Abstract
Background
Nodular melanoma (NM) is more likely to be fatal compared with other melanoma subtypes, an effect attributed to its greater Breslow thickness.
Methods
Clinicopathological features of NM and superficial spreading melanoma (SSM) diagnosed in 17 centers in Europe (n = 15), the United States, and Australia between 2006 and 2015, were analyzed by multivariable logistic regression analysis, with emphasis on thin (T1 ≤ 1.0 mm) melanomas. Cox analysis assessed melanoma-specific survival. All statistical tests were two sided.
Results
In all, 20 132 melanomas (NM: 5062, SSM: 15 070) were included. Compared with T1 SSM, T1 NM was less likely to have regression (odds ratio [OR] = 0.46, 95% confidence interval [CI] = 0.29 to 0.72) or nevus remnants histologically (OR = 0.60, 95% CI = 0.42 to 0.85), and more likely to have mitoses (OR = 1.97, 95% CI = 1.33 to 2.93) and regional metastasis (OR = 1.77, 95% CI = 1.02 to 3.05). T1 NM had a higher mitotic rate than T1 SSM (adjusted geometric mean = 2.2, 95% CI = 1.9 to 2.5 vs 1.6, 95% CI = 1.5 to 1.7 per mm2, P < .001). Cox multivariable analysis showed a higher risk for melanoma-specific death for NM compared with SSM for T1 (HR = 2.10, 95% CI = 1.24 to 3.56) and T2 melanomas (HR = 1.30, 95% CI = 1.01 to 1.68), and after accounting for center heterogeneity, the difference was statistically significant only for T1 (HR = 2.20, 95% CI = 1.28 to 3.78). The NM subtype did not confer increased risk within each stratum (among localized tumors or cases with regional metastasis).
Conclusions
T1 NM (compared with T1 SSM) was associated with a constellation of aggressive characteristics that may confer a worse prognosis. Our results indicate NM is a high-risk melanoma subtype that should be considered for inclusion in future prognostic classifications of melanoma.
The incidence of cutaneous melanoma is increasing worldwide in white populations, with projected continuous increases in cases for the next several decades (1). Melanoma incidence is highest in Queensland, Australia, with a rate of 72 per 100 000 per year (2010–2014) (2). In the United States, Surveillance, Epidemiology, and End Results (SEER)-9 registry data (1989–2009) indicated increasing incidence (from 13.94 to 21.87 per 100 000 person-years) across melanomas of all thicknesses (3). A similar increase in incidence of invasive melanoma was observed in European cancer registry data (1995–2012), mostly attributed to the increasing incidence of thin tumors (≤1 mm) (4).
Melanoma is a heterogeneous tumor that can be classified into four major subtypes: superficial spreading melanoma (SSM, frequency 41–57%), nodular melanoma (NM, 14–17%), lentigo maligna melanoma (6–14%), and acral lentiginous melanoma (1–7%) (5–8). NM represents a considerable proportion of thicker and ultimately fatal melanomas (9) and exhibits aggressive clinicopathological features considered as proxies of increased Breslow thickness, such as ulceration, rapid growth rate, and increased mitotic rate (MR) (6, 10–12).
Although patients with thin melanomas have high survival rates overall, the number of patients with fatal T1 melanomas is greater than the number with fatal T4 melanomas because the vast majority of melanoma patients present with early-stage disease (13). There is growing interest in the predictors of aggressive thin (T1) melanomas, but there are limited data on thin NMs (≤1 mm). It has been proposed that patients with thin melanomas should be evaluated by more refined criteria to determine their individual prognosis (14). Characteristics that may determine the prognosis of thin melanoma include ulceration, location on the head and neck (15), higher MR (16), and NM subtype (17, 18). We conducted a large international collaborative study to investigate the clinical, histological, and prognostic parameters of T1 NM vs T1 SSM, and provide evidence of whether NM represents a melanoma subtype affecting patient survival independent of Breslow thickness.
Methods
Study Patients
Our study included retrospective, deidentified data of patients diagnosed with primary cutaneous melanoma from 2006 to 2015 at 15 European melanoma centers comprising a collaborative network within the European Association of Dermato-Oncology (EADO), one center in Sydney, Australia (Melanoma Institute Australia [MIA]), and one center in the United States (The University of Texas MD Anderson Cancer Center [MD Anderson], Houston, TX). Eligible cases included patients older than 16 years with a diagnosis of primary cutaneous melanoma of NM or SSM subtype. Melanomas in situ were excluded. Only the index case was included for patients with multiple primary melanomas. Institutional ethics and/or review board approval was obtained by all participating centers.
Variables of Interest
Variables of interest at initial diagnosis included patient age and sex, the tumor’s anatomic site, and histological characteristics including Breslow thickness, the presence of an associated nevus, ulceration, regression, presence of mitoses, and MR per square millimeter. All participating centers used the established definition for NM (19): dermal invasion with intraepidermal growth not extending three rete ridges beyond the underlying dermal component. If this growth extended beyond three rete ridges in any section, with no features of another subtype, the tumor was classified as SSM. Breslow thickness was recalculated to the nearest 0.1 mm according to the current eighth American Joint Committee on Cancer (AJCC) staging system (20). More than 95% of all cases in Europe and Australia were reported as non-Hispanic whites; MD Anderson reported rates of 90%. Centers classified the tumor spread of melanomas at initial presentation as localized (ie, with no evidence of nodal involvement, satellite lesions, or in-transit metastases), regional disease (including locoregional and nodal metastasis), and distant metastasis. To confirm the correct classification of T2 through T4 clinically node-negative melanomas as localized, as individual patient data for sentinel lymph node biopsy (SLNB) were not collected, all centers confirmed the routine use of SLNB for all T2 to T4 melanomas, except for the Bucharest study center and a group of patients with T4 melanoma from the Turin study center, as previously published (21). Survival data included status at last observation (alive or dead), follow-up duration, and melanoma-specific mortality.
Statistical Analysis
Continuous data were compared using Student t test for comparisons between NMs and SSMs. The mean of log-transformed MRs (geometric mean) of NM adjusted for age, sex, thickness, and ulceration were compared with those of SSM using linear regression.
