Abstract
Development of a comprehensive regulatory T cell compartment in the thymus is required to maintain immune homeostasis and prevent autoimmunity. Here, we review cellular and molecular determinants of Treg cell development in the thymus. We focus on the evidence for a self-antigen focused Treg cell repertoire, as well as the APCs responsible for presenting self-antigens to developing thymocytes. We also cover the contribution of different cytokines to thymic Treg development and the cellular populations that produce these cytokines. Finally, we update the originally proposed “two-step” model of thymic Treg differentiation by incorporating new evidence demonstrating that Treg cells develop from two Treg progenitor populations and discuss the functional importance of Treg cells generated via either progenitor pathway.
Introduction
Adaptive immunity evolved as a powerful defense mechanism to eliminate foreign pathogens and eradicate transformed cells. This system relies on two chief capabilities- extensive repertoire diversity and the ability to discriminate “self” versus “non-self” (1). In T cells, diversity is derived from random rearrangements of the TCR alpha and beta loci (2, 3). However, diversity comes at a cost, as some of these rearrangements will generate self-reactive T cells capable of initiating pathogenic immune responses. The thymus acts as a training ground for T cells and plays a role in ensuring a diverse, “non-self” focused, TCR repertoire capable of eliminating pathogens. The process of generating a diverse TCR repertoire also leads to the development of many autoreactive T cells. Many of these autoreactive T cells are eliminated via clonal deletion in the thymus. However, many self-reactive T cells do escape clonal deletion and, when left uncontrolled, elicit detrimental autoimmune diseases. While several mechanisms evolved to control autoimmune responses, a specialized subset of suppressor CD4+ T cells, termed regulatory T cells (Treg), plays a particularly important role in maintaining immune homeostasis.
Over the past 20 years tremendous progress has been made in the identification and understanding of Treg cells. This relatively small population, ~1% of developing CD4 single positive thymocytes and ~10-15% of CD4+ T cells in secondary lymphoid organs, is responsible for maintaining immune homeostasis and is crucial for survival (4–9). Treg cells are an incredibly diverse population with regard to both TCR repertoire and function. Treg cells regulate numerous physiologic processes, including maternal-fetal conflict (10–17), germ cell tolerance (18), stem cell differentiation in the skin (19), muscle repair (20), adipocyte homeostasis and function (21–25), and retinal inflammation (26). In addition, Treg cells also regulate effector immune responses in disease states such as germinal center reactions (27, 28), inhibit overzealous T cell responses during infection (29–34), enhance effector T cell differentiation and memory formation to pathogens (35–37), inhibit tumor immunity (38, 39), and promote tolerance to environmental and commensal antigens (40–42). The burden of regulating these diverse processes has led the field to propose two broad functional classes of Treg cells defined by their ontogeny- peripheral- (pTreg) and thymic- (tTreg) derived Treg cells. In this review we focus on tTreg cell development.
Why the thymus?
The thymus has been an organ of immense curiosity for immunologists for some time. While initial thymectomy experiments failed to reveal immunological consequences (43), subsequent work revealed a central function in immune responses (44–46). Work as early as 1962 by Jacques Miller suggested a role in immune tolerance, as day 3 thymectomized (d3Tx) mice succumbed to an autoimmune wasting disease by 3 months of age (47). A seminal study in 1969 described that day 3, but not day 7 or later, thymectomized mice developed autoimmunity of the ovary that could be rescued by a thymus transplant (48). Work by Gershon and Kondo subsequently showed that thymocytes could produce dominant tolerance during immune responses to sheep red blood cells and coined the term “suppressor T cells” (49–51). Together, this work suggested the existence of a population of thymus-derived suppressive T cells that had delayed kinetics of thymic export.
Although the concept of immune suppression was clearly correct, early models to explain this process proved unsatisfactory. Most notably, it was suggested that “suppressor T cells” could function via a soluble factor encoded in the MHC locus, I-J (52). However, the I-J locus was eventually found not to encode a unique protein (53). This led many to reject the concept of a unique population of T cells capable of immune suppression (54). Despite these controversies, work in the early 1980’s already suggested the presence of a subpopulation of T cells, defined by anti-Lyt-1 (later described as CD5) antibody positivity, that were capable of suppressing autoimmunity in d3Tx mice (55). A seminal study by Sakaguchi in 1995 discovered that CD25+ T cells were necessary and sufficient for suppressing autoimmune responses. The identification of CD25 as a marker of suppressive T cells was critical to add legitimacy to the field (4). A follow-up study connected this concept to autoimmunity observed in d3Tx experiments, as d3Tx prevented accumulation of CD25+ cells in the periphery of mice. Transfer of CD25+ cells into d3Tx mice was able to rescue autoimmunity, while transfer of CD25-depleted splenocytes caused autoimmunity in athymic mice, revealing that thymically derived CD25+ T cells were critical controllers of autoimmunity (56). Groundbreaking studies in humans, suffering from immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX), and scurfy mice, identified a critical role for the transcription factor FOXP3 in Treg cells (6, 7, 57). This led to the generation of a series of reporter mice to track FOXP3 expression in live cells (58–60), enabling functional Treg transfer experiments. Additionally, protocols were developed to detect intracellular FOXP3 by flow cytometry that enabled tracking and quantification of Treg cells in non-reporter mice and humans (61). The identification of CD25 and FOXP3 as useful markers of Treg cells led to an explosion of studies seeking to understand Treg cell development and function.
Two-step model of thymic Treg cell development
The prevailing paradigm of thymic Treg cell development involves a two-step process (62, 63). Step one is driven by strong TCR stimulation in developing CD4 single positive thymocytes. This causes the upregulation of the high affinity IL-2 receptor, CD25, as well as TNF receptor superfamily (TNFRSF) members GITR, OX40 and TNFR2, thereby generating CD25+FOXP3- Treg cell progenitors (TregP). The second step is driven by cytokine-dependent conversion of TregP into mature Treg cells via upregulation of FOXP3. These CD25+FOXP3+ cells are mature Treg cells that emigrate from the thymus and mediate tolerance. More recent studies have implicated an alternative, CD25-Foxp3lo TregP cell population (64); differentiation of these TregP depends on the same two-step process (65). In this review we focus on the mechanisms that drive Treg cell development in the thymus and summarize current evidence on how the thymus shapes the Treg repertoire and function to maintain comprehensive immune tolerance.
TCR signals as an instructive cue for thymic Treg cell development
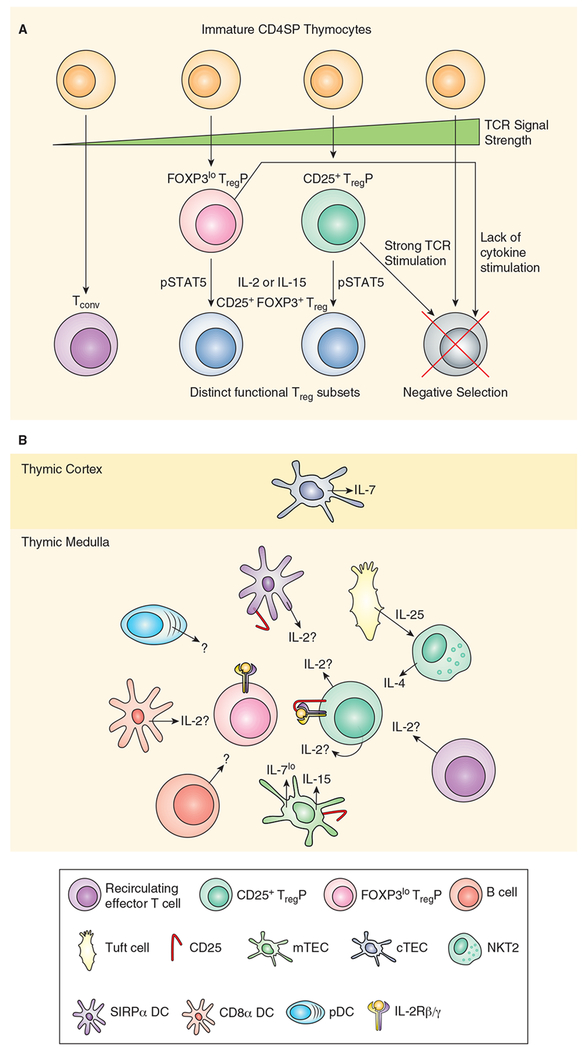
Whether the tTreg cell TCR repertoire is enriched in self-reactive TCRs was initially controversial. For example, one group found extensive overlap between TCRs in Tconv and Treg cells and suggested that Treg cells respond to “non-self” antigens (66). Likewise, analysis of AND TCR transgenic mice observed that inducing antigen expression increased Treg cell proportion but not numbers in the thymus, suggesting that engagement of cognate self-antigen was not driving Treg cell development (67). Nevertheless, other studies have provided evidence that the Treg cell TCR repertoire is more self-reactive than its conventional counterpart, and that acquisition of agonist TCR stimulation is important in Treg cell development. This view originated from early experiments observing the presence of CD25+ cells in the thymus of wildtype mice, but not those expressing a transgenic TCR specific for foreign antigen (68). This hypothesis was confirmed in later studies showing that TCR transgenics could drive thymic Treg cell development only when the cognate antigen was also expressed in the thymus (69). Further, TCR sequencing experiments on mice with reduced TCR repertoires observed that Treg TCRs are largely distinct from conventional T cell TCRs (70, 71), but overlap with TCRs expressed by pathogenic self-reactive T cells in Foxp3−/− mice (72). In addition, a series of experiments observed that intraclonal competition for cognate antigen limits Treg cell differentiation (73, 74) suggesting that interaction with antigen, presumably self-antigen, is important for Treg cell development. Later work used TCR transgenics with varying affinity for OVA and observed a linear relationship between TCR affinity and Treg cell development (75). OVA-specific Treg cells develop in RIP-mOVA thymi with TCRs spanning a broad 3 log fold response range. While lower affinity TCRs can drive Treg induction, TCR affinity and Treg cell niche size are directly correlated with higher affinity TCRs driving increased numbers of Treg cells (75). Further, analysis of Nur77-GFP transgenic reporter mice, in which GFP is expressed coordinately with TCR signal strength, observed that Treg cells were interacting more strongly with self-antigens (76). For example, lower proportions of TCR transgenic thymocytes in chimeric mice led to increased CD25+ cell proportions and higher Nur77-GFP signal, confirming that developing Treg cells compete for self-antigen during lineage commitment. TCR signal strength has also been related to the competency of developing TregP cells to respond to low levels of intrathymic IL-2, suggesting another mechanism that would bias a Treg cell repertoire towards self-reactivity (65). More recent studies have shown that intermediate dwell times for TCR-peptide:MHC complexes facilitate Treg differentiation, while shorter dwell times preferentially drive positive selection and longer dwell times lead to clonal deletion (77). This evidence collectively suggests that Treg cell interaction with thymically presented antigen, at some elevated threshold (Figure 1a), is necessary for initiating Treg cell development.
