J. Nucl. Med 2017, 58, 1545–1552. [DOI] [PubMed] [Google Scholar]
- [8].Rowe SP, Gorin MA, Allaf ME, Pienta KJ, Tran PT, Pomper MG, Ross AE, Cho SY, Prostate Cancer Prostatic Dis. 2016, 19, 223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Patel A, Salarian M, Xue S, Qiao J, Feng J, Tan S, Pu F, Li X, Mamouni K, Hekmatyar K, et al. , Nanoscale 2016, 8, 12668–12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Banerjee SR, Ngen EJ, Rotz MW, Kakkad S, Lisok A, Pracitto R, Pullambhatla M, Chen Z, Shah T, Artemov D, et al. , Angew. Chem. Int. Ed. Engl 2015, 54, 10778–10782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Banerjee SR, Chen Z, Pullambhatla M, Lisok A, Chen J, Mease RC, Pomper MG, Bioconjug Chem 2016, 27, 1447–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Foss CA, Pullambhatla M, Byun Y, Nimmagadda S, Banerjee SR, Green G, Fox JJ, Lupold SE, Mease RC, Pomper MG, Angew. Chem. Int. Ed. Engl 2011, 50, 9167–9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wu X, Yu G, Lindner D, Brady-Kalnay SM, Zhang Q, Lu Z-R, Am J Nucl Med Mol Imaging 2014, 4, 525–536. [PMC free article] [PubMed] [Google Scholar]
- [14].Grubmüller B, Baltzer PA, Hartenbach S, D’Andrea D, Helbich TH, Haug A, Goldner G, Wadsak W, Pfaff S, Mitterhauser M, et al. , Clin Cancer Res 2018, clincanres.0768.2018. [DOI] [PubMed] [Google Scholar]
- [15].Chang SS, Rev Urol 2004, 6 Suppl 10, S13–18. [PMC free article] [PubMed] [Google Scholar]
- [16].Bařinka C, Rojas C, Slusher B, Pomper M, Curr. Med. Chem 2012, 19, 856–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Eder M, Eisenhut M, Babich J, Haberkorn U, Eur J Nucl Med Mol Imaging 2013, 40, 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bychkov A, Vutrapongwatana U, Tepmongkol S, Keelawat S, Sci Rep 2017, 7, 5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fragomeni RAS, Amir T, Sheikhbahaei S, Harvey SC, Javadi MS, Solnes LB, Kiess AP, Allaf ME, Pomper MG, Gorin MA, et al. , J Nucl Med 2018, 59, 871–877. [DOI] [PubMed] [Google Scholar]
- [20].Byun Y, Foss CA, Castanares M, Mease RC, Banerjee SR, Fox JJ, Hilton J, Lupold SE, Kozikowski AP, Pomper MG, J. Med. Chem 2008, 51, 4504–4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Joyal JL, Hillier SM, Femia FJ, Keith D, Barone C, Maresca KP, Zimmerman CN, Kozikowski AP, Barrett JA, Eckelman WC, et al. , J. Med. Chem 2009, 52, 347–357. [DOI] [PubMed] [Google Scholar]
- [22].Foss CA, Banerjee SR, Mease RC, Rowe SP, Rao A, Kiess AP, Chen Y, Yang X, Cho SY, Nimmagadda S, et al. , Q J Nucl Med Mol Imaging 2015, 59, 241–268. [PMC free article] [PubMed] [Google Scholar]
- [23].Metcalfe P, Liney GP, Holloway L, Walker A, Barton M, Delaney GP, Vinod S, Tome W, Technol. Cancer Res. Treat 2013, 12, 429–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mazaheri Y, Shukla-Dave A, Muellner A, Hricak H, J Magn Reson Imaging 2011, 33, 258–274. [DOI] [PubMed] [Google Scholar]
- [25].Grubmüller B, Senn D, Kramer G, Baltzer P, D’Andrea D, Grubmüller KH, Mitterhauser M, Eidherr H, Haug AR, Wadsak W, et al. , Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1063–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Brücher E, Tircsó G, Baranyai Z, Kovács Z, Sherry AD, in The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, John Wiley & Sons, Ltd, 2013, pp. 157–208. [Google Scholar]
- [27].Sherry AD, Caravan P, Lenkinski RE, J Magn Reson Imaging 2009, 30, 1240–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Zhou Z, Lu Z-R, Wiley Interdiscip Rev Nanomed Nanobiotechnol 2013, 5, 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schmitthenner HF, Beach S, Weidman C, Barrett T, Modular Imaging Agents Containing Amino Acids and Peptides, 2015, US20150038672A1. [Google Scholar]
- [30].Schmitthenner HF, Sweeney-Jones AM, Williams S, Transmetalation Methods for the Synthesis of PET and SPECT Imaging Agents, 2016, US20160251378A1. [Google Scholar]
- [31].De León-Rodríguez LM, Kovacs Z, Bioconjugate Chemistry 2008, 19, 391–402. [DOI] [PubMed] [Google Scholar]
- [32].León‐Rodriguez LMD, Kovacs Z, Dieckmann GR, Sherry AD, Chemistry – A European Journal 2004, 10, 1149–1155. [DOI] [PubMed] [Google Scholar]
- [33].Azhdarinia A, Ghosh P, Ghosh S, Wilganowski N, Sevick-Muraca EM, Mol Imaging Biol 2012, 14, 261–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Benešová M, Schäfer M, Bauder-Wüst U, Afshar-Oromieh A, Kratochwil C, Mier W, Haberkorn U, Kopka K, Eder M, J. Nucl. Med 2015, 56, 914–920. [DOI] [PubMed] [Google Scholar]
- [35].André JP, Tóth É, Fischer H, Seelig A, Mäcke HR, Merbach AE, Chemistry – A European Journal 1999, 5, 2977–2983. [Google Scholar]
- [36].Cacheris WP, Nickle SK, Sherry AD, Inorg. Chem 1987, 26, 958–960. [Google Scholar]
- [37].Chang CA, Liu Y-L, Journal of the Chinese Chemical Society 2000, 47, 1001–1006. [Google Scholar]
- [38].Maresca KP, Hillier SM, Femia FJ, Keith D, Barone C, Joyal JL, Zimmerman CN, Kozikowski AP, Barrett JA, Eckelman WC, et al. , J. Med. Chem 2009, 52, 347–357. [DOI] [PubMed] [Google Scholar]
- [39].Armor T, Coleman RE, Goldsmith SJ, Vallabhajosula S, Petry NA, Cho S, Barrett JA, Stubbs JB, Maresca KP, Stabin MG, et al. , J Nucl Med 2013, 54, 380–387. [DOI] [PubMed] [Google Scholar]
- [40].Fan H, Mena E, Rowe SP, Plyku D, Nidal R, Eisenberger MA, Antonarakis ES, Szabo Z, Dannals RF, Chen Y, et al. , Mol Imaging Biol 2015, 17, 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.