Categorical clinical and histological characteristics associated with NM or SSM were investigated by exploratory analysis using the χ2 test or Fisher exact test. Multivariable logistic regression analysis was adjusted for Breslow thickness and for possible confounders. A stratified multiple logistic regression analysis of the characteristics of thin NM compared with thin SSM was conducted.
The prognostic role of the NM or SSM subtype with respect to melanoma-specific survival (MSS) was investigated by the Kaplan-Meier estimator used to calculate survival curves, and potential differences were evaluated using the log-rank test. Patient survival time was calculated as the time from the date of the primary tumor diagnosis to the date of melanoma-related death or last follow-up visit. Patients with an unknown cause of death or death not related to melanoma were censored. The Cox proportional hazards model was used for the analysis of MSS. The proportional hazard assumption was checked by the scaled Schoenfeld residuals. Because tumor spread is an intermediate variable between the effect of NM on survival, it was not included in the multivariate Cox model to avoid overadjustment bias (22), but a stratified analysis by tumor spread was carried out. A shared frailty model was used to assume that the proportional hazards assumption holds conditionally on an unobserved cluster center-specific random effect for the 17 centers (23). The Akaike Information Criterion and Bayesian Information Criterion were used as a measure of model fit.
All P values were two sided and the statistical significance level was less than .05. Analyses were carried out using STATA, version 13 (StataCorp 2013, Stata Statistical Software: Release 13. College Station, TX).
Results
Participating Centers and Included Cases
Data from 25 776 deidentified melanoma cases were obtained from 17 participating centers. To group the data by region, we pooled data from the 15 European centers (EADO centers) while keeping the data from the other 2 centers (United States and Australia) separate. Among the 21 025 melanomas that were of the NM or SSM subtype, 893 cases were excluded (exclusion reasons are given in Supplementary Table 1, available online).
Among the eligible 20 132 cases, complete data were available for gender, age, melanoma subtype, and Breslow thickness. MR was missing for 72.1% of EADO cases, and consequently analysis of this variable in the linear regression analysis of the geometric mean MR was restricted to those from MD Anderson and MIA Sydney. Mitosis present (yes or no) was also missing for 56.7% of EADO cases, but was included in all analyses. Apart from the absence of data on MR from EADO cases, there were no other systematic associations among cases with any missing data. For the survival analysis, 1759 cases were excluded because of missing follow-up data, leading to 18 373 eligible cases.
Characteristics of NM Compared With SSM
Among the 20 132 melanomas (EADO: 10 400, MIA/Sydney: 6109, MD Anderson/USA: 3623), there were 5062 NM (25.1%) and 15 070 SSM (74.9%). Characteristics of NM compared with SSM overall are presented in Table 1. At diagnosis, NM patients had a median age of 62.8 years (interquartile range [IQR] = 50.1–73.9) compared with 55.8 years (IQR = 44.1–67.1) for SSM patients (P < .001). The median Breslow thickness was statistically significantly higher for NM compared with SSM (3.1 mm [IQR = 2.0–5.2] vs 0.8 mm [IQR = 0.5–1.4], P < .001). An identified nevus remnant was present in statistically significantly fewer patients with NM compared with SSM (17.0% vs 36.0%, respectively, P < .001). The characteristics of NM compared to SSM were similar by participating region (EADO, Australia, United States, data not shown).
Table 1.
Clinical and histological characteristics of NM and SSM in overall cases (N = 20 132)
| Variable | NM No. (%) | SSM No. (%) | P |
|---|---|---|---|
| Total | 5062 (100.0) | 15 070 (100.0) | |
| Age, median (IQR), y | 62.8 (50.1−73.9) | 55.8 (44.1−67.1) | <.001* |
| Age, y | |||
| ≤50 | 1253 (24.8) | 5656 (37.5) | <.001† |
| >50 | 3809 (75.2) | 9414 (62.5) | |
| Sex | |||
| Male | 3051 (60.3) | 7880 (52.3) | <.001† |
| Female | 2011 (39.7) | 7190 (47.7) | |
| Anatomic site of melanoma | |||
| Head/neck | 1042 (20.8) | 1589 (10.7) | <.001† |
| Trunk | 1842 (36.8) | 6734 (45.2) | |
| Upper extremities | 1038 (20.7) | 2879 (19.3) | |
| Lower extremities | 1082 (21.6) | 3700 (24.8) | |
| Missing | 58 | 168 | |
| Breslow thickness, mm | |||
| ≤1.0 | 297 (5.9) | 9384 (62.3) | <.001† |
| 1.1–2.0 | 1082 (21.4) | 3484 (23.1) | |
| 2.1–4.0 | 1823 (36.0) | 1547 (10.3) | |
| >4.0 | 1860 (36.7) | 655 (4.3) | |
| Breslow thickness, median (IQR), mm | 3.1 (2.0, 5.2) | 0.8 (0.5, 1.4) | <.001* |
| Ulceration | |||
| Present | 2370 (49.0) | 1976 (13.7) | <.001† |
| Absent | 2464 (51.0) | 12 422 (86.3) | |
| Missing | 228 | 672 | |
| Regression | |||
| Present | 420 (9.7) | 3782 (28.1) | <.001† |
| Absent | 3918 (90.3) | 9657 (71.9) | |
| Missing | 724 | 1631 | |
| Nevus remnants | |||
| Present | 627 (17.0) | 4014 (36.0) | <.001† |
| Absent | 3051 (83.0) | 7140 (64.0) | |
| Missing | 1384 | 3916 | |
| Mitoses | |||
| Present | 3616 (94.9) | 6384 (63.9) | <.001† |
| Absent | 193 (5.1) | 3613 (36.1) | |
| Missing | 1253 | 5073 | |
| Tumor spread (initial diagnosis) | |||
| Localized | 3066 (69.8) | 11 802 (87.5) | <.001† |
| Regional metastasis | 1159 (26.4) | 1592 (11.8) | |
| Distant metastasis | 170 (3.9) | 98 (0.7) | |
| Missing | 667 | 1578 |
P value was calculated using a two-sided Mannã Whitney test. IQR = interquartile range; NM = nodular melanoma; SSM = superficial spreading melanoma.