Figure 1.
A, Model of thymic Treg cell development. CD4 single positive thymocytes interact with a range of different affinity for self-antigens presented by thymic APC subsets including, SIRPα+ and CD8α+ DC, pDC, mTEC, B cells and perhaps macrophages. TCR signal strength initiates fate decisions. Weak TCR signaling is required to develop Tconv, while strong TCR stimulation drives clonal deletion. Intermediate TCR signaling drives Treg cell commitment; stronger TCR signals lead to upregulation of CD25 generating a CD25+ TregP while weaker TCR stimulation causes upregulation of FOXP3 and produces a FOXP3lo TregP. Some CD25+ TregP still undergo clonal deletion, likely due to the high TCR signal strength experienced by this population and FOXP3 expression in FOXP3lo TregP drives clonal deletion unless counterbalanced by survival signals mediated by engagement of γC cytokines. When either TregP bind IL-2, or IL-15, this activates STAT5 and completes the differentiation of mature tTreg cells, defined by dual expression of CD25 and FOXP3. B, Cytokine producing cells in Treg cell development. Various cells in the thymus contribute cytokines to the thymic microenvironment. cTEC produce IL-7 which may function as a survival factor for developing thymocytes in the cortex. mTEC have been shown to produce IL-15 as well as low levels of IL-7. mTEC also express CD25 which may function to transpresent IL-2 to developing TregP or deplete local IL-2 from TregP. DC derived IL-2 may be produced by CD8α+ DC or SIRPα+ DC however, SIRPα+ DC also express CD25 which may modulate local IL-2 availability. It is unknown if pDC produce Treg inducing cytokines. Similarly, it is unknown if thymic B cells contribute any cytokines capable of driving Treg differentiation or serve only as a antigen presenting cell. Tuft cells produce IL-25 which acts on NKT2 cells to produce intrathymic IL-4. IL-4 plays a role in promoting survival and/or differentiation of Foxp3lo TregP. Finally, T cells represent the critical source of IL-2 required to drive Treg cell differentiation, however, it is unclear if the IL-2 is being produced by CD25+ TregP or a subset of recirculating effector T cells.
APCs
Medullary thymic epithelial cells (mTECs)
Thymic selection is defined by a cellular dilemma - without the presence of specialized cell subsets, such as pancreatic beta cells, how is the T cell repertoire pruned of reactivity to tissue specific antigens (TSA) uniquely encoded by these cells? This led to the hypothesis that these specialized self-antigens were in fact expressed at some low level in the thymus, an idea first corroborated by human data correlating thymic insulin expression and susceptibility to the development of diabetes (78, 79). Subsequent work revealed evidence of broad “promiscuous” gene expression in the thymus and attributed mTEC with the sole ability to produce these TSAs (80). These studies also correlated expression of the transcriptional modulator Autoimmune Regulator (AIRE), a gene previously linked to polysymptomatic autoimmunity (81, 82), with the presence of TSA expression in mTECs. This supposition was confirmed in a set of ground-breaking experiments, showing AIRE expression was necessary for tissue specific gene expression in mTECs. Mice that lacked thymic expression of these TSAs had increased numbers of autoreactive T cells in peripheral lymphoid organs, which led to multiorgan immune destruction and generation of autoantibodies (83). Likewise, HEL reactive TCR transgenic T cells underwent clonal deletion when HEL was expressed under the control of the rat insulin promoter, an AIRE responsive locus in mTEC. The proportion of CD25+ thymocytes increased; however, since there was no change in absolute number of these cells, the authors dismissed a role for Treg cell development to these antigens (84). These observations led to the hypothesis that the main role of AIRE in central tolerance was due to clonal deletion of tissue specific effector T cells.
While some controversy exists, numerous studies have now defined a role for AIRE-mediated ectopic antigen expression in mTECs in tTreg cell development. Early studies in humans patients with Autoimmune Polyendocrinopathy Candidiasis and Ectodermal Dysplasia, a disorder caused by mutations in AIRE, documented a loss of Treg cells and alterations in their TCR repertoire (85). Further, expression of hemagglutinin (HA) via the AIRE promoter in mice led to the development of HA-specific Treg cells, which was dependent on MHC-II expression on mTECs (86). However, a follow-up study in AIRE-OVA mice produced a counterpoint to this hypothesis, as MHC-II knockdown on mTECs caused an increase in OVA-specific Treg cell development (87). This finding suggested that low levels of high affinity antigens drive tTreg differentiation, while higher expression of these same antigens resulted in clonal deletion. In addition, another study observed AIRE-dependent prostate-reactive Treg cell development in the thymus (88). Interestingly, analysis of the TCR repertoire of Tconv and Treg cells in wildtype and Aire−/− mice found that cells normally directed towards the Treg cell lineage were instead found in the Tconv lineage in Aire−/− mice (89), suggestive of Treg cell agonist selection via AIRE driven antigens. A similar phenomenon is observed in human patients harboring AIRE mutations in which TCRs normally found in Treg cells are found in the Tconv compartment (90). In addition to AIRE, the transcription factor FEZF2 also regulates expression of TSA in the thymus. Fezf2−/− mice also developed multiorgan autoimmunity, but the spectrum of organs targeted was distinct from Aire−/− mice (91). Fezf2−/− mice have fewer Treg cells in the thymus and an altered TCR repertoire, reiterating a role for TSA expression in Treg cell development. These results point to a crucial role for mTEC-derived TSA in central tolerance and Treg cell development.
Recently, a distinct stromal cell involved in initiating type II mucosal immune responses, the Tuft cell, has been identified in the thymus. Tuft cells were found to resemble mTEC and produce IL-25, a major inducer of IL-4 production (92, 93). Tuft cells contribute to the Hassall’s corpuscle, a structure in the thymus previously associated with Treg cell generation in humans via licensing thymic dendritic cells (DC) to produce CD80 and CD86 via TSLP stimulation (94). Interestingly, we observed that mice lacking the transcription factor POU2F3, which is required for Tuft cell development, have reduced numbers of FOXP3lo TregP suggesting that Tuft cells can influence Treg cell differentiation (95). Although the mechanism for this remains unclear, it may be due to IL-25 production or the expression of unique TSAs by Tuft cells such as taste receptors (93).
Dendritic cells
The thymic DC compartment consists of conventional DC, including SIRPα+ and CD8α+ DC, and plasmacytoid DC (pDC) (96). Earlier studies suggested that DC favor clonal deletion over Treg cell development (86, 97). However, experiments using MHC-II−/− bone marrow chimeras clearly implicated a role for bone marrow-derived DC in both clonal deletion and Treg cell induction (98). Other data using in vitro models of Treg cell development also observed efficient Treg generation by conventional DC, and to a lesser extent pDC (98–100). While the role of DCs in Treg development has become clearer, the antigens they present, required for inducing tolerance, remain blurry. This is due to the paradox that tolerance to AIRE-driven antigens are frequently dependent on DCs (101). Mechanistic insight to this paradox was revealed in studies documenting antigen transfer from AIRE-expressing mTEC to medullary DC (102, 103). Interestingly, AIRE+ mTEChi cells produce the chemokine XCL1 that recruits thymic CD8α+ DCs to the medulla, and Xcl1−/− mice exhibit defects in Treg generation (104). CD8α+ DC are the dominant cross-presenting thymic DC subtype; thus, in addition to producing intrathymic antigens (105), AIRE also mediates recruitment of APC populations to the thymic medulla required for efficient Treg induction. Subsequent work used TCR sequencing and TCR transgenics derived from TCRs isolated from Treg cells to determine the relative contributions of DCs and mTECs on central tolerance (105). This study observed that for some antigens, mTEC and DC played non-redundant roles in Treg cell differentiation and clonal deletion. However, for other antigens, mTEC and DC played redundant roles in Treg cell selection due to transfer of antigen from mTEC to DC. Indeed, more recent studies using a prostate reactive TCR transgenic observed that DC were required to generate Treg cells in the thymus, despite expression of the antigen being AIRE dependent (106). These experiments highlight the complex interconnections between thymic DC and mTEC necessary for broad induction of antigen-specific thymic Treg cells
The contribution of SIRPα+ DC and pDC in Treg cell polarization is particularly interesting as these represent migratory DC populations, capable of trafficking peripheral antigens to the thymus and inducing Treg cell differentiation (96, 107, 108). pDC also survey the gut via a CCR9 dependent mechanism (109), a chemokine receptor also required for pDC thymic localization and induction of central tolerance to peripheral antigens (110). This could represent a mechanism to transport gut-derived environmental or commensal antigens to the thymus. However, the contribution of endogenous peripheral self- or non-self-antigen trafficking to the thymus in Treg development remains an open question.
B cells
The presence of non-transformed B cells in the thymus was observed more than 30 years ago (111). Early studies observed that B cell-deficient animals failed to delete Mtv-9 specific T cells, but reconstitution of these mice with B cells rescued this deletion (112). Further, in vitro studies observed efficient deletion of thymocytes by thymic but not splenic B cells (113). More recent studies have confirmed a role for thymic B cells in deletional tolerance to self-antigens (114, 115). For example, B cells induce clonal deletion of KRN autoreactive TCR transgenic T cells (116). The role of intrathymic B cells in Treg cell development is less clear. The first evidence that thymic B cells affect tTreg cell development came from the observation that BAFF-Tg mice had more tTreg cells than WT mice, due to an increase in thymic B cells. However, tTreg cell development was decreased when thymic B cells were derived from hen egg lysozyme specific transgenic B cells, suggesting that a broad, self-reactive B cell repertoire was required to promote tTreg cell development (117). Using in vitro differentiation models, it was also observed that B cells isolated from the thymus were able to polarize CD4+ thymocytes to the Treg cell lineage in a contact, CD80/86, and MHC-II dependent manner (118). These experiments suggested that B cells increase the presence of CD25+ TregP cells but do not facilitate the subsequent conversion of TregP cells to mature Treg cells.
T cells reactive to B cell encoded proteins (such as Ig) are deleted by thymic B cells (119–121). There is some evidence that Treg cells may also be generated to BCR antigens (120), although whether this happens in the thymus is unclear. In mouse and humans, AID- and CD40L-deficiency results in autoimmunity that correlates with a decrease in the proportion Treg cells (122). These studies, combined with observations that thymic B cells induce Treg cell development in an MHC-II dependent manner, suggest that thymic B cell-induced Treg cell generation is critical for comprehensive immune homeostasis. Moreover, it was observed that self-antigens drive thymic B cell class-switching, which was required for inducing tolerance to self-antigens and was dependent on AID (123). A thymic B cell licensing process has also been described wherein interactions with T cell-derived CD40L increases antigen presentation on thymic B cells and induces AIRE expression on these B cells (124). This raises the possibility that thymic B cells have a parallel function to mTEC in producing TSA. However, it is still unclear what specificities of tTreg cells are dependent on thymic B cells and whether interactions with thymic B cells preferentially promote Treg cell development via CD25+ or Foxp3lo TregP cells.
Cytokines in thymic Treg cell development
Prior to the identification of CD25 as a marker for Treg cells there were hints that IL-2 receptor signaling was important for immune tolerance. In 1993, Il2−/− mice were generated; these mice had increased numbers of activated T cells and developed colitis-like disease (125). Similar observations were made in Il2ra−/− and Il2rb−/− mice (126, 127). This was initially puzzling as IL-2 is a known T cell growth factor. Subsequent studies revealed that expression of IL2Rβ specifically in the thymus was sufficient to rescue the autoimmune phenotype observed in Il2rb−/− mice, suggesting a role for IL2R signaling during tTreg development (128). These findings were questioned by studies showing development of CD25-FOXP3+ Treg cells in Il2−/− mice (129–131) and that transfer of T cells from Il2−/−mice could protect against experimental autoimmune encephalomyelitis (EAE) (132). However, further analysis observed that while Il2−/− do develop a small population of CD25-Foxp3+ Treg cells, IL2Rβ−/− have a larger block in Treg cell development (131, 133). Further experiments observed that IL2Rβ binding cytokines, IL-2 and IL-15, were the major inducers of Treg cell development (131), although IL-7 had limited capacity to induce FOXP3 expression (134, 135). These latter findings reconciled previous reports of Treg cell development in Il2−/− mice, suggesting that in the absence of IL-2 other cytokines drive Treg development, although not as efficiently as IL-2. Further, Stat5−/− T cells are unable to differentiate into Treg cells, while constitutive activation of STAT5 in STAT5b-CA transgenic mice led to a striking increase in Treg cell differentiation (136, 137). Together, these findings confirm the critical role STAT5 plays in Treg cell development.
Other γC cytokines have also been evaluated for their effect on Treg cell development. IL-4 potently inhibits induced Treg cell generation, and IL-4 blockade increased Treg cell differentiation both in vitro and in vivo (138). Moreover, IL-4 is unable to induce STAT5 activation in CD25+ TregP cells and Il4ra−/− mice show no obvious defect in Treg cell generation in the thymus (134). However, more recent work has observed that IL-4 stimulation of Foxp3lo TregP maintains FOXP3 expression and upregulates CD25. Further, Itk−/− mice, which exhibit elevated IL-4 production, exhibited an IL4Rα-dependent increase in FOXP3lo TregP and mature Treg cells. Consistent with this observation, BALB/c mice also have increased tTreg cell production that is diminished on the Cd1d−/− background (95), which eliminates NKT2 cells responsible for producing excess IL-4 in BALB/c mice (139, 140). Thus, IL-4 may function as a survival factor, or provide a direct differentiation stimulus, for FOXP3lo TregP. However, the mechanism by which IL-4 promotes tTreg cell development and the significance of this pathway remain unclear.
The cellular sources of cytokines needed for tTreg development remain incompletely understood. T cells and dendritic cells represent the most likely cellular sources of IL-2 for tTreg differentiation. Recent studies have observed that DC-derived IL-2 was particularly important for inducing Treg cell development in ex vivo thymic slice models (141). These experiments suggested that DCs create a niche for efficient Treg cell development by providing the antigenic stimulation for TregP cell generation and the cytokine responsible for driving Treg cell maturation. However, more recent work, using Il2fl/fl mice crossed to T cell (Cd4-Cre), DC (Cd11c-Cre) or B cell (Cd79a-Cre) specific CRE-recombinases, observed that T cell-derived IL-2 is necessary and sufficient to drive tTreg cell development (142). Further, autocrine production of IL-2 was not required for conversion of TregP into mature Treg cells. It remains unclear what subset of T cells is producing the intrathymic IL-2 necessary for Treg cell development. FOXP3 blocks Il2 transcription (143), likely precluding FOXP3lo TregP as producers of IL-2. However, CD25+ TregP may be competent to produce intrathymic IL-2 as these cells are receiving strong TCR stimulation. Alternatively, IL-2 may also be generated by activated recirculating T cells in the thymus (Figure 1b). Future studies are necessary to pinpoint the specific cellular sources of IL-2 in tTreg cell development.
Generation of IL-7 and IL-15 reporter mice has provided initial insight into the cellular players producing these cytokines in the thymus. Using IL-7-GFP knock-in mice, it was observed that IL-7 is present in both the thymic cortex and medulla. However, on a per-cell basis cortical thymic epithelial cells produced more IL-7 than mTECs (144). The lack of robust IL-7 production in the thymic medulla may explain the negligible effect of IL-7 on Treg development (134). IL-15-CFP reporter-mice produced the opposite result; IL-15 was preferentially found in the thymic medulla (145). Interestingly, IL-15 production was highest in mTEChi cells, the most robust antigen-presenting subset of mTECs defined by high expression of AIRE. More work is required to understand the cellular sources of IL-15 that may be contributing to tTreg cell development.
Transcriptional regulation
Transcriptional regulation of Foxp3 and the broader Treg epigenetic signature is essential for proper tTreg cell development. Experiments to reverse engineer the Treg cell transcriptional network surprisingly revealed a highly redundant system (146). It was revealed that FOXP3 alone was insufficient to drive the stable Treg cell transcriptional landscape. However, FOXP3 plus any one of a quintet of other transcription factors - EOS, IRF4, SATB1, LEF1 or GATA1 - was sufficient to solidify the Treg cell transcriptional signature. Deletion of EOS or LEF1 had no effect on Treg development by themselves (147, 148), while the effects of IRF4 or GATA1 deletion on Treg development remain unstudied. However, subsequent studies observed a critical role for SATB1 in tTreg cell development. SATB1 deletion at the CD4+CD8+ thymocyte stage prevented subsequent establishment of Treg cell super-enhancers and caused inefficient Foxp3 expression during later Treg cell differentiation (149). Early work suggested that TCR stimulation also facilitates Treg cell epigenetic signatures (150, 151). However, more recent experiments using an Il2ra mutant mouse provide evidence that IL-2 signaling is important for initiating the Treg epigenetic signature (152). Specifically, SATB1 positioning throughout the genome was interrupted in developing T cells in Il2ra mutant mice. These results suggest that IL-2 signaling is also important for SATB1 to establish the Treg epigenetic signature. Finally, deletion of the transcription factors Nr4a1-3 almost completely blocks tTreg generation (153, 154). Whether Nr4a family members, or other transcription factors, act in concert with SATB1 to establish a permissive state prior to Foxp3 upregulation remains an open question.
Several studies have shown a crucial role for NFκB activation in Treg cell development. In particular, c-Rel activation is required for Treg cell development (155–158). c-Rel, but not NFκB1, activation downstream of CD28 is required for developing T cells to become CD25+ TregP (156). However, Foxp3lo TregP are highly dependent on both c-REL and NFκB1 expression (95). Moreover, p65 (RELA) deficient thymi also contain decreased amounts of CD25+ TregP and mature Treg cells (159). RELA and c-REL play partially redundant roles in maintaining Treg cell transcriptional signature and homeostasis, although deletion of RELA resulted in a more severe autoimmune phenotype than deletion of c-REL (159). These findings suggest that NFkB family members may also be important in locking in a stable Treg cell phenotype, although the precise function of each NFκB member during tTreg development in establishing the Treg cell transcriptional signature is still uncertain.
A key step in the development of tTreg cells is stable upregulation of Foxp3. Much effort has focused on the factors and regulatory elements that control Foxp3 expression. Several conserved regulatory regions in the Foxp3 locus have been identified. These include the Foxp3 promoter, three intronic enhancers (Cns1-3) (158) and the Foxp3 pioneer enhancer element Cns0 (149). Cns0 is targeted by the transcriptional regulator SATB1 and acts to poise the Foxp3 locus for active transcription (149). Later during Treg cell selection, Cns3 acts as a pioneer regulatory element in the Foxp3 locus to drive de novo Foxp3 expression. This pioneer function is dependent on agonist TCR stimulation- and CD28-induced activation of c-Rel and binding of c-Rel to Cns3 (157, 158). c-Rel targeting to the Foxp3 locus arranges an enhanceosome including several other transcription factors important for Foxp3 expression including RELA, NFAT, SMAD and CREB (160). Cns3−/− Treg cells are biased towards higher self-reactivity suggesting that c-Rel targeting of Cns3 is required to sensitize the Foxp3 locus to TCR stimulation (161). Additionally, Cns3−/− thymi are devoid of the less self-reactive Foxp3lo TregP cell population (95). These experiments suggest that Cns3 evolved in part to expand the repertoire of Treg cells. Interestingly, deletion of an Il2ra enhancer element CaRE4 (162), that has been linked to autoimmune SNPs in humans (163–165), causes a mild block in CD25+ TregP and mature tTreg development (95). Thus, regulatory regions inside the Foxp3 locus as well as those outside of Foxp3 are required for proper Treg cell development. Future studies will need to identify other enhancer elements critical for tTreg cell development and determine the specific role these enhancers play in generating the mature Treg cell repertoire and transcriptome.
Cellular models of thymic Treg cell development
Studies of early Treg cell ontogeny (58) illustrated that CD25 expression precedes FOXP3 expression and the thymic CD4+CD25+ compartment is comprised of both FOXP3+ and FOXP3- cells (166). This data provided the first hint that CD4+ CD25+ FOXP3- thymocytes may represent cellular progenitors for mature CD25+ FOXP3+ Treg cells. Subsequent studies illustrated that CD25+FOXP3- thymocytes represent the direct cellular progenitors of mature Treg cells (62, 63). These studies provided a “two-step” model of thymic Treg cell differentiation (Figure 1a). In step one agonist TCR stimulation generates a CD25+ TregP cell, while in step two IL-2/STAT5-converts CD25+ TregP into mature Treg cells. Later studies connected these two steps, finding that TCR signal strength correlated with expression of three TNFRSF members, GITR, OX40 and TNFR2, and signaling via these TNFRSF members renders developing TregP cells much more sensitive to IL-2 (65). Thus, higher TCR self-reactivity imputes a selective advantage for developing TregP by allowing these cells to compete more effectively for IL-2, thereby biasing the Treg cell repertoire towards self-reactivity.
More recently, an alternative TregP population was identified, defined by low FOXP3 and lack of detectable CD25 expression (Foxp3lo TregP). Initial reports demonstrated that Foxp3lo TregP cells efficiently develop into mature Treg cells either in vitro to high dose IL-2 (200 U/mL) or in vivo in the periphery of mice. However, this paper also suggested that FOXP3 is normally a pro-apoptotic protein and must be counterbalanced by γC cytokine stimulation, such as IL-2, in order for TregP to survive thymic selection (167). Despite the lack of CD25 expression, Foxp3lo TregP cells are able to differentiate into mature Treg cells in response to low-dose IL-2 (0.2-1 U/mL) (65, 95) or intrathymic transfer (95, 168). Interestingly, in competitive intrathymic transfer experiments, CD25+ and Foxp3lo TregP both differentiated into mature Treg cells at similar efficiencies - it remains unclear how Foxp3lo TregP are capable of such IL-2 sensitivity while lacking CD25 expression. CD25+ TregP experience greater TCR stimulation, as measured by NUR77-GFP signal intensity, than Foxp3lo TregP during thymic selection (95, 168). The TCR repertoire of these two TregP populations overlap significantly with mature Treg cells but much less so with each other (95). These observations suggested that these were unique TregP populations selected by distinct interactions with self-antigens and contributed unique TCRs to the mature Treg cell repertoire. Remarkably, Treg cells derived from CD25+ TregP, but not Foxp3lo TregP, could protect mice from EAE while Treg cells derived from Foxp3lo TregP were able to consistently suppress colitis. Collectively, these data provide an updated model of tTreg cell development in which both CD25+ and Foxp3lo TregP contribute quantitatively equivalently, but qualitatively distinctly, to the mature Treg cell repertoire.