†P value was calculated using a two-sided χ2 test.
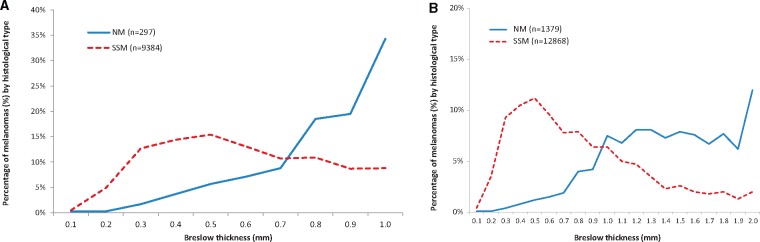
We examined the clinical, histological, and prognostic characteristics separately for T1 (≤1 mm), T2 (>1–2 mm), T3 (>2–4 mm), and T4 (>4 mm) melanomas (Supplementary Table 2, available online). T1 melanomas (n = 9681) consisted mainly of SSM (96.8%); only 3.2% were NM. T2 melanomas consisted of 76.3% SSM and 23.7% NM, whereas melanomas thicker than 2 mm consisted mainly of NM (62.6%) and fewer SSM (37.4%). Among T1 melanomas, there was a striking difference in the distribution of NM by increasing thickness up to 1.0 mm, with most T1 NM being 0.8 mm or thicker (72.4%) compared with SSM that were mostly less than 0.5 mm (Figure 1, A and B).
Figure 1.
Percentage of melanomas by nodular melanoma (NM) vs superficial spreading melanoma (SSM) histological subtype for increasing Breslow thickness by decimal point. A) In T1 melanomas. B) In T1 or T2 melanomas.
Multivariable logistic regression analysis of NM compared with SSM stratified for T1 and T2 thickness is presented in Table 2 and for T3 and T4 in Supplementary Table 3 (available online). T1 NM compared with T1 SSM was associated with presence on the head/neck (OR = 2.16, 95% CI = 1.27 to 3.69) and the presence of mitoses (OR = 1.97, 95% CI = 1.33 to 2.93), and T1 NM were less likely to have regression (OR = 0.46, 95% CI = 0.29 to 0.72) or nevus remnants present (OR = 0.60, 95% CI = 0.42 to 0.85). Similarly, for T2, T3, and T4 melanomas, NM compared with SSM was also associated with lack of regression and nevus remnants, and mitoses (present/absent) were not associated with NM compared with SSM among T3 and T4 melanomas. Regional metastasis was independently associated with the NM subtype for T1 melanomas only (OR = 1.77, 95% CI = 1.02 to 3.05). Similar associations were found for T1 and T2 in localized melanomas (Supplementary Table 4, available online).
Table 2.
Multivariable logistic regression analysis of the characteristics of NM compared with SSM stratified by T1 and T2 Breslow thickness*
| Variable | Breslow thickness |
|||
|---|---|---|---|---|
| T1: ≤1 mm (n = 4229) |
T2: 1.1–2.0 mm (n = 2232) |
|||
| OR (95% CI) | P † | OR (95% CI) | P † | |
| Sex | ||||
| Female | 1.00 (Ref) | 1.00 (Ref) | ||
| Male | 1.35 (0.95 to 1.91) | .09 | 1.16 (0.94 to 1.44) | .17 |
| Age, y | ||||
| ≤50 | 1.00 (Ref) | 1.00 (Ref) | ||
| >50 | 1.03 (0.73 to 1.46) | .85 | 1.06 (0.85 to 1.32) | .62 |
| Log Breslow thickness, mm | 13.06 (6.59 to 25.91) | <.001 | 7.74 (4.59 to 13.05) | <.001 |
| Anatomic site | ||||
| Lower extremities | 1.00 (Ref) | 1.00 (Ref) | ||
| Head/neck | 2.16 (1.27 to 3.69) | .005 | 1.42 (1.02 to 2.00) | .04 |
| Trunk | 1.14 (0.72 to 1.81) | .59 | 1.08 (0.82 to 1.42) | .60 |
| Upper extremities | 1.16 (0.71 to 1.91) | .56 | 1.56 (1.17 to 2.07) | .002 |
| Ulceration | ||||
| Absent | 1.00 (Ref) | 1.00 (Ref) | ||
| Present | 1.37 (0.75 to 2.49) | .31 | 1.15 (0.89 to 1.47) | .28 |
| Regression | ||||
| Absent | 1.00 (Ref) | 1.00 (Ref) | ||
| Present | 0.46 (0.29 to 0.72) | .001 | 0.45 (0.34 to 0.60) | <.001 |
| Nevus remnants | ||||
| Absent | 1.00 (Ref) | 1.00 (Ref) | ||
| Present | 0.60 (0.42 to 0.85) | .004 | 0.57 (0.45 to 0.71) | <.001 |
| Mitoses | ||||
| Absent | 1.00 (Ref) | 1.00 (Ref) | ||
| Present | 1.97 (1.33 to 2.93) | .001 | 2.43 (1.57 to 3.75) | <.001 |
| Tumor spread (initial diagnosis) | ||||
| Localized | 1.00 (Ref) | 1.00 (Ref) | ||
| Regional metastasis | 1.77 (1.02 to 3.05) | .04 | 0.78 (0.60 to 1.01) | .06 |
| Distant metastasis | 21.35 (4.94 to 92.25) | <.001 | 1.33 (0.52 to 3.36) | .55 |
Multivariable analysis adjusted for all variables included in this table. CI = confidence interval; NM = nodular melanoma; SSM = superficial spreading melanoma.
All statistical tests are two sided.
In linear regression analysis, the adjusted geometric mean MR was statistically significantly higher for NM compared with SSM for T1 (2.2 vs 1.6, respectively, P < .001), T2, and T3 melanomas but not for T4 tumors (Table 3). Similar results were shown when sensitivity analysis was restricted to localized melanomas (Supplementary Table 5, available online).
Table 3.