Future considerations
Despite decades of research directed at understanding the development of tTreg cells, many questions remain unanswered. While two cellular progenitors have been described that contribute to the mature Treg cell repertoire, the precursors to each of these populations have not been effectively described. Preliminary reports have identified a CD122+ GITR+CD25-Foxp3- TregP precursor that can give rise to CD25+ TregP via a c-REL dependent mechanism (169). However, whether this population also represents the precursors to Foxp3lo TregP remains unclear. Defining the signals and relevant antigens that commit Treg cell development via either TregP pathway will be important for understanding the role each pathway plays in immune tolerance.
Cytokine signaling is clearly required for Treg cell generation. However, more nuanced effects of cytokines on Treg selection remain poorly defined. CD25 can be expressed on thymic DC and mTEC (142); however, it is unclear if CD25 trans presentation (170) occurs in the thymus and if so what affect this has on Treg cell selection. The role of IL-4 is also unclear; mice of different background produce distinct amounts of IL-4 (95, 139, 140), which could influence tTreg cell TCR repertoire and possibly susceptibility to different types of autoimmunity. Further, certain subsets of thymic APC produce different cytokines, such as IL-2 from DC (141) and IL-15 from mTEC (145). Future studies directed at understanding how distinct cytokines affect Treg development will likely produce interesting insight into how cytokine stimulation affects Treg cell repertoires.
Another mystery in Treg cell development is how Treg cells develop that enforce tolerance to transitory states, such as inflammation, puberty, estrous, or distinct metabolic states. Certainly for B cell immune responses there is evidence of thymus induced Treg tolerance to Ig antigens (119–122), and loss of Tuft cells leads to the development of anti-IL-25 antibodies (93). Further, development of inflammation specific Treg cells has been observed in the thymus (171). Interestingly, testosterone levels regulate AIRE-mediated TSA production (172), which may explain resistance to various forms of autoimmunity in males. Prepubertal males and females have similar levels of testosterone (173); thus, any differences imposed by this hormone likely occur after puberty has initiated in humans. The broad specificity of tTreg cells needed to provide tolerance in transitory states is still poorly understood.
The tTreg pool is composed of recently differentiated cells but also Treg cells that have been retained following development (resident) or have recirculated to the thymus from the periphery (174–176). Studies with Rag2-GFP mice demonstrate that older GFP-negative Treg cells progressively accumulate in the thymus as mice age and represent the majority of thymic Treg cells by about 8 weeks of age (176, 177). However, the origin of these Treg cells is debated with some suggesting that they are mostly resident cells that never left the thymus (174) and others proposing that they are primarily recirculating cells (176). It has been difficult to distinguish between these two populations to determine their relative contributions to the tTreg cell pool. Thymus transplantation studies demonstrate that Treg cells migrate from the periphery to the thymus preferentially by comparison to conventional T cells (178). Additionally, mature RAG2-GFP- Treg cells in the thymus have a similar gene expression profile to splenic Treg cells and their TCR repertoire shows evidence of peripheral modification supporting the possibility that these cells are recirculating (176). Resident and recirculating Treg cells have been shown to compete with developing thymic Treg cells for access to IL-2 and limit their differentiation to the Treg cell lineage (141, 176). The immunological benefit of restricting new Treg cell development is unclear. It is possible that these older Treg cells also compete with thymocytes for antigen, co-stimulatory ligands, and TNFRSF ligands necessary for Treg cell development. The presence of a large population of recirculating or resident Treg cells represents both an opportunity to understand the biological importance of these recirculating Treg cells and a problem, as these RAG2-GFP- Treg cells contaminate analysis of de novo Treg cell development. Cellular phenotypes for “old” contaminating Treg cells have been proposed, including CCR6+CCR7- (178) as well as CD73+ (95); these markers should be used to exclude “old” Treg cells in studies de novo tTreg development.
Finally, despite years of debate, controversy still exists over the relative role of tTreg cells and pTreg cells. The hypothetical requirement for pTreg is at mucosal surfaces (179) where diverse antigens are being surveyed or during pregnancy where ectopic alloantigens are contributed by the male gamete (180). Several studies suggest a role for thymic deletion and Treg cell selection in mucosal tolerance (66, 181–183) while other studies argue for the importance of pTreg generation (40, 179, 184–187). More recent studies have suggested that some populations of thymic Treg cells are required to polarize Tconv to pTreg cells, perhaps relating these disparate findings (184, 188). Likewise, Treg cells derived from thymic FOXP3lo TregP were able to suppress colitis, suggesting tolerance to commensal organisms can be induced by specific tTreg cell subsets (95). Further experimentation is required to conclusively delineate the unique and overlapping responsibilities of pTreg and tTreg in immune tolerance.
Conclusions
The evolutionary constraints placed on T cell selection in the thymus are immense - exogenous pressure from pathogens places a high priority on TCR diversity, while endogenous pressure requires removal of self-reactive and potentially pathogenic T cells. Thus, Treg cell development represents a mechanism that allows this leaky selection system to persist and focus effector T cell responses on “non-self” antigens. Future studies defining endogenous Treg cell antigenic targets, and the thymic populations required to produce these antigens, will be required to understand the complex processes that govern the selection of a competent repertoire of tTreg cells. Further, understanding the role of antigen specificity of Treg cells in homeostatic, inflammatory, or autoimmune contexts will be crucial in linking thymic selection to peripheral homeostasis.
Acknowledgements
We thank Dalton Hermans for critical comments on the manuscript.
Funding
DLO and LES were supported by NIH T32 training grant 2T32AI007313. MAF was supported by NIH grant AI124512.
References
- 1.Burnet FM 1957. A Modification of Jerne’s Theory of Antibody Production using the Concept of Clonal Selection. Austrailian J. Sci. 20: 67–69. [DOI] [PubMed] [Google Scholar]
- 2.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE 1992. RAG-1-Deficient Mice Have No Mature B and T Lymphocytes. Cell 68: 869–877. [DOI] [PubMed] [Google Scholar]
- 3.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, and Alt FW. 1992. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68: 855–867. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, and Toda M 1995. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 155: 1151–1164. [PubMed] [Google Scholar]
- 5.Takahashi T, Tagami T, Yamazaki S, Uede T, Shimizu J, Sakaguchi N, Mak TW, and Sakaguchi S. 2000. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192: 303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Dagna Bricarelli F, Byrne G, McEuen M, Proll S, Appleby M, and Brunkow ME. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 27: 18–20. [DOI] [PubMed] [Google Scholar]
- 7.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, and Ramsdell F. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 27: 68–73. [DOI] [PubMed] [Google Scholar]
- 8.Malek TR, Yu A, Vincek V, Scibelli P, and Kong L. 2002. CD4 regulatory T cells prevent lethal autoimmunity in IL-2Rβ-deficient mice: Implications for the nonredundant function of IL-2. Immunity 17: 167–178. [DOI] [PubMed] [Google Scholar]
- 9.Kim JM, Rasmussen JP, and Rudensky AY. 2007. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat. Immunol. 8: 191–197. [DOI] [PubMed] [Google Scholar]
- 10.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, and Rudensky AY. 2012. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal-fetal conflict. Cell 150: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aluvihare VR, Kallikourdis M, and Betz AG. 2004. Regulatory T cells mediate maternal tolerance to the fetus. Nat. Immunol. 5: 266–271. [DOI] [PubMed] [Google Scholar]
- 12.Jasper MJ, Tremellen KP, and Robertson SA. 2006. Primary unexplained infertility is associated with reduced expression of the T-regulatory cell transcription factor Foxp3 in endometrial tissue. Mol. Hum. Reprod. 12: 301–308. [DOI] [PubMed] [Google Scholar]
- 13.Rowe JH, Ertelt JM, Aguilera MN, Farrar MA, and Way SS. 2011. Foxp3 + regulatory T cell expansion required for sustaining pregnancy compromises host defense against prenatal bacterial pathogens. Cell Host Microbe 10: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahn DA, and Baltimore D. 2010. Pregnancy induces a fetal antigen-specific maternal T regulatory cell response that contributes to tolerance. Proc. Natl. Acad. Sci. 107: 9299–9304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shima T, Sasaki Y, Itoh M, Nakashima A, Ishii N, Sugamura K, and Saito S. 2010. Regulatory T cells are necessary for implantation and maintenance of early pregnancy but not late pregnancy in allogeneic mice. J. Reprod. Immunol. 85: 121–129. [DOI] [PubMed] [Google Scholar]
- 16.Teles A, Thuere C, Wafula PO, El-Mousleh T, Zenclussen ML, and Zenclussen AC. 2013. Origin of Foxp3+ cells during pregnancy. Am J Clin Exp Immunol 2: 222–233. [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe JH, Ertelt JM, Xin L, and Way SS. 2012. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 490: 102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tung KSK, Harakal J, Qiao H, Rival C, Li JCH, Paul AGA, Wheeler K, Pramoonjago P, Grafer CM, Sun W, Sampson RD, Wong EWP, Reddi PP, Deshmukh US, Hardy DM, Tang H, Cheng CY, and Goldberg E. 2017. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Invest. 127: 1046–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali N, Zirak B, Rodriguez RS, Pauli ML, Truong H-A, Lai K, Ahn R, Corbin K, Lowe MM, Scharschmidt TC, Taravati K, Tan MR, Ricardo-Gonzalez RR, Nosbaum A, Bertolini M, Liao W, Nestle FO, Paus R, Cotsarelis G, Abbas AK, and Rosenblum MD. 2017. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 169: 1119–1129. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, and Mathis D. 2013. A Special Population of Regulatory T Cells Potentiates Muscle Repair. Cell 155: 1282–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cipolletta D, Feuerer M, Li A, Kamei N, Lee J, Shoelson SE, Benoist C, and Mathis D. 2012. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue T reg cells. Nature 486: 549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, and Mathis D. 2009. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat. Med. 15: 930–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolodin D, van Panhuys N, Li C, Magnuson AM, Cipolletta D, Miller CM, Wagers A, Germain RN, Benoist C, and Mathis D. 2015. Antigen- and Cytokine-Driven Accumulation of Regulatory T Cells in Visceral Adipose Tissue of Lean Mice. Cell Metab. 21: 543–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bapat SP, Myoung Suh J, Fang S, Liu S, Zhang Y, Cheng A, Zhou C, Liang Y, LeBlanc M, Liddle C, Atkins AR, Yu RT, Downes M, Evans RM, and Zhang Y. 2015. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 528: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, DiSpirito JR, Zemmour D, Spallanzani RG, Kuswanto W, Benoist C, and Mathis D. 2018. TCR Transgenic Mice Reveal Stepwise, Multi-site Acquisition of the Distinctive Fat-Treg Phenotype. Cell 174: 285–299. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deliyanti D, Talia DM, Zhu T, Maxwell MJ, Agrotis A, Jerome JR, Hargreaves EM, Gerondakis S, Hibbs ML, Mackay F, and Wilkinson-Berka JL. 2017. Foxp3+ Tregs are recruited to the retina to repair pathological angiogenesis. Nat. Commun. 8: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang Y-H, Lim H, Reynolds JM, Zhou X-H, Fan H-M, Liu Z-M, Neelapu SS, and Dong C. 2011. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 17: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KGC, and Vinuesa CG. 2011. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 17: 975–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanteri MC, O’Brien KM, Purtha WE, Cameron MJ, Lund JM, Owen RE, Heitman JW, Custer B, Hirschkorn DF, Tobler LH, Kiely N, Prince HE, Ndhlovu LC, Nixon DF, Kamel HT, Kelvin DJ, Busch MP, Rudensky AY, Diamond MS, and Norris PJ. 2009. Tregs control the development of symptomatic West Nile virus infection in humans and mice. J. Clin. Invest. 119: 3266–3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmitz I, Schneider C, Fröhlich A, Frebel H, Christ D, Leonard WJ, Sparwasser T, Oxenius A, Freigang S, and Kopf M. 2013. IL-21 Restricts Virus-driven Treg Cell Expansion in Chronic LCMV Infection. PLoS Pathog. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suvas S, Kumaraguru U, Pack CD, Lee S, and Rouse BT. 2003. CD4 + CD25 + T Cells Regulate Virus-specific Primary and Memory CD8 + T Cell Responses. J. Exp. Med. 198: 889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, and Rudensky AY. 2015. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell 162: 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suvas S, Azkur AK, Kim BS, Kumaraguru U, and Rouse BT. 2004. CD4+CD25+ Regulatory T Cells Control the Severity of Viral Immunoinflammatory Lesions. J. Immunol. 172: 4123–4132. [DOI] [PubMed] [Google Scholar]
- 34.Oldenhove G, Bouladoux N, Wohlfert EA, Hall JA, Chou D, Dos santos L, O’Brien S, Blank R, Lamb E, Natarajan S, Kastenmayer R, Hunter C, Grigg ME, and Belkaid Y. 2009. Decrease of Foxp3+ Treg Cell Number and Acquisition of Effector Cell Phenotype during Lethal Infection. Immunity 31: 772–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandiyan P, Conti HR, Zheng L, Peterson AC, Mathern DR, Hernández-Santos N, Edgerton M, Gaffen SL, and Lenardo MJ. 2011. CD4+CD25+Foxp3+ Regulatory T Cells Promote Th17 Cells In Vitro and Enhance Host Resistance in Mouse Candida albicans Th17 Cell Infection Model. Immunity 34: 422–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y, Haines CJ, Gutcher I, Hochweller K, Blumenschein WM, McClanahan T, Hämmerling G, Li MO, Cua DJ, and McGeachy MJ. 2011. Foxp3+ Regulatory T Cells Promote T Helper 17 Cell Development In Vivo through Regulation of Interleukin-2. Immunity 34: 409–421. [DOI] [PubMed] [Google Scholar]
- 37.Laidlaw BJ, Cui W, Amezquita RA, Gray SM, Guan T, Lu Y, Kobayashi Y, Flavell RA, Kleinstein SH, Craft J, and Kaech SM. 2015. Production of IL-10 by CD4+regulatory T cells during the resolution of infection promotes the maturation of memory CD8+T cells. Nat. Immunol. 16: 871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrikant P, Khoruts A, and Mescher MF. 1999. CTLA-4 blockade reverses CD8+T cell tolerance to tumor by a CD4+T cell- and IL-2-dependent mechanism. Immunity 11: 483–493. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu J, Yamazaki S, and Sakaguchi S. 1999. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J. Immunol. 163: 5211–8. [PubMed] [Google Scholar]
- 40.Sefik E, Geva-Zatorsky N, Oh S, Konnikova L, Zemmour D, McGuire AM, Burzyn D, Ortiz-Lopez A, Lobera M, Yang J, Ghosh S, Earl A, Snapper SB, Jupp R, Kasper D, Mathis D, and Benoist C. 2015. Individual intestinal symbionts induce a distinct population of ROR + regulatory T cells. Science (80-. ). 349: 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim KS, Hong SW, Han D, Yi J, Jung J, Yang BG, Lee JY, Lee M, and Surh CD. 2016. Dietary antigens limit mucosal immunity by inducing regulatory T cells in the small intestine. Science (80-. ). 351: 858–863. [DOI] [PubMed] [Google Scholar]
- 42.Harrison OJ, Linehan JL, Shih HY, Bouladoux N, Han SJ, Smelkinson M, Sen SK, Byrd AL, Enamorado M, Yao C, Tamoutounour S, Van Laethem F, Hurabielle C, Collins N, Paun A, Salcedo R, O’Shea JJ, and Belkaid Y. 2019. Commensal-specific T cell plasticity promotes rapid tissue adaptation to injury. Science (80-. ). 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maclean LD, Zak SJ, Varco RL, and Good RA. 1957. The role of the thymus in antibody production; an experimental study of the immune response in thymectomized rabbits. Transpl. Bull 4: 21–2. [PubMed] [Google Scholar]
- 44.Jankovic BD, Waksman BH, and Arnason BG. 1962. Role of the Thymus in Immune Reactions in Rate. J. Exp. Med. 116: 159–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller JFAP 1961. Immunological Function of the Thymus. Lancet 278: 748–749. [DOI] [PubMed] [Google Scholar]
- 46.Good RA, Dalmasso AP, Martinez C, Archer OK, Pierce JC, and Papermaster BW. 1962. The role of the Thymus in Development of Immunologic Capacity in Rabbits and Mice. J. Exp. Med. 116: 773–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller JFAP 1962. Effect of Neonatal Thymectomy on the Immunological Responsiveness of the Mouse. Proc. R. Soc. B Biol. Sci. 156: 415–428. [Google Scholar]
- 48.Nishizuka Y, and Sakakura T. 1969. Thymus and Reproduction : Sex-Linked Dysgenesia of the Gonad after Neonatal Thymectomy in Mice. Science (80-.). 166: 753–755. [DOI] [PubMed] [Google Scholar]
- 49.Gershon RK, and Kondo K. 1970. Cell interactions in the induction of tolerance: the role of thymic lymphocytes. Immunology 18: 723–37. [PMC free article] [PubMed] [Google Scholar]
- 50.Gershon RK, and Kondo K. 1971. Infectious immunological tolerance. Immunology 21: 903–14. [PMC free article] [PubMed] [Google Scholar]
- 51.Gershon RK, Cohen P, Hencin R, and Liebhaber SA. 1972. Suppressor T Cells. J. Immunol. 108: 586–590. [PubMed] [Google Scholar]
- 52.Benacerraf B, and Germain RN. 1981. A single major pathway of T-lymphocyte interactions in antigen-specific immune suppression. Scand. J. Immunol. 13: 1–10. [DOI] [PubMed] [Google Scholar]
- 53.Kronenberg M, Steinmetz M, Kobori J, Kraig E, Kapp JA, Pierce CW, Sorensen CM, Suzuki G, Tada T, and Hood L. 1983. RNA transcripts for I-J polypeptides are apparently not encoded between the I-A and I-E subregions of the murine major histocompatibility complex. Proc. Natl. Acad. Sci. 80: 5704–5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Möller G 1988. Do suppressor T cells exist? Scand. J. Immunol. 27: 247–250. [DOI] [PubMed] [Google Scholar]
- 55.Sakaguchi S, Takahashi T, and Nishizuka Y. 1982. Study on cellular events in post-thymectomy autoimmune oophoitis in mice. J. Exp. Med. 156: 1577–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asano M, Toda M, Sakaguchi N, and Sakaguchi S 1996. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 184: 387–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, and Ochs, H D. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 27: 20–1. [DOI] [PubMed] [Google Scholar]
- 58.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, and Rudensky AY. 2005. Regulatory T cell lineage specification by the forkhead transcription factor Foxp3. Immunity 22: 329–341. [DOI] [PubMed] [Google Scholar]
- 59.Wan YY, and Flavell RA. 2005. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc. Natl. Acad. Sci. 102: 5126–5131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Haribhai D, Lin W, Relland LM, Truong N, Williams CB, and Chatila TA. 2007. Regulatory T Cells Dynamically Control the Primary Immune Response to Foreign Antigen. J. Immunol. 178: 2961–2972. [DOI] [PubMed] [Google Scholar]
- 61.Roncador G, Brown PJ, Maestre L, Hue S, Martínez-Torrecuadrada JL, Ling KL, Pratap S, Toms C, Fox BC, Cerundolo V, Powrie F, and Banham AH. 2005. Analysis of FOXP3 protein expression in human CD4+CD25+ regulatory T cells at the single-cell level. Eur. J. Immunol. 35: 1681–1691. [DOI] [PubMed] [Google Scholar]