Mitotic rate for NM (median and adjusted geometric mean) compared with SSM stratified for T1, T2, T3, and T4 melanomas: normal regression analysis restricted to cases from MD Anderson and MIA/Sydney
| Breslow thickness | Total No. | Mitotic rate per mm2 |
||||||
|---|---|---|---|---|---|---|---|---|
| NM |
SSM |
P * , † | ||||||
| No. | GM* (95% CI) | Median (25th, 75th) | No. | GM* (95% CI) | Median (25th, 75th) | |||
| T1: ≤1.0 mm | 1776 | 142 | 2.2 (1.9 to 2.5) | 2.0 (1.0, 3.0) | 1634 | 1.6 (1.5 to 1.7) | 0.0 (0.0, 1.0) | <.001 |
| T2: 1.1–2.0 mm | 1964 | 563 | 3.2 (3.0 to 3.5) | 4.0 (2.0, 6.0) | 1401 | 2.6 (2.4 to 2.8) | 3.0 (1.0, 5.0) | <.001 |
| T3: 2.1–4.0 mm | 1654 | 921 | 4.6 (4.2 to 5.1) | 6.0 (3.0, 11.0) | 733 | 4.2 (3.8 to 4.7) | 5.0 (3.0, 10.0) | .03 |
| T4: >4.0 mm | 1164 | 891 | 6.2 (5.4 to 7.1) | 9.0 (5.0, 15.0) | 273 | 6.6 (5.6 to 7.7) | 10.0 (5.0, 16.0) | .28 |
Normal regression analysis adjusted for sex, age, and ulceration. CI = confidence interval; GM = geometric mean; MD Anderson = The University of Texas MD Anderson Cancer Center; MIA = Melanoma Institute Australia; NM = nodular melanoma; SSM = superficial spreading melanoma.
All statistical tests are two sided.
Survival Analysis of NM Compared With SSM
For the survival cohort (n = 18 373), the median follow-up was 32.1 months (IQR = 12.7–59.1) (NM: 29.9 [IQR = 13.4–56.1], SSM: 32.8 [IQR = 12.3–60.2]). The univariate five-year MSS rate was 75.4% for NM compared with 91.0% for SSM, respectively (P < .001) (Supplementary Table 6, available online). There were statistically significantly worse 5-year MSS rates for T1 NM compared with T1 SSM (88.5% vs 96.7%, P < .001) and for T2 NM compared with T2 SSM (P = .009), but not for T3 or T4 tumors (Supplementary Figure 1, A–C, available online). Focusing on T1 melanomas, the MSS rates were worse for NM compared with SSM for melanomas thinner than 0.8 mm (T1a AJCC eighth edition) as well as for melanomas 0.8–1.0 mm (T1b AJCC eighth edition) (Supplementary Figure 2, available online).
Multivariable Cox survival analysis adjusting for age, sex, Breslow thickness, and ulceration showed that the risk of melanoma-specific death was statistically significantly higher for the NM subtype compared with SSM in T1 (HR = 2.10, 95% CI = 1.24 to 3.56) and T2 melanomas (HR = 1.30, 95% CI = 1.01 to 1.68), with no statistically significant difference for T3 or T4 melanomas (Table 4). However, the statistically significant difference for T2 was lost after fitting a shared frailty model that accounted for unobserved center-specific heterogeneity. After considering center frailty, the Akaike Information Criterion/Bayesian Information Criterion values were lower, supporting a better model fit (Supplementary Table 7, available online). This analysis showed a statistically significant effect of the risk of NM vs SSM subtype for melanoma-specific death only for T1 melanomas (HR = 2.20, 95% CI = 1.28 to 3.78) (Table 4). Adjustment for time period at diagnosis (2006–2010 vs 2011–2015) to evaluate the potential effect of novel therapeutic agents in advanced melanoma showed similar results for NM vs SSM, so this variable was not included in the final parsimonious model (data not shown).
Table 4.
Cox proportional hazard models for the risk of death from melanoma for NM vs SSM stratified for T1, Τ2, Τ3, and T4 melanomas*
| Cox proportional hazard models | T1 |
T2 |
T3 |
T4 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | HR (95% CI) | P † | No. | HR (95% CI) | P † | No. | HR (95% CI) | P † | No. | HR (95% CI) | P † | |
| All cases | ||||||||||||
| Univariate | 8748 | 3.06 (1.88 to 4.96) | <.001 | 4170 | 1.37 (1.08 to 1.74) | .009 | 3135 | 0.89 (0.74 to 1.07) | .22 | 2320 | 0.99 (0.82 to 1.18) | .88 |
| Multivariable 1 (adjusted for age, sex, Breslow thickness) | 8748 | 2.36 (1.44 to 3.89) | .001 | 4170 | 1.30 (1.02 to 1.65) | .04 | 3135 | 0.88 (0.73 to 1.06) | .19 | 2320 | 0.93 (0.77 to 1.11) | .40 |
| Multivariable 2 (adjusted for age, sex, Breslow thickness, ulceration) | 8370 | 2.10 (1.24 to 3.56) | .006 | 3977 | 1.30 (1.01 to 1.68) | .04 | 3023 | 0.88 (0.73 to 1.06) | .17 | 2230 | 0.93 (0.77 to 1.12) | .44 |
| Multivariable 2 with frailty for center | 8370 | 2.20 (1.28 to 3.78) | .004 | 3977 | 1.23 (0.95 to 1.60) | .11 | 3023 | 0.84 (0.69 to 1.03) | .10 | 2230 | 0.96 (0.79 to 1.17) | .68 |
| Stratified: localized melanomas | ||||||||||||
| Univariate | 7391 | 2.76 (1.40 to 5.43) | .003 | 3039 | 1.21 (0.84 to 1.72) | .30 | 1938 | 0.79 (0.59 to 1.06) | .12 | 1095 | 0.93 (0.66 to 1.32) | .68 |
| Multivariable 1 (adjusted for age, sex, Breslow thickness) | 7391 | 1.98 (0.99 to 3.98) | .05 | 3039 | 1.09 (0.76 to 1.57) | .64 | 1938 | 0.80 (0.60 to 1.08) | .14 | 1095 | 0.86 (0.60 to 1.22) | .40 |
| Multivariable 2 (adjusted for age, sex, Breslow thickness, ulceration) | 7142 | 1.61 (0.77 to 3.38) | .20 | 2914 | 1.14 (0.78 to 1.67) | .50 | 1884 | 0.83 (0.62 to 1.13) | .24 | 1056 | 0.91 (0.63 to 1.30) | .60 |
| Multivariable 2 with frailty for center | 7142 | 1.57 (0.74 to 3.32) | .24 | 2914 | 1.02 (0.70 to 1.51) | .90 | 1884 | 0.80 (0.58 to 1.10) | .17 | 1056 | 1.09 (0.74 to 1.61) | .68 |
Median follow-up: T1, 32.6 months (IQR = 12.2–60.8); T2, 34.5 months (IQR = 14.9–62.4); T3, 33.1 months (IQR = 16.3–58.5); T4, 25.2 months (IQR = 12.4–48.4). CI = confidence interval; HR = hazard ratio; NM = nodular melanoma; SSM = superficial spreading melanoma.