- 62.Lio CWJ, and Hsieh CS. 2008. A Two-Step Process for Thymic Regulatory T Cell Development. Immunity 28: 100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Burchill MA, Yang J, Vang KB, Moon JJ, Chu HH, Lio CWJ, Vegoe AL, Hsieh CS, Jenkins MK, and Farrar MA. 2008. Linked T Cell Receptor and Cytokine Signaling Govern the Development of the Regulatory T Cell Repertoire. Immunity 28: 112–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, Singer D, and Singer A. 2013. Foxp3 Transcription Factor Is Proapoptotic and Lethal to Developing Regulatory T Cells unless Counterbalanced by Cytokine Survival Signals. Immunity 38: 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahmud SA, Manlove LS, Schmitz HM, Xing Y, Wang Y, Owen DL, Schenkel JM, Boomer JS, Green JM, Yagita H, Chi H, Hogquist KA, and Farrar MA. 2014. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 15: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, and Ignatowicz L. 2007. Nonself-Antigens Are the Cognate Specificities of Foxp3 + Regulatory T Cells. Immunity 27: 493–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Santen H-M, Benoist C, and Mathis D. 2004. Number of T Reg Cells That Differentiate Does Not Increase upon Encounter of Agonist Ligand on Thymic Epithelial Cells. J. Exp. Med. 200: 1221–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Itoh M, Takahashi T, Sakaguchi N, Kuniyasu Y, Shimizu J, Otsuka F, Sakaguchi S 1999. CD25 +CD4+ Naturally Anergic and Thymus and Autoimmunity: Production of Suppressive T Cells as a Key Function of the Thymus in Maintaining Immunologic Self-Tolerance. J. Immunol. 162: 5317–5326. [PubMed] [Google Scholar]
- 69.Jordan Martha S., Boesteanu Alina, Reed Amy J., Petrone Andria L., Holenbeck Andrea E., Lerman Melissa A., Naji Ali, Caton Andrew J., Jordan MS, Boesteanu A, Reed AJ, Petrone AL, Holenbeck AE, Lerman MA, Naji A, and Caton AJ. 2001. Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2: 301–6. [DOI] [PubMed] [Google Scholar]
- 70.Hsieh C, Liang Y, Tyznik AJ, Self SG, Liggitt D, and Rudensky AY. 2004. Recognition of the Peripheral Self by Naturally Arising CD25. Immunity 21: 267–277. [DOI] [PubMed] [Google Scholar]
- 71.Pacholczyk R, Ignatowicz H, Kraj P, and Ignatowicz L. 2006. Origin and T Cell Receptor Diversity of Foxp3+CD4+CD25+ T Cells. Immunity 25: 249–259. [DOI] [PubMed] [Google Scholar]
- 72.Hsieh CS, Zheng Y, Liang Y, Fontenot JD, and Rudensky AY. 2006. An intersection between the self-reactive regulatory and nonregulatory T cell receptor repertoires. Nat. Immunol. 7: 401–410. [DOI] [PubMed] [Google Scholar]
- 73.Leung MWL, Shen S, and Lafaille JJ. 2009. TCR-dependent differentiation of thymic Foxp3 + cells is limited to small clonal sizes . J. Exp. Med. 206: 2121–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bautista JL, Lio CWJ, Lathrop SK, Forbush K, Liang Y, Luo J, Rudensky AY, and Hsieh CS. 2009. Intraclonal competition limits the fate determination of regulatory T cells in the thymus. Nat. Immunol. 10: 610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee HM, Bautista JL, Scott-Browne J, Mohan JF, and Hsieh CS. 2012. A Broad Range of Self-Reactivity Drives Thymic Regulatory T Cell Selection to Limit Responses to Self. Immunity 37: 475–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moran AE, Holzapfel KL, Xing Y, Cunningham NR, Maltzman JS, Punt J, and a Hogquist K. 2011. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208: 1279–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stadinski BD, Blevins SJ, Spidale NA, Duke BR, Huseby PG, Stern LJ, and Huseby ES. 2019. A temporal thymic selection switch and ligand binding kinetics constrain neonatal Foxp3+ Treg cell development. Nat. Immunol. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pugliese A, Zeller M, Jr AF, Laura J, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth G, Bennett ST, and Patel DD. 1997. The insulin gene is transcribed in the human thymus and transcription levels correlate with allelic variation at the INS VNTR-lDDM2 susceptibility locus for type 1 diabetes. Nat. gen 15: 293–297. [DOI] [PubMed] [Google Scholar]
- 79.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, and Polychronakos C. 1997. Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat. Genet. 15: 289–292. [DOI] [PubMed] [Google Scholar]
- 80.Derbinski J, Schulte A, Kyewski B, and Klein L. 2001. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat. Immunol. 2: 1032–1039. [DOI] [PubMed] [Google Scholar]
- 81.Stenbit AE, Tsa T, Lr ING, Burcelin R, Geenen DL, Factor SM, Houseknecht K, Katz EB, and Charron MJ. 1997. An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat. Genet. 17: 399–403. [DOI] [PubMed] [Google Scholar]
- 82.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, Krohn KJE, Lalioti MD, Mullis PE, Antonarakis SE, Kawasaki K, Asakawa S, Ito F, and Shimizu N. 1997. Positional cloning of the APECED gene. Nat. Genet. 17: 393–398. [DOI] [PubMed] [Google Scholar]
- 83.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, von Boehmer H, Bronson R, Dierich A, Benoist C, and Mathis D. 2002. Projection of an Immunological Self Shadow Within the Thymus by the Aire Protein. Science (80-. ). 298: 1395–1401. [DOI] [PubMed] [Google Scholar]
- 84.Liston A, Lesage S, Wilson J, Peltonen L, and Goodnow CC. 2003. Aire regulates negative selection of organ-specific T cells. Nat. Immunol. 4: 350–354. [DOI] [PubMed] [Google Scholar]
- 85.Kekalainen E, Tuovinen H, Joensuu J, Gylling M, Franssila R, Pontynen N, Talvensaari K, Perheentupa J, Miettinen A, and Arstila TP. 2007. A Defect of Regulatory T Cells in Patients with Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. J. Immunol. 178: 1208–1215. [DOI] [PubMed] [Google Scholar]
- 86.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, Rolink A, and Klein L. 2007. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat. Immunol. 8: 351–358. [DOI] [PubMed] [Google Scholar]
- 87.Hinterberger M, Aichinger M, Da Costa OP, Voehringer D, Hoffmann R, and Klein L. 2010. Autonomous role of medullary thymic epithelial cells in central CD4 + T cell tolerance. Nat. Immunol. 11: 512–519. [DOI] [PubMed] [Google Scholar]
- 88.Malchow S, Leventhal DS, Nishi S, Fischer BI, Shen L, Paner GP, Amit AS, Kang C, Geddes JE, Allison JP, Socci ND, and Savage PA. 2013. Aire-Dependent Thymic Development of Tumor-Associated Regulatory T Cells. Science (80-. ). 339: 1219–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malchow S, Leventhal DS, Lee V, Nishi S, Socci ND, and Savage PA. 2016. Aire Enforces Immune Tolerance by Directing Autoreactive T Cells into the Regulatory T Cell Lineage. Immunity 44: 1102–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sng J, Ayoglu B, Chen JW, Schickel J-N, Ferre EMN, Glauzy S, Romberg N, Hoenig M, Cunningham-Rundles C, Utz PJ, Lionakis MS, and Meffre E. 2019. AIRE expression controls the peripheral selection of autoreactive B cells. Sci. Immunol. 4: eaav6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takaba H, Morishita Y, Tomofuji Y, Danks L, Nitta T, Komatsu N, Kodama T, and Takayanagi H. 2015. Fezf2 Orchestrates a Thymic Program of Self-Antigen Expression for Immune Tolerance. Cell 163: 975–987. [DOI] [PubMed] [Google Scholar]
- 92.Bornstein C, Nevo S, Giladi A, Kadouri N, Pouzolles M, Gerbe F, David E, Machado A, Chuprin A, Tóth B, Goldberg O, Itzkovitz S, Taylor N, Jay P, Zimmermann VS, Abramson J, and Amit I. 2018. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 559: 622–626. [DOI] [PubMed] [Google Scholar]
- 93.Miller CN, Proekt I, von Moltke J, Wells KL, Rajpurkar AR, Wang H, Rattay K, Khan IS, Metzger TC, Pollack JL, Fries AC, Lwin WW, Wigton EJ, Parent AV, Kyewski B, Erle DJ, Hogquist KA, Steinmetz LM, Locksley RM, and Anderson MS. 2018. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 559: 627–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Watanabe N, Wang YH, Lee HK, Ito T, Wang YH, Cao W, and Liu YJ. 2005. Hassall’s corpuscles instruct dendritic cells to induce CD4 +CD25+ regulatory T cells in human thymus. Nature 436: 1181–1185. [DOI] [PubMed] [Google Scholar]
- 95.Owen DL, Mahmud SA, Sjaastad LE, Williams JB, Spanier JA, Simeonov DR, Ruscher R, Huang W, Proekt I, Miller CN, Hekim C, Jeschke JC, Aggarwal P, Broeckel U, LaRue RS, Henzler CM, Alegre ML, Anderson MS, August A, Marson A, Zheng Y, Williams CB, and Farrar MA. 2019. Thymic regulatory T cells arise via two distinct developmental programs. Nat. Immunol. 20: 195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li J, Park J, Foss D, and Goldschneider I. 2009. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J. Exp. Med. 206: 607–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liston A, Nutsch KM, Farr AG, Lund JM, Rasmussen JP, Koni PA, and Rudensky AY. 2008. Differentiation of regulatory Foxp3+ T cells in the thymic cortex. Proc. Natl. Acad. Sci. 105: 11903–11908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D’Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, and Wu L. 2008. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc. Natl. Acad. Sci. 105: 19869–19874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guerri L, Peguillet I, Geraldo Y, Nabti S, Premel V, and Lantz O. 2013. Analysis of APC Types Involved in CD4 Tolerance and Regulatory T Cell Generation Using Reaggregated Thymic Organ Cultures. J. Immunol. 190: 2102–2110. [DOI] [PubMed] [Google Scholar]
- 100.Wirnsberger G, Mair F, and Klein L. 2009. Regulatory T cell differentiation of thymocytes does not require a dedicated antigen-presenting cell but is under T cell-intrinsic developmental control. Proc. Natl. Acad. Sci. 106: 10278–10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gallegos AM, and Bevan MJ. 2004. Central Tolerance to Tissue-specific Antigens Mediated by Direct and Indirect Antigen Presentation. J. Exp. Med. 200: 1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Koble C, and Kyewski B. 2009. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J. Exp. Med. 206: 1505–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hubery F, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZF, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR 2011. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood 118: 2462–2472. [DOI] [PubMed] [Google Scholar]