All statistical tests are two sided.
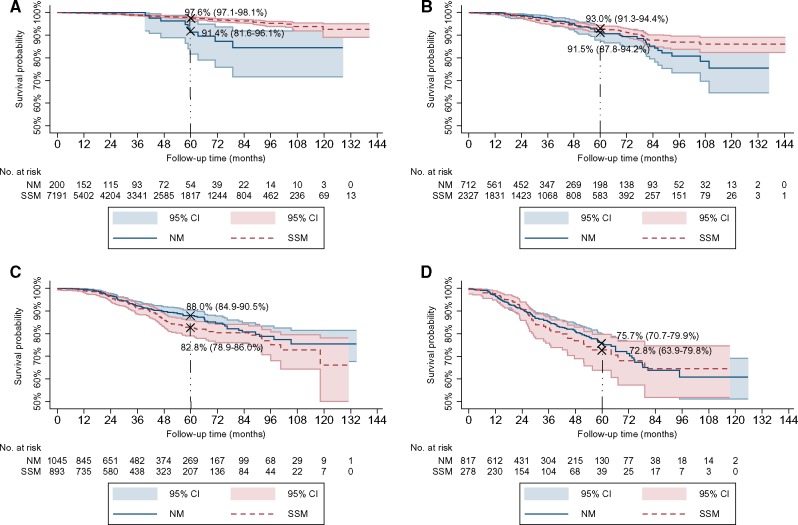
In stratified Kaplan-Meier analysis for localized melanomas, there were statistically significantly worse 5-year MSS rates for T1 NM compared with T1 SSM (91.4%, 95% CI = 81.6% to 96.1%] vs 97.6%, 95% CI = 97.1% to 98.1 %], P = .002), but not for T2, T3, and T4 melanomas (Figure 2, A–D). In multivariable Cox analysis stratified for tumor spread, there was no increased risk for melanoma-specific death for NM compared to SSM among each stratum for localized melanomas (OR = 1.08, 95% CI = 0.89 to 1.30) (Table 4) or cases with regional metastasis (OR = 1.11, 95% CI = 0.94 to 1.32) (data not shown).
Figure 2.
Kaplan–Meier plots for melanoma-specific survival (MSS) rates for nodular melanoma (NM) compared with superficial spreading melanoma (SSM) for localized melanomas. A) In T1 cases: statistically significantly worse MSS for NM vs SSM (P = .002). B) In T2 cases (P = .29). C) In T3 cases (P = .12). D) In T4 cases (P = .66). P values were calculated using a two-sided log-rank test. Shaded areas indicate 95% confidence intervals. Vertical bold-dashed line indicates 5-year rates.
Discussion
This large international multicenter study including more than 20 000 NM and SSM cases from participating centers in Europe, the United States, and Australia showed that NM is a distinct melanoma subtype with a constellation of aggressive biological characteristics that may confer worse prognosis, even for Τ1 (<1 mm) melanomas. Even though we focused on thin melanomas, we included all T tumors (T1–T4) in our analysis in order to explore the effect of Breslow thickness among different T categories.
Previous reports have documented distinct features of NM compared with SSM. NM occurs more frequently on the head/neck and lower extremities (24), has higher growth kinetics and MR (11, 25), has distinct features with dermoscopy (26) and in vivo reflectance confocal microscopy (27), and often has clinical characteristics that can make early detection difficult, including amelanosis, symmetry, and border regularity (11, 12, 24, 26, 28, 29). Compared with SSM, our study further showed that after adjusting for Breslow thickness, NMs were more likely to be ulcerated and less likely to have regression or be histologically associated with a nevus. The absence of histologic regression and of nevus remnants (the latter defining de novo melanomas) are considered high-risk characteristics of worse clinical outcome. A meta-analysis showed that regression was associated with a lower likelihood of having a positive sentinel lymph node (30) and was a protective factor for survival, likely due to an early activation of the host immune system against melanoma (31). In a prospective cohort study, de novo melanomas were associated more frequently with the NM subtype and had a worse overall survival vs nevus-associated melanomas (32), whereas there was no difference in survival in a retrospective study after multivariable adjustment (33).
With limited data on T1 NM in the literature, we focused our analysis on these tumors to study the clinicopathological profile of NM at an early phase of its evolution. Compared with T1 SSM, T1 NM was less likely to have regression or be histologically associated with a nevus, yet more likely to be located on the head/neck and have a higher MR. Although MR is not a staging criterion for T1 melanomas in the latest (eighth edition) melanoma staging system (20), the AJCC continues to consider MR an important prognostic factor for clinical care and strongly recommends that MR data continue to be collected (20, 34, 35). Furthermore, MR is a high-risk characteristic in Τ1 melanomas, associated with lymph node positivity (36) and worse disease-free survival (37). Herein, T1 NMs were associated with increased risk for regional metastasis compared with T1 SSMs, an effect that was not shown for thicker melanomas, suggesting the effect of the NM subtype on tumor spread during the early stages of melanoma evolution.