- 104.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bösl MR, Holländer GA, Hayashi Y, de Waal Malefyt R, Nitta T, and Takahama Y. 2011. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J. Exp. Med. 208: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Perry JSA, Lio CWJ, Kau AL, Nutsch K, Yang Z, Gordon JI, Murphy KM, and Hsieh CS. 2014. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 41: 414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Leventhal DS, Gilmore DC, Berger JM, Nishi S, Lee V, Malchow S, Kline DE, Kline J, Vander Griend DJ, Huang H, Socci ND, and Savage PA. 2016. Dendritic Cells Coordinate the Development and Homeostasis of Organ-Specific Regulatory T Cells. Immunity 44: 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Atibalentja DF, Byersdorfer CA, and Unanue ER. 2009. Thymus-Blood Protein Interactions Are Highly Effective in Negative Selection and Regulatory T Cell Induction. J. Immunol. 183: 7909–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Atibalentja DF, Murphy KM, and Unanue ER. 2011. Functional Redundancy between Thymic CD8 + and Sirp + Conventional Dendritic Cells in Presentation of Blood-Derived Lysozyme by MHC Class II Proteins. J. Immunol. 186: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wendland M, Czeloth N, Mach N, Malissen B, Kremmer E, Pabst O, and Forster R. 2007. CCR9 is a homing receptor for plasmacytoid dendritic cells to the small intestine. Proc. Natl. Acad. Sci. 104: 6347–6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, and Butcher EC. 2012. Plasmacytoid Dendritic Cells Transport Peripheral Antigens to the Thymus to Promote Central Tolerance. Immunity 36: 438–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Isaacson PG, Norton AJ, and Addis BJ. 1987. The Human Thymus Contains a Novel Population of B Lymphocytes. Lancet 330: 1488–1491. [DOI] [PubMed] [Google Scholar]
- 112.Gollob KJ, and Palmer E. 1993. Aberrant induction of T cell tolerance in B cell suppressed mice. J. Immunol. 150: 3705–12. [PubMed] [Google Scholar]
- 113.Ferrero I, Anjuère F, Martín P, Del Hoyo GM, Fraga ML, Wright N, Varona R, Márquez G, and Ardavín C. 1999. Functional and phenotypic analysis of thymic B cells: Role in the induction of T cell negative selection. Eur. J. Immunol. 29: 1598–1609. [DOI] [PubMed] [Google Scholar]
- 114.Fujihara C, Williams JA, Watanabe M, Jeon H, Sharrow SO, and Hodes RJ. 2014. T Cell–B Cell Thymic Cross-Talk: Maintenance and Function of Thymic B Cells Requires Cognate CD40–CD40 Ligand Interaction. J. Immunol. 193: 5534–5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Frommer F, and Waisman A. 2010. B cells participate in thymic negative selection of murine auto-reactive CD4+ T cells. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Perera J, Meng L, Meng F, and Huang H. 2013. Autoreactive thymic B cells are efficient antigen-presenting cells of cognate self-antigens for T cell negative selection. Proc. Natl. Acad. Sci. 110: 17011–17016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Walters SN, Webster KE, Daley S, and Grey ST. 2014. A Role for Intrathymic B Cells in the Generation of Natural Regulatory T Cells. J. Immunol. 193: 170–176. [DOI] [PubMed] [Google Scholar]
- 118.Lu FT, Yang W, Wang YH, Di Ma H, Tang W, Yang JB, Li L, Ansari AA, and Lian ZX. 2015. Thymic B cells promote thymus-derived regulatory T cell development and proliferation. J. Autoimmun. 61: 62–72. [DOI] [PubMed] [Google Scholar]
- 119.Rudensky AY, Mazel SM, and Yurin VL. 1990. Presentation of endogenous immunoglobulin determinant to immunoglobulin‐recognizing T cell clones by the thymic cells. Eur. J. Immunol. 20: 2235–2239. [DOI] [PubMed] [Google Scholar]
- 120.Detanico T, Heiser RA, Aviszus K, Bonorino C, and Wysocki LJ. 2011. Self-Tolerance Checkpoints in CD4 T Cells Specific for a Peptide Derived from the B Cell Antigen Receptor. J. Immunol. 187: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Munthe LA, Corthay A, Os A, Zangani M, and Bogen B. 2014. Systemic Autoimmune Disease Caused by Autoreactive B Cells That Receive Chronic Help from Ig V Region-Specific T Cells. J. Immunol. 175: 2391–2400. [DOI] [PubMed] [Google Scholar]
- 122.Meyers G, Ng Y-S, Bannock JM, Lavoie A, Walter JE, Notarangelo LD, Kilic SS, Aksu G, Debre M, Rieux-Laucat F, Conley ME, Cunningham-Rundles C, Durandy A, and Meffre E. 2011. Activation-induced cytidine deaminase (AID) is required for B-cell tolerance in humans. Proc. Natl. Acad. Sci. 108: 11554–11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perera J, Zheng Z, Li S, Gudjonson H, Kalinina O, Benichou JIC, Block KE, Louzoun Y, Yin D, Chong AS, Dinner AR, Weigert M, and Huang H. 2016. Self-Antigen-Driven Thymic B Cell Class Switching Promotes T Cell Central Tolerance. Cell Rep. 17: 387–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yamano T, Nedjic J, Hinterberger M, Steinert M, Koser S, Pinto S, Gerdes N, Lutgens E, Ishimaru N, Busslinger M, Brors B, Kyewski B, and Klein L. 2015. Thymic B Cells Are Licensed to Present Self Antigens for Central T Cell Tolerance Induction. Immunity 42: 1048–1061. [DOI] [PubMed] [Google Scholar]
- 125.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, and Horak I. 1993. Ulcerative Colitis-like Disease in Mice with a Disrupted Interleukin-2 Gene. Cell 75: 253–261. [DOI] [PubMed] [Google Scholar]
- 126.Suzuki H, Kündig TM, Furlonger C, Wakeham A, Matsuyama T, Schmits R, Simard JJL, Ohashi PS, Taniguchi T, Paige CJ, and Mak TW. 1995. Deregulated T Cell Activation and Autoimmunity in Mice Lacking Interleukin- 2 Receptor β Published by : American Association for the Advancement of Science Stable. Science (80-. ). 268: 1472–1476. [DOI] [PubMed] [Google Scholar]
- 127.Willerford DM, Chen J, Ferry JA, Davidson L, Ma A, Alt FW 1995. Interleukin-2 Recptor a Chain Regulates the size and content of the peripheral lymphoid compartment. Immunity 3: 521–530. [DOI] [PubMed] [Google Scholar]
- 128.Malek TR, Porter BO, Codias EK, Scibelli P, and Yu A. 2000. Normal Lymphoid Homeostasis and Lack of Lethal Autoimmunity in Mice Containing Mature T Cells with Severely Impaired IL-2 Receptors. J. Immunol. 164: 2905–2914. [DOI] [PubMed] [Google Scholar]
- 129.D’Cruz LM, and Klein L. 2005. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat. Immunol. 6: 1152–1159. [DOI] [PubMed] [Google Scholar]
- 130.Fontenot JD, Rasmussen JP, Gavin MA, and Rudensky AY. 2005. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat. Immunol. 6: 1142–1151. [DOI] [PubMed] [Google Scholar]
- 131.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, and Farrar MA. 2007. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178: 280–90. [DOI] [PubMed] [Google Scholar]
- 132.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, and Lafaille JJ. 2002. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J. Exp. Med. 196: 851–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soper DM, Kasprowicz DJ, and Ziegler SF. 2007. IL-2Rβ links IL-2R signaling with Foxp3 expression. Eur. J. Immunol. 37: 1817–1826. [DOI] [PubMed] [Google Scholar]
- 134.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, and Farrar MA. 2008. IL-2, −7, and −15, but Not Thymic Stromal Lymphopoeitin, Redundantly Govern CD4+Foxp3+ Regulatory T Cell Development. J. Immunol. 181: 3285–3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bayer AL, Lee JY, de la Barrera A, Surh CD, and Malek TR. 2008. A Function for IL-7R for CD4+CD25+Foxp3+ T Regulatory Cells. J. Immunol. 181: 225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, and O’Shea JJ. 2007. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109: 4368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burchill MA, Goetz CA, Prlic M, O’Neil JJ, Harmon IR, Bensinger SJ, Turka LA, Brennan P, Jameson SC, and Farrar MA. 2003. Distinct Effects of STAT5 Activation on CD4+ and CD8+ T Cell Homeostasis: Development of CD4+CD25+ Regulatory T Cells versus CD8+ Memory T Cells. J. Immunol. 171: 5853–5864. [DOI] [PubMed] [Google Scholar]
- 138.Wei J, Duramad O, Perng OA, Reiner SL, Liu Y-J, and Qin FX-F. 2007. Antagonistic nature of T helper ½ developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. 104: 18169–18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lai D, Zhu J, Wang T, Hu-Li J, Terabe M, Berzofsky JA, Clayberger C, and Krensky AM. 2011. KLF13 sustains thymic memory-like CD8 + T cells in BALB/c mice by regulating IL-4–generating invariant natural killer T cells . J. Exp. Med. 208: 1093–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, and Hogquist KA. 2013. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 14: 1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Weist BM, Kurd N, Boussier J, Chan SW, and Robey EA. 2015. Thymic regulatory T cell niche size is dictated by limiting IL-2 from antigen-bearing dendritic cells and feedback competition. Nat. Immunol. 16: 635–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Owen DL, Mahmud SA, Vang KB, Kelly RM, Blazar BR, Smith KA, and Farrar MA. 2018. Identification of Cellular Sources of IL-2 Needed for Regulatory T Cell Development and Homeostasis. J. Immunol. 200: 3926–3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hori S, Nomura T, and Sakaguchi S. 2003. Control of regulatory T cell development by the transcription factor Foxp3.[see comment]. Science. 299: 1057–1061. [DOI] [PubMed] [Google Scholar]
- 144.Hara T, Shitara S, Imai K, Miyachi H, Kitano S, Yao H, Tani-ichi S, and Ikuta K. 2012. Identification of IL-7-Producing Cells in Primary and Secondary Lymphoid Organs Using IL-7-GFP Knock-In Mice. J. Immunol. 189: 1577–1584. [DOI] [PubMed] [Google Scholar]
- 145.Cui G, Hara T, Simmons S, Wagatsuma K, Abe A, Miyachi H, Kitano S, Ishii M, Tani-ichi S, and Ikuta K. 2014. Characterization of the IL-15 niche in primary and secondary lymphoid organs in vivo. Proc. Natl. Acad. Sci. 111: 1915–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fu W, Ergun A, Lu T, Hill JA, Haxhinasto S, Fassett MS, Gazit R, Adoro S, Glimcher L, Chan S, Kastner P, Rossi D, Collins JJ, Mathis D, and Benoist C. 2012. A multiply redundant genetic switch “locks in” the transcriptional signature of regulatory T cells. Nat. Immunol. 13: 972–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rieder SA, Metidji A, Glass DD, Thornton AM, Ikeda T, Morgan BA, and Shevach EM. 2015. Eos Is Redundant for Regulatory T Cell Function but Plays an Important Role in IL-2 and Th17 Production by CD4+ Conventional T Cells. J. Immunol. 195: 553–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang BH, Wang K, Wan S, Liang Y, Yuan X, Dong Y, Cho S, Xu W, Jepsen K, Feng GS, Lu LF, Xue HH, and Fu W. 2019. TCF1 and LEF1 Control Treg Competitive Survival and Tfr Development to Prevent Autoimmune Diseases. Cell Rep. 27: 3629–3645. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kitagawa Y, Ohkura N, Kidani Y, Vandenbon A, Hirota K, Kawakami R, Yasuda K, Motooka D, Nakamura S, Kondo M, Taniuchi I, Kohwi-Shigematsu T, and Sakaguchi S. 2017. Guidance of regulatory T cell development by Satb1-dependent super-enhancer establishment. Nat. Immunol. 18: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Josefowicz SZ, Wilson CB, and Rudensky AY. 2009. Cutting Edge: TCR Stimulation Is Sufficient for Induction of Foxp3 Expression in the Absence of DNA Methyltransferase 1. J. Immunol. 182: 6648–6652. [DOI] [PubMed] [Google Scholar]