We found statistically significantly worse MSS for NM compared with SSM for overall cases at 5 years and a higher risk for melanoma-specific death associated with T1 NM, independent of age, sex, thickness, and ulceration. In multivariable Cox survival analysis stratified for tumor spread, the histologic NM vs SSM subtype did not confer increased risk for melanoma-specific death within each stratum, that is, among localized tumors or among cases with regional metastasis. There was a trend toward T1 NM lesions having worse prognosis compared with SSM when stratifying by localized melanomas. The lack of a statistically significant difference may be related to the relatively few T1 NMs. However, in the model stratified by regional metastasis, T1 NMs were no longer statistically significantly different from T1 SSMs. This is likely because it is offset by a model that includes tumor thickness and regional metastasis, of which SSMs are the dominant subtype.
The NM subtype was associated with a higher risk of death in a Victorian Cancer Registry study (1989–2004) (6) and in a SEER Registry study (1973–2012) of stage I–III melanomas (38). Our findings are consistent with previous evidence that NM independently increased the risk of death among 26 736 patients with T1 melanoma in an Australian population-based, prospective melanoma registry (17, 39). Interestingly, in the present study, the MSS rates for thin NMs compared with thin SSMs were worse both in T1a (<0.8 mm) and T1b (0.8–1.0 mm) melanomas, supporting the different behavior of NM even in the earliest phases of its evolution. These findings suggest that NM is a biologically distinct melanoma subtype at its outset, characterized by aggressive histological characteristics that influence clinical course and survival rates. Our results also emphasize the importance of studying thin NM to identify the early steps of its progression. The worse survival rates for T1 NM compared with T1 SSM and the striking finding that the majority of T1 NMs are greater than 0.8 mm in thickness underscore the effect of NM on overall mortality and imply that there is a potential benefit of aggressive screening for earlier detection of this subtype.
Limitations of our study include, first, its retrospective nature and center-based design. Second, central pathology review was not available, potentially leading to misclassification bias, even though the cases were from major melanoma centers using established definitions of NM and SSM. The fact that the proportion of NM to SSM patients was relatively similar between EADO cases vs those from MIA and MD Anderson mitigates this concern. Third, individual patient data on SLNB and pathological staging for T2 to T4 cases were not collected; however, nearly all centers confirmed that their practice was to perform SLNB for all T2 to T4 cases at an institutional level. Fourth, the median survival follow-up time was limited. Fifth, additional factors that might affect survival such as molecular characteristics and therapeutic interventions were not studied. However, localized melanomas were all treated with surgical excision, and including an adjustment for the time period in the survival models showed no differences. Also, a shared frailty model accounted for unobserved heterogeneity that possibly resulted, at least in part, from these factors. Strengths of our study include the large number of cases from referral centers in Europe, the United States, and Australia contributing to the largest combined database to date for the investigation of thin T1 NM.
Documenting the histopathological subtype of melanoma is recommended by some but not all international melanoma pathology reporting guidelines (40, 41). In contrast, others have emphasized the importance of documenting melanoma subtype in pathology reports because it allows clinicopathologic correlation to accurately classify melanoma (42, 43). The present study, involving a large patient population, showed that thin NMs do occur and can be diagnosed; are associated with features portending an aggressive clinical behavior; and have a prognostic significance among thin melanomas, independent of tumor thickness. We conclude that the NM subtype is a distinct, high-risk entity that should continue to be included in histopathological reporting and may be considered in the future prognostic classification of melanoma.
Funding
Τhe study was partially funded by the Institute of Dermatologic Research and Education.
CD performed this research implemented through the IKY scholarships program and co-financed by the European Union (European Social Fund [ESF]) and Greek national funds through the action entitled “Reinforcement of Postdoctoral Researchers,” in the framework of the Operational Program “Human Resources Development Program, Education and Lifelong Learning” of the National Strategic Reference Framework (NSRF) 2014–2020.
For JEG, this work was supported in part by the National Institutes of Health Specialized Program of Research Excellence (SPORE) melanoma grant no. P50 CA93459 (to The University of Texas MD Anderson Cancer Center); by a Melanoma Research Alliance Team Science Award; by the Robert and Lynne Grossman Family Foundation; and by the Michael and Patricia Booker Melanoma Research Endowment.
RAS is supported by an Australian NHMRC Fellowship. JFT is supported by The Melanoma Foundation of the University of Sydney. RAS and JFT are also supported by an Australian NHMRC Program Grant.
EL is funded by the National Cancer Institute through grants DP2CA225433 and R21CA212201 and the National Institute on Aging K76AG054631.
The Swiss Melanoma Register Project is partially funded by BMS and Novartis.
The research at the Melanoma Unit in Barcelona is partially funded by grants PI15/00716 and PI15/00956 from Fondo de Investigaciones Sanitarias, Spain; by the CIBER de Enfermedades Raras of the Instituto de Salud Carlos III, Spain; by the AGAUR 2014_SGR_603 of the Catalan Government, Spain; by the European Commission under the 6th Framework Programme, contract no. LSHC-CT-2006–018702 (GenoMEL), under the 7th Framework Programme (Diagnoptics); by the MARATÓ de TV3 Foundation; by the Asociación Española Contra el Cáncer AECC; and by the Leo Messi Foundation.