- 151.Ohkura N, Hamaguchi M, Morikawa H, Sugimura K, Tanaka A, Ito Y, Osaki M, Tanaka Y, Yamashita R, Nakano N, Huehn J, Fehling HJ, Sparwasser T, Nakai K, and Sakaguchi S. 2012. T Cell Receptor Stimulation-Induced Epigenetic Changes and Foxp3 Expression Are Independent and Complementary Events Required for Treg Cell Development. Immunity 37: 785–799. [DOI] [PubMed] [Google Scholar]
- 152.Chorro L, Suzuki M, Chin SS, Williams TM, Snapp EL, Odagiu L, Labrecque N, and Lauvau G. 2018. Interleukin 2 modulates thymic-derived regulatory T cell epigenetic landscape. Nat. Commun. 9: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sekiya T, Kashiwagi I, Inoue N, Morita R, Hori S, Waldmann H, Rudensky AY, Ichinose H, Metzger D, Chambon P, and Yoshimura A. 2011. The nuclear orphan receptor Nr4a2 induces Foxp3 and regulates differentiation of CD4+ T cells. Nat. Commun. 2: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sekiya T, Hibino S, Saeki K, Kanamori M, Takaki S, and Yoshimura A. 2018. Nr4a Receptors Regulate Development and Death of Labile Treg Precursors to Prevent Generation of Pathogenic Self-Reactive Cells. Cell Rep. 24: 1627–1638. e6. [DOI] [PubMed] [Google Scholar]
- 155.Isomura I, Palmer S, Grumont RJ, Bunting K, Hoyne G, Wilkinson N, Banerjee A, Proietto A, Gugasyan R, Wu L, McNally A, Steptoe RJ, Thomas R, Shannon MF, and Gerondakis S. 2009. c-Rel is required for the development of thymic Foxp3 + CD4 regulatory T cells. J. Exp. Med. 206: 3001–3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vang KB, Yang J, Pagán AJ, Li L-X, Wang J, Green JM, a Beg A, and a Farrar M. 2010. Cutting edge: CD28 and c-Rel-dependent pathways initiate regulatory T cell development. J. Immunol. 184: 4074–4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Long M, Park SG, Strickland I, Hayden MS, and Ghosh S. 2009. Nuclear Factor-κB Modulates Regulatory T Cell Development by Directly Regulating Expression of Foxp3 Transcription Factor. Immunity 31: 921–931. [DOI] [PubMed] [Google Scholar]
- 158.Zheng Y, Josefowicz S, Chaudhry A, Peng XP, Forbush K, and Rudensky AY. 2010. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463: 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oh H, Grinberg-Bleyer Y, Liao W, Maloney D, Wang P, Wu Z, Wang J, Bhatt DM, Heise N, Schmid RM, Hayden MS, Klein U, Rabadan R, and Ghosh S. 2017. An NF-κB Transcription-Factor-Dependent Lineage-Specific Transcriptional Program Promotes Regulatory T Cell Identity and Function. Immunity 47: 450–465.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, and Chen YH. 2009. Development of Foxp3+ Regulatory T Cells Is Driven by the c-Rel Enhanceosome. Immunity 31: 932–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Feng Y, Van Der Veeken J, Shugay M, Putintseva EV, Osmanbeyoglu HU, Dikiy S, Hoyos BE, Moltedo B, Hemmers S, Treuting P, Leslie CS, Chudakov DM, and Rudensky AY. 2015. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature 528: 132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Simeonov DR, Gowen BG, Boontanrart M, Roth TL, Gagnon JD, Mumbach MR, Satpathy AT, Lee Y, Bray NL, Chan AY, Lituiev DS, Nguyen ML, Gate RE, Subramaniam M, Li Z, Woo JM, Mitros T, Ray GJ, Curie GL, Naddaf N, Chu JS, Ma H, Boyer E, Van Gool F, Huang H, Liu R, Tobin VR, Schumann K, Daly MJ, Farh KK, Ansel KM, Ye CJ, Greenleaf WJ, Anderson MS, Bluestone JA, Chang HY, Corn JE, and Marson A. 2017. Discovery of stimulation-responsive immune enhancers with CRISPR activation. Nature 549: 111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Huang J, Ellinghaus D, Franke A, Howie B, and Li Y. 2012. 1000 Genomes-based imputation identifies novel and refined associations for the Wellcome Trust Case Control Consortium phase 1 Data. Eur. J. Hum. Genet. 20: 801–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang H, Fang M, Jostins L, Umićević Mirkov M, Boucher G, Anderson CA, Andersen V, Cleynen I, Cortes A, Crins F, D’Amato M, Deffontaine V, Dmitrieva J, Docampo E, Elansary M, Farh KKH, Franke A, Gori AS, Goyette P, Halfvarson J, Haritunians T, Knight J, Lawrance IC, Lees CW, Louis E, Mariman R, Meuwissen T, Mni M, Momozawa Y, Parkes M, Spain SL, Théâtre E, Trynka G, Satsangi J, Van Sommeren S, Vermeire S, Xavier RJ, Weersma RK, Duerr RH, Mathew CG, Rioux JD, McGovern DPB, Cho JH, Georges M, Daly MJ, and Barrett JC. 2017. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547: 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Onengut-Gumuscu S, Chen WM, Burren O, Cooper NJ, Quinlan AR, Mychaleckyj JC, Farber E, Bonnie JK, Szpak M, Schofield E, Achuthan P, Guo H, Fortune MD, Stevens H, Walker NM, Ward LD, Kundaje A, Kellis M, Daly MJ, Barrett JC, Cooper JD, Deloukas P, Todd JA, Wallace C, Concannon P, and Rich SS. 2015. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat. Genet. 47: 381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fontenot JD, Dooley JL, Farr AG, and Rudensky AY. 2005. Developmental regulation of Foxp3 expression during ontogeny. J. Exp. Med. 202: 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tai X, Erman B, Alag A, Mu J, Kimura M, Katz G, Guinter T, McCaughtry T, Etzensperger R, Feigenbaum L, Singer DS, and Singer A. 2013. Foxp3 Transcription Factor Is Proapoptotic and Lethal to Developing Regulatory T Cells unless Counterbalanced by Cytokine Survival Signals. Immunity 38: 1116–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Marshall D, Sinclair C, Tung S, Marshall D, Sinclair C, Tung S, and Seddon B. 2014. Differential Requirement for IL-2 and IL-15 during Bifurcated Development of Thymic Regulatory T Cells. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schuster M, Plaza-Sirvent C, Matthies A-M, Heise U, Jeron A, Bruder D, Visekruna A, Huehn J, and Schmitz I. 2017. c-REL and IκB Govern Common and Independent Steps of Regulatory T Cell Development from Novel CD122-Expressing Pre-Precursors. J. Immunol. 199: 920–930. [DOI] [PubMed] [Google Scholar]
- 170.Wuest SC, Edwan JH, Martin JF, Han S, a Perry JS, Cartagena CM, Matsuura E, Maric D, a Waldmann T, and Bielekova B. 2011. A role for interleukin-2 trans-presentation in dendritic cell-mediated T cell activation in humans, as revealed by daclizumab therapy. Nat. Med. 17: 604–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Stadinski BD, Blevins SJ, Spidale NA, Duke BR, Huseby PG, Stern LJ, and Huseby ES. 2019. A temporal thymic selection switch and ligand binding kinetics constrain neonatal Foxp3+ Treg cell development. Nat. Immunol. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhu M, Bakhru P, Conley B, Nelson JS, Free M, Martin A, Starmer J, Wilson EM, Su MA 2016. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 7: 11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ostatníková D, Pastor K, Putz Z, Dohnányiová M, Mat’ašeje A, and Hampl R. 2002. Salivary testosterone levels in preadolescent children. BMC Pediatr. 2: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yang E, Zou T, Leichner TM, Zhang SL, and Kambayashi T. 2014. Both retention and recirculation contribute to long-lived regulatory T-cell accumulation in the thymus. Eur. J. Immunol. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhan Y, Bourges D, Dromey JA, Harrison LC, and Lew AM. 2007. The origin of thymic CD4+CD25+ regulatory T cells and their co-stimulatory requirements are determined after elimination of recirculating peripheral CD4+ cells. Int. Immunol. 19: 455–463. [DOI] [PubMed] [Google Scholar]
- 176.Thiault N, Darrigues J, Adoue V, Gros M, Binet B, Perals C, Leobon B, Fazilleau N, Joffre OP, Robey EA, Van Meerwijk JPM, and Romagnoli P. 2015. Peripheral regulatory T lymphocytes recirculating to the thymus suppress the development of their precursors. Nat. Immunol. 16: 628–634. [DOI] [PubMed] [Google Scholar]
- 177.McCaughtry TM, Wilken MS, and Hogquist KA. 2007. Thymic emigration revisited. J. Exp. Med. 204: 2513–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Cowan JE, McCarthy NI, and Anderson G. 2016. CCR7 Controls Thymus Recirculation, but Not Production and Emigration, of Foxp3+ T Cells. Cell Rep. 14: 1041–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y, Umetsu DT, and Rudensky AY. 2012. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 482: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, and Rudensky AY. 2012. Extrathymic Generation of Regulatory T Cells in Placental Mammals Mitigates Maternal-Fetal Conflict. Cell 150: 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Cebula A, Seweryn M, a Rempala G, Pabla SS, a McIndoe R, Denning TL, Bry L, Kraj P, Kisielow P, and Ignatowicz L. 2013. Thymus-derived regulatory T cells contribute to tolerance to commensal microbiota. Nature 497: 258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Nedjic J, Aichinger M, Emmerich J, Mizushima N, and Klein L. 2008. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455: 396–400. [DOI] [PubMed] [Google Scholar]
- 183.Glabraith RF, Summerskill WH, Murray J 1964. Systemic lupus erythematosus, cirrhosis and ulcerative colitis after thymectomy for myasthenia gravis. N. Engl. J. Med. 270: 229–232. [DOI] [PubMed] [Google Scholar]
- 184.Haribhai D, Lin W, Edwards B, Ziegelbauer J, Salzman NH, Carlson MR, Li S-H, Simpson PM, Chatila TA, and Williams CB. 2009. A Central Role for Induced Regulatory T Cells in Tolerance Induction in Experimental Colitis. J. Immunol. 182: 3461–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haribhai D, Williams JB, Jia S, Nickerson D, Schmitt EG, Edwards B, Ziegelbauer J, Yassai M, Li SH, Relland LM, Wise PM, Chen A, Zheng YQ, Simpson PM, Gorski J, Salzman NH, Hessner MJ, Chatila TA, and Williams CB. 2011. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 35: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lathrop SK, Bloom SM, Rao SM, Nutsch K, Lio C, Santacruz N, Peterson DA, Stappenbeck TS, Hsieh C 2011. Peripheral education of the immune system by the colonic microbiota. Nature 478: 250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, and Rudensky AY. 2013. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504: 451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wyss L, Stadinski BD, King CG, Schallenberg S, Mccarthy NI, Lee JY, Kretschmer K, Terracciano LM, Anderson G, Surh CD, Huseby ES, and Palmer E. 2016. Affinity for self antigen selects Treg cells with distinct functional properties. Nat. Immunol. 17: 1093–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]