Notes
Affiliations of authors: 1st Department of Dermatology-Venereology, National and Kapodistrian University of Athens, Andreas Sygros Hospital, Athens, Greece (CD, AS, GGC, MP, AJS); Department of Hygiene and Epidemiology, University of Ioannina Medical School, Ioaninna, Greece (ND, EE); Department of Social and Behavioral Sciences, Harvard T.H. Chan School of Public Health, Boston, MA (ACG); Melanoma Institute Australia, The University of Sydney, Sydney, NSW, Australia (SL, JFT, RAS); Centre for Dermatooncology, Department of Dermatology, Eberhard Karls University, Tuebingen, Germany (UK, CG); Department of Surgical Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX (JEG, LEH); Department of Medical Sciences, Section of Dermatology, University of Turin, Turin, Italy (SR, PQ); Dermatology Department, Melanoma Unit, Hospital Clinic Barcelona, University of Barcelona, IDIBAPS, Barcelona, Spain (SP, JM); CIBERER, Instituto de Salud Carlos III, Barcelona, Spain (SP, JM); Department of Dermatology, Medical Faculty, Military Medical Academy, Belgrade, Serbia (LKS, TR); Department of Dermatology, Venereology and Allergology, Goethe-University Hospital, Frankfurt am Main, Germany (RK, LM); Departments of Dermatology and Pathology, Instituto Valenciano de Oncología, Valencia, Spain (EN, VT); Onco-Dermatology Department, CHU Nantes, CIC 1413, CRCINA, University Nantes, Nantes, France (BD, EV); Dermatology Department, Hospital Universitario Virgen Macarena, Seville, Spain (DMR); Department of Dermatology, University Hospital of Zurich, University of Zurich, Zurich, Switzerland (RD, JM); Department of Dermatology and Venerology, University Hospital of Schleswig-Holstein, Campus Kiel, Germany (AH, FE); Institute of Dermatology, Fondazione Policlinico Universitario A. Gemelli IRCCS – Catholic University, Rome, Italy (KP, LDR); Department of Oncologic Dermatology and Allergology, Elias University Hospital (AMF), and Department of Pathology, Colentina Hospital (SAZ), Carol Davila University of Medicine and Pharmacy, Bucharest, Romania; Coimbra Hospital and Universitary Centre, Coimbra, Portugal (RV, AB); Dermatology Clinic, Maggiore Hospital, University of Trieste, Trieste, Italy (IZ); Division of Dermatology and Venerology, Medical University of Graz, Graz, Austria (TD); Program for Clinical Research, Department of Dermatology, University of California, San Francisco, CA (EL); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (EE); Sydney Medical School, The University of Sydney, Sydney, Australia (JFT, RAS); Royal Prince Alfred Hospital, Camperdown, Sydney, Australia (JFT, RAS).
The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
The authors have no conflicts of interest to disclose related to this study.
KP and LDR gratefully acknowledge support from colleagues at Institute of Dermatology, Fondazione Policlinico Universitario A. Gemelli IRCCS – Catholic University (Rome, Italy). SP and JM acknowledge the members of the Skin Cancer Unit at the Hospital Clinic of Barcelona for their work in the management of melanoma patients and collection of data at this center (Barcelona, Spain). JFT and RAS gratefully acknowledge support from colleagues at MIA and Royal Prince Alfred Hospital (Sydney, Australia).
Supplementary Material
References
- 1. Whiteman DC, Green AC, Olsen CM.. The growing burden of invasive melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136(6):1161–1171. [DOI] [PubMed] [Google Scholar]
- 2. Aitken JF, Youlden DR, Baade PD, et al. Generational shift in melanoma incidence and mortality in Queensland, Australia, 1995–2014. Int J Cancer. 2018;142(8):1528–1535. [DOI] [PubMed] [Google Scholar]
- 3. Shaikh WR, Dusza SW, Weinstock MA, et al. Melanoma thickness and survival trends in the United States, 1989 to 2009 . J Natl Cancer Inst. 2016;108(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sacchetto L, Zanetti R, Comber H, et al. Trends in incidence of thick, thin and in situ melanoma in Europe. Eur J Cancer. 2018;92:108–118. [DOI] [PubMed] [Google Scholar]
- 5. Minini R, Rohrmann S, Braun R, et al. Incidence trends and clinical-pathological characteristics of invasive cutaneous melanoma from 1980 to 2010 in the Canton of Zurich, Switzerland. Melanoma Res. 2017;27(2):145–151. [DOI] [PubMed] [Google Scholar]
- 6. Mar V, Roberts H, Wolfe R, et al. Nodular melanoma: a distinct clinical entity and the largest contributor to melanoma deaths in Victoria, Australia. J Am Acad Dermatol. 2013;68(4):568–575. [DOI] [PubMed] [Google Scholar]
- 7. Teramoto Y, Keim U, Gesierich A, et al. Acral lentiginous melanoma: a skin cancer with unfavourable prognostic features. A study of the German Central Malignant Melanoma Registry (CMMR) in 2050 patients. Br J Dermatol. 2018;178(2):443–451. [DOI] [PubMed] [Google Scholar]
- 8. Brunssen A, Jansen L, Eisemann N, et al. Long-term relative survival from melanoma in Germany 1997–2013. J Am Acad Dermatol. 2019;80(4):938–946. [Google Scholar]
- 9. Demierre MF, Chung C, Miller DR, et al. Early detection of thick melanomas in the United States: beware of the nodular subtype. Arch Dermatol. 2005;141(6):745–750. [DOI] [PubMed] [Google Scholar]
- 10. Shaikh WR, Xiong M, Weinstock MA.. The contribution of nodular subtype to melanoma mortality in the United States, 1978 to 2007. Arch Dermatol. 2012;148(1):30–36. [DOI] [PubMed] [Google Scholar]
- 11. Liu W, Dowling JP, Murray WK, et al. Rate of growth in melanomas: characteristics and associations of rapidly growing melanomas. Arch Dermatol. 2006;142(12):1551–1558. [DOI] [PubMed] [Google Scholar]
- 12. Dessinioti C, Geller AC, Stergiopoulou A. , et al. Association of skin examination behaviors and thinner nodular vs superficial spreading melanoma at diagnosis. JAMA Dermatol. 2018;154(5):544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Whiteman DC, Baade PD, Olsen CM.. More people die from thin melanomas (1 mm) than from thick melanomas (>4 mm) in Queensland, Australia. J Invest Dermatol. 2015;135(4):1190–1193. [DOI] [PubMed] [Google Scholar]
- 14. Elder DE. Thin melanoma. Arch Pathol Lab Med. 2011;135(3):342–346. [DOI] [PubMed] [Google Scholar]
- 15. Tas F, Erturk K.. Scalp melanoma is associated with high mitotic rate and is a poor prognostic factor for recurrence and outcome. Melanoma Res. 2017;27(4):387–390. [DOI] [PubMed] [Google Scholar]
- 16. Maurichi A, Miceli R, Camerini T, et al. Prediction of survival in patients with thin melanoma: results from a multi-institution study. J Clin Oncol. 2014;32(23):2479–2485. [DOI] [PubMed] [Google Scholar]
- 17. Green AC, Baade P, Coory M, et al. Population-based 20-year survival among people diagnosed with thin melanomas in Queensland, Australia. J Clin Oncol. 2012;30(13):1462–1467. [DOI] [PubMed] [Google Scholar]
- 18. Green AC, Viros A, Hughes MC, et al. Nodular melanoma: a histopathologic entity? Acta Derm Venereol. 2018;98(4):460–462. [DOI] [PubMed] [Google Scholar]
- 19. Clark WH Jr, From L, Bernardino EA, et al. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969;29(3):705–727. [PubMed] [Google Scholar]
- 20. Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ribero S, Osella-Abate S, Sanlorenzo M, et al. Sentinel lymph node biopsy in thick-melanoma patients (n=350): what is its prognostic role? Ann Surg Oncol. 2015;22(6):1967–1973. [DOI] [PubMed] [Google Scholar]
- 22. Schisterman EF, Cole SR, Platt RW.. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20(4):488–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Austin PC. A tutorial on multilevel survival analysis: methods, models and applications. Int Stat Rev. 2017;85(2):185–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chamberlain AJ, Fritschi L, Giles GG, et al. Nodular type and older age as the most significant associations of thick melanoma in Victoria, Australia. Arch Dermatol. 2002;138(5):609–614. [DOI] [PubMed] [Google Scholar]
- 25. Shen S, Wolfe R, McLean CA, et al. Characteristics and associations of high-mitotic-rate melanoma. JAMA Dermatol. 2014;150(10):1048–1055. [DOI] [PubMed] [Google Scholar]
- 26. Kalkhoran S, Milne O, Zalaudek I, et al. Historical, clinical, and dermoscopic characteristics of thin nodular melanoma. Arch Dermatol. 2010;146(3):311–318. [DOI] [PubMed] [Google Scholar]
- 27. Segura S, Pellacani G, Puig S, et al. In vivo microscopic features of nodular melanomas: dermoscopy, confocal microscopy, and histopathologic correlates. Arch Dermatol. 2008;144(10):1311–1320. [DOI] [PubMed] [Google Scholar]
- 28. Chamberlain AJ, Fritschi L, Kelly JW.. Nodular melanoma: patients' perceptions of presenting features and implications for earlier detection. J Am Acad Dermatol. 2003;48(5):694–701. [DOI] [PubMed] [Google Scholar]
- 29. Greenwald HS, Friedman EB, Osman I.. Superficial spreading and nodular melanoma are distinct biological entities: a challenge to the linear progression model. Melanoma Res. 2012;22(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ribero S, Gualano MR, Osella-Abate S, et al. Association of histologic regression in primary melanoma with sentinel lymph node status: a systematic review and meta-analysis. JAMA Dermatol. 2015;151(12):1301–1307. [DOI] [PubMed] [Google Scholar]
- 31. Gualano MR, Osella-Abate S, Scaioli G, et al. Prognostic role of histological regression in primary cutaneous melanoma: a systematic review and meta-analysis. Br J Dermatol. 2018;178(2):357–362. [DOI] [PubMed] [Google Scholar]
- 32. Cymerman RM, Shao Y, Wang K, et al. De novo vs nevus-associated melanomas: differences in associations with prognostic indicators and survival. J Natl Cancer Inst. 2016;108(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin-Gorgojo A, Requena C, Garcia-Casado Z, et al. Dysplastic vs. common naevus-associated vs. de novo melanomas: an observational retrospective study of 1, 021 patients. Acta Derm Venereol. 2018;98(6):556–562. [DOI] [PubMed] [Google Scholar]
- 34. Gershenwald JE, Scolyer RA.. Melanoma staging: American Joint Committee on Cancer (AJCC) 8th edition and beyond. Ann Surg Oncol. 2018;25(8):2105–2110. [DOI] [PubMed] [Google Scholar]
- 35. Scolyer GJ, R.A Hess KR, et al. Melanoma of the Skin In: Amin MB, Edge S, Greene F , et al. , eds. AJCC Cancer Staging Manual. Switzerland: Springer; 2017:563–585. [Google Scholar]
- 36. Wheless L, Isom CA, Hooks MA, et al. Mitotic rate is associated with positive lymph nodes in patients with thin melanomas. J Am Acad Dermatol. 2018;78(5):935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. von Schuckmann LA, Celia BHM, Lee R, et al. Survival of patients with early invasive melanoma down-staged under the new eighth AJCC edition. J Am Acad Dermatol. 2019;80(1):272–274. [DOI] [PubMed] [Google Scholar]
- 38. Lattanzi M, Lee Y, Simpson D, et al. Primary melanoma histologic subtype: impact on survival and response to therapy. J Natl Cancer Inst. 2019;111(2):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Balch CM, Balch GC, Sharma RR.. Identifying early melanomas at higher risk for metastases. J Clin Oncol. 2012;30(13):1406–1407. [DOI] [PubMed] [Google Scholar]
- 40. Scolyer RA, Judge MJ, Evans A, et al. Data set for pathology reporting of cutaneous invasive melanoma: recommendations from the International Collaboration on Cancer Reporting (ICCR). Am J Surg Pathol. 2013;37(12):1797–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.College of American Pathologists. Protocol for the examination of specimens from patients with melanoma of the skin http://www.cap.org/ShowProperty? nodePath=/UCMCon/ContributionFolders/WebContent/pdf/cp-skin-melanoma-17protocol-4010.pdf. Accessed October 12, 2018.
- 42. Scolyer RA, Long GV, Thompson JF.. Evolving concepts in melanoma classification and their relevance to multidisciplinary melanoma patient care. Mol Oncol. 2011;5(2):124–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kelly JW, Chamberlain AJ, Staples MP, et al. Nodular melanoma. No longer as simple as ABC. Aust Fam Physician. 2003;32(9):706–709. